Translate this page into:
Antioxidant activities of seeds and sprouts of Momordica charantia and their antibacterial efficacies against pathogenic bacteria isolated from hospitalized patients
⁎Corresponding author. rph.shihab@gmail.com (Md. Shihab Uddin Sohag)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
During sprouting, significant chemical transformations frequently transpire, leading to variations in the concentrations of diverse bioactive compounds present in the seeds. Consequently, seeds and sprouts may have altered bioactivity. The current study aimed to evaluate the in vitro antioxidant activities of crude hydroalcoholic (70 % ethanol and 30 % distilled water) extracts of Momordica charantia seeds and sprouts and their antibacterial efficacies against morbific bacteria sourced from hospitalized patients. Utilizing three assays- DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid), and ferric ions reducing power), the antioxidant activities were evaluated. The results revealed that the sprout extract significantly outperformed the seed extract in terms of Fe3+ ions reducing power, DPPH radical scavenging activity (IC50 = 163.37 ± 23.91 μg/ml), and ABTS radical scavenging activity (IC50 = 30.56 ± 6.90 µg/ml). The antibacterial efficacies against five morbific bacteria (Enterobacter cloacae, Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, and Klebsiella oxytoca) isolated from hospitalized patients were evaluated by the agar disc diffusion method. The findings indicated that the extracts derived from both sprouts and seeds exerted potent antibacterial efficacy by suppressing the growth of several clinical isolates. Both extracts possessed statistically significant (p < 0.05) antibacterial activity against Enterobacter cloacae and Escherichia coli. Seed extract had greater antibacterial activity than sprout extract against Enterococcus faecalis. Pseudomonas aeruginosa exhibited the lowest and identical susceptibility at three distinct concentrations (200 μg, 400 μg, and 800 μg) of seed extract. No extracts have shown any antibacterial action against Klebsiella oxytoca. The results of the study indicate that Momordica charantia sprouts exhibit a greater capacity to scavenge free radicals as antioxidants, whereas seeds possess more potent and broader antibacterial efficacy compared to sprouts.
Keywords
Momordica charantia
Antioxidant activity
Antibacterial activity
Sprout extract
Seed extract
Agar disc diffusion
Bitter gourd
- MCSE
-
Momordica charantia Seed Extract
- MCSPE
-
Momordica charantia Sprout Extract
- STD
-
Standard Drug
- DPPH
-
2, 2-diphenyl-1-picrylhydrazyl
- ABTS
-
2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)
Abbreviations
1 Introduction
Sprouting stimulates the biosynthesis process (Liu et al., 2019) and causes a major change in the phytochemistry of the seeds, particularly in the nutritional profile and bioactive chemicals (Mir et al., 2021). Sprouting is an old habit that was adopted thousands of years ago (around 3000BCE) by the ancient Egyptians (Szopa et al., 2023). Higher amounts of bioactive chemicals are frequently found in microgreens and sprouted seeds compared to raw seeds (Butkutė et al., 2019). The health-related phytochemicals (natural antioxidants and glucosinolates) enhance along with palatability and nutrient bioavailability, while the amount of antinutritive compounds (phytic acid, tannin, pentosan, cyanides, and trypsin inhibitor) decreases during the germination process (Ebert, 2022) and generates more antioxidant and antibacterial effects compared to seeds (Salah et al., 2024). Historically, plants have been the most plentiful source of effective and safe remedies for humans and other animals (Akhtar, 2022). Essential bioactive molecules found in plants are categorized as alkaloids, carotenoids, phenolics, organosulfur compounds, etc., based on their chemistry. These molecules are then reclassified based on their pharmacological actions as antioxidants, anticancer, detoxifying agents, cardioactive agents, immunity-potentiating agents, analgesics, and neuropharmacological agents, etc. (Ugboko et al., 2020).
The majority of plants contain antioxidants, which are believed to shield against the harm caused by the free radicals in a variety of ways, including by directly scavenging the radicals and inhibiting the enzymes that generate them. Consuming cheaper and more secure sources of antioxidants from natural sources, particularly plants, is becoming more popular due to increased concerns about the safety of taking synthetic antioxidants (Stanković et al., 2016).
The primary tool used in the treatment of bacterial illnesses is the antibiotic. Plants have long been utilized by traditional healers to treat or avert infectious diseases. A wide range of bioactive compounds, such as terpenoids, saponins, polyphenols (phenolic acids, tannins, flavonoids, etc.), polyines, coumarins, withanolides, thiophenes, furils, sulfides, alkaloids, glycosinolates, β-sitosterol, resins, essential oils, polysaccharides, fatty oils, proteins, peptides, etc., are plentiful in plants and have been previously shown to possess antibacterial properties in vitro (AlSheikh et al., 2020; Céspedes-Méndez et al., 2021; Hochma et al., 2021; Gayathry & John, 2022). The use of plant-origin antimicrobial compounds may help reduce the need for antibiotics and lower the likelihood that foodborne morbific bacteria would develop antibiotic resistance (AlSheikh et al., 2020). The increasing incidence of antimicrobial resistance and the rise of unwanted effects caused by certain antibiotics have motivated researchers to seek new antibacterial medications, particularly those obtained from medicinal plants.
