Translate this page into:
New record of Phylloteles hyalipennis (Baranov, 1934) (Diptera: Miltogramminae) from North India, with morphology, life cycle and SEM study of immature stages
⁎Corresponding author. madhu_zology@pbi.ac.in (Madhu Bala)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
First time from North India, the immature stages of a species of the genus Phylloteles Loew, 1844, are described, with a redescription of the adults. The research explored the morphological characteristics and distribution of Phylloteles hyalipennis (Baranov, 1934) from India. This study aims to enhance species identification and potentially revise classification of this scientifically important group.
Methods
Gravid females of this species were collected from the decomposing chicken meat from the Union territory Jammu and Kashmir (India), and larvae were reared under optimal conditions to get a pure colony. The immature stages were examined under light as well as scanning electron microscope (SEM).
Results
The results included descriptions of adult morphology, systematic classification, and detailed analysis of immature stages. The Pseudocephalon, maxillary palpus, anterior and posterior spiracles, and variations of thoracic and abdominal segments, anal region, and details of cephaloskeleton of immatures were utilized as key characters for species identification in case of immature stages and presented along with illustrations.
Conclusion
The study provides valuable insights into the poorly understood larval morphology of Miltogramminae, specifically in Oriental taxa. This information can stipulate new characteristics for species identification in case of immature stages and enhances systematics of Indian Sarcophagidae.
Keywords
Miltogramminae
Immature stages
Ultra morphology
Scanning micrographs
Phylloteles
1 Introduction
Within the diverse world of Sarcophagids (Diptera), the subfamily Miltogramminae stands out as a moderately diverse group comprising approximately 600 known species with a unique ecological niche (Verves, 1989). These flies are important in the veterinary, medical, and forensic sciences, as well as being potential bioindicators for environmental impact assessments (Dufek et al., 2020). Within the Miltogramminae, the genus Phylloteles Loew, 1844 has scientific relevance across multiple disciplines (Szpila et al., 2010; Sinha, 2012). Species of Phylloteles have been reported to rear on dead insects and colonized on buried vertebrate carrion (Szpila and Pape, 2007; Szpila et al., 2010). Phylloteles hyalipennis (Baranov, 1934) was also discovered near the ground nests of several sphecid and nyssomid wasps (Sinha, 2012). This fly's interaction with wasps may reveal additional information that should be researched in the future. First instar larvae of Phylloteles Loew, have a brief 'free-living' period during which they locate the food source, which appears to have resulted in a remarkable diversity of integument architecture (Szpila and Pape, 2007; Szpila and Pape, 2008; Kutty et al., 2010). There was no more information available when the genera were first defined and identified other than their adult morphology, and there is insufficient information on larvae to provide additional evidence supporting generic monophyly. Therefore, non-larval characteristics have typically been used to classify Miltogramminae and determine monophyly (Verves, 1989, 1994; Pape, 1996).
Miltogramminae, Paramacronychiinae, and Sarcophaginae are three subfamilies of the Sarcophagidae based on the morphology and molecular data of adult flies (Piwczyński et al., 2017). While Miltogramminae and Paramacronychiinae show significant radiation in the Palaearctic, Sarcophaginae underwent much of their diversification in the Neotropics (Yan et al., 2021). According to the phylogenetic hypothesis, the subfamily Miltogramminae is considered sister to Paramacronychiinae (Piwczyński, et al., 2014; Piwczyński et al., 2017; Yan et al., 2017). Some species of Miltogramminae are kleptoparasites of solitary bees and wasps, feeding on the stored food (insects, spiders, and pollen) that these hymenopterans supply for their offspring (Pape, 1996; Szpila and Pape, 2007; Sinha, 2012). Some of these flies’ deposit eggs or larvae on the wasp host before the host carries them into the nest. There has been limited study on species of Miltogramminae in India. Nandi (2002) reported 41 species from 22 genera in India, with most of the species reported from Jammu and Kashmir (Sinha, 2012). Only a small fraction of genera and species of Miltogrammine larvae have been studied (Szpila and Pape, 2005a, 2005b). However, larval morphology is unusually diverse (when compared to the other sarcophagids) and has a high potential for aligning adult characters in phylogenetic analyses (Szpila and Pape, 2007; Johnston et al., 2024). Future research should focus on adult and larval characteristics, given the benefits of such combined analyses and the fact that newly hatched first instars can be procured easily from gravid females because all species are ovoviviparous (Meier et al., 1999).
Some species of this genus, such as Phylloteles pictipennis, are recognized as forensically important (Szpila et al., 2015). This species can also be significant from a forensic point of view as it is associated with decaying carcasses. During the present study also, adults were captured from decaying chicken carcasses. As a member of the Sarcophagidae family and the presence of this species on decomposing animal remains can make it a potential forensic indicator to aid in estimating postmortem interval. Acknowledging these research, present study utilized light and scanning electron microscopy (SEM) to contribute to the understanding of Phylloteles hyalipennis within the Miltogramminae subfamily. By incorporating both adult and immature characteristics in a diagnostic context, the study intends to facilitate more accurate identification and potentially revise existing classification within this remarkable fly group.
