Translate this page into:
Modulatory effects of glutamic acid on growth, photosynthetic pigments, and stress responses in olive plants subjected to cadmium stress
⁎Corresponding authors at: Department of Environmental Sciences and Engineering, Government College University Allama Iqbal Road, Faisalabad 38000, Pakistan (S. Ali). muhammadanas@bs.qau.edu.pk (Muhammad Anas), shafaqataligill@yahoo.com (Shafaqat Ali), mirmazloum.seyediman@uni-mate.hu (Iman Mirmazloum)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cadmium (Cd) is a toxic heavy metal that severely impacts plant growth and photosynthesis and induces oxidative stress. This study investigates the modulatory effects of glutamic acid (GA) on Olea europaea (olive) seedlings subjected to cadmium stress. The experiment included control, Cd-stressed, GA-treated, and combined Cd and GA-treated groups. Cd exposure significantly reduced plant growth, as evidenced by decreased root length (3.5 cm) and shoot length (9 cm) compared to control plants (5 cm and 12 cm, respectively). Additionally, Cd stress led to a reduction in chlorophyll content (16.2 mg/g fresh weight) and elevated oxidative markers like H2O2 and MDA. The application of GA significantly improved plant growth and physiological parameters, with statistically significant increases in root length (up to 6.5 cm) and shoot length (up to 14 cm) in the combined treatment group (p ≤ 0.05). Furthermore, GA treatment led to a marked elevation in total chlorophyll content (up to 27.5 mg/g fresh weight), compared to 16.2 mg/g in Cd-stressed plants (p ≤ 0.05), reflecting a significant improvement in photosynthetic efficiency. GA also elevated the antioxidant enzyme activity catalase (CAT) and peroxidase (POD), reducing oxidative stress by decreasing hydrogen peroxide and MDA levels. The findings suggest that glutamic acid effectively mitigates Cd-induced phytotoxicity, enhancing stress resistance and promoting plant growth. This research provides valuable insights into using glutamic acid as a possible approach to mitigate heavy metal stress in plants, offering implications for agriculture and environmental management in Cd-contaminated areas. Specific applications may include its use in phytoremediation practices or as a supplement in agricultural management to improve crop resilience in polluted environments. Further research could explore the molecular mechanisms underlying GA’s protective effects and its potential synergy with other biostimulants to enhance heavy metal tolerance in a broader range of crops.
Keywords
Cadmium
Glutamic acid
Oxidative stress
Olea europaea
Phytoremediation
1 Introduction
Glutamic acid (Glu) is a non-critical amino acid in plants that is crucial for multiple physiological and biochemical processes, particularly in modulating plant responses to abiotic stresses like heavy metal toxicity, such as cadmium (Cd) exposure (Liao et al., 2022). Cd is a pervasive environmental pollutant that negatively impacts plant growth and development, causing growth retardation, reduced photosynthetic capacity, and oxidative stress resulting from reactive oxygen species (ROS) buildup (Genchi et al., 2020; Ma et al., 2022; Okla et al., 2023). Recent studies have shown that glutamic acid can alleviate the harmful effects of Cd in plants by enhancing nutrient uptake, modulating phytohormone levels, and increasing antioxidant enzyme activities, which together promote better growth under Cd stress (Alfosea-Simón et al., 2021, Shan et al., 2022). Moreover, glutamic acid supplementation has been observed to improve photosynthetic efficiency by stabilizing chlorophyll pigment synthesis and protecting the photosynthetic apparatus from Cd-induced damage, thereby countering the inhibition of chlorophyll biosynthesis and interference with the photosynthetic system (Gill and Tuteja 2010, Grajek et al., 2020). The enhancement of photosynthetic machinery is critical as it directly affects plant energy metabolism and biomass accumulation, which are often compromised under heavy metal stress (Afzal et al., 2020; Imran et al., 2023).
Beyond its role in photosynthesis and nutrient uptake, glutamic acid is involved in complex signaling networks and gene regulation processes that contribute to plant stress resilience. It may activate distinct signaling cascades, including pathways like mitogen-activated protein kinase (MAPK), that modulate gene expression related to stress defense mechanisms, including the upregulation of genes encoding for metallothioneins and phytochelatins. These molecules are essential for detoxifying Cd by sequestrating it and facilitating its compartmentalization within plant cells (Rennenberg and Herschbach 2014, Qiu et al., 2019). Additionally, glutamic acid enhances the synthesis of glutathione (GSH), a critical antioxidant that maintains redox homeostasis under stress conditions (Aoyama and Nakaki 2015). Increased GSH levels contribute to better management of oxidative stress induced by Cd, thus preserving cellular integrity and function by reducing lipid peroxidation and protecting cellular membranes (Cuypers et al., 2010). Recent research has also highlighted glutamic acid's role in epigenetic regulation and the involvement of small and long non-coding RNAs in Cd-induced transcriptional responses, suggesting new avenues for genetic improvements in plants against heavy metal stress through advanced techniques like CRISPR/Cas9 and genome-wide association studies (GWAS) (Al-Khayri et al., 2023, Li et al., 2023). These findings underscore the potential for glutamic acid not only as a biochemical modulator but also as a target for genetic manipulation to enhance plant resilience.
While the role of glutamic acid in mitigating Cd stress has been explored in various plant species, its specific effects on Olea europaea remain under-researched. Olea europaea, commonly known as the olive tree, is economically significant and valued for its ability to thrive in harsh environments. However, like other plants, it is vulnerable to heavy metal contamination, which can severely affect its growth and productivity. This study is the first to comprehensively examine the combined effects of glutamic acid on growth, photosynthetic efficiency, and antioxidant activity in Olea europaea under cadmium stress, thereby filling a significant gap in current research. The research focuses on understanding how glutamic acid modulates integrated physiological, biochemical, and molecular responses in olive trees exposed to Cd stress, including its impact on plant biomass, chlorophyll content, and antioxidant defense mechanisms. By highlighting these aspects, this study provides valuable insights into how biostimulants like glutamic acid can be utilized to improve crop resilience in Cd-contaminated environments, offering practical applications for sustainable agricultural practices and contributing to broader strategies for enhancing plant stress tolerance (Jiang et al., 2020). Future research could build on these findings by exploring the synergistic effects of glutamic acid with other biostimulants and its potential applications in other economically important crops.
