Translate this page into:
Dysregulation of gene expression of PTEN and AKT signaling pathway in patients of ovarian cancer: A pilot study
⁎Corresponding author. sabakhaliq@uhs.edu.pk (Saba Khaliq)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
PI3K/AKT/mTOR signaling pathway has a crucial role in chemo- and radiotherapy resistance, and tumorigenesis of ovarian cancer. Some of the genes of this pathway are also linked with progression of disease in association of different miRNAs. The main objective of the current research was to determine the level of mRNA expression of different genes along with their respective microRNA (miRNAs) in ovarian cancer and apparently healthy women.
Methods
Samples from 46 ovarian cancer and 46 healthy females were obtained from a local cancer hospital. mRNA and miRNAs were extracted and gene expression of mRNA and miRNA were analyzed by qPCR. All data was analyzed using SPSS version 22 and CFX96 manager software for comparative gene analysis.
Results
The relative mRNA expression levels of AKT, PI3K, PDK1, NFkB, and mTOR were high (2.14, 1.93, 1.93, 3.31, and 1.82 folds, respectively) as compared to healthy controls, whereas expression of PTEN was down-regulated (0.10-fold) in patients. There was a significant difference (p = 0.0019) in serum levels of phosphorylated-Akt in patients and controls. The expression of miR-29b was up-regulated in patients of ovarian cancer.
Conclusion
Gene expression of PTEN/AKT pathway is deregulated in ovarian cancer patients. Moreover, the phosphorylated level of AKT, expression of mTOR or miRNAs may be a marker for the disease.
Keywords
Ovarian cancer (OC)
miRNA (micro RNA)
Phosphoinositide3-kinase (PI3K)
Phosphatase and tensin (PTEN)
Pyrunate deghydrogenase kinase 1(PDK1)
1 Introduction
Globally ovarian cancer (OC) is the seventh most common type of cancer among women (Reid and Sellers, 2017). This is the deadly tumor of female reproductive system and ranked second as the most frequent gynecological malignancy in females (Ranjbar et al. 2015). It has poor diagnosis with a high mortality rate (Coburn et al., 2017). Distinct geographic areas have a variable frequency of OC i.e., low incidences observed in Asian countries whereas Western Europe and North America showed a high frequency of OC (Holschneider and Berek, 2000). Among women 184,799 deaths were recorded due to ovarian cancer in 2018 (Bray et al., 2018). On the basis of grade and physiology, OC is categorized into 2 different types; one is indolent and 2nd is vigorous. Type 1 include mucinous, clear cell, low grade serous, and endometrioid cancers. On the other hand, type 2 includes carcinosarcomas,undifferentiated cancers, and high-grade serous (Jayson et al., 2014).
Cellular processes like metabolism, transcription, translation, growth, and proliferation have involvement of PI3K/AKT/mTOR signaling pathway (Carnero et al., 2008). Different genetic or epigenetic changes within this pathway may lead to different kinds of human cancer (Hennessy et al., 2005). Tumor formation, invasion, and cancer cell migration all are linked with PI3K signaling pathway (Li et al., 2014). PI3K/AKT/mTOR signaling pathway also has a crucial role in chemo and radiotherapy resistance and tumorigenesis of OC (Dobbin et al., 2013; Gasparri et al., 2017). Various cellular pathways are regulated directly/indirectly by the AKT protein that has a role in cell cycle progression, cellular growth, and survival (Vara et al., 2004). In high-grade serous ovarian carcinomas, AKT pathway is activated along with down-regulation of PTEN gene expression (Altomare et al., 2004; Stocker et al., 2002). mTOR has a vital role in cell growth and differentiation, it is responsible for any pathological anomaly and progression of different cancers like prostate, liver, and breast (Saxton and Sabatini, 2017).
