Translate this page into:
Borneol facilitates the whitening and anti-wrinkle effect of the essential oil extracted from Abies koreana needles
⁎Corresponding author. sam1017@ish.ac.kr (Seahyoung Lee)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
It has been reported that the essential oil extracted from Abies koreana has skin-improving activity, such as anti-wrinkle and whitening effect. However, the key ingredients that facilitate such biological activity has not been fully elucidated. In the present study, to identify the key skin-improving component(s) of the A. koreana essential oil, 10 sequentially fractioned essential oil from A. koreana pine by hydrodistillation were examined for their effect on tyrosinase activity. Out of 10 fractions, only 1 fraction significantly suppressed tyrosinase activity, and subsequent GC/MS analysis indicated that borneol and its acetate ester, bornyl acetate, were unique to the effective fraction, suggesting they are responsible for the skin-improving activity of the essential oil from A. koreana. The biological activity of borneol was evaluated, and borneol significantly inhibited ultraviolet irradiation-induced tyrosinase and matrix metalloproteinase 1 activity while maintain collagen type I synthesis. The results of this study suggest that borneol is a key component of A. koreana essential oil that has potential for mediating whitening and anti-wrinkle effects.
Keywords
Abies Koreana
Essential oil
Whitening
Anti-wrinkle
1 Introduction
Abies koreana belongs to genus Abies (Pinaceae) and is an endemic alpine plant grows in South Korea (Sato et al., 2019). Despite the commercial use of its needle oil as a component of cosmetic products, there are not many studies on its detailed chemical composition and/or biological activity. Currently, Pubmed search using A. koreana as a key word brings up only a handful of studies, and less than 10 papers examined the composition and/or biological activity of the essential oils extracted from A. koreana. According to the previous studies, the main components of the A. koreana essential oil include monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, and oxygenated sesquiterpenes, as well as trace amounts of diterpenes (Jeong et al., 2007; Wajs-Bonikowska et al., 2013). The most well-known biological activity of the A. koreana essential oil is antimicrobial activity (Jeong et al., 2007). Previous studies have reported that the essential oil of A. koreana was effective against strains of Bacillus, Staphylococcus, Pseudomonas, and Enterobacter (Lee and Hong, 2009). Additionally, its anti-inflammatory effect has been reported as well (Yoon et al., 2009).
Recently, it has been reported the anti-wrinkle and whitening effect of the essential oil extracted from A. koreana (Song et al., 2018). However, the results were obtained by using the whole essential oil extracted from A. koreana needles, and the active chemical ingredients responsible for the observed anti-wrinkle and whitening effects were not identified. Since the amount of active ingredients in natural product such as essential oils is generally small, extraction and isolation of the active ingredients can be a time-consuming process, and this hinders the application of essential oils in commercial use, creating an urgent need for finding effective and selective methods for the extraction and isolation of natural bioactive ingredients (Zhang et al., 2018). However, such an optimized extraction and isolation protocol should be preceded by the identification of active ingredients.
Therefore, in the present study, the whole essential oil extracted from A. koreana needles was further separated into 10 sequential fractions using hydrodistillation. As one of the classical methods for the extraction of essential oils and bioactive compounds from plant materials, hydrodistillation can be conducted in 3 different ways, and we used water distillation method where hydro-diffusion is the main physicochemical process involved (Azmir et al., 2013). The 10 fractions were individually screened for the anti-wrinkle and whitening effect to select the most effective fraction, and the active compounds contained only in the most effective fraction were identified by using gas chromatography-mass spectrometry (GC/MS). The anti-wrinkle and whitening effect of the identified compounds were also evaluated to confirm their bioactivity.
2 Materials and methods
2.1 Preparation of primary whole essential oil from A. Koreana
A. koreana trees with a height of approximately 3 m were harvested from Chuncheon province of South Korea. Washed pine needles (1 kg) were thoroughly minced in 3L distilled water using a blender. The mincing product was subjected to a hydrodistillation to obtain a primary whole essential oil. The initial temperature of a heat block was set to 140℃, and then the temperature was decreased to 120℃ as the first drop of essential oil was collected. With this process, approximately 80 ml of primary whole essential oil was obtained within 150 min. The primary whole essential oil was gradually cooled down to ambient temperature, and then it was stored in a 4℃ refrigerator using amber glass bottles to avoid heat and light, until further sequential fraction.