2 Methods
2.1 Plant selection and preparation
The olive tree (Olea europaea L.) was chosen as the focus of this study due to its symbolic importance within the Spanish flora and its economic significance in agriculture. To ensure a controlled environment free from contamination by any external organisms, we produced an aseptic, soilless medium for early development phases in vitro. The seeds were disinfected with a 1-minute rinse in 70 % ethanol, followed by a 10-minute soak in 5 % sodium hypochlorite, and finished with a thorough wash in sterile water to remove any remaining residue. The trays and all tools used for handling were autoclaved at 121 °C for 20 min. The seeds were sown in sterilized trays under a laminar flow hood to maintain sterility and allowed to germinate under controlled conditions at 25 °C with 70 % relative humidity. A 16/8-hour light–dark cycle was employed to simulate natural daylight patterns, which is crucial for the physiological processes of germination and early seedling development (Yin et al., 2021). All subsequent handling and transfer of seedlings were performed under sterile conditions to prevent contamination.
2.2 Experimental setup
The study was divided into four groups: the control group, Cd stress group, glutamic acid group, and combined treatment group. The control treatments were used as the baseline, without the application of cadmium or glutamic acid treatment. The Cd stress group was induced by the application of 50 µM CdCl2 to seedlings, which caused Cd stress but no lethality, allowing the seedlings to show a visible stress response. In the third group, seedlings were treated with 1 mM glutamic acid without Cd stress. The combined treatment group, which was of significant importance in this study, involved seedlings grown in the presence of both 50 µM CdCl2 and 1 mM glutamic acid to examine the ability of glutamic acid to mitigate Cd-induced stress. Each group consisted of 3 replicates, with each replicate containing 5 seedlings, resulting in a total of 15 seedlings per group and 60 seedlings tested across all groups. All treatments were arranged in a completely randomized design to minimize variability and ensure accurate results.
2.3 Growth conditions
The growth conditions of Olea europaea L. seedlings were maintained entirely within a controlled growth chamber designed to simulate optimal environmental conditions. The chamber was set up to maintain a consistent environment that supports the physiological needs of the seedlings, without any comparison to an open field condition. The temperature inside the chamber was regulated with a light/dark program to mimic a natural daily temperature cycle (day: 24 °C, night: 20 °C) that corresponds to the natural habitat of Olea europaea L. The chamber was also set to maintain 60 % relative humidity and a photoperiod of 16 h of light and 8 h of darkness, which simulated a long-day photoperiod suitable for the growth of Olea europaea L. A uniform light intensity of 200 µmol m−2 s−1 was employed, which was sufficient for photosynthesis without causing photoinhibition or being considered stress-inducing light.
2.4 Data collection points
Root and shoot lengths (cm) were recorded after 30 days of application using a standard scale with a precision of 0.1 cm to ensure accurate measurement of growth metrics. Fresh weights of roots and shoots were assessed with a balance with 4-digit precision (0.0001 g), and the immediate biomass was recorded without any moisture weight. This level of precision was consistently applied across all fresh weight measurements, including roots, shoots, and leaves. Dry mass was determined after desiccating samples at 70 °C for 48 h to determine the actual organic matter content in the plant tissues. Of particular interest were leaf traits because of their important functions in photosynthesis and stress response. Leaf length (cm) was measured with a precision of 0.1 cm as a proxy for leaf expansion and development. Fresh weight measurements of the leaves, which represented the immediate biomass quantity, were obtained using the same balance with 4-digit precision (0.0001 g), and the dry weight was calculated after drying in the oven.
3 Biochemical analyses
3.1 Chlorophyll and carotenoids
Chlorophyll and carotenoids were extracted in acetone, centrifuged at 12,000 x g for 10 min, and absorbance was measured at 663 nm for chlorophyll a, 645 nm for chlorophyll b, and 470 nm for carotenoids, following the method of Taffouo et al., (2017). These wavelengths correspond to the peaks of light absorption for these pigments, facilitating their detection and quantification.
3.2 Soluble sugars and proteins
Soluble sugars were measured using the anthrone method Hayat et al., (2024) with absorbance at 620 nm. Protein content was determined via the Bradford assay by incubating leaf extract with dye, then reading absorbance at 595 nm.
3.3 Secondary metabolites and stress markers
Phenolics and flavonoids, key metabolites in plant defense, were measured by colorimetric assays. Phenolics were assessed using the Folin-Ciocalteu method (Berhow 2002), with absorbance read at 750 nm. Flavonoid levels were quantified via the aluminum chloride assay (Berhow, 2002), measuring absorbance at 510 nm. Hydrogen peroxide content followed the method of Velikova et al., (2000), with tissue extracted in TCA and centrifuged at 12,000 x g. MDA was determined using a modified method by Heath and Packer (1968) with TCA, and absorbance was read at 532 and 600 nm.
4 Enzyme activity assays
4.1 Catalase (CAT) and peroxidase (POD) activity assay
The catalase (CAT) assay, based on Hadwan (2018), involved freshly prepared enzyme extracts from plant tissue, homogenized in a phosphate buffer with EDTA and PVP to prevent oxidation, followed by centrifugation. The reaction setup included phosphate buffer and H2O2, with the enzyme extract added to start the reaction. Absorbance at 240 nm was checked every 30 s for 3 min at ambient temperature (25 °C), where a faster decline indicated higher CAT activity.
For the peroxidase (POD) assay, following Anas et al., (2023), fresh extracts were made in phosphate buffer with EDTA and PVP, then centrifuged. The reaction mix contained phosphate buffer, guaiacol, and H2O2. Absorbance at 470 nm was observed every 20 s for 3 min, with higher readings signaling increased POD activity and enhanced stress resistance.