Besides mRNAs, both normal and pathologic states of any cell/tissue are regulated by some key molecular component such as microRNA (miRNA) (Ebert and Sharp, 2012). miRNA are almost 22-nucleotides long noncoding RNAs with a role in post-transcriptional gene regulation. The miRNA binds with the 3‘untranslated region (UTR) of target mRNA and regulates gene expression negatively (Lewis et al., 2003). The invasion, metastasis, and manifestation of cancer correlate with circulating miRNA (Shen et al., 2013). Expression of different miRNAs was dysregulated in OC tissues (Banno et al., 2014). Therefore, this study was aimed to analyze the expression levels of genes and circulating miRNAs in OC patients.
2 Materials and methods
2.1 Study subjects
Patients of ovarian cancer were recruited from the INMOL Cancer Hospital Lahore after approval from Ethical review board of University of Health Sciences, Lahore (UHS/REG-17/ERC/4659). This study population comprised of 46 ovarian cancer and 46 healthy females. Inclusion criteria for cases included diagnosed cases of ovarian cancer of age group 20–70 years, and patients tentatively planned for any of the surgical procedure (unilateral/bilateral) i.e., Oophorectomy, Salpingo oophorectomy either by laparotomy or laproscopically, subtotal resection or removal of the tumor in fragments, and Hysterectomy with salpingo-Oophorectomy. The patients undergoing chemotherapy were excluded from the study. Healthy females (20–70 years) with no record of any cancer were included in the study as controls. Healthy women who had previously undergone any abdominal surgery, family history of any cancer, or taking any hormonal treatment for any reason were excluded from the study.
2.2 mRNA and miRNA extraction
Three (3) ml of venous blood from each patient was drawn with a sterile 5 ml syringe and collected in a tube for RNA extraction. Samples were transported to the laboratory in an icebox within 3 h of sample collection. Total mRNA and miRNA were extracted using mirVana™ miRNA Isolation Kit (Catalog number: AM1561, Ambion). The kit was used to isolate total RNA and miRNA from WBCs and Plasma according to the protocol given by the manufacturer, respectively. This kit is capable of extracting both RNA and miRNA from the given sample which was eluted in 50 μl of nuclease-free water. The quantity of mRNA and miRNA was checked using Nanodrop 2000 (ND-2000 by Thermo Scientific) and the purity was assessed by the A260/A280 value. cDNA was synthesized using 1 µg of total mRNA via Revert Aid First Strand cDNA synthesis kit (Thermo scientific, USA) following the manufacturer’s instructions. cDNA from miRNA was synthesized using miScript II RT Kit (Qiagen) according to the instructions. The cDNA of both mRNA and miRNA was stored at −20 °C.
2.3 Expression analysis
Real-time PCR CFX 96 (Biorad,USA) was used for the analysis of gene expression of selected genes in controls and cases. The reaction mixture of 25 µl contained 12.5 µl of 2X SYBR Green Real-Time PCR master-mix, 1 µl of forward and reverse primers, RNAse-free water, and 200 ng cDNA for gene expression, and 20 ng for miRNA quantification. Each sample was analyzed in duplicate along with three housekeeping genes (HKGs). The fold change in gene expression was calculated using the ddCt method with an average of HKGs (18S rRNA, b-actin, and GAPDH) as the internal control for mRNA expressions and U6 for miRNA expression (Tables 1 and 2).