2.2 Cell culture
Primary Human dermal fibroblast (HDF) cells (PCS-201-012, American Type Culture Collection, Manassas, VA, USA) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 1% penicillin and streptomycin (P/S) in a humidified 5% CO2 atmosphere at 37 °C. The seeding density of HDF was 1 × 104 cells/well, 1 × 105 cells/well, and 3.5 × 105 cells/well for a 96 well plate, 6 well plate, and 100 mm culture dish, respectively. The mouse melanoma B16F10 cell line was purchased from the Korean Cell Line Bank (Seoul, Korea). B16F10 cells were grown in DMEM with 4.5 g/l glucose and 4 mM L-glutamine, supplemented with 10% fetal calf serum. The seeding density of B16F10 was 3 × 105 cells/well, 8 × 105 cells/well, and 1 × 106 for a 6 well plate, 60 mm culture dish, and 100 mm culture dish, respectively. The cells reached approximately 80% confluency in every 2–3 days so they were passaged accordingly. All the culture media contained phenol red to monitor the pH of the media, and the pH of the culture medium was maintained in between pH 7.3–7.6 during the cell culture.
2.3 Cell viability assay (cell counting kit8, CCK8)
To determine cell viability, a WST-8 {2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt} solution (CCK-8, Dojindo, Japan) was added to each well (10% (v/v)) and incubated at 37 °C for 2 h to allow for the formation of WST-8 formazan. The absorbance of a water soluble formazan dye was measured at 450 nm using a microplate reader (Molecular Devices, USA). Briefly, HDFs were seeded in a 96 well culture plate at a density of 1 × 104 cells/ml (100 μl/well) and cultured for 24 h. For treatment, the cells were treated with 103–107 fold diluted whole essential oil in serum free media for 24 h before sampling.
2.4 Tyrosinase inhibition assay for whitening effect
For tyrosinase inhibition assay, B16F10 cells were seeded in a 6 well culture plate at a density of 3 × 105 cells/well, and the cells were cultured in a 5% CO2 incubator at 37 °C for 24 h. For the assay, the cells were treated with 10 different fractioned essential oils (fraction 1–10: F1–F10, 105 fold diluted) in serum free media and cultured for 48 h. After 48 h of treatment, the cells were washed 3 times with PBS and lysed in a lysis buffer containing 0.1 M sodium phosphate m phosphate (pH 6.8) and 1% Triton X-100. The lysed samples were centrifuged at 13,000 rpm for 25 min, and the supernatants were collected and protein concentration was determined. Each sample protein (20 μg) was mixed with 180 μl of L-DOPA (L-3,4-dihydroxyphenylalanine) buffer (2 mg/ml in 0.1 M sodium phosphate) and reacted in a 96 well culture plate at 37 °C for 3 h. In this assay, L-DOPA served as a substrate for tyrosinase, and it is converted to dopachrome which is detected at 475 nm. For a negative control that increases tyrosinase activity, 100 nM of α-MSH (Melanin Stimulating Hormone) was used. TGF-β (Transforming growth factor beta, 5 ng/ml) was used as a positive control that decreases tyrosinase activity.
2.5 Gas chromatography/mass spectrometry (GC/MS)
GC/MS analysis was conducted by Yonsei Center for Research Facilities (YCRF, Seoul, Korea) using a Trace GC gas chromatograph coupled to a quadrupole mass spectrometer (Thermo, Austin, TX, USA). The GC-system was equipped with an injection port held at 250 °C. The samples were separated on a HP-5MS column (Agilent, Santa Clara, CA, USA), and 2 ml of sample was injected with an injection speed of 50 ml/sec into the inlet with a split ratio of 10:1. During the GC run, a constant flow rate (0.8 ml/min) of carrier gas (helium) was maintained. The GC program started at 40 °C for 4 min, and the temperature increased to 100 °C at a rate of 5 °C per minute, followed by an increase at 8 °C per minute to 230 °C, with a final holding time of 8 min. The temperature of the interface to the mass spectrometer interface was set at 250 °C. The mass spectra were obtained by electron impact (EI) ionization at 70 eV at 200 °C. The detector was set in TIC mode from m/z 33–450amu. The detector voltage and filament emission current were 350 V and 200 mA, respectively. Qualitative analysis of compounds presented in sample was done by comparing the mass spectrum of each peak to a standard library.
2.6 Melanin contents assay
For melanin assay, a mouse melanin ELISA kit (SL0925Mo, Sunlong Biotech, Hangzhou, China) was used following the protocol provided by the manufacturer. B16F10 cells were seeded in a 60 mm culture dish at a density of 8 × 105 cells/dish, and the cells were cultured in a 5% CO2 incubator at 37 °C for 24 h. For the assay, the cells were irradiated with UV (60 mJ/Cm2) using a UV lamp (VL-215-LM, Vilber, Eberhardzell, Germany) for 3 h. The cells were then treated with varying concentrations of borneol (50 nM–10 μM) in serum free media for 48 h prior to assay procedure.
2.7 Collagen contents assay
For evaluating collagen contents assay, a pro-collagen type I C-peptide ELISA kit (MK101, Takara Bio, Japan) was used following the protocol provided by the manufacturer. HDFs were seeded in a 6 well culture plate at a density of 1 × 105 cells/well, and the cells were cultured in a 5% CO2 incubator at 37 °C for 24 h. For the assay, the cells were irradiated with UV (60 mJ/Cm2) for 3 h. The cells were then treated with either 105 fold diluted F1 or 10 μM of borneol in serum free media for 48 h prior to assay procedure. The culture medium was centrifuged at 14,000 rpm for 10 min, and the supernatants were subjected to the assay.