4.2 Statistical analysis
The variations across treatments (Control, Cd alone, Glutamic acid alone, Combined treatment) were assessed through one-way ANOVA (Table 1). Data normality was checked with the Shapiro-Wilk test, and variance uniformity was examined with Levene’s test to meet ANOVA requirements. The independent variable was treatment category, and the dependent variables included biochemical and growth measures. Statistical significance for group differences was set at a p-value of ≤ 0.05. Tukey’s HSD test was applied for post-hoc comparisons among group means to control Type I error (p < 0.05). Outliers, identified via Grubbs’ test, were removed if significant (p < 0.05). Listwise deletion addressed any missing entries to keep dataset consistency. *Cd = Cadmium *GA = Glutamic acid. S1: Control, S2: Glutamic Acid 0.5 mM, S3: Glutamic Acid 0.75 mM, S4: Cd 250 µM, S5: Cd 500 µM, S6: Cd 250 µM + Glutamic Acid 0.5 mM, S7: Cd 250 µM + Glutamic Acid 0.75 mM, S8: Cd 500 µM + Glutamic Acid 0.5 mM, S9: Cd 500 µM + Glutamic Acid 0.75 mM.
Parameter
S1: Control
S2: GA 0.5 mM
S3: GA 0.75 mM
S4: Cd 250 µM
S5: Cd 500 µM
S6: Cd 250 µM + GA 0.5 mM
S7: Cd 250 µM + GA 0.75 mM
S8: Cd 500 µM + GA 0.5 mM
S9:Cd 500 µM + GA 0.75 mM
Root Length (cm)
5
6
6.5
4
3.5
5.5
6
4.5
5
Shoot Length (cm)
12
13
14
10
9
11.5
12.5
10.5
11
Root Fresh Weight (g)
50
55
60
45
40
52
57
47
53
Shoot Fresh Weight (g)
100
110
120
90
80
105
115
95
106
Leaf Fresh Weight (g)
30
35
40
25
20
33
38
28
32
Root Dry Weight (g)
10
11
12
9
8
10.5
11.5
9.5
10.7
Shoot Dry Weight (g)
20
22
24
18
16
21
23
19
21.4
Leaf Dry Weight (g)
6
7
8
5
4
6.5
7.5
5.5
6.7
5 Results
The application of glutamic acid markedly improved the growth attributes of Olea europaea L, with significant increases observed in root and shoot lengths, signifying a robust vertical growth response (Fig. 1a, b). This enhancement extended to the biomass of the plants, as evidenced by notable gains in both fresh and dry weights of roots, shoots, and leaves (Fig. 1c-j). The distinctions in growth parameters were statistically significant, as indicated by the variation in alphabetical letters denoting group means, affirming the efficacy of glutamic acid in fostering plant development under stress conditions.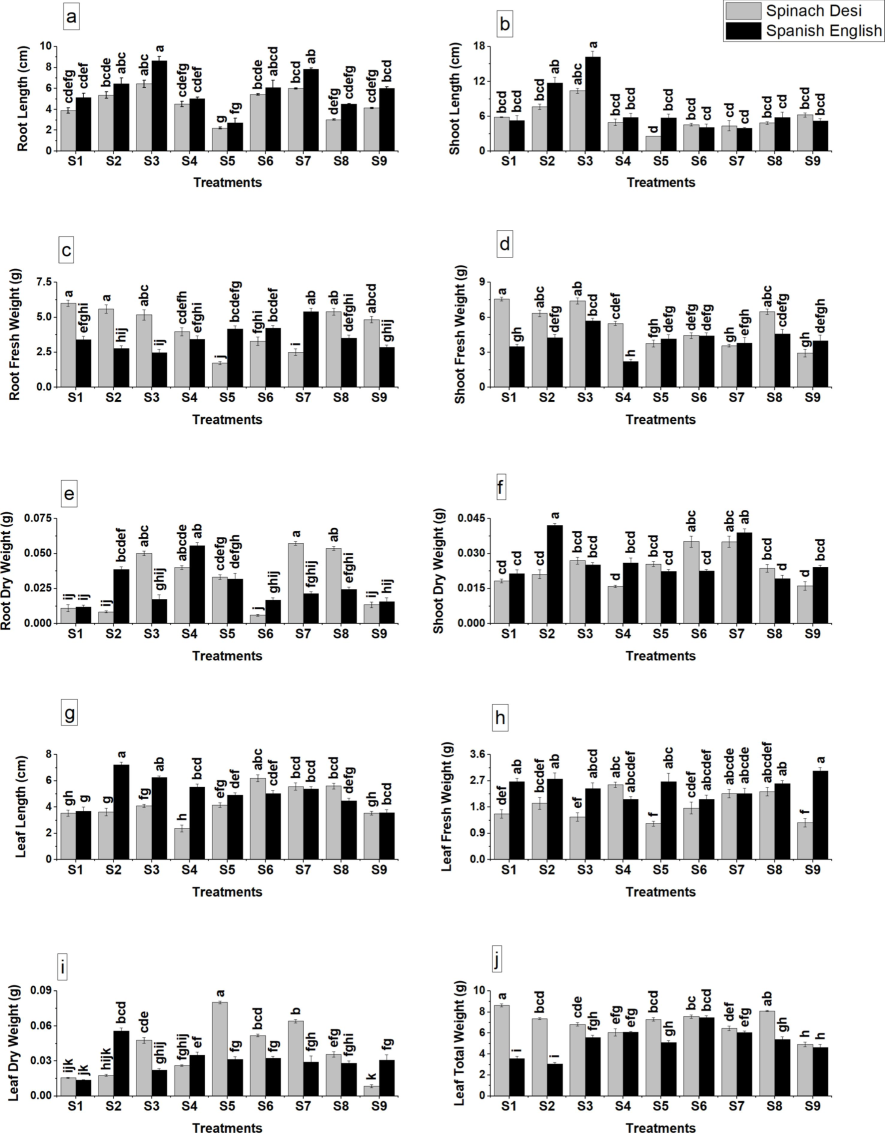
Impact of glutamic acid treatment on (a) root length, (b) shoot length, (c) fresh root weight, (d) fresh shoot weight, (e) dry root weight, (f) dry shoot weight, (g) leaf length, (h) fresh leaf weight, (i) dry leaf weight, and (j) total leaf weight of spinach olive plants under cadmium stress. Values are presented as mean ± SE. Bars with distinct letters denote significant differences at p ≤ 0.05. S1: Control, S2: Glutamic Acid 0.5 mM, S3: Glutamic Acid 0.75 mM, S4: Cd 250 µM, S5: Cd 500 µM, S6: Cd 250 µM + Glutamic Acid 0.5 mM, S7: Cd 250 µM + Glutamic Acid 0.75 mM, S8: Cd 500 µM + Glutamic Acid 0.5 mM, S9: Cd 500 µM + Glutamic Acid 0.75 mM.