Gene
Forward primer
Reverse primer
AKT
TCTATGGCGCTGAGATTGTG
CTTAATGTGCCCGTCCTTGT
PTEN
CGACGGGAAGACAAGTTCAT
AGGTTTCCTCTGGTCCTGGT
mTOR
CCTGCCACTGAGAGATGACA
TCCGGCTGCTGTAGCTTATT
PI3K
TGGATGCTCTACAGGGCTTT
GTCTGGGTTCTCCCAATTCA
NFκB
TACTCTGGCGCAGAAATTAGGTC
CTGTCTCGGAGCTCGTCTATTTG
PDK1
CTGTGATACGGATCAGAAACCG
TCCACCAAACAATAAAGAGTGCT
GAPDH
ACGGATTTGGTCGTATTGGG
CGCTCCTGGAAGATGGTGAT
βactin
TCCACCTTCCAGCAGATGTG
GCATTTGCGGTGGACGAT
18 s
AGAAACGGCTACCACATCCAA
CCTGTATTGTTATTTTTCGTCACTACCT
Gene
Primer
U6-F
5′-CTC GCT TCG GCA CA-3′
U6-R
5′-AAC GCT TCA CGA ATT TGC GT-3′
Uni-R
5′GTG CAG GGT CCG AGG T-3′
Mi29b-1-5p
5′-GGG CTG GTT TCA TAT G-3′
Mi21-5p
5′-GTT TGG TAG CTT ATC AGA CTG-3′
Phosphorylated Akt (p-Akt) was measured using AKT [pS473] ELISA Kit (Invitrogen), which is a solid-phase sandwich Enzyme-Linked Immunosorbent Assay (ELISA). This assay is designed to detect and quantify the level of AKT protein that is phosphorylated at serine residue 473 in cell lysates. The ELISA was performed according to the manufacturer’s instructions in duplicate samples.
2.4 Statistical analysis
SPSS (22.0 version) was used for RT-PCR data evaluation. CFX96 manager software performed the comparative gene analysis. Standard error and the significance level were calculated. Normally distributed quantitative variables are presented with Mean ± SD, whereas interquartile range is given for non-normally distributed quantitative variables. A paired t-test was used to determine the significance of differential mRNA and miRNA expression between controls and ovarian cancer samples obtained by qRT-PCR. The relation between multiple groups was calculated with the aid of Annova. A p-vaue < 0.05 was considered statistically significant.
3 Results
Out of the total 46 patients, maximum patients (21 %) were diagnosed with serous carcinoma, whereas only 2 (4 %) patients were diagnosed with Clear cell carcinoma. Majority of patients enrolled in this study were at II and III TNM stages (41 % and 32 %, respectively) (Table 3). Gene expression of PI3K signaling pathway was analyzed as a fold change in both control and ovarian cancer patient group. mRNA expression of AKT gene was 2.14-fold high and PTEN was low with 0.10 folds in cases. mTOR and NF-κB mRNA expression were high as 1.82 and 3.31-folds, respectively, in ovarian cancer patients. PDK1 and PI3K expression was high in ovarian cancer patients with 1.93 folds. The p-value < 0.001 for all results showed that results were highly significant (Fig. 1).
Parameter
Groups
N (%)
Age at diagnosis
<40 years
30 (65 %)
>40 years
16 (34 %)
TNM Stages
I
5(10 %)
II
19 (41 %)
III
15(32 %)
IV
7(15 %)
Histological Tumor Type
Adeno
4 (8 %)
Endometrioid adeno
4 (8 %)
Serous
10 (21 %)
Borderline serous
6 (13 %)
Papillary serous
5 (10 %)
Mucinous
5 (10 %)
Cyto-ademas
4 (8 %)
Endometrioid
6(13 %)
Clear cell
2 (4 %)
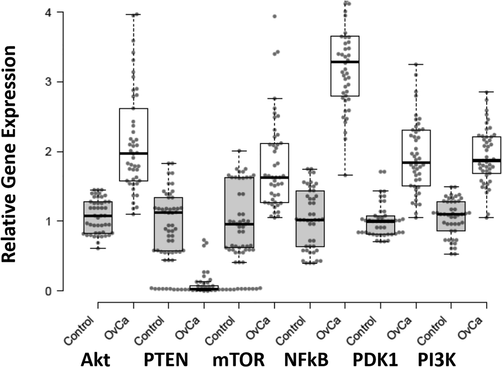
Expression pattern of AKT, PTEN, mTOR, NFkB, PDK1, and PI3K in Ovarian Cancer and healthy control samples. X-axis presenting the target genes along with control and patients’ group. Y-axis is presenting the fold increase or decrease in expression. Error bars indicating the standard deviation from the mean.