Momordica charantia L., usually referred to as bitter gourd or bitter melon, is a tropical climbing plant classified under the Cucurbitaceae family. Tropical and subtropical locations, mainly in India, Bangladesh, Malaysia, Southern Asia, Africa, China, South America, the Caribbean, and Mediterranean countries, commercially produce it for its fruits, which are utilized for culinary and medicinal purposes (Gayathry & John, 2022). It has been reported that bitter gourd has therapeutic properties, such as antidiabetic (Çiçek, 2022), antioxidant, and antibacterial activities (Yaldız et al., 2015; Jabeen & Khanum, 2017). Momordica charantia is abundant in bioactive chemicals, including over sixty phytomedicines that exhibit broad activity toward over thirty ailments, even diabetes and cancer (Gayathry & John, 2022). Its typical primary metabolites include polysaccharides, proteins, polypeptides (polypeptide-p, polypeptide-k), fatty acids, and chlorophyll, and prevalent secondary metabolites include phenolic compounds (phenolic acids, phlobatannins, flavonoids), carotenoids, cardiac glycosides, curcubitane, 196 triterpenoids (charantin, momordicine I, II, and III, karavilagenin A-E, kuguacins A-S), alkaloids, phytosterols, saponins, and essential oils that spread throughout the plant's various parts (Sun et al., 2021; Gayathry & John, 2022; Mishra et al., 2022).
Bitter gourd (Momordica charantia) seeds are abundant in carbohydrates, proteins, peptides, vitamins, minerals, and bioactive compounds such as triterpenoid saponins, polypeptide-k, conjugated fatty acids, essential oils, glycosides, flavonoids, and phenolic compounds (Jia et al., 2017; Gayathry & John, 2022). An HPLC-MS analysis of the hydroalcoholic extract of bitter gourd seeds revealed the presence of apigenin, epicatechin, catechin, p-coumaric acid, ferulic acid, chlorogenic acid, kaempferol, rutin, caffeic acid, gallic acid, ethyl gallate, and naringenin (Hussain et al., 2024). An HPLC analysis conducted recently demonstrated that bitter gourd seeds contain α-eleostearic acid, flavonoids such as rutin, quercetin, kaempferol, luteolin, and catechin, together with phenolics including benzoic acid, chlorogenic acid, cinnamic acid, caffeic acid, ferulic acid, gallic acid, salicylic acid, syringic acid, and pyrogallol. Moreover, these bioactive compounds may also serve to mitigate neurological disorders, reduce the risk of some malignancies, promote weight reduction, and protect cells against oxidative damage (Kai et al., 2014; Ardia et al., 2023). The GC/MS analysis of M. charantia seeds identified 25 compounds, which make up 90.90 % of the total oil; the chief components found were apiole, germacrene D, trans-nerolidol, and cis-dihydrovarveol (Gayathry & John, 2022). In another study of SPME-GC/MS that identified 21 compounds in bitter gourd seed, five main compounds were alcohols, aldehydes, esters, monoterpenes, and monoterpenoids (Altun & Orhan, 2023). A gas chromatography investigation detected α-eleostearic acid, α-linoleic acid, β-sitosterol, campesterol, stigmasterol, and stearic acid in the seeds (Yoshime et al., 2016).
No antibacterial and antioxidant study was conducted by sprout extract and the least investigated on seeds extract of M. charantia. The aim of the current study was to evaluate and compare the antioxidant activities of Momordica charantia seeds and sprout hydroalcoholic extract, and their antibacterial efficacy against morbific bacteria obtained from hospitalized patients.
2 Material and Methods
2.1 Collection and Authentication
The seeds of Momordica charantia (bitter gourd or bitter melon) were procured from the local farmland located at 24.3174° N, 89.5622° E (Bangladesh) in October 2023. Plant samples were processed and deposited as herbarium voucher specimens at the National Herbarium, Ministry of Environment and Forests, Government of the People's Republic of Bangladesh, Mirpur, Dhaka (DACB Accession No: 99080).
2.2 Preparation of sprouts
The seed surface was unsullied by running tap water for 20 min. After rinsing the seeds once with distilled water, immerse them in distilled water for 24 h. After that, the moistened seeds were roofed with a moistened cloth and left in an open container for a period of 10 to 12 days. After the sprouting process, sprouted seeds were sliced into small pieces of 10–30 mm and shade-dried for 3 days.
2.3 Preparation of seed and sprout extract
Maceration and solvent evaporation were employed for extraction. The dried seeds and dried, sliced sprouts were crushed in an electronic grinder separately. Coarse powders of Momordica charantia seeds and sprouts were soaked in hydroalcoholic solvent (70 % ethanol and 30 % distilled water) with a ratio of 1:4 in stopper amber glass bottles separately. It was then permitted to stand for 48 h at 25–35°C while being shaken frequently by hand. After passing through cotton, the extracts were filtrated through grade F1001 filter paper [CHM Lab, Spain] and condensed using a rotating evaporator [Senco, China] at − 55 to − 65 cmHg pressure at 55 °C. The extracts were then dried in an oven [Daihan LabTech, South Korea] at 55 °C. The extracts of seeds (MCSE) and sprouts (MCSPE) were preserved at 4 °C in tightly sealed containers.
2.4 Chemicals and reagents
For extraction, distilled water and ethanol [Merck KGaA, Germany] were utilized. Methanol [Merck KGaA, Germany], Ascorbic acid, ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt) [SRL, India], DPPH (2,2-diphenyl-1-picrylhydrazyl) [Research-Lab Fine Chem Industries, India], Iron (III) chloride, K3[Fe(CN)6], and Trichloroacetic acid were used to test antioxidant activity. Analytical-grade reagents and chemicals were utilized in all cases.