Photosynthetic pigments, crucial for the plant's energy capture and stress adaptation mechanisms, showed significant improvement. In Fig. 2, the number of leaves per plant was recorded across nine different treatment scenarios (S1-S9). Both the control group (Spanish Des) and the experimental group (Spanish English) displayed variable responses, with significant differences marked by disparate alphabetical letters above the bars at the p ≤ 0.05 significance level. Chlorophyll a (graph b) and chlorophyll b (graph c) concentrations followed a similar pattern, showcasing the impact of the treatments with fluctuations in content across the groups (Fig. 2b-e). This enhancement suggests a fortified photosynthetic apparatus, essential under stress conditions. The chlorophyll a/b ratio (graph d), total chlorophyll content (graph e), total soluble sugar (graph f), total soluble protein (graph g), and anthocyanin content (graph h) also revealed significant alterations under different treatment conditions (Fig. 2f, g), highlighting an improved metabolic status conducive to stress resilience. The increase in anthocyanin content (Fig. 2h) further underscores the role of glutamic acid in activating stress defense pathways.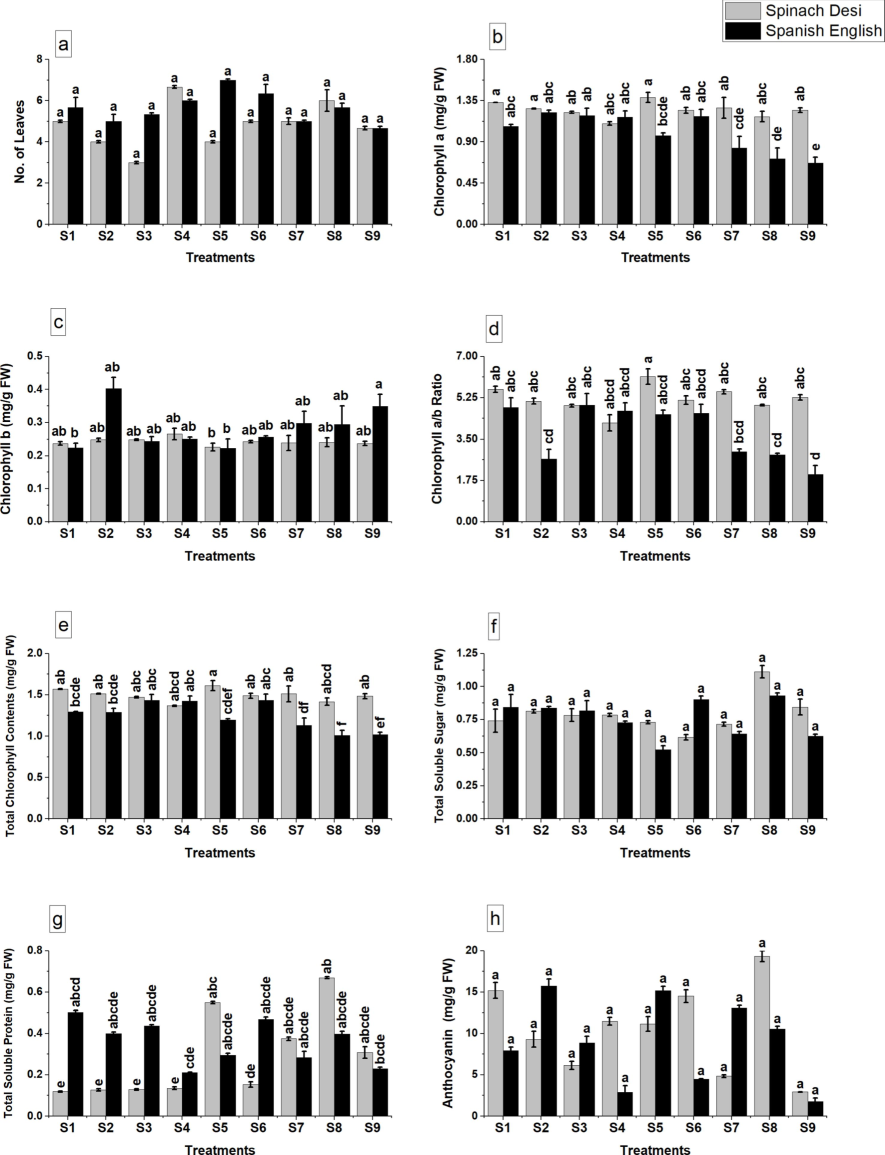
Effect of application of glutamic acid on a: no. of leaves, b: chlorophyll a, c: chlorophyll b, d: chlorophyll a/b ratio, e: total chlorophyll contents, f: total soluble sugar, g: total soluble protein, h: anthocyanin of olive plants under Cd stress. Data are mean value ± SE. Bars with different letters are significantly different at p ≤ 0.05 level.
S1: Control, S2: Glutamic Acid 0.5 mM, S3: Glutamic Acid 0.75 mM, S4: Cd 250 µM, S5: Cd 500 µM, S6: Cd 250 µM + Glutamic Acid 0.5 mM, S7: Cd 250 µM + Glutamic Acid 0.75 mM, S8: Cd 500 µM + Glutamic Acid 0.5 mM, S9: Cd 500 µM + Glutamic Acid 0.75 mM.