In the studied population a left tail distribution of p-Akt was observed ranging from 0 to 1.02U/mg total protein, with a median of 0.38U/mg. In cancer patients, 72 % of population showed > 0.51U/mg and 28 % population showed lower levels of p-Akt, with a median of 0.63U/mg. The p-Akt levels in healthy controls were < 0.36U/mg with median of 0.21U/mg. P-value after student’s t-test was 0.0019 which is statistically significant. miR-29b was up-regulated in patients with fold 1.36. The p-value < 0.001 showed that the results were highly significant (Fig. 2). The expression of miR-21 which targets PTEN gene was also checked but there was no significant difference in expression of this miRNA in both patients and healthy controls.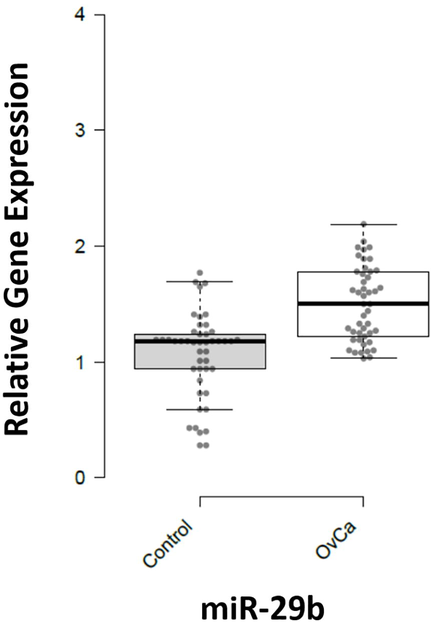
Expression pattern of miR-29b in Ovarian Cancer and healthy controls. X-axis presenting the miRNAs along with the control and patients’ group. Y-axis is presenting the fold increase or decrease in expression. Error bars indicating the standard deviation from the mean.
4 Discussion
PTEN/PI3K/AKT pathway has important role in propagation of human cancer. Poor diagnosis of this cancer has been linked with aberrant expression of this pathway (Gutierrez et al., 2009). In Ovarian cancer cases low expression of PTEN is also observed (Cai et al., 2014). It was reported that low level of PTEN was associated with high level of pAKT (Kurose et al., 2001). In current research, expression of PI3K, AKT, and PTEN were examined in 46 patients and healthy controls. PI3K, AKT and pAKT expression was upregulated in patients. A High frequency of changes/variations in the PI3K pathway in different gynecological cancers are reported in different studies (Courtney et al., 2010). High expression of PI3K was connected with poor survival in epithelial ovarian cancer patients. Central node of PI3K pathway is AKT gene (Liu et al., 2009). All the isomers of this gene have crucial role in different cancers. Many studies revealed that HGSC exhibited high expression of AKT and p-AKT (Yuan et al., 2000; Carpten et al., 2007). AKT and pAKT expression was high in patient, and low expression of PTEN gene was observed in patients. The p < 0.001 proved that results were highly significant.
PDK1/AKT/mTOR expression was up regulated in ovarian cancer patients (Peng et al., 2010). This complex is involved in cell growth and death. High level of PDK1 expression is exhibited in distinct grades of OC. Serous Ovarian carcinoma had elevated expression of PDK1 (Lohneis et al., 2015). Current investigation showed high level of PDK1 in ovarian cancer patients. mTOR have role in cellular survival and proliferation (Mabuchi et al., 2015). Almost 55 % of epithelial ovarian cancer showed hyperactivity of mTORC1 complex. Many cancer models proved activity of mTOR in different types of ovarian cancer (Liu et al., 2018). mTOR expression was also high in our study.