2.8 MMP-1 activity assay
To examine MMP-1 activity, en contents assay, a human active MMP-1 Fluorokine E kit (F1M00, R&D systems, Minneapolis, MN, USA) was used following the protocol provided by the manufacturer. HDFs were seeded in a 6 well culture plate at a density of 1 × 105 cells/well, and the cells were cultured in a 5% CO2 incubator at 37 °C for 24 h. For the assay, the cells were irradiated with UV (60 mJ/Cm2) for 3 h. The cells were then treated with either 105 fold diluted F1 or 10 μM of borneol in serum free media for 48 h prior to assay procedure. The culture medium was centrifuged at 14,000 rpm for 10 min, and the supernatants were subjected to the assay.
2.9 Western blot
For western blot, the cells cultured in 100 mm culture dishes were lysed using a RIPA lysis buffer (89901, Thermo Scientific Pierce. Rockford, IL, USA) containing protease inhibitor and phosphatase inhibitor, and 40 μg of cell lysates per lane was used for SDS-polyacrylamide gel electrophoresis (PAGE). The proteins were transferred to a polyvinylidene fluoride membranes (PVDF, Santa Cruz Biotechnology, Santa Cruz, CA, USA). After blocking the membrane with 5% nonfat dried milk in TBS-T (Tris-buffered saline-Tween 20, 0.1% Tween 20) for 1 h at room temperature, the membrane was washed twice with TBS-T and incubated with primary antibody (1:1000 dilution) for 1 h at room temperature or overnight at 4 °C. For secondary antibody, the membrane was washed three times with TBS-T for 10 min and incubated for 1 hr at room temperature with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology). After extensive washing, immuno-labeled bands were visualized using an enhanced chemiluminescence system (RPN2232, GE Healthcare, Chicago, IL, USA). The following antibodies were used for the study; anti-collagen I antibodies (ab34710, Abcam, Cambridge, UK), anti-MMP-1 antibodies (ab134184, Abcam), and anti-tyrosinase antibodies (LS‑C192923, LSBio, Seattle, WA, USA).
2.10 Reverse transcriptase-PCR
Total RNA was extracted from the cells cultured in 60 mm culture dishes by adding 500 µl of TRIzol reagent (Sigma Aldrich, St. Louise, MO, USA) and 100 µl of chloroform to each sample. One microgram of total RNA was reverse-transcribed to produce complementary DNA (cDNA) in a 20 µl reaction mixture containing 5 mM MgCl2, 10 mM Tris-HCl, 50 mM KCl, 0.1% Triton X-100, 1 mM each dNTP, 1 unit/µl RNase inhibitor, 0.5 µg of oligo (dT)15 primer, and 15 units/µg reverse transcriptase for 15 min at 42 °C; the reaction was terminated by heating at 99 °C for 5 min. One microgram of cDNA, and 10 pM of each primer (sequence listed down below), 0.1 mM of a dNTP mixture, 1.25 U Taq polymerase, and 10X reaction buffer were mixed with nuclease free water to give a final volume of 25 μl. PCR was conducted using a thermal cycler (C1000 thermal cycler, Bio-Rad, Hercules, CA, USA) and PCR conditions were as follows; Initial denaturation at 94 °C for 3 min, 25 cycles of denaturation at 94 °C for 30 s, annealing at 58–60 °C for 30 s, and elongation at 72 °C for 30 s. Finally, the temperature was held at 72 °C for 10 min before PCR reaction was terminated. The primers used for RT-PCR are as follows; human collagen type I (223 bp, Forward: 5′- AGG GCT CCA ACG AGA TCG AG-3′, Reverse: 5′- TAC AGG AAG CAG ACA GGG CC-3′), human MMP-1 (207 bp, Forward: 5′- GGT CTC TGA GGG TCA AGC AG-3′, Reverse: 5′- AGT TCA TGA GCT GCA ACA CG-3′), and mouse tyrosinase (287 bp, Forward: 5′- TTA TGC GAT GGA ACA CCT GA-3′, Reverse: 5′- GAG CGG TAT GAA AGG AAC CA-3′).
2.11 Statistical analysis
All data were compared via one-way analysis of variance (ANOVA) using the Statistical Package for the Social Sciences (SPSS, version 14.0 K) program. The data are expressed as means ± standard deviation. Group means were considered significantly different at p < 0.05, as determined by the protected least-significant difference (LSD) test when ANOVA indicated an overall significant treatment effect (p < 0.05).