2.5 Bacteria, Media, and disc
The bacterial strains of Enterobacter cloacae, Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, and Klebsiella oxytoca were received from Khwaja Yunus Ali Medical College & Hospital, Enayetpur, Sirajganj-6751, Bangladesh. Nutrient agar media [Himedia, India], Blank disc [BioMaxima SA, Poland], Azithromycin 15 μg (AZM-15), Amoxicillin + Clavulanic acid 20 + 10 μg (AMC-30) disc [Bioanalyse, Turkey] were employed to examine antibacterial activity.
2.6 Antioxidant activities
2.6.1 DPPH radicals scavenging assay
In vitro antioxidant activities of the MCSE & MCSPE extracts were investigated using the DPPH free radical scavenging assay (Rahman et al., 2015) with a slight adjustment. After preparing a 0.1 mM DPPH in methanol solution, 2.4 ml of this solution was added to 1.6 ml of extract (or standard) in methanol solution at various concentrations (5, 10, 20, 40, 80, 160, and 320 μg/ml). After carefully vortexing the reaction mixture, it was left at RT for 30 mins in the dark. Using spectrophotometry, the mixture's absorbance was calculated at 517 nm. The reference antioxidant was ascorbic acid. The percentage of DPPH radical scavenging activity was calculated using this formula: where AC = absorbance of the control (DPPH solution, 2.4 ml + methanol, 1.6 ml) and AS = absorbance of the sample (DPPH solution, 2.4 ml + extracts/standard solution, 1.6 ml).
2.6.2 ABTS radical scavenging assay
To assess the scavenging activity of the ABTS radical of the MCSE & MCSPE extracts, the ABTS radical scavenging assay (González-Palma et al., 2016) with slight adjustment was adopted. A 7 mM ABTS solution and 2.45 mM potassium persulfate solution were separately prepared in dH2O. To generate the ABTS radical cation (ABTS∙+) solution, an equal volume of both solutions (1:1) was mixed and left in the dark to react for 16 h at RT. An absorbance of 0.70 (±0.05) at 734 nm was achieved by diluting the ABTS∙+ solution with 50 % ethanol. 1 ml extract in 50 % ethanol at each concentration (1, 2, 5, 10, 20, 40, 60, 80, 100 μg/ml) was reacted with 3 ml of the diluted ABTS∙+ solution and left for 6 mins. Using the spectrophotometer [VWR, USA], absorbance was measured at 734 nm. The reference standard was ascorbic acid. ABTS radical scavenging activity of both MCSE and MCSPE was calculated by this formula: where AC = Absorbance of control (1 ml of 50 % ethanol + 3 ml of the diluted ABTS∙+ solution) and AS = Absorbance of sample after 6 mins (1 ml extracts/standard in 50 % ethanol + 3 ml of the diluted ABTS∙+ solution).
2.6.3 Ferric ion reducing power (FRP) assay
The reducing power of MCSE and MCSPE was investigated by the Fe3+ to Fe2+ transformation as performed by Quy Diem Do et al. (Do et al., 2014) with minor modification. 1 ml of each extract (MCSE and MCSPE) at several concentrations (2.5, 5, 10, 20, 40, 80, and 160 μg/ml) was combined with 0.2 M phosphate buffer (2.5 ml, pH 6.6) and 2.5 ml of potassium ferricyanide (1 % w/v) in a test tube. After that, the test tube was incubated for 20 min at 50 °C. Trichloroacetic acid (2.5 ml, 10 % w/v) was added to the test tube after the incubation period, and it was centrifuged for 10 min at 3,000 rpm. 0.5 ml of freshly produced iron (III) chloride (0.1 % w/v) was added to the supernatant (2.5 ml) after it had been diluted with 2.5 ml of distilled water. After meticulous vortexing, a UV–VIS spectrophotometer was used to measure the absorbance of the mixture at 700 nm in order to determine the reducing power of the samples. A higher reducing power was demonstrated by the higher absorbance of the test sample. Ascorbic acid served as the reference standard (STD) in this instance.