2 Materials and methods
Phylloteles hyalipennis (Baranov, 1934) was collected during a survey tour of the family Sarcophagidae in village Maila 489 m (32°28′34.3″N 75°16′07.0″E), District Kathua, Jammu and Kashmir (India). Gravid females were collected from decaying chicken meat (2.5 Kg), which was used as bait to lure flesh flies. The collected flies were kept in rearing boxes (1′ x 1′ x 1′). Females were fed with 15 g of powdered sugar and water in a 200-ml conical flask with a cotton wick. Females were also provided with 10 g of chicken liver in a Petri dish as an oviposition medium. After eclosion of first-instar larvae were transferred into a 1L glass rearing jar with a husk-based pupation medium and provided with 20 g of chicken meat as a food source. The glass container was sealed with a piece of muslin fabric and a rubber band to stop the larvae from escaping. Total fifteen adults were collected, killed, and examined after their emergence. Out of which four male and four female specimens were stretched, and pinned, and their genitalia were dissected to study their characteristics. Nandi (2002) keys were utilized to identify the specimens. Structures like fifth sternite, cercus, surstylus, and phallus were separated. Photographs were captured using a Canon EOS 1200D DSLR Camera with 18 MP and a 5x optical zoom.
For light micrographs immature stages (n = 8) were retrieved at regular intervals from rearing jars, killed by boiling water (95 °C), and stored in 70 % alcohol to minimize deformities. To examine the cephaloskeleton, anterior spiracles, and posterior spiracles larval instars (n = 4) were dissected. With the aid of light microscope (Leica DM, 2000 with 4x to 20x magnification), photography was done.
For SEM analyses, third-instar larvae (n = 3) and puparia (n = 3) were procured following the method given by Szpila and Pape (2007) and Kumar et al. (2021) to explore the detailed external morphology of the immature stages. SEM pretreatment included dehydration in 30, 60, 80, 90, and 99.5 % ethanol and critical point drying in CO2. The immatures were then sputter-coated with gold, and SEM images were obtained using a JOEL JSM-6510LV scanning electron microscope with 5x to 300,000x magnification. For specimen characterization, the terminology given by Szpila and Pape (2007), Kurahashi and Samerjai (2018), and Szpila et al. (2021) were applied.
3 Results
Light microscopy was employed to identify adults based on their morphological characteristics. In contrast, the identification of immature stages of Phylloteles hyalipennis (Baranov), such as larvae and puparia, was done using both light and electron microscopy.
3.1 Systematic account
Subfamily: Miltogramminae Brauer and Bergenstamm, 1889.
Diagnosis: Medium-sized flies with blackish color and silvery, greyish, or golden pollen; frequently distinguished by conspicuously black abdominal bands and spots; either viviparous or ovoviviparous (Nandi, 2002).
Bionomics:
The lower miltogrammines contain species that are saprophagous or predatory on reptile eggs and buried vertebrate carrion (Piwczyński et al., 2017) or are termite-associated (Pape, 2006). The higher miltogrammines are predominantly kleptoparasites in the nests of aculeate Hymenoptera (Piwczyński et al., 2017).
Distribution: Worldwide.
Tribe: PHYLLOTELINI Rohdendorf.
Subtribe: Phyllotelina Rohdendorf.
Genus: Phylloteles Loew, 1844.
Diagnosis: In profile, small to medium sized flies; head is somewhat projecting forward; narrow frons; parafacial region bare or with short setae; antennae short; distinctly flattened short and tapered arista; orbital bristles 3–5 in number and proclinate, vibrissae absent or shorter than antennae, gena often with less setae; phallus large with short and compact paraphallus; ejaculatory apodeme broad, epiphallus flat and wide.
Biology: Most Indian species were collected from the herbaceous vegetation in the sub-tropical region (Nandi, 2002; Sinha, 2012). Species of Phylloteles have been reported to have been bred from eggs-pods of acridid grasshoppers (Schistocerca sp.) (Zumpt, 1973), nests of sphecids (Philantus triangulum) (Charykuliev and Myartseva, 1964), and infested sea turtle (Caretta caretta) nests (Krohn, 2007).