3 Results
3.1 Cytotoxicity of whole extraction oil from a. Koreana needles
In a previous study, the whole essential oil extracted from A. koreana needles did not show any significant cytotoxicity when diluted more than 104 folds in B16F10 melanoma cells (Song et al., 2018). In the present study, cytotoxicity of the whole essential oil was evaluated in human dermal fibroblast (HDF) using dilution factors ranging from 103 and 107. According to our data, when the whole essential oil was diluted more than 105 folds, it did not cause any apparent morphological changes. However, less diluted whole essential oil treatment caused apparent detachment of the cells (Fig. 1A). The results of cytotoxicity assay also indicated that sufficiently diluted whole essential oil (dilution factor ≥ 105) did not cause any significant cytotoxicity against HDFs (Fig. 1B). Based on this finding, the dilution factor of 105 was used for further experiments.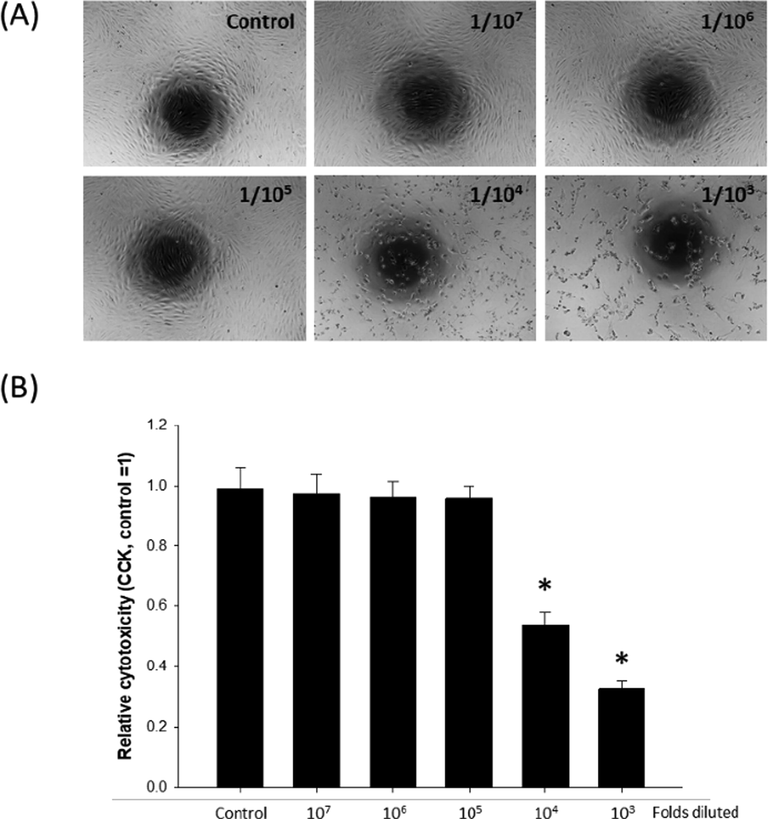
Cytotoxicity of whole essential oil extract on human dermal fibroblasts (HDF). HDFs were treated with increasing concentrations of whole essential oil extract, and (A) gross morphology and (B) cytotoxicity were examined. *p < 0.05 compared to control, n = 4.
3.2 Sequential fractionation of whole essential oil using hydrodistillation
For further fractionation of whole essential oil, 10 ml of the whole essential oil was thoroughly mixed with 90 ml of distilled water and gradually heated until 10 fractions were obtained. Initially, a heat block was set to 120 °C, and after 15 min, temperature of the bifurcation to cooling condenser unit reached 90 °C without actual condensation inside the condenser unit (Fig. 2A). Thereafter, the temperature was increased at a rate of 5 °C/min throughout the process. The very first fraction (fraction 1: F1) was started to be collected when the temperature of the bifurcation reached 93 °C, and when the volume of fraction reached approximately 1.5 ml (approximately 1 ml of oil phase and 0.5 ml of aqueous phase, inset Fig. 2A), next fraction (fraction 2: F2) was started to be collected. The fractioned essential oil was colorless in visual examination (inset picture of Fig. 2A). Rest of the fractions (fraction 3 to 10: F3-F10) were obtained as the temperature gradually increased. Detailed conditions for temperature and duration of each fraction are shown in Fig. 2B.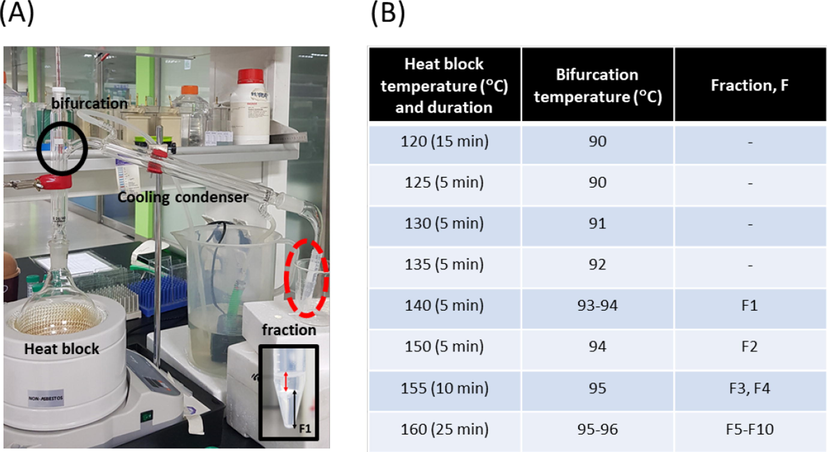
Hydrodistillation apparatus and detailed conditions for collecting fractions. (A) During hydrodistillation, the temperature of the heat block and the bifurcation (indicated with black circle) was recorded. Inset image: typical composition of a fraction; red arrows indicates oil phase and black arrows indicates aqueous phase. F1: fraction 1. (B) Temperature changes and duration for each fraction.