2.7 Antibacterial efficacy
The agar disc diffusion method (Kirby-Bauer test) was applied to measure the antibacterial efficacy of the Momordica charantia hydroalcoholic extracts of seeds (MCSE) and sprouts (MCSPE). Nutrient agar was utilized throughout the experiment, and the extracts' inhibitory zones were examined (Bauer et al., 1966) (Ikram et al., 2021). After the bacterial strains were collected, they were homogenized separately in a test tube using 5 ml of sterile nutritional broth (13 g in 1000 ml dH2O) and incubated for 16 h at 37 °C (80 rpm) in a shaking water bath [Taisite, USA]. Once the bacteria had been incubated, the cultures were diluted and standardized using 0.5 McFarland (turbidity) standards. A suspension of nutrient agar media was prepared (28.0 g in 1000 ml dH2O) and sterilized using an autoclave [Taisite, USA] at 121 °C (103.4 kPa pressure) for 15 min. Pour into a sterile 150 mm Petri dish after cooling to 45–50 °C. After solidification, the cultures of bacteria (1000 µl) were evenly distributed on each petri dish using a spreader and left to dry under a biological safety cabinet [Labconco, USA] for 20–30 min. Three distinct concentrations of MCSE and MCSPE extracts were prepared separately in sterile dH2O, resulting in final concentrations of 200 µg/25 µl, 400 µg/25 µl, and 800 µg/25 µl for each extract. A 0.20 μm syringe filter [Sartorius Biotech, Germany] was used to filter the extract solution in order to sterilize it before impregnation. 25 μl of the MCSE and MCSPE extracts of each concentration were loaded onto a sterile black paper disc (6 mm in diameter) separately, which were then placed on the previously bacterium-inoculated surface of the nutrient agar dish by the flamed forceps. The discs were then gently pressed to ensure perfect contact between the discs and the nutrient agar dish's surface. After a 30-minute diffusion period, all petri dishes were turned upside down and placed in an incubator at 37˚C for 48 h [Daihan LabTech, South Korea]. After a 48-hour incubation period, a distinct inhibition zone was measured in millimeters around each disc using a slide caliper. Larger zones state higher antibacterial efficacy. Azithromycin 15 μg (AZM-15) and amoxicillin/clavulanic acid 20/10 μg (AMC-30) discs were utilized as reference standards. Bacterial strains considered resistant to an extract or antibiotic will reach the disc's margin, implying that the absence of an inhibitory zone around paper discs hydrated with extract or antibiotics on a lawn of bacteria planted on the surface of an agar medium indicates resistance (Bubonja-Šonje et al., 2020).
2.8 Statistical analysis
The means ± standard deviations (SD) were employed to express the data. Every test was performed in triplicate. IC50 was computed using Fit spline/LOWESS. One-way ANOVA- Tukey’s test was used for statistical comparisons, and p values < 0.05 were considered significant. Microsoft Excel 2013 (USA) and GraphPad Prim 8.4.3 (USA) were both utilized for data analysis.
3 Results and Discussion
3.1 Antioxidant activities
3.1.1 DPPH radical scavenging activity
The most prevalent, well-recognized, and precise technique for assessing antioxidant activity is the DPPH free radical scavenging assay. Being a free radical, DPPH only has one electron and can take on additional electrons or hydrogen ions. An interaction between molecules of antioxidants and the DPPH radicals causes a decrease in absorbance at 517 nm. This fall in absorbance causes the radical to be scavenged by hydrogen donation, turning the solution from violet to pale yellow. A decrease in absorbance level indicates a rise in antioxidant capacity (Wang et al., 2024). The % of DPPH radical scavenging activity of the hydroalcoholic seed and sprout extracts (MCSE and MCSPE) was depicted in Fig. 1 (A). At varying concentrations (5 μg/ml to 320 μg/ml), the test samples MCSE, MCSPE, and STD exhibited a percentage inhibition of 15.39 ± 3.731 to 59.84 ± 1.64, 21.67 ± 1.23 to 71.22 ± 3.40, and 35.06 ± 5.36 to 91.71 ± 4.66, respectively. They exhibited potent in vitro DPPH radical scavenging potentials that were concentration-dependent.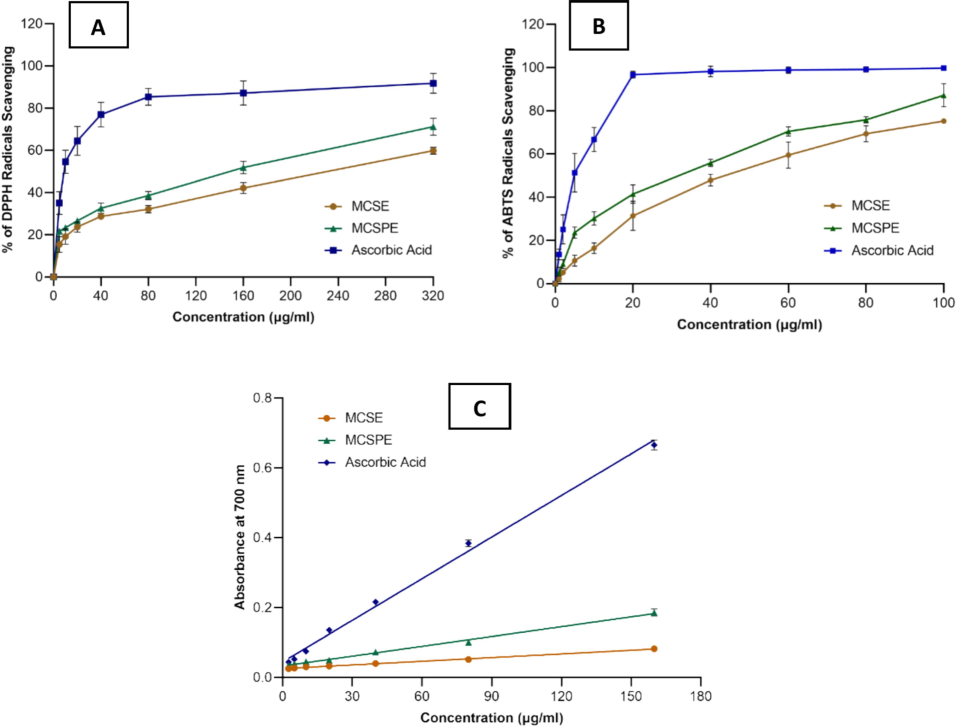
Antioxidant activities of seeds (MCSE), sprouts (MCSPE) of Momordica charantia, and reference standard using (A) the DPPH radical scavenging activity, (B) the ABTS radical scavenging activity, and (C) Ferric Ion Reducing Power (FRP) activity.