Distribution: Oriental, Palearctic, and Afrotropical regions.
Phylloteles hyalipennis (Baranov)
Adults
Male Diagnosis (Fig. 1): Male length 4.0–5.5 mm in size.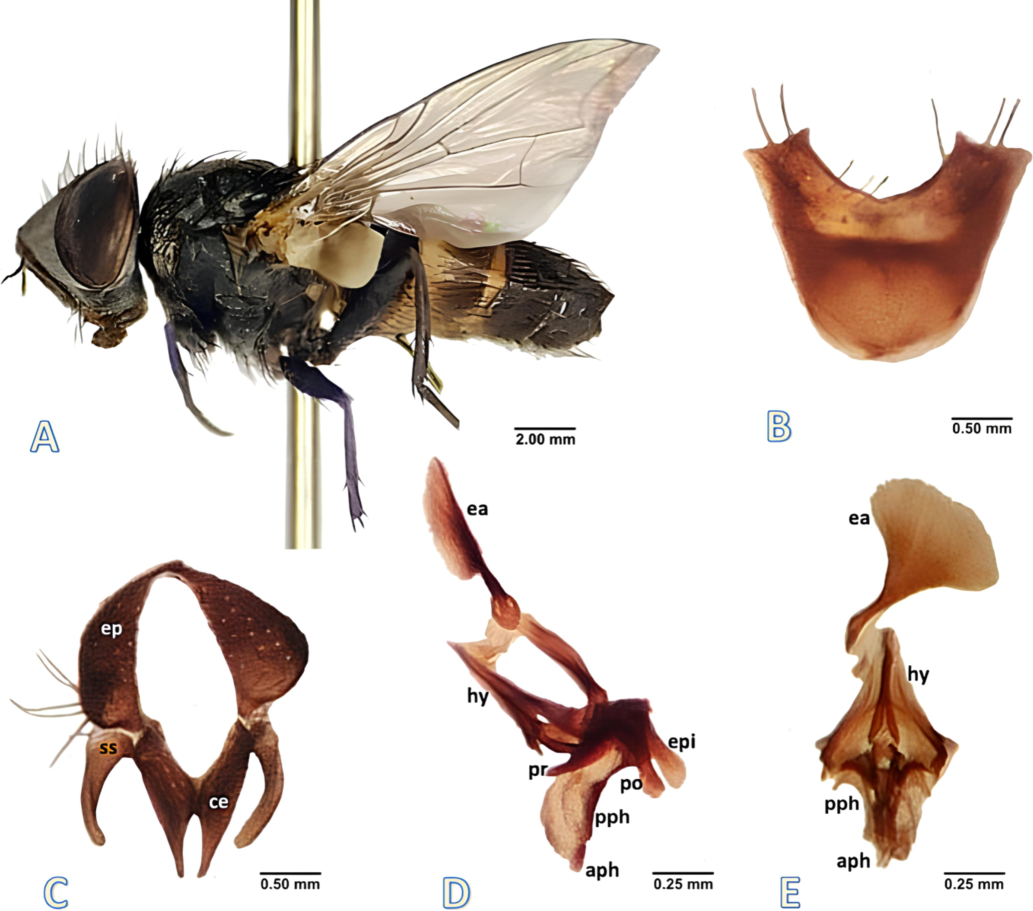
Phylloteles hyalipennis (Baranov, 1934) adult male. A male habitus, lateral view, B fifth sternite, ventral view. C cercus and surstylus, caudal view. D phallus, lateral view. E phallus, ventral view. Abbreviations: aph-acrophallus, ce- cercus, ea-ejaculatory apodeme, ep- epandrium, epi- epiphallus, hy- hypandrium, pph- paraphallus, po- postgonite, pr- pregonite, ss- surstylus.
Head with red eyes; dark frontal vitta; greyish parafrontal with silvery pollen but no setae; parafacial silvery pollen with sparse short setae; antennae elongated and greyish-brown, arista dilated, brownish-black; third antennal segment shorter than arista, about 12 frontal bristles, posterior 6 reclinate and anterior 3 proclinate; 3 proclinate orbital bristles; gena greyish with silvery pollen and short white setae, outer vertical bristles half the length of inner vertical; ocellar region with about 4 pairs of short setae, ocellar bristle as long as inner vertical.
Thorax grey with three black stripes on the dorsal surface, the middle stripe stretching to scutellum; dorsocentral 3 + 3; acrostichal 3 + 2; presutural 1; intra-alar 0 + 2; humeral 2; posthumeral 1; notopleural 2; supra-alar 2; postalar 2; sternopleural 1 + 1; mesopleural 6; hypopleural 8; propleura silver white and without spine; scutellum greyish with a pair of short discoscutellar, 2 pairs of lateroscutellar, and a pair of stout apicoscutellar bristles.
Transparent wings; black epaulet; yellowish basicostal scale; first costal section (CS1) with long bristles at the edge of costaginal break, second costal section (CS2) with humeral break; Sc bare above with spike of stout spines; R1 bare; R4+5 dorsally with a row of 3–4 short setae on basal node to r-m, ventrally 2 short setae on basal node of R4+5; fifth costal segment doubles that of second; yellowish white squama; halter yellowish brown; bend of m somewhat obtuse.
Legs black; forefemur with 2 parallel rows of long bristles run along the posterodorsal surface, ventrally a row of bristles runs along the posterior margin; fore tibia posterodorsally with 1 bristle on distal one-third and 1 bristle posteroventral distally; mid femur having a row of 3–4 bristles present anterodorsally on middle portion, long setae with few short bristles present on posteroventral and anteroventral surfaces with 2 bristles terminally on posterodorsal surface; hind femur with a row of stout bristles present both on anterodorsal and anteroventral surfaces and posteroventrally with few fine bristles on basal one-third; claws and pulvilli short; claw pointed and strongly curved apically.
Dark greyish abdomen, tapered posteriorly, anteriorly with a row of visible shining yellowish-white band on third and fourth abdominal tergites; tergites 1 + 2 and 3 yellowish black; 4 and 5 dark black; second tergite with 2 lateral marginal bristles, median marginal bristles absent; third tergite with short median and 3 lateral marginal bristles; forth with a row of short marginal bristles; fifth with a row of 12 strong marginal bristles; sternites 1–4 covered by long setae with a pair of strong marginal bristles posteriorly; fifth V-shaped without window, the lateral arms blunt and have few setae (Fig. 1B); Inner and outer forceps narrow terminally, inner forceps slightly hairy with pointed end (Fig. 1C); outer forceps more broader than the inner and blunt terminally (Fig. 1C); ejaculatory apodeme broad and flat (Fig. 1D, E); pregonite large, broad basely, gradually curved, and terminally pointed (Fig. 1D); postgonite flat, dilated structure, wide basely, slightly rounded, and narrow terminally (Fig. 1D); paraphallus divaricated, long and curved, gradually narrowed distally with hook-like spiky acrophallus (Fig. 1D, E); epiphallus laterally flat, elongated, and wide apically (Fig. 1D).