3.3 Selection of the fraction containing active compounds
To select the fraction containing active compounds, tyrosinase inhibition assay that can predict the whitening effect of a compound being tested was used (Uchida et al., 2014). When B16F10 cells were treated with 105 fold diluted whole essential oil it did not suppressed tyrosinase activity, while the negative control (α-MSH) and the positive control (TGF-β) increased and decreased the tyrosinase activity, respectively. On the other hand, when the cells were treated with each fraction diluted 105 fold, only the fraction 1 (F1) significantly decreased the tyrosinase activity, suggesting active compounds that facilitates the reported anti-wrinkle and whitening effect of the essential oil extracted from A. Koreana might be present in the F1 (Fig. 3). Therefore, a detailed composition of the F1 was further examined.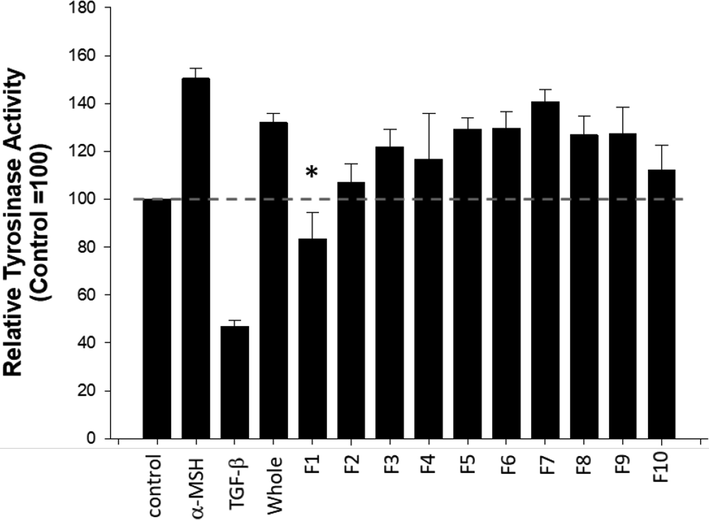
Tyrosinase inhibitory effect of each fraction. Bl6F10 melanoma cells were treated with each fraction diluted 105 folds for 48 h. Cell lysates were subjected to tyrosinase assay. α-melanin stimulating hormone (α-MSH, 100 nM) and TGF-β (5 ng/ml) were used as a negative control and a positive control, respectively. *p < 0.05 compared to control, n = 4. F1-F10: fraction 1–10.
3.4 GC/MS analysis of F1 for identification of active compound candidates
To identify compounds presented only in the fraction 1 (F1), GC/MS analysis on the 10 different fractions (F1-F10) was conducted. Depending on fractions, 15 to 27 peaks were detected by GC/MS (Supplementary Fig. S1-S9), and the number of peaks detected in the F1 was 15 (Fig. 4A). Among