No antioxidant study was conducted on sprouts of bitter gourd (Momordica charantia). Winarti et al. reported that the IC50 of bitter melon seed oil was 1310 ± 0.77 μg/ml (Winarti et al., 2021). In this investigation, the IC50 of MCSE and MCSPE was also assessed and displayed in Table 1. Sprout extract (MCSPE) was shown to have DPPH radical activity (IC50 = 163.37 ± 23.91 μg/ml), which was significantly greater potency than (MCSE) seed extract (IC50 = 244.11 ± 15.44 μg/ml) as well as vitamin E (IC50 = 172.21 μg/ml) (Wu & Ng, 2008). The greater IC50 meant the extracts had less capacity to scavenge DPPH radicals. It was discovered that the IC50 sequence was MCSE < MCSPE < STD.
Methods
Extracts
Positive control
MCSE
MCSPE
Ascorbic Acid
DPPH/ IC50 (µg/mL)
244.11 ± 15.44c
163.37 ± 23.91b
13.72 ± 0.89a
ABTS/ IC50 (µg/mL)
42.40 ± 6.79c
30.56 ± 6.90b
6.37 ± 0.17a
3.1.2 ABTS radical scavenging activity
This test presents crucial data about a substance's potential health benefits and antioxidant activity by examining its resistance to oxidative stress and damage generated by free radicals (Skrovankova et al., 2022). When ABTS and potassium persulfate interacted, the blue-green ABTS cationic radical was formed. When antioxidant components reacted with ABTS cationic radicals, the system turned discolored, which was a sign of the material's antioxidant capacity. The scavenging activity of MCSE, MCSPE, and STD on the ABTS radical was shown in Fig. 1(B). From the concentration of 1 μg/ml to 100 μg/ml of the MCSE, MCSPE, and STD, the % ABTS scavenging was 2.3 ± 1.32 to 75.21 ± 0.75, 5.13 ± 2.40 to 87.18 ± 5.31, and 13.48 ± 2.50 to 99.67 ± 0.23, respectively. A quantitative link was revealed by the strong scavenging action of all samples on the ABTS radical with increasing concentration. MCSPE's ability to scavenge ABTS radical was superior to MCSE's at a concentration. The hydroalcoholic MCSE, MCSPE extracts, and STD exhibited IC50 values of 42.40 ± 6.79 µg/ml, 30.56 ± 6.90 µg/ml, and 6.37 ± 0.17 µg/ml, respectively, as shown in Table 1. Compared to MCSE, MCSPE was found to have the highest scavenging activity on ABTS radicals. The extract’s greatest capacity to scavenge ABTS radicals was demonstrated by the lowest IC50.
3.1.3 Ferric Ion reducing power (FRP) activity
Reducing power study is an accepted technique used to assess the electron-donating capacity of antioxidants (Yıldırım et al., 2000). Within this evaluation, the capacity of MCSE and MCSPE to convert Fe3+ to Fe2+ was ascertained. The presence of antioxidants in the hydroalcoholic extracts resulted in the transformation of the ferric cyanide complex (Fe3+) into the ferrous cyanide form (Fe2+). During the reducing power evaluation, antioxidants prompt the conversion of Fe3+ to Fe2+, consequently altering the solution's color to various hues ranging from green to blue, contingent upon the compounds' reducing power (Ferreira et al., 2007). Nevertheless, potent reducing agents created Perl's Prussian blue hue and absorbed light at 700 nm. Previous studies have suggested that a substance's ability to give hydrogen may be the origin of its reducing power (Shimada et al., 1992). An elevated absorbance in the reaction mixture is indicative of an increased reducing capability. The MCSE, MCSPE, and STD at concentrations of 2.5 μg/ml to 160 μg/ml exhibited absorbance values of 0.025 ± 0.001 to 0.082 ± 0.004, 0.035 ± 0.002 to 0.186 ± 0.010, and 0.044 ± 0.003 to 0.665 ± 0.014, respectively. A direct correlation was seen between the concentration of the extracts and the increase in reduction power of MCSE and MCSPE. MCSE demonstrated a lower level of Fe3+ reduction in comparison to MCSPE. The reducing power of STD (Ascorbic acid) surpassed that of all the examined extracts. Fig. 1(C) portrayed the reducing capabilities of MCSE and MCSPE from Momordica charantia in contrast to ascorbic acid as the standard. The reducing power displayed a strong linear relationship in both the standard (R2 = 0.995) and MCSE (R2 = 0.9863) and MCSPE (R2 = 0.986). The reducing power was observed in the sequence of STD > MCSPE > MCSE.
Multiple studies indicate that bitter gourd is a valuable natural reservoir of antioxidants. In this work, the results indicated that sprouts of bitter gourd exhibited superior antioxidant activities compared to seeds. Sprouts are rich in plant secondary phytochemicals, particularly polyphenols, polysaccharides, and saponins. Elevated concentrations of these bioactive chemicals have been conferred as promising antioxidant efficacy (Jia et al., 2017; Szopa et al., 2023).