Female Diagnosis (Fig. 2): Female length 5.0–6.5 mm.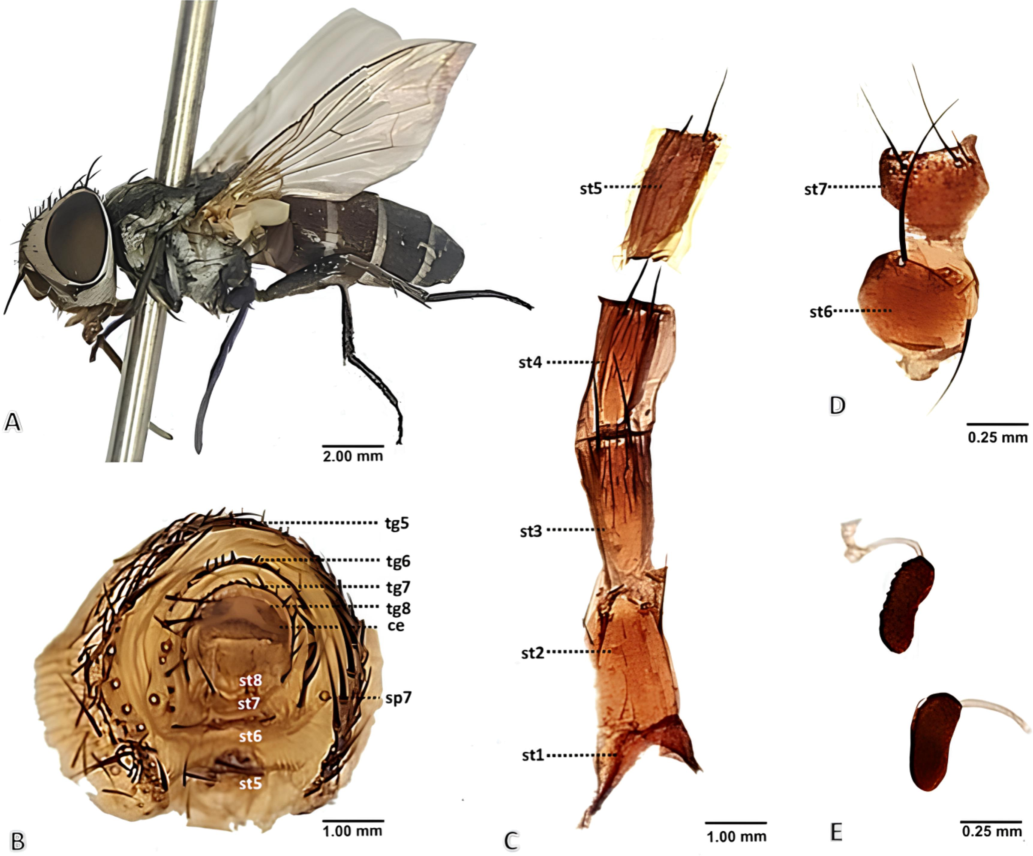
Phylloteles hyalipennis (Baranov, 1934) adult female. A female habitus, dorsal view. B terminalia, posterior view. C sternites 1–5, ventral view. D sternites 6 + 7, ventral view. E spermathecae. Abbreviations: ce- cercus, sp- spiracle, st- sternite, tg- tergite.
Head with reddish brown eyes; frontal vitta reddish anteriorly, gradually dark and widened posteriorly; parafrontal broad, grey, and silvery tinted, bare; parafacial silver with reddish pollen, frons, and parafacial broader; antennae short, oval-shaped, first and second antennal segments with short setae, third segment entirely reddish-brown, arista not dilated; third antennal segment shorter than arista, facial ridge with few white setae, 10–12 frontal bristles; 4 orbital bristles, anterior 3 proclinate, posterior 1 reclinate; gena silvery pollen with long white setae; outer vertical bristles half the length of inner vertical; ocellar region silver-white with about 6 pairs of short setae, and ocellar bristles are as long as outer vertical.
Thorax black with white–grey coating but with well-defined median and lateral black stripes; dorsocentral 3 + 4; acrostichal 1 + 2; presutural 1; intra-alar 0 + 3; humeral 2; posthumeral 1; notopleural 2; supra-alar 2; postalar 2; sternopleural 1 + 1; mesopleural 6; hypopleural 6; propleura silver white and without spine; scutellum greyish with a pair of short discoscutellar, 2 pairs of lateroscutellar and a pair of stout apicoscutellar bristles, discoscutellar bristle short, apical and latero-cutellar bristles share the same size.
Transparent wings; black epaulet with 2 long bristles; basicostal scale yellowish; CS1 with a long bristle at the edge of costaginal break, CS2 with a humeral break; SC bare above with spike of a stout spine; R1 bare; R4+5 dorsally with a row of 3–5 short setae on basal node to r-m, ventrally 2–3 short setae on basal node of R4+5; r4+5 closed; second costal segment doubles that of fifth; yellowish white squama; halter yellowish brown; bend of m somewhat obtuse; m and cu slightly curved.
Legs black; forefemur has a row of bristles on the dorsal side; posterodorsal surface with two parallel rows of long bristles; ventrally, a row of bristles runs along the posterior margin; foretibia has a bristle at the middle and distal end of the posterodorsal surface, posterodorsal and posteroventral regions each have one bristle distally; mid femur has a row of 3–4 bristles along the subbasal region posteroventrally, mid tibia anterodorsally with 1 bristle medially and 1 bristle distally, posterodorsal surface has 1 bristle at subdistal region and 1 bristle distally, 1 bristle present at midpoint posteriorly; hind femur with a row of bristles anterodorsally and posteriorly with a bristle at the subbasal region; hind tibia with a row of bristles along the anterodorsal region, 2 bristles anteroventrally present at the subdistal and distal region; claws and pulvilli short; claw sharp but less curved apically.