the 15 peaks of the F1, only 2 of them were unique to the F1, and they were borneol ((1S-endo)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-ol, CAS No. 464-45-9) and bornyl acetate (CAS No. 76-49-3) (Fig. 4B). This data indicated that borneol and/or bornyl acetate might be the active compounds responsible for the reported anti-wrinkle and whitening effect of the essential oil extracted from A. Koreana.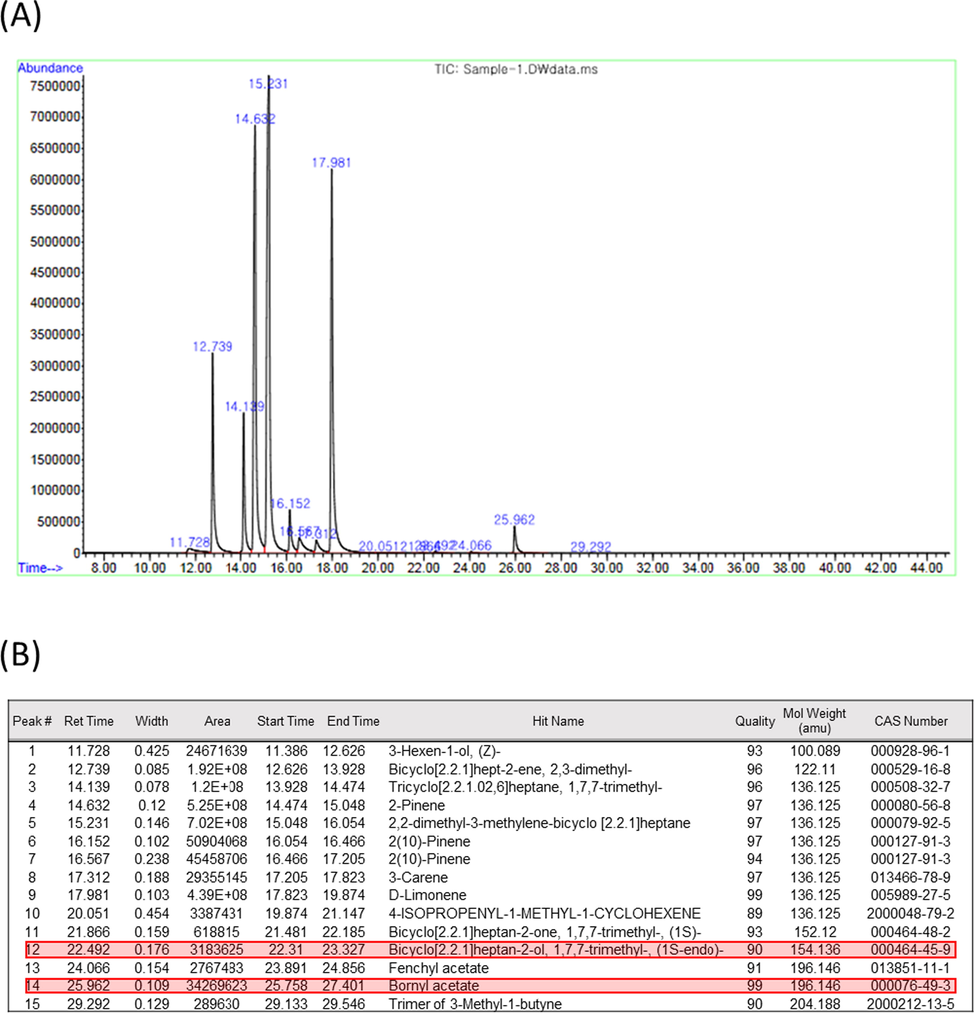
GC/MS analysis of fractions. (A) GC/MS peaks of fraction 1 (F1). (B) Identification of 15 peaks detected in F1. Compounds only presented in F1, namely borneol and bornyl acetate, but not in other 9 fractions are indicated with red shades.
According to the additional quantitative GC/MS analysis, concentrations of borneol and bornyl acetate in the fraction 1 (F1) was 24.53 ppm (μg/ml) and 70.27 ppm, respectively. Since the molecular weight of borneol is 154.25 (Fig. 5A), 24.53 ppm of borneol is approximately equivalent to 0.16 M of borneol. Therefore, considering the dilution factor (105) used, the concentration of borneol used for the tyrosinase inhibition assay can be approximately estimated as 1.6 μM. Similarly, the molecular weight of bornyl acetate is 196.288 (Fig. 5B), and the approximated concentration of bornyl acetate used for the tyrosinase inhibition assay was estimated as 3.6 μM.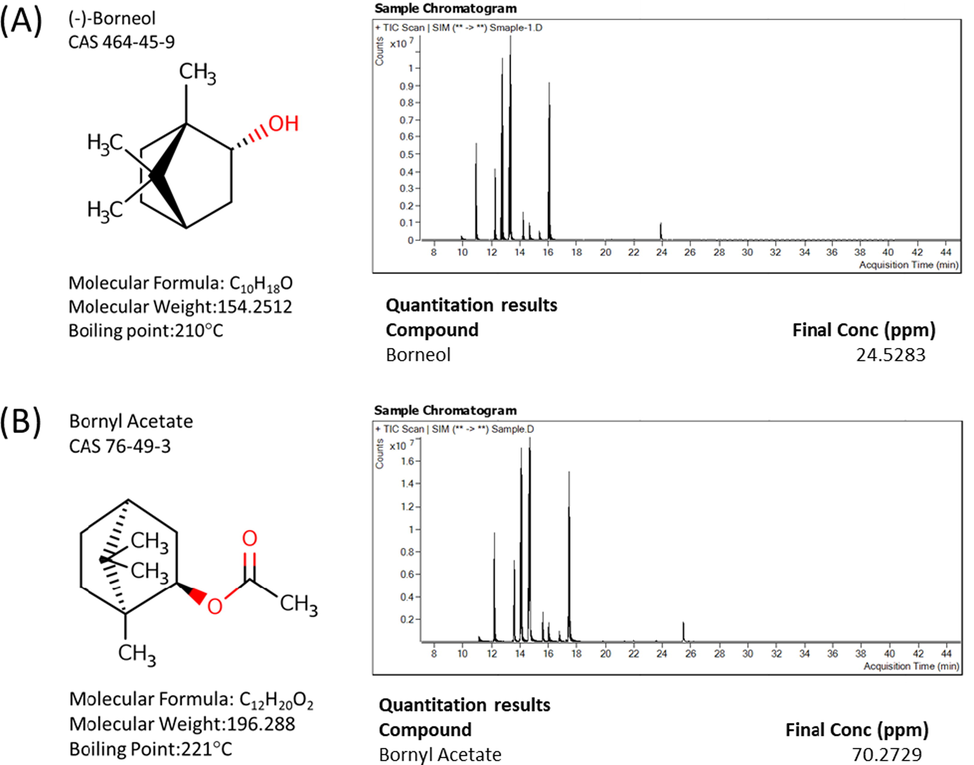
Structure and amount of borneol and derivative contained in fraction 1 (F1). (A) Basic characteristics of borneol and its quantification by GC/MS analysis of F1. (B) Basic characteristics of bornyl acetate and its quantification by GC/MS analysis of F1.