3.2 Antibacterial efficacy
A wide variety of bitter gourd species has been documented to exhibit strong antibacterial properties (Gayathry & John, 2022). This study was investigated to assess the antibacterial activities of extracts of seeds (MCSE) and sprouts (MCSPE) against five bacteria (Enterobacter cloacae, Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, Klebsiella oxytoca) extracted from infected individuals in a hospital setting. The strains exhibited pathogenicity and frequently displayed several traits, including antibiotic resistance, virulence determinants, and genetic variability, which were dependent upon the infection and the patient's state. The antibacterial activity of hydroalcoholic extracts of seeds (MCSE) and sprouts (MCSPE) of bitter gourd was displayed in Table 2 and Fig. 2. Although both extracts exhibited antibacterial activity against Enterobacter cloacae, Escherichia coli, and Enterococcus faecalis, only MCSE demonstrated antibacterial effectiveness against Pseudomonas aeruginosa, and none of the extracts showed antibacterial activity against Klebsiella oxytoca. Enterobacter cloacae was highly susceptible to both MCSE and MCSPE extracts in a concentration-dependent manner. Note: AZM-15 (Azithromycin 15 μg) and AMC-30 (Amoxicillin + Clavulanic acid 20 + 10 μg), (Zone of Inhibition in Mean ± SD), “0″ sign indicated no zone of inhibition. Clear zone was taken in diameter (mm). A row of values with similar alphabetic letters is not statistically significant, while values containing distinct alphabetic letters are (P < 0.05) significant.
Morbific bacteria
Inhibitory Zone in Diameter (mm) (Mean ± SD)
MCSE
MCSPE
Standard Antibiotics
200 μg
400 μg
800 μg
200 μg
400 μg
800 μg
AMC-30
AZM-15
Enterobacter cloacae
8.6d ± 0.20
10.28c ± 0.23
11.9b ± 0.30
7.2e ± 0.10
9.3d ± 0.17
11.27b ± 0.42
0f
16.28a ± 0.68
Escherichia coli
7.4c ± 0.10
7.63c ± 0.06
8.22b ± 0.25
6.4d ± 0.17
6.7d ± 0.10
7.57b ± 0.21
0e
15.9a ± 0.62
Enterococcus faecalis
7.1c ± 0.44
9.33b ± 0.63
10.3b ± 0.92
6.58c ± 0.13
6.6c ± 0.17
7.1c ± 0.56
0d
17.27a ± 1.04
Pseudomonas aeruginosa
6.57b ± 0.12
6.63b ± 0.23
6.6b ± 0.20
0c
0c
0c
0c
13.73a ± 2.80
Klebsiella oxytoca
0a
0a
0a
0a
0a
0a
0a
0a
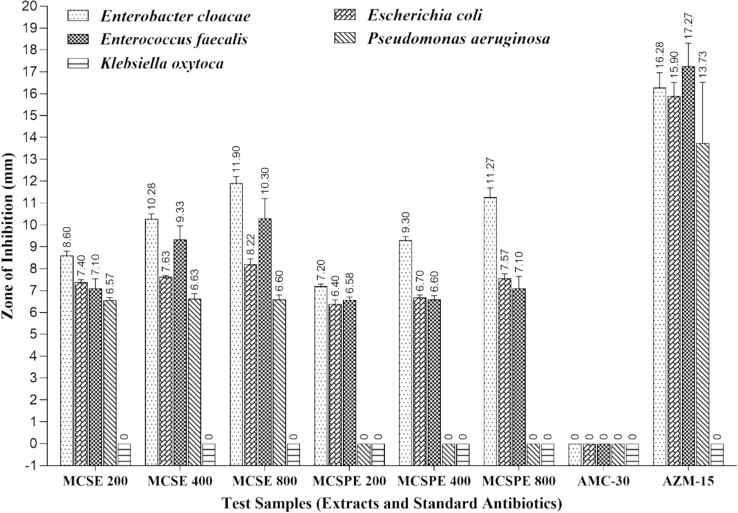
Antibacterial activity of seed and sprout extracts of Momordica charantia against morbific bacteria isolated from hospitalized patients.
A report documented that the crude seed extract of M. charantia displayed the highest activity against Escherichia coli (12.66 ± 1.15 mm) and the lowest activity against Pseudomonas aeruginosa (8.00 ± 0.00 mm) (Jabeen & Khanum, 2017), which was comparable with this study. Yaldız et al. reported that the bitter melon seed extract had a potent antibacterial impact on Escherichia coli and Salmonella typhi but had a less pronounced antibacterial result on C. albicans and Pseudomonas aeruginosa (Yaldız et al., 2015). In another study by Altun & Orhan, it was found that the seed extract showed antibacterial properties against E. coli, E. faecalis, P. aeruginosa, S. epidermidis, S. agalactiae, P. vulgaris, S. pyogenes, B. subtilis, and S. aureus (Altun & Orhan, 2023). In our study, seed extract (MCSE) demonstrated the highest activity against Enterobacter cloacae (8.6 ± 0.20 mm to 11.9 ± 0.30 mm), followed by Enterococcus faecalis (7.1 ± 0.44 mm to 10.3 ± 0.92 mm) and Escherichia coli (7.4 ± 0.10 mm to 8.22 ± 0.25 mm), and it exhibited the lowest activity against Pseudomonas aeruginosa (6.57 ± 0.12 to 6.6 ± 0.20 mm) as well as resistance to Klebsiella oxytoca (0.0 ± 0.0 mm). Conversely, MCSPE displayed antibacterial activity against Enterobacter cloacae (7.2 ± 0.10 mm to 11.3 ± 0.42 mm), then Escherichia coli (6.4 ± 0.17 mm to 7.57 ± 0.21 mm), and Enterococcus faecalis (6.58 ± 0.13 mm to 7.1 ± 0.56 mm), while exhibiting resistance towards Pseudomonas aeruginosa and Klebsiella oxytoca. Pseudomonas aeruginosa showed the lowest and similar susceptibility at 3 different concentrations (200 μg, 400 μg, and 800 μg) of MCSE.