Biochemical assays revealed a significant impact of glutamic acid on stress markers. There was a notable reduction in stress-induced hydrogen peroxide and malondialdehyde (MDA) levels (Fig. 3c, d), indicating decreased oxidative stress. Conversely, beneficial compounds like phenolics, flavonoids, and proline were augmented (Fig. 3a, b, e), suggesting enhanced stress defense. The activities of antioxidant enzymes catalase (CAT) and peroxidase (POD) were also elevated (Fig. 3f, g), further corroborating the ameliorative effects of glutamic acid on stress responses.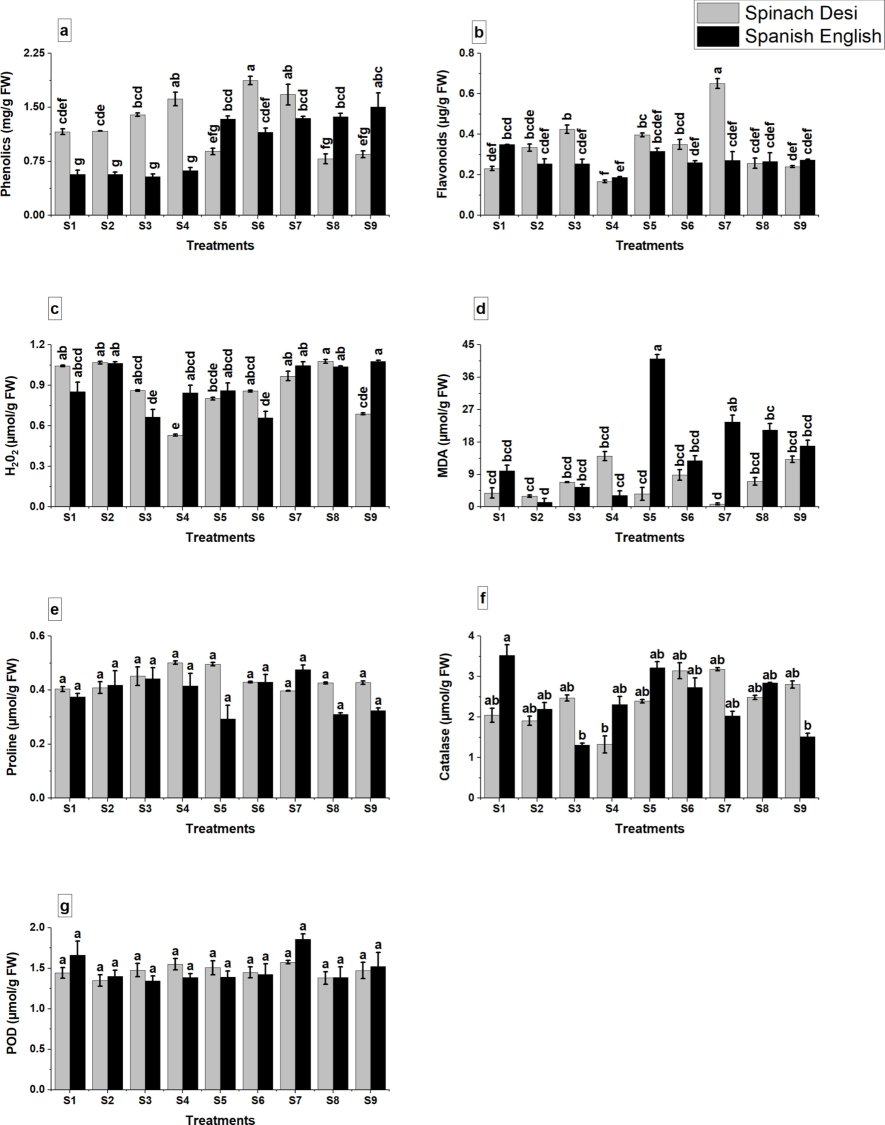
Effect of application of glutamic acid on a: phenolics, b: flavonoids, c: hydrogen peroxide, d: MDA, e: proline, f: catalase, g: POD of olive plants under Cd stress. Data are mean value ± SE. Bars with different letters are significantly different at p ≤ 0.05 level.
The principal components analysis (PCA) outlined in Fig. 4 provided a holistic view of the treatment effects, illustrating the relationships between different plant parameters under the influence of glutamic acid and Cd stress. The PCA vectors revealed how each trait contributed to the plant's overall response to the treatments. In this analysis, two principal components—PC1 and PC2—capture a combined total of 33.5 % of the variance within the dataset, with PC1 accounting for 18.1 % and PC2 for 15.4 %, traits such as chlorophyll content (Chl A, Chl B, and Total Chl), total soluble proteins (TSP), and leaf total weight (LTW) are strongly aligned with PC1, suggesting a high correlation with this component. In contrast, other traits like root fresh weight (RFW), shoot fresh weight (SFW), and malondialdehyde (MDA) content are more closely associated with PC2 (Fig. 4).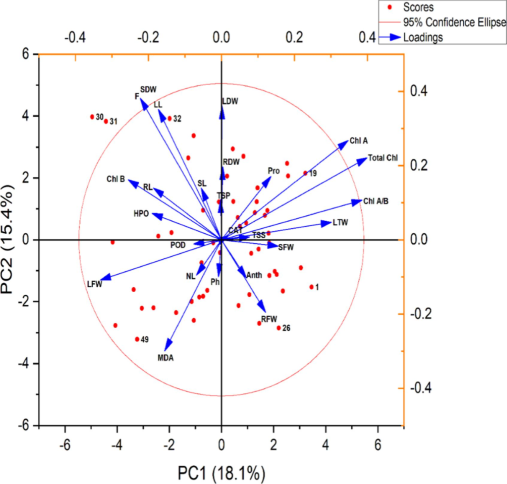
Principal components analysis (PCA) showing the relationship of various parameters of olive plant grown under treatment of glutamic acid and Cd stress. Variables (olive plants' traits) used for the PCA are displayed with their vector.
Abbreviations of traits used.