3.5 Evaluation of the whitening effect of borneol
As shown in the Fig. 5, borneol and bornyl acetate share same backbone having similar structure. Furthermore, it has been suggested that the acetylation of borneol to make bornyl acetate occurs in the plastids of plants (Thiel and Adam, 2002). Therefore, between the 2 compounds, borneol was further scrutinized for its whitening effect for the present study.
According to the results of melanin contents assay, ultraviolet irradiation (UV, 60 mJ/cm2) increased the melanin contents of Bl6F10 cells (242.09 ± 41.14 and 277.62 ± 10.90 pg/ml for untreated control and UV control, respectively), and positive control TGF-β, which is known to decrease melanin synthesis (Kim et al., 2004), suppressed the UV-induced increase of melanin contents (237.52 ± 22.43 pg/ml). These data indicated that our in vitro system was functional.
Furthermore, varying concentrations of borneol (50 nM–10 μM) also suppressed UV-induced increase of melanin contents, with 10 μM of borneol showing most significant effect (230.20 ± 17.14 pg/ml, Fig. 6A). Additionally, the effect of borneol on tyrosinase expression was examined, and the results indicated that borneol effectively suppressed the expression of tyrosinase, an oxidizing enzyme facilitating melanin synthesis, both at mRNA (Fig. 6B) and protein level (Fig. 6C).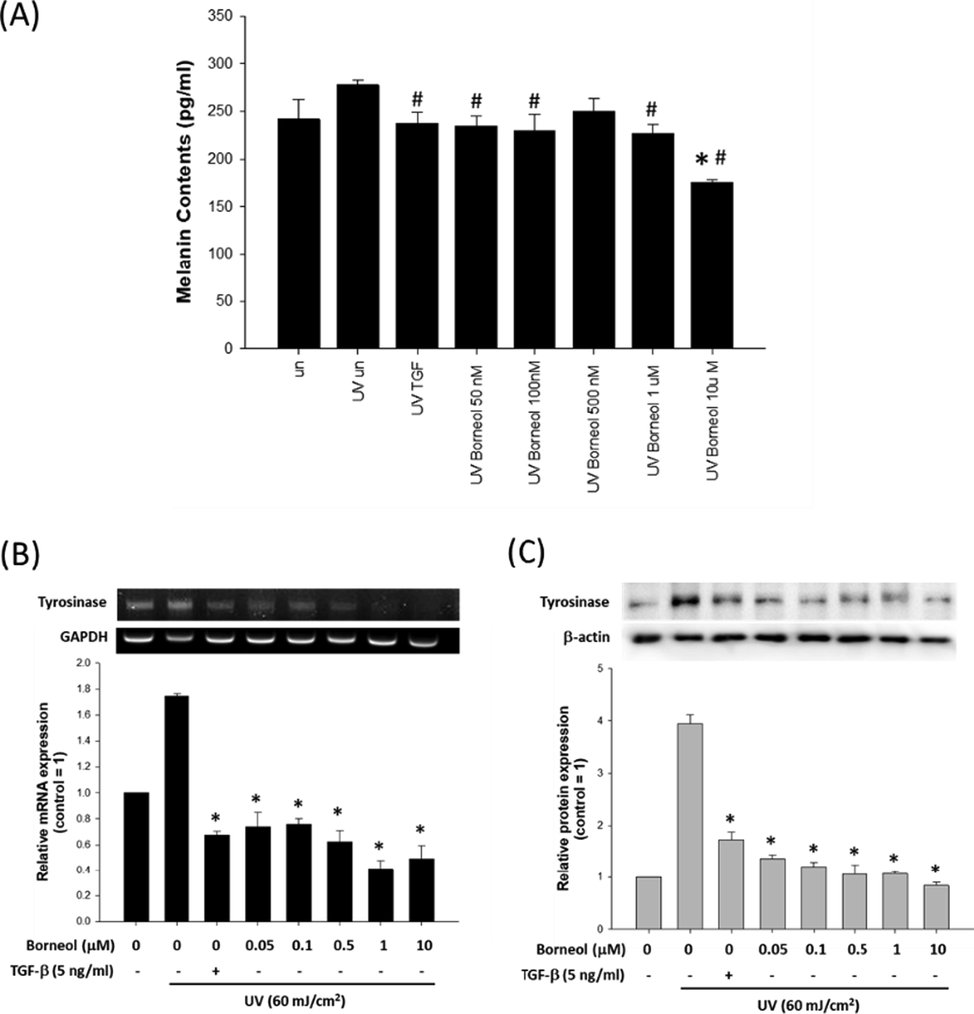
Effect of borneol on melanin synthesis. (A) Effect of varying concentrations of borneol on melanin production in UV stimulated (60 mJ/cm2) Bl6F10 cells using melanin contents assay. Data are represented as a mean ± standard deviation (n = 4). #p < 0.05 compared to UV treated control and *p < 0.05 compared to untreated control. Expression of tyrosinase was evaluated at mRNA level (B) and protein level (C), with or without borneol treatment. GAPDH and β-actin were used as internal controls. TGF-β was used as positive control. Data are represented as a mean ± standard deviation (n = 3) *p < 0.05 compared to UV treated control.