Three distinct concentrations of MCSE and MCSPE were tested along with two common antibiotics. The results of the antibacterial efficacy of these antibiotics against the identical variety of bacterial isolates as shown in Table 2. AZM-15 (Azithromycin 15 μg) as a control revealed the highest antibacterial efficacy against all tested bacteria, excluding Klebsiella oxytoca. On the other hand, AMC-30 (amoxicillin + clavulanic acid 20 + 10 μg) as the second control showed resistance to all tested bacteria collected from hospitalized patients.
A high concentration of saponin, triterpenoids, alkaloids, germacrene-D, trans-nerolidol, cis-dihydrovarveol, α-linoleic acid, coumarins, polypeptide (polypeptide-p), flavonoids, phenolics, essential oils, polysaccharides, fatty acids, and β-sitosterol are present in the hydroalcoholic extract of Momordica charantia seeds, which contribute to its potent antibacterial properties than sprouts (Saeed et al., 2018, Sun et al., 2021, Gayathry & John, 2022). In this study, the sprout extract showed higher antioxidant activity than the seed extract but comparatively weaker antibacterial activity against some bacteria. It has been well established that reactive oxygen species (ROS) have the ability to eradicate harmful microorganisms. High antioxidant capabilities shown by the sprout extract (MCSPE) could suppress the effects of reactive oxygen species (ROS), thereby contributing to its limited antibacterial efficacy (Yıldırım et al., 2000). In addition, the sprouts retain a less bitter taste in comparison to the seeds since they contain fewer amounts of saponin, triterpenoids, and alkaloids (Jia et al., 2017). Possibly due to the absence of these compounds and the reduced quantity of essential oils and fatty acids, the antibacterial action was moderately compromised.
4 Conclusion
The present study demonstrated that Momordica charantia sprout extract exhibited higher antioxidant activity compared to the seed extract, as evidenced by its ability to scavenge DPPH and ABTS radicals and its reducing power activity. Moreover, both sprout and seed extracts displayed antibacterial efficacy against a range of pathogenic bacteria isolated from hospitalized patients, with the seed extract showing broader and stronger activity. Based on these findings, Momordica charantia seed and sprout could serve as natural antioxidant and antibacterial agents. However, more research is still needed to verify its effectiveness as a safe dietary supplement.
Ethical approval
Ethical approval was taken from the ethical committee of Khwaja Yunus Ali University, ethical clearance certificate number: KYAU/DEAN/EGC/2024/001.
Funding
No outside funding was available for this research work.
CRediT authorship contribution statement
Md. Shihab Uddin Sohag: Writing – review & editing, Writing – original draft, Visualization, Supervision, Project administration, Methodology, Formal analysis, Data curation, Conceptualization. Md. Al-Monsur: Investigation, Funding acquisition, Data curation. Md. Mushfiqur Rahman: Investigation, Funding acquisition, Data curation. Md. Al Amin Sarker: Investigation, Funding acquisition, Data curation. Sanjay Dutta: Writing – review & editing, Supervision, Formal analysis, Data curation. Fazle Rabbi Shakil Ahmed: Writing – review & editing, Supervision, Software, Resources, Project administration.
Acknowledgment
The authors are grateful to Prof. Dr. Abdullah Akhtar Ahmed, Professor and HOD of Laboratory Services, Department of Microbiology, Khwaja Yunus Ali Medical College, for providing the bacterial strains isolated from hospitalized patients for this research work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anti-inflammatory medicinal plants of bangladesh—a pharmacological evaluation. Front. Pharmacol.. 2022;13:809324
- [CrossRef] [Google Scholar]
- Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics. 2020;9(8):480.
- [CrossRef] [Google Scholar]
- Determination of phytochemical and antibacterial properties of Momordica charantia seed extracts. Celal Bayar Üniversitesi Fen Bilimleri Dergisi. 2023;19(4):309-313.
- [CrossRef] [Google Scholar]
- Chemical and nutritional evaluation of bitter melon seeds and their use in the preparation of tahini. J. Advances in Agricu. Res.. 2023;28(3):560-570.
- [CrossRef] [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol.. 1966;45(4_ts):493-496.
- [CrossRef] [Google Scholar]
- Challenges to antimicrobial susceptibility testing of plant-derived polyphenolic compounds. Arch. Ind. Hyg. Toxicol.. 2020;71(4):300-311.
- [CrossRef] [Google Scholar]
- Small-seeded legumes as a novel food source. variation of nutritional, mineral and phytochemical profiles in the chain: raw seeds-sprouted seeds-microgreens. Molecules. 2019;24(1)
- [CrossRef] [Google Scholar]
- Secondary metabolites and biological profiles of Datura genus. J. Chil. Chem. Soc.. 2021;66(2):5183-5189.
- [CrossRef] [Google Scholar]
- Momordica charantia L.—diabetes-related bioactivities, quality control, and safety considerations. Front. Pharmacol.. 2022;13:904643
- [CrossRef] [Google Scholar]
- Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal.. 2014;22(3):296-302.