SL: shoot length, RL: root length, RFW: root fresh weight, RDW: root dry weight, SFW: shoot fresh weight, SDW: shoot dry weight Chl.a: chlorophyll a, Chl. b: chlorophyll b, Chl a/b: chlorophyll a/b ratio, total Chl: total chlorophyll contents, C: carotenoids, Anth: anthocyanin, NL: number of leaves, LL:leaf length, LFW: leaf fresh weight, LDW: leaf dry weight, LTW: leaf total weight, TSS: total soluble sugar, TSP: total soluble protein, TFAA: total free amino acids, F: flavonoids, SOD: superoxide dismutase, POD: peroxidase, APX: Ascorbate peroxidase, HPO: H2O2, Ph: phenolics, TSP: total soluble protein, Pro: proline, F: flavonoids, cat: catalase, MDA: malondialdehyde.
Prominent positive correlations are observed among various growth traits such as root length (RL), shoot length (SL), and fresh and dry weights of roots (RFW, RDW), shoots (SFW, SDW), and leaves (LFW, LDW, LTW). This suggests that as one of these traits increases, the others tend to increase as well, which is typical for the overall growth of the plant. Biochemical parameters such as chlorophyll a and b (Chl a, Chl b), total chlorophyll (total Chl), and the chlorophyll a/b ratio (Chl a/b) also display strong inter-relationships, indicative of coordinated changes in photosynthetic pigments in response to the experimental treatments. Interestingly, some biochemical traits like anthocyanin (Anth), total soluble protein (TSP), phenolics (Ph), and hydrogen peroxide (HPO) show significant negative correlations with certain growth parameters, implying that an increase in these biochemical compounds could be associated with a stress response, potentially leading to reduced growth metrics. (Fig. 5).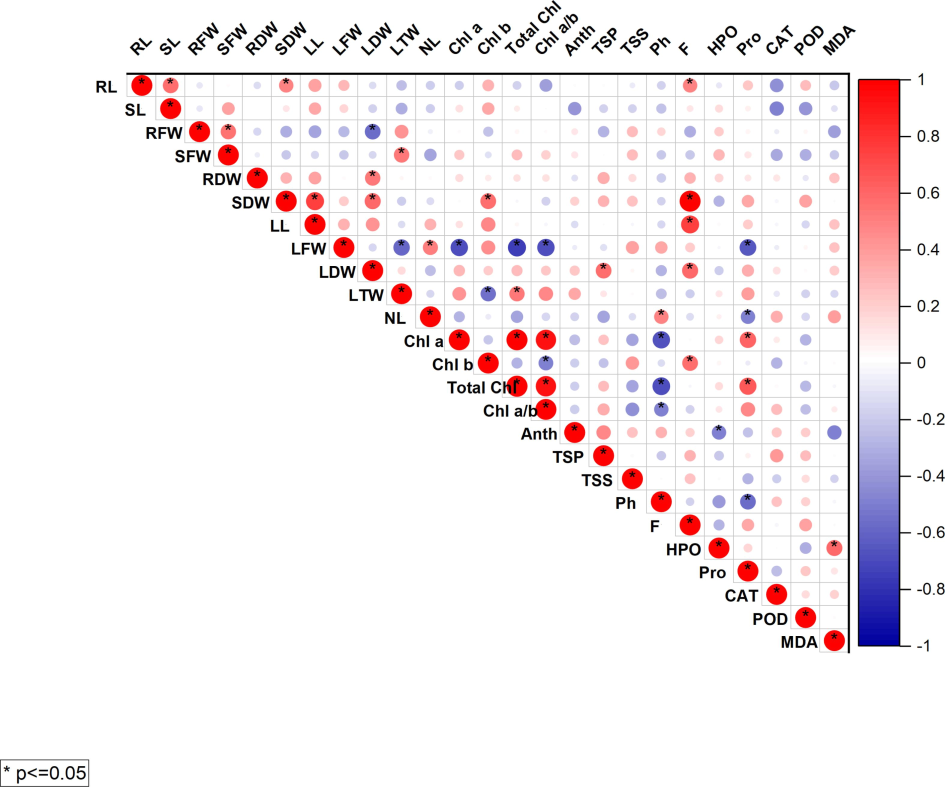
Pearson’s correlation ploted between various parameters of olive plants grown under treatment of glutamic acid and Cd stress. Different colors represent positive (blue) or negative correlations (red), and color intensity represents Pearson’s correlation coefficient.
Abbreviations of traits used.
SL: shoot length, RL: root length, RFW: root fresh weight, RDW: root dry weight, SFW: shoot fresh weight, SDW: shoot dry weight Chl.a: chlorophyll a, Chl. b: chlorophyll b, Chl a/b: chlorophyll a/b ratio, total Chl: total chlorophyll contents, C: carotenoids, Anth: anthocyanin, NL: number of leaves, LL:leaf length, LFW: leaf fresh weight, LDW: leaf dry weight, LTW: leaf total weight, TSS: total soluble sugar, TSP: total soluble protein, TFAA: total free amino acids, F: flavonoids, SOD: superoxide dismutase, POD: peroxidase, APX: Ascorbate peroxidase, HPO: H2O2, Ph: phenolics, TSP: total soluble protein, Pro: proline, F: flavonoids, cat: catalase, MDA: malondialdehyde.
6 Discussion
The exploration of the role of glutamic acid in enhancing the tolerance of Olea europaea L. to Cd stress opens a new avenue in the field of plant physiology and stress management. The general discussion draws on the findings presented, situating them within the broader context of current scientific understanding, and gradually transitions towards a conclusion that encapsulates the essence and implications of the study. The findings of this study demonstrate significant improvements in growth parameters, photosynthetic pigments, and stress markers in Olea europaea L. upon glutamic acid treatment under Cd stress, which is consistent with the growing body of evidence supporting amino acids as critical modulators of plant stress responses. Among the compounds used in growth and phytohormones, the biosynthesis of glutamic acid, a major precursor in nitrogen metabolism, has been studied extensively (Forde and Lea 2007, Singh et al., 2015). The longer roots, taller shoots, and greater biomass and leaf metrics (Fig. 1) observed with glutamic acid treatment highlight the prospect of using glutamic acid to improve nutrient uptake and use, which is critical during stress (Nawaz et al., 2024).