Abdomen slightly brownish-grey, oval, anteriorly with white band on third to fifth abdominal tergites; third tergite with short median marginal bristles; fourth tergite with median and 4 short lateral marginal bristles; fifth with a row of 16–18 strong marginal bristles (Fig. 2B); sixth entire with many stout marginal bristles (Fig. 2B), seventh well sclerotized, with a row of short marginal bristles (Fig. 2B); eight membranous (Fig. 2B); ninth sclerotized with lateral lobes and membranous middle portion (Fig. 2B); sternites first to fifth with closely set short setae and a pair of long bristles on posterior margin (Fig. 2C); sternite sixth rounded, slightly grooved posteriorly (Fig. 2D); sternite seventh somewhat triangular shaped (Fig. 2D); both with two pair of strong bristle posteriorly (Fig. 2D); sternite eight membranous and well differentiated, anal cercus somewhat rectangular with short setae posteriorly (Fig. 2B); spermathecae elongated oval structure with striated segmental constrictions (Fig. 2E).
3.2 Difference between male and female specimens
Male is like female but can be differentiated by the following characteristics. In female, head with more orbital bristles to male; arista not dilated; frons, and parafacial broader than male; antennae oval-shaped; female gena hairier compared to male; claw in legs sharp but less curved apically to male; sternite 5th to 7th in female posteriorly with a pair of long bristles.
Immature stages (Fig. 3).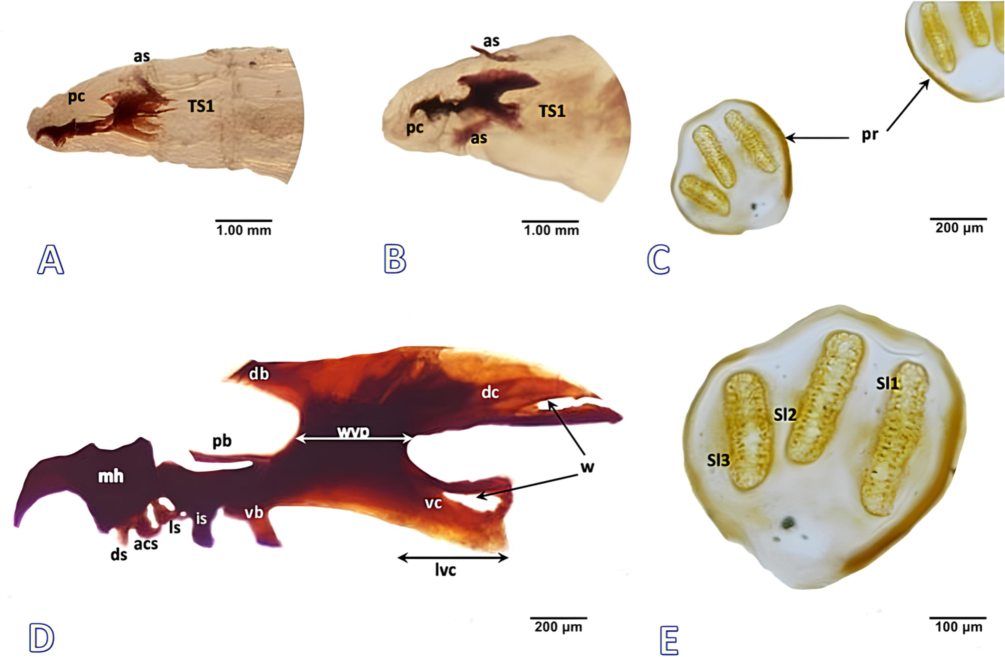
Larvae of Phylloteles hyalipennis (Baranov, 1934). A First instar, anterior view. B Second instar, anterior view. C Posterior spiracle, third instar. D Cephaloskeleton, third instar. E Left posterior spiracle, third instar. Abbreviations: acs- accessory stomal sclerite, as- anterior spiracle, db- dorsal bridge, dc- dorsal cornu, ds- dental sclerite, is- intermediate sclerite, ls- labial sclerite, lvc- length of ventral cornu, mh- mouthhooks, pb- parastomal bar, pc- pseudocephalon, sl- spiracular slit, TS- thoracic segment, vb- ventral bridge, vc- ventral cornu, w- window, wvp- width of vertical plate.
First instar: Length, 2.0–2.5 mm; diameter, 1.0–1.5 mm.
Pseudocephalon (Fig. 3A). Antennal complex short and oval throughout, antennal dome and antennal basal ring undifferentiated; maxillary palpus shaped as a plane disc not clearly distinguished from surrounding cuticle and lack proper central and dorsal region; labial organs are bulky and fleshy; grooved oral ridges lacking margin anteriorly; pseudocephalon with cuticular ridges behind the antennal complex.
Cephaloskeleton (Fig. 3A). Mouth hooks short, slender anterior part of mouth hooks shorter than the broader basal part, hooks in the anterior part of the mouth bend downward with pointed tips; In lateral view, intermediate sclerite is a long, thin structure located below parastomal bars; parastomal bars moderately long; dorsal bridge absent, dorsal cornua window is wide open, and the ventral cornua window is indistinct; vertical plate width shorter to the ventral cornua.
Second instar: Length, 5.0–6.0 mm; diameter, 2.5–3.0 mm.