- [CrossRef] [Google Scholar]
- Sprouts and microgreens—novel food sources for healthy diets. Plants. 2022;11(4)
- [CrossRef] [Google Scholar]
- Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: individual cap and stipe activity. Food Chem.. 2007;100(4):1511-1516.
- [CrossRef] [Google Scholar]
- A comprehensive review on bitter gourd (Momordica charantia L.) as a gold mine of functional bioactive components for therapeutic foods. Food Prod. Process. Nutr.. 2022;4(1):10.
- [CrossRef] [Google Scholar]
- Evaluation of the antioxidant activity of aqueous and methanol extracts of pleurotus ostreatus in different growth stages. Front. Microbiol.. 2016;7
- [CrossRef] [Google Scholar]
- Antimicrobial effect of phytochemicals from edible plants. Processes. 2021;9(11)
- [CrossRef] [Google Scholar]
- Evaluation of peel, flesh and seeds of bitter gourd (Momordica charantia L.) for biologically active components, through development of powders and ethanolic extracts. Discover Appl. Sci.. 2024;6(8):432.
- [CrossRef] [Google Scholar]
- Antimicrobial and antioxidant activities of methanolic extract and fractions of epilobium roseum (Schreb.) against bacterial strains. Am. J. Plant Sci.. 2021;12(3)
- [CrossRef] [Google Scholar]
- Isolation and characterization of potential food preservative peptide from Momordica charantia L. Arab. J. Chem.. 2017;10:S3982-S3989.
- [CrossRef] [Google Scholar]
- Recent advances in momordica charantia: functional components and biological activities. Int. J. Mol. Sci.. 2017;18(12):2555.
- [CrossRef] [Google Scholar]
- Identification of a bioactive compound against adult T-cell leukaemia from bitter gourd seeds. Plants. 2014;3(1):18-26.
- [CrossRef] [Google Scholar]
- Effects of elicitation on bioactive compounds and biological activities of sprouts. J. Funct. Foods. 2019;53:136-145.
- [CrossRef] [Google Scholar]
- An overview of sprouts nutritional properties, pathogens and decontamination technologies. LWT. 2021;141:110900
- [CrossRef] [Google Scholar]
- NMR-based metabolomic profiling of the differential concentration of phytomedicinal compounds in pericarp, skin and seeds of Momordica charantia (bitter melon) Nat. Prod. Res.. 2022;36(1):390-395.
- [CrossRef] [Google Scholar]
- In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC. Res. Notes. 2015;8(1):621.
- [CrossRef] [Google Scholar]
- Bitter melon (Momordica charantia): A natural healthy vegetable. Int. J. Food Prop.. 2018;21(1):1270-1290.
- [CrossRef] [Google Scholar]
- Improvement of phenolic profile and biological activities of wild mustard sprouts. Sci. Rep.. 2024;14(1):10528.
- [CrossRef] [Google Scholar]
- Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem.. 1992;40(6):945-948.
- [CrossRef] [Google Scholar]
- Diversity of phytochemical and antioxidant characteristics of black mulberry (Morus nigra L.) Fruits from Turkey. Antioxidants. 2022;11(7)
- [CrossRef] [Google Scholar]
- Antibacterial and antioxidant activity of traditional medicinal plants from the balkan peninsula. NJAS: Wageningen J. Life Sci.. 2016;78(1):21-28.
- [CrossRef] [Google Scholar]
- The triterpenoids of the bitter gourd (Momordica Charantia) and their pharmacological activities: a review. J. Food Compos. Anal.. 2021;96:103726
- [CrossRef] [Google Scholar]
- Chia sprouts and as a new nutraceutical raw materials and their health-promoting impact in modern dietetics. Current Issues in Pharmacy and Medical Sci.. 2023;36(1):33-44.
- [CrossRef] [Google Scholar]
- Antimicrobial importance of medicinal plants in nigeria. Scientific World Journal 2020e7059323
- [CrossRef] [Google Scholar]
- Antioxidant activities of sea buckthorn polysaccharides and their potential application in cosmetic industry. J. Dermatologic Sci. Cosmetic Technol. 2024100023
- [CrossRef] [Google Scholar]
- Radical scavenging activity and acute toxicity of bitter melon (Momordica Charantia L.) Seed Oil. Majalah Obat Tradisional. 2021;26(1)
- [CrossRef] [Google Scholar]
- Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia Linn. Var. Abbreviata Ser.) in Taiwan. LWT Food Sci. Technol.. 2008;41(2):323-330.
- [CrossRef] [Google Scholar]
- Antimicrobial activity and agricultural properties of bitter melon (Momordica charantia L.) grown in northern parts of Turkey: a case study for adaptation. Nat. Prod. Res.. 2015;29(6):543-545.
- [CrossRef] [Google Scholar]
- Comparison of antioxidant and antimicrobial activities of tilia (Tilia Argentea Desf Ex DC), Sage (Salvia Triloba L.), and black tea (Camellia Sinensis) extracts. J. Agric. Food Chem.. 2000;48(10):5030-5034.
- [CrossRef] [Google Scholar]
- Bitter gourd (Momordica charantia L.) seed oil as a naturally rich source of bioactive compounds for nutraceutical purposes. Nutrire. 2016;41(1):12.
- [CrossRef] [Google Scholar]