This suggests the importance of glutamate for photosynthesis in promoting the expression of enzymes necessary for chlorophyll biosynthesis to maintain normal photosynthetic levels under stress (Yang et al., 2021; Munawar et al., 2022). This is of particular importance considering the fact that Cd stress hampers chlorophyll synthesis and disturbs the photosynthetic process (Sharma and Dietz 2006; Sun et al., 2021). The effective countering effect of glutamic acid on these activity reductions supports the idea that this carboxylate plays a protective role in maintaining the stability of the photosynthetic machinery. Moreover, the reduction in oxidative stress markers, namely hydrogen peroxide and MDA, accompanied by the potation of antioxidant enzyme activities (CAT and POD) and accumulation of phenolics, flavonoids, and proline (Fig. 3), suggests an overall strengthening of the antioxidant defense system. This is in agreement with previous studies (Kuznetsov and Shevyakova 1999, Szabados and Savouré 2010; Bi et al., 2024), where the role of amino acids in plant stress resistance to oxidative damage is well documented, and different types of pathways and biochemical mechanisms are thought to be activated by plants due to the summation of specific amino acids (Saleem et al., 2024).
PCA and Pearson correlation analyses demonstrated a detailed relationship between a variety of physiological and biochemical indices, indicating that plant responses to glutamic acid treatment are x under Cd stress. The positive correlations between growth parameters and photosynthetic pigments, along with the negative correlations with oxidative stress markers, indicated a balanced modulation of growth and stress mitigation processes by glutamic acid. The PCA plot displays two principal components, PC1 and PC2, which together explain a certain percentage of the total variance in the data: 18.1 % by PC1 and 15.4 % by PC2, as indicated on the axes. The longer a vector, the more the variable contributes to the components in the analysis (Fig. 4). The observed enhancements in root and shoot lengths, as well as fresh and dry biomass in glutamic acid-treated Olea europaea L. (Fig. 1), are consistent with previous research indicating that amino acids can serve as precursors for growth-promoting phytohormones and other essential metabolites (Singh et al., 2015). For instance, glutamic acid has been implicated in the regulation of key genes involved in nitrogen metabolism, which are critical for protein synthesis and plant growth (Forde and Lea 2007). The significant increase in biomass accumulation in response to glutamic acid treatment under Cd stress suggests that glutamic acid may enhance the efficiency of nutrient uptake and assimilation, a phenomenon also observed in other plant species subjected to heavy metal stress (Aftab 2023).
In this context, an increase in chlorophyll a, chlorophyll b, and total chlorophyll content (Fig. 2) in glutamic acid-treated plants is indicative of the significant role of glutamic acid in the maintenance of photosynthetic activity under stress conditions. This is exemplified by the results obtained by Villalobos-López et al., (2022), who found a stabilizing role for exogenous amino acid treatment (except glutamic acid) in chlorophyll molecules and improved photosynthesis efficiency in plants under abiotic stresses. This elevated chlorophyll content may be associated with the role of glutamic acid in reducing the toxic effects of Cd on the chlorophyll biosynthesis pathways (Sharma and Dietz 2006; Saleem et al., 2022). The decrease in the activities of hydrogen peroxide and MDA (Fig. 3), and the increase in the activities of antioxidant enzymes (CAT and POD), phenolics, and flavonoids also indicate a strong antioxidant defense system induced by glutamic acid. This observation is consistent with the modulation of antioxidant capacity by exogenous amino acids in plants, as reported previously (Kuznetsov and Shevyakova 1999). Moreover, the increase in proline and osmo-protectant levels also demonstrates the role of glutamic acid in stress tolerance through osmotic adjustment and protection of cell structures (Szabados and Savouré 2010).
PCA and Pearson correlation analyses illustrated a comprehensive perspective of the complex interaction between different parameters, showing a series of insights into the physiological and biochemical changes associated with Cd stress and glutamic acid treatment (Figs. 4, 5). Together, these analyses reflect the diversity of plant stress responses and the diverse roles of glutamic acid in the regulation of such responses. It advocates the role of glutamic acid in both growth and stress mitigation through positive correlations of growth parameters with photosynthetic pigments and negative correlations of growth parameters with oxidative stress markers. In summary, the results of this study revealed the beneficial effects of glutamic acid in inducing the regeneration of Cd-stressed Olea europaea L. plants, suggesting that this free amino acid can maximize the promotion of leaf growth, improve photosynthetic attributes, and re-enforce antioxidant defenses. These results have deepened our understanding of the mechanisms underlying glutamic acid-induced mitigation of oxidative stress and laid an important foundation for further improving the tolerance of plants to heavy metals. The authors note the relevance of their findings to agriculture and environmental management beyond Olea europaea L., especially in regions experiencing heavy metal pollution. Elucidation of the molecular basis of glutamic acid will be beneficial for understanding the unique function of this amino acid in abiotic stress tolerance, which could be further eabioperable, leading to its application in agriculture for crop improvement and increasing sustainable agriculture under harsh conditions.
CRediT authorship contribution statement
Muhammad Hamzah Saleem: Writing – review & editing, Writing – original draft, Visualization, Validation, Resources. Sadia Zafar: Writing – review & editing, Writing – original draft, Supervision, Software. Sadia Javed: Writing – review & editing, Writing – original draft, Supervision, Software. Muhammad Anas: Visualization, Validation, Methodology, Investigation. Temoor Ahmed: Writing – review & editing, Validation, Resources, Project administration. Shafaqat Ali: Writing – review & editing, Writing – original draft, Software. Iman Mirmazloum: Writing – original draft, Supervision, Software, Funding acquisition. Ajaz Ahmad: Writing – review & editing, Writing – original draft, Software, Resources.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2024R350), King Saud University, Riyadh, Saudi Arabia
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- New Frontiers in Plant-Environment Interactions: Innovative Technologies and Developments. Springer Nature; 2023.