Pseudocephalon (Fig. 3B). Antennal complex differentiated, antennal dome oval with distinctly rounded tip; maxillary palpus distinguished from surrounding cuticle with separate central and dorsal region; although, all sensilla are undifferentiated; labial organ increased size; oral ridges apparent as rows of irregular processes with anterior border.
Cephaloskeleton (Fig. 3B). Mouth hooks elongated, anterior part of mouth hooks comparable with basal part; In lateral view, intermediate sclerite increased size; parastomal bars long and straight; dorsal bridge visible, dorsal cornua window indistinct, and the ventral cornua window visible; vertical plate with comparable width to ventral cornua.
Scanning electron micrographs of third-instar larvae and puparia were obtained and scrutinized to provide a complete description of this species.
Third instar: Length, 8.0–9.0 mm; diameter, 3.0–4.0 mm.
Pseudocephalon (Fig. 4A, B). Antennal complex more pronounced, antennal dome fixed in socket of prominent antennal basal ring; maxillary palpus flattened with the lobed protuberance, positioning anteriorly on pseudocephalon; six different sensilla grouped in maxillary palpus's central region as a separate lobe, only three of which placed in swelling sockets; all three sensilla coeloconica located in distinct sockets and similar in size; two more sensilla coeloconica (ns1, ns2) positioned on maxillary palpus’s separate dorsal lobe, ns2 broader than the ns1; oral ridges originating from the bottom of functional mouth; oral ridges differentiated laterally and posteriorly by edge of cheek organ; Keilin’s organ with three somewhat elongated sensilla.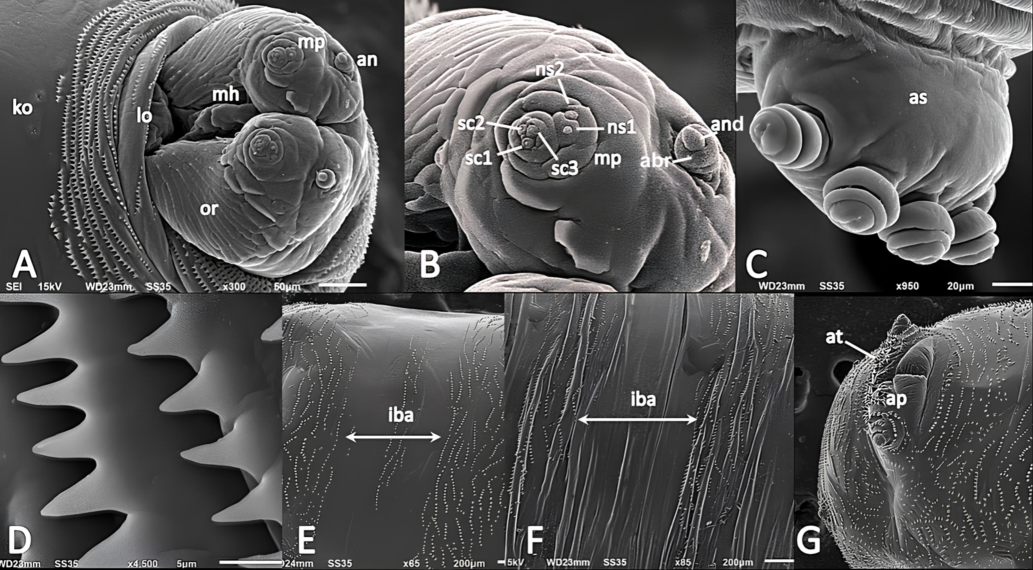
SEM analysis of Phylloteles hyalipennis (Baranov, 1934) third instar larva. A anterior end, antero-ventral view. B antennal complex and maxillary Palpus. C anterior spiracle. D anterior spines. E abdomen, ventral view. F abdomen, dorsal view. G posterior end, ventral view. Abbreviations: abr- antennal basal ring, an- antennal complex, and- antennal dome, as- anterior spiracle, at- anal tuft, ap- anal pad, iba- inter-band area, ko- Keilin’s organ, lo- labial organ, mh- mouth hook, mp- maxillary palpus; ns1- first additional sensillum coeloconicum; ns2- second additional sensillum coeloconicum, or- oral ridges, sc1–3- sensilla coeloconica 1–3.
Cephaloskeleton (Fig. 3D). Mouth hooks short, curved anteriorly downward with pointed tips; mouth hooks mid-dorsally with a tiny, rounded noticeable protrusion; dental sclerite short and straight; accessory stomal sclerite coma shaped and broader than dental sclerite; labial sclerite distinct and analogous to the accessory stomal sclerite; parastomal bars long, slender, and pointed upward; dorsal bridge narrow, somewhat straight with a pointed end; dorsal bridge extended halfway to parastomal bars; intermediate sclerite and ventral bridge appeared as thick tubercles of comparable size in lateral view; dorsal cornu is curved gradually toward apex and twice as long as ventral cornu; dorsal cornu windows are quite large and open; ventral cornu is equivalent in width to vertical plate; ventral cornu windows are wide and closed.
3.3 Puparium
Length, 6.0–7.0 mm; diameter, 3.0–3.5 mm; fusiform shape; initially light reddish-black, darkening as it approaches adult emergence; segmentation indistinct; anterior spiracles dark, exhibiting a fan-shaped structure with 5–7 rounded lobes; posterior spiracular plate arranged in very shallow depression; spiracular slits darker and more sclerotized than larval instars; abdominal segments display regular set of transverse cuticular ridges across entire surface; puparium and third instar larva share majority of similar characteristics; spine configurations on the spinose bands more visible in puparium, spine appeared shrunk and smaller in puparium.