High percentage of death has been perceived due to PI3K and NFĸB crosstalk (Annunziata et al., 2010). p50 and p65 subunits of NFĸB presented high ratio in EOC. Tumor growth can be increased by the NFĸB signaling in ovarian cancer. Chemo-resistance were connected with expression level of this gene. Poor diagnosis of this cancer has been observed due to the elevated frequency of this nuclear factor gene (Giopanou et al., 2014). PTEN low expression is linked with high expression level of NFĸB, that is another aspect of chemo-resistance in ovarian cancer (Lili and Wang, 2015). Our results showed high expression of NFĸB with low expression of PTEN.
miRNAs have an important part in development of OC proved by many studies (Chong et al., 2015). miR-19b suppressed the expression of PTEN. High expression of miR-19b has been detected in many cases. PTEN/AKT pathway could improve by the miR-19b (Dan-Tong, 2018). miR-205-5p is involved in inhibition of PTEN and regulation of AKT. This miRNA may have role in cisplatin-resistance of these cancerous cells (Shi et al., 2018). Expression level of PTEN decreased due to overexpression of miR-216a. Phosphorylation of AKT changed according to the suppression of PTEN which prove that miR-216a could elevate the epithelia mesenchymal transition of ovarian cancer (Xiaoxue et al., 2017).
miR-29b have role in apoptosis, cancer proliferation and cell cycle control (Guo et al., 2010). Our results showed high expression of miR-29b in ovarian cancer patients as compared to healthy controls. miR-21 target the PTEN negatively in epithelial ovarian cancer. miR-21 may have role in chemotherapy of epithelial ovarian cancer. (Yu et al., 2017). In our case miR-21 expression remained undifferentiated in both groups.
Late diagnosis is the key factor for the increasing death rate due to ovarian cancer. Better understanding of PI3K signaling pathway may improve the diagnosis of ovarian cancer. Phosphorylated level of AKT, expression of mTOR or miRNAs may be used as biomarker for this cancer.
5 Statement of ethics
An informed written consent was obtained with all the relevant details such as age, clinical findings, and therapeutic history in the structured questionnaire after approval from the institutional ethical review committee (UHS/REG-17/ERC/4659).
Acknowledgements
Statements made herein are solely the responsibility of the authors. We also acknowledge the support provided by Higher Education Commission of Pakistan through the Problem-based Applied Interdisciplinary Research Program Grant (PBAIRP) # 22-04.
Author contribution
LZ contributed towards literature survey, sampling, extraction, expression analysis and first draft of manuscript write up. RH helped in literature research, sample collection and data analysis. HU carried out lab analysis. NN helped in histological identification of patient data, and manuscript write up. MSM help in sample collection, data analysis and write-up of first draft of manuscript. SK and UAA conceived the idea, guide the experimental design and lab work, statistically analyzed the data, and proof read the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 2004;23(34):5853-5857.
- [Google Scholar]
- Nuclear factor κB transcription factors are coexpressed and convey a poor outcome in ovarian cancer. Cancer. 2010;116(13):3276-3284.
- [Google Scholar]
- Application of microRNA in diagnosis and treatment of ovarian cancer. Biomed Res. Int.. 2014;2014:1-6.
- [Google Scholar]
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.. 2018;68(6):394-424.
- [Google Scholar]
- The role of the PTEN/PI3K/Akt pathway on prognosis in epithelial ovarian cancer: a meta-analysis. Oncologist. 2014;19(5):528-535.
- [Google Scholar]
- The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets. 2008;8:187-198.
- [Google Scholar]
- A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448(7152):439-444.
- [Google Scholar]
- Differential microRNA expression profiles in primary and recurrent epithelial ovarian cancer. Anticancer Res.. 2015;35:2611-2617.
- [Google Scholar]
- International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int. J. Cancer. 2017;140(11):2451-2460.
- [Google Scholar]
- The PI3K pathway as drug target in human cancer. J. Clin. Oncol.. 2010;28(6):1075-1083.
- [Google Scholar]
- MicroRNA-19b promotes the migration and invasion of ovarian cancer cells by inhibiting the PTEN/AKT signaling pathway. Oncol. Lett.. 2018;16:559-565.