- Role of ferrous sulfate (FeSO4) in resistance to cadmium stress in two rice (Oryza sativa L.) genotypes. Biomolecules. 2020;10(12):1693.
- [Google Scholar]
- Physiological, nutritional and metabolomic responses of tomato plants after the foliar application of amino acids aspartic acid, glutamic acid and alanine. Front. Plant Sci.. 2021;11:581234
- [Google Scholar]
- Cadmium toxicity in medicinal plants: An overview of the tolerance strategies, biotechnological and omics approaches to alleviate metal stress. Front. Plant Sci.. 2023;13
- [Google Scholar]
- Histological and ionomics assessment to elucidate tolerance mechanisms of nickel-tolerant and sensitive cultivars of bread wheat (Triticum aestivum L.) Plant Stress. 2023;10 100277
- [CrossRef] [Google Scholar]
- Glutathione in cellular redox homeostasis: association with the excitatory amino acid carrier 1 (EAAC1) Molecules. 2015;20(5):8742-8758.
- [Google Scholar]
- Modern analytical techniques for flavonoid determination. Flavonoids in Cell Function. 2002:61-76.
- [Google Scholar]
- Effects of Bacillus subtilis on cotton physiology and growth under water and salt stress. Agricultural Water Management. 2024;303:109038.
- [Google Scholar]
- Glutamate in plants: metabolism, regulation, and signalling. J. Exp. Bot.. 2007;58(9):2339-2358.
- [Google Scholar]
- The effects of cadmium toxicity. Int. J. Environ. Res. Public Health. 2020;17(11):3782.
- [Google Scholar]
- Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav.. 2010;5(1):26-33.
- [Google Scholar]
- Cadmium ion-chlorophyll interaction–Examination of spectral properties and structure of the cadmium-chlorophyll complex and their relevance to photosynthesis inhibition. Chemosphere. 2020;261:127434
- [Google Scholar]
- Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem.. 2018;19:1-8.
- [Google Scholar]
- Comparative morpho-physiological traits, antioxidant defense and nutritional profiling under Cd stress of japonica-indica elite rice (Oryza sativa L.) cultivars. Journal of Crop Science. Biotechnology. 2024;27(2):175-186.
- [Google Scholar]
- Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry Biophysics.. 1968;125(1):189-198.
- [Google Scholar]
- Nitric oxide confers cadmium tolerance in fragrant rice by modulating physio-biochemical processes, yield attributes, and grain quality traits. Ecotoxicology and Environmental Safety. 2023;261:115078.
- [Google Scholar]
- Glutamate alleviates cadmium toxicity in rice via suppressing cadmium uptake and translocation. J. Hazard. Mater.. 2020;384:121319
- [Google Scholar]
- Kuznetsov, V. V. and N. Shevyakova, 1999. Proline under stress: biological role, metabolism, and regulation.
- Transcriptional regulatory network of plant cadmium stress response. Int. J. Mol. Sci.. 2023;24(5):4378.
- [Google Scholar]
- Response of cauliflower (Brassica oleracea L.) to nitric oxide application under cadmium stress. Ecotoxicology and Environmental Safety. 2022;243:113969.
- [Google Scholar]
- Attenuation of cadmium induced oxidative stress in cucumber seedlings by modulating the photosynthesis and antioxidant machinery through foliar applied glutamic acid. Hortic. Sci.. 2022;49(1)
- [Google Scholar]
- Nitric oxide reduces cadmium uptake in wheat (Triticum aestivum L.) by modulating growth, mineral uptake, yield attributes, and antioxidant profile. Environmental Science and Pollution Research. 2024;31(6):9844-9856.
- [Google Scholar]
- Foliar application of iron-lysine to boost growth attributes, photosynthetic pigments and biochemical defense system in canola (Brassica napus L.) under cadmium stress. BMC Plant Biology. 2023;23(1):648.
- [Google Scholar]
- A detailed view on sulphur metabolism at the cellular and whole-plant level illustrates challenges in metabolite flux analyses. J. Exp. Bot.. 2014;65(20):5711-5724.
- [Google Scholar]
- Silicon fertigation regimes attenuates cadmium toxicity and phytoremediation potential in two maize (Zea mays L.) cultivars by minimizing its uptake and oxidative stress. Sustainability. 2022;14(3):1462.
- [Google Scholar]
- Alleviation of cadmium toxicity in pea (Pisum sativum L.) through Zn− Lys supplementation and its effects on growth and antioxidant defense. Environmental Science and Pollution Research. 2024;31(7):10594-10608.
- [Google Scholar]
- Cereals and Phytohormones Under UV Stress. Sustainable Remedies for Abiotic Stress in Cereals. Springer 2022:425-441.
- [Google Scholar]
- The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot.. 2006;57(4):711-726.
- [Google Scholar]
- Natural allelic diversity in OsDREB1F gene in the Indian wild rice germplasm led to ascertain its association with drought tolerance. Plant Cell Rep.. 2015;34:993-1004.
- [Google Scholar]
- Ultralight and superhydrophobic perfluorooctyltrimethoxysilane modified biomass carbonaceous aerogel for oil-spill remediation. Chemical Engineering Research and Design. 2021;174:71-78.
- [Google Scholar]
- Effects of salt stress on plant growth, nutrient partitioning, chlorophyll content, leaf relative water content, accumulation of osmolytes and antioxidant compounds in pepper (Capsicum annuum L.) cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca.. 2017;45(2):481-490.
- [Google Scholar]
- Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci.. 2000;151(1):59-66.
- [Google Scholar]
- Biotechnological advances to improve abiotic stress tolerance in crops. Int. J. Mol. Sci.. 2022;23(19):12053.
- [Google Scholar]
- OsTTG1, a WD40 repeat gene, regulates anthocyanin biosynthesis in rice. The Plant Journal. 2021;107(1):198-214.
- [Google Scholar]
- Optimization of light exposure and sleep schedule for circadian rhythm entrainment. PLoS One. 2021;16(6)
- [CrossRef] [Google Scholar]