3.4 Detailed morphology of third instar and puparia (Fig. 4)
Anterior spiracles (Fig. 4C):
Anterior spiracle is an elongated, oval-shaped structure with five to seven finger-like lobes; lobes are small, knobby, and segmented into striated circular constrictions; the lobes are arranged in a single regular row.
Shape and arrangement of spines (Fig. 4C, D, E, F):
Spines well developed; anterior spines initially appeared on spinose band, posterior spines present on inner-band region; inter-band areas on thoracic segment devoid of spine; spine appeared conical shaped with a broad base and pointed apex; laterally and ventrally the anterior spines arranged in serrated clusters and fused basally in regular rows; spine ranges from single to bifurcate tip; entire anal segment covered by spines, ventrally anal pad lack spines; anal papillae covered by circular rings of spines; anal tuft with several large, sturdy spines.
Spiracular field and accompanying structures (Fig. 4G):
Spiracular cavity weakly developed; spiracular field surrounded by a symmetrical ring of spines; papillae enclosing spiracular field small and visible as cone-shaped protuberances; posterior spiracles distinctly visible as sclerotized plates from posterior view; anal segment posteriorly between spiracular field and anal pad with circular depression; anal papilla entirely rounded, robust structure rests ventrally on fleshy anal pad (Fig. 4G).
Posterior spiracles (Fig. 3C, E):
Two symmetrical posterior spiracular plates located in spiracular field; under light microscope, posterior spiracles with three ventrally elongated, golden-brown spiracular slits; peritreme nearly circular; spiracular slits oval and straight; outer and middle slits (sl3 + sl2) comparable in length; while inner one (sl1) larger than both, sl2 is oriented basally toward the sl3; button indistinct; the dorsal arc of peritreme more defined than the ventral arc.
4 Discussion
The predatory behavior of Sarcophagidae appears to have evolved from predominantly invertebrate sarcosaprophagy in subfamily Sarcophaginae, vertebrate sarcosaprophagy in subfamily Miltogramminae, or general sarcosaprophagy in vertebrates and invertebrates in subfamily Paramacronychiinae (Yan et al., 2021). In adult males, the shape of the cercus, surstylus, fifth sternite, and phallus exhibits interspecific variation and is used to identify miltogramminae species along with some other characters (i.e., head, fore tarsus, and wings) (Nandi, 2002; Zhang et al., 2014). Since this subfamily's adults have been the subject of ample research over the last ten years, morphological data for practically all of the world's taxa are now readily available (Szpila et al., 2021).
Over the last two decades, studies conducted worldwide contributed to a notable increase in data about the identification of immature stages of Miltogramminae (Szpila and Pape, 2005b; Szpila and Pape, 2007; Szpila et al., 2015; Szpila et al., 2021). However, in the case of Oriental taxa immature stages morphology remains unavailable, and the Indian sarchophagid fauna stands out in morphological data on Miltogramminae immature stages. The larvae of subfamily Miltogramminae exhibit a strikingly high degree of morphological variation compared to other sarcophagid subfamilies (Szpila and Pape, 2005b; Szpila and Pape, 2007; Buenaventura et al., 2020). Among the Miltogramminae genera, the larvae of the genus Phylloteles display considerable morphological similarity that makes its species more difficult to identify (Szpila et al., 2015). The third instar larvae of Phylloteles hyalipennis shows many morphological resemblances to the Phylloteles pictipennis Loew, 1844, including number of lobes of anterior spiracles, short window in cornua of cephaloskeleton etc. However, differences are observed e.g., the conical shaped integumental spines at P. hyalipennis in contrast to the warts or wart-shaped spines in P. pictipennis. Additionally, spiracular slits in posterior spiracles of P. hyalipennis are relatively more separate and do not point toward opening of peritreme, unlike in P. pictipennis (Szpila and Pape, 2007).
Since several species in the genus Phylloteles are known to be associated with decomposing organic matter, such as buried vertebrate carrion and chicken carcasses, these findings reveal their importance as forensic indicators. Further research on the adult and immature stages of Indian Miltogramminae is required, as it could enlighten new information about these flies.
5 Conclusion
The present new record of Phylloteles hyalipennis (Baranov, 1934) is extremely important regarding the Miltogramminae species of India. This research intends to be more accurate in identifying this species by using combined adult and immature characteristics documented through light and electron microscopy. This study fills a gap regarding larval morphology of subfamily Miltogramminae and Oriental taxa and contributes towards the world’s databases about immature stages. A detail study of Phylloteles hyalipennis with all its developmental stages gives us an idea of morphological variation amongst the genera, laying down an important groundwork for investigations on adult and immature stages of Indian Miltogramminae. This study can open the way for further information on ecological functions, and in veterinary, medical and forensic science as well as in evaluating environmental changes to some extent.
Authors contribution- MB- conceptualized, framed and analysed the research proposal, RK-conducted the experiment, curated data and prepared original draft, AHS- Analysed experimentation, Final analysis of manuscript and Funding acquisition.
CRediT authorship contribution statement
Rohit Kumar: Writing – original draft, Validation, Resources, Methodology, Investigation. Althaf Hussain Shaik: Writing – review & editing, Visualization, Validation, Project administration, Funding acquisition. Madhu Bala: Writing – review & editing, Validation, Supervision, Investigation, Formal analysis, Data curation, Conceptualization.
Acknowledgments
The authors are thankful to the Department of Zoology and Environmental Sciences, Punjabi University, Patiala (India) for providing a laboratory facility and King Saud University, Riyadh, Saudi Arabia for funding, project no (RSP2024R371).