- [Google Scholar]
- The importance of the PI3K/AKT/MTOR pathway in the progression of ovarian cancer. Int. J. Mol. Sci.. 2013;14(4):8213-8227.
- [Google Scholar]
- Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149(3):515-524.
- [Google Scholar]
- PI3K/AKT/mTOR pathway in ovarian cancer treatment: are we on the right track? Geburtshilfe Frauenheilkd.. 2017;77(10):1095-1103.
- [Google Scholar]
- Metadherin, p50, and p65 expression in epithelial ovarian neoplasms: an immunohistochemical study. Biomed Res. Int.. 2014;2014:1-8.
- [Google Scholar]
- Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835-840.
- [Google Scholar]
- High frequency of PTEN, PI3K, and AKT abnormalities in T-cell acute lymphoblastic leukemia. Blood. 2009;114(3):647-650.
- [Google Scholar]
- Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discovery. 2005;4(12):988-1004.
- [Google Scholar]
- Ovarian cancer: epidemiology, biology, and prognostic factors. Semin. Surg. Oncol.. 2000;19(1):3-10.
- [Google Scholar]
- Frequent loss of PTEN expression is linked to elevated phosphorylated Akt levels, but not associated with p27 and cyclin D1 expression, in primary epithelial ovarian carcinomas. Am. J. Pathol.. 2001;158(6):2097-2106.
- [Google Scholar]
- PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Arch. Gynecol. Obstet.. 2014;290(6):1067-1078.
- [Google Scholar]
- Expression of NF-κB and PTEN in primary epithelial ovarian carcinoma and the correlation with chemoresistance. Int. J. Clin. Exp. Path.. 2015;8:10953.
- [Google Scholar]
- Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov.. 2009;8(8):627-644.
- [Google Scholar]
- The role of mTOR in ovarian Neoplasms, polycystic ovary syndrome, and ovarian aging. Clin. Anat.. 2018;31(6):891-898.
- [Google Scholar]
- PDK1 is expressed in ovarian serous carcinoma and correlates with improved survival in high-grade tumors. Anticancer Res.. 2015;35:6329-6334.
- [Google Scholar]
- The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol. Oncol.. 2015;137(1):173-179.
- [Google Scholar]
- Role of the Akt/mTOR survival pathway in cisplatin resistance in ovarian cancer cells. Biochem. Biophys. Res. Commun.. 2010;394(3):600-605.
- [Google Scholar]
- R. Ranjbar F. Nejatollahi A.S. Nedaei Ahmadi H. Hafezi A. Safaie Expression of Vascular Endothelial Growth Factor (VEGF) and Epidermal Growth Factor Receptor (EGFR) in Patients With Serous Ovarian Carcinoma and Their Clinical Significance Iran J Cancer Preven 8 4.
- Reid and Sellers. (2017) Epidemiology of ovarian cancer: a review. Cancer biology & medicine 14: 9.
- MicroRNAs as potential biomarkers in human solid tumors. Cancer Lett.. 2013;329(2):125-136.
- [Google Scholar]
- miR-205-5p mediated downregulation of PTEN contributes to cisplatin resistance in C13K human ovarian cancer cells. Front. Genet.. 2018;9
- [Google Scholar]
- Living with lethal PIP3 levels: viability of flies lacking PTEN restored by a PH domain mutation in Akt/PKB. Science. 2002;295(5562):2088-2091.
- [Google Scholar]
- MicroRNA-216a promotes the metastasis and epithelial–mesenchymal transition of ovarian cancer by suppressing the PTEN/AKT pathway. Oncol Targets Ther.. 2017;10:2701.
- [Google Scholar]
- miRNA-21 enhances chemoresistance to cisplatin in epithelial ovarian cancer by negatively regulating PTEN. Oncol. Lett.. 2017;14(2):1807-1810.
- [Google Scholar]
- Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene. 2000;19(19):2324-2330.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102378.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