The authors are grateful for valuable comments made by two reviewers.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anchored hybrid enrichment challenges the traditional classification of flesh flies (Diptera: Sarcophagidae) Syst. Entomol.. 2020;45(2):281-301.
- [Google Scholar]
- The natural enemies of Philantus triangulum. Kolkhozno-Sovkhoznoye Proizvedeniya Turkmenii 1964 [1964](5).84–88
- [Google Scholar]
- The effect of anthropization on Sarcophagidae (Diptera: Calyptratae) community structure: an assessment on different types of habitats in the Humid Chaco ecoregion of Argentina. J. Med. Entomol.. 2020;57(5):1468-1479.
- [Google Scholar]
- Anchored phylogenomics and revised classification of the Miltogramminae (Diptera: Sarcophagidae) Syst. Entomol.. 2024;49(1):138-155.
- [Google Scholar]
- Krohn, C. 2007. Okologische Untersuchungen zur Insektenfauna in Nestern der Unechten Karettschildkrote, Caretta caretta, in der Bucht von Kyparissia, Griechenland. Master’s Thesis, Kiel University, Kiel.
- Kumar, R., Sayed, S., Bala, M., et al. 2021. Ultramorphological study of immature stages and male genitalia of forensically significant flesh fly Sarcophaga dux thomson, 1868 (Diptera: Sarchophagidae). Journal of King Saud University, Science 33, 101460. https://doi.org/10.1016 /j.jksus.2021.101460.
- Revised keys to the flesh flies of Thailand, with the establishment of a new genus (Diptera: Sarcophagidae) Med. Entomol. Zool. 2018;69(2):67-93.
- [CrossRef] [Google Scholar]
- Molecular phylogeny of the Calyptratae (Diptera: Cyclorrhapha) with an emphasis on the superfamily Oestroidea and the position of Mystacinobiidae and McAlpine’s fly. Syst. Entomol.. 2010;35:614-635.
- [CrossRef] [Google Scholar]
- Fauna of India: Diptera: Sarcophagidae. Published by Zoological Survey of India.. 2002;10:270-274.
- [Google Scholar]
- Catalogue of the Sarcophagidae of the world (Insecta: Diptera) Memoirs Entomology International. 1996;8:1-558.
- [Google Scholar]
- A new species of Hoplacephala Macquart (Diptera: Sarcophagidae) from Namibia, with a discussion of generic monophyly. Zootaxa. 2006;1183(1):57-68.
- [Google Scholar]
- A large-scale molecular phylogeny of flesh flies (Diptera: Sarcophagidae) Syst. Entomol.. 2014;39:783-799.
- [Google Scholar]
- Molecular phylogeny of Miltogramminae (Diptera: Sarcophagidae): implications for classification, systematics, and evolution of larval feeding strategies. Mol. Phylogenetic Evol.. 2017;116:49-60.
- [Google Scholar]
- Report of some satellite flies (Diptera, Miltogramminae) in West Bengal, India, for the first time. National Bengal Univ. J. Anim. Sci.. 2012;6:1-7.
- [Google Scholar]
- The first instar larva of Apodacra pulchra (Diptera: Sarcophagidae, Miltogramminae) Insect Systematics Evol.. 2005;36:293-300.
- [Google Scholar]
- Comparative morphology of the first instar larva of three species of Metopia Meigen (Diptera: Sarcophagidae) Acta Zool.. 2005;86:119-134.
- [Google Scholar]
- Rediscovery, redescription and reclassification of Beludzhia phylloteliptera (Diptera: Sarcophagidae: Miltogramminae) Eur. J. Entomol.. 2007;104(1):119-137.
- [Google Scholar]
- Morphological diversity of first instar larvae in Miltogramma subgenus Pediasiomyia (Diptera: Sarcophagidae, Miltogramminae) Zoological Anzeiger. 2008;247:259-273.
- [Google Scholar]
- A new dipteran forensic indicator in buried bodies. Med. Vet. Entomol.. 2010;24:278-283.
- [Google Scholar]
- First instar larvae of endemic Australian Miltogramminae (Diptera: Sarcophagidae) Sci. Rep.. 2021;11(1):1-12.
- [Google Scholar]
- The phylogenetic systematics of the miltogramminae flies (Diptera: Sarcophagidae) of the world. Japan. J. Med. Sci. Biol.. 1989;42:111-126.
- [Google Scholar]
- A key to genera and subgenera of Palaearctic Miltogramminae (Diptera: Sarcophagidae) with a description of a new genus. Diptera Res.. 1994;5:239-247.
- [Google Scholar]
- First mitogenome for the subfamily Miltogramminae (Diptera: Sarcophagidae) and its phylogenetic implications. Eur. J. Entomol.. 2017;114:422-429.
- [Google Scholar]
- A phylotranscriptomic framework for flesh fly evolution (Diptera, Calyptratae, Sarcophagidae) Cladistics. 2021;37(5):540-558.
- [Google Scholar]
- Taxonomic review of the Sphecapatodes ornata group (Diptera: Sarcophagidae: Miltogramminae), with description of one new species. Zool. Stud.. 2014;53(1):1-12.
- [Google Scholar]
- The Genus Phylloteles Loew (Diptera: Sarcophagidae; Miltogramminae) in Africa and Europe. Bulletin & Annales De La Société Royale D'entomologie De Belgique. 1973;109:308-319.
- [Google Scholar]