3.6 Assessment of the anti-wrinkle effect of borneol
To examine the effect of borneol on collagen type I synthesis, pro-collagen type I C-peptide assay was conducted. For a comparison purpose, and as well as for empirical verification of the estimation of F1 contained borneol concentration, the effects of both 105 fold diluted F1 and 10 μM of borneol on the collagen type I synthesis in UV irradiated HDFs were examined. As shown in Fig. 7A, UV irradiation decreased approximately 50% of collagen contents (349.52 ± 18.44 vs. 171.74 ± 33.49 ng/ml for untreated control and UV treated group, respectively), and diluted F1 prevented such drastic decrease of collagen contents resulting in about 25% of reduction of collagen contents (240.96 ± 32.97 ng/ml). Nevertheless, 10 μM of borneol was most effective in preventing the UV-induced reduction of collagen contents (314.45 ± 18.94 ng/ml), indicating the estimation of borneol in F1 was not significantly deviated or incorrect. The effect of borneol on collagen type I synthesis was also examined, and the results indicated that both F1 and borneol significantly prevented the UV-induced drastic decrease of collagen type I mRNA synthesis (Fig. 7B). Furthermore, both F1 and borneol recovered the UV-induced decrease of collagen type I protein synthesis (Fig. 7C). However, only the recovery by borneol was statistically significant.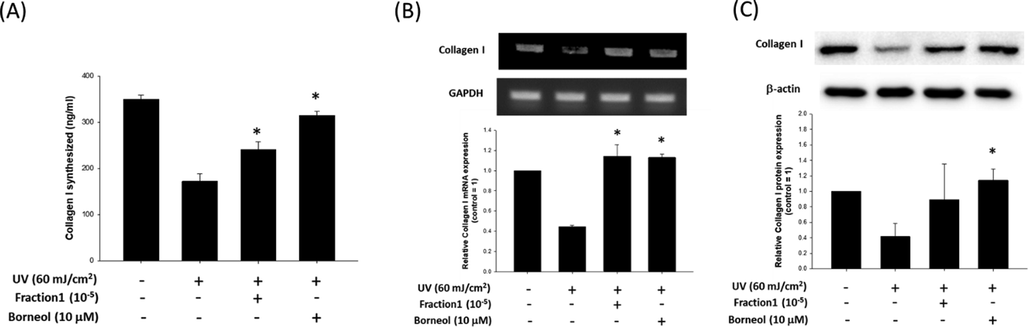
Effect of borneol on collagen synthesis. (A) Effect of 105 fold diluted F1 and borneol (10 μM) on collagen type I contents of HDFs was evaluated by using pro-collagen type I C-peptide assay. Data are represented as a mean ± standard deviation (n = 4). Expression of collagen type I was evaluated at mRNA level (B) and protein level (C), with or without diluted F1 or borneol treatment. GAPDH and β-actin were used as internal controls. Data are represented as a mean ± standard deviation (n = 3). *p < 0.05 compared to UV only treated group.
To further evaluate anti-wrinkle effect of borneol, its effect on matrix metalloproteinase 1 (MMP-1) production was examined. The result of human active MMP-1 ELISA demonstrated that UV irradiation increased the amount of MMP-1 released into culture medium (74.01 ± 7.24 vs. 185.11 ± 16.64 ng/ml for untreated control and UV treated group, respectively), and both diluted F1 and borneol significantly suppressed the UV-induced release of MMP-1 (69.54 ± 4.06 and 47.23 ± 3.46 ng/ml for F1 and borneol, respectively) (Fig. 8A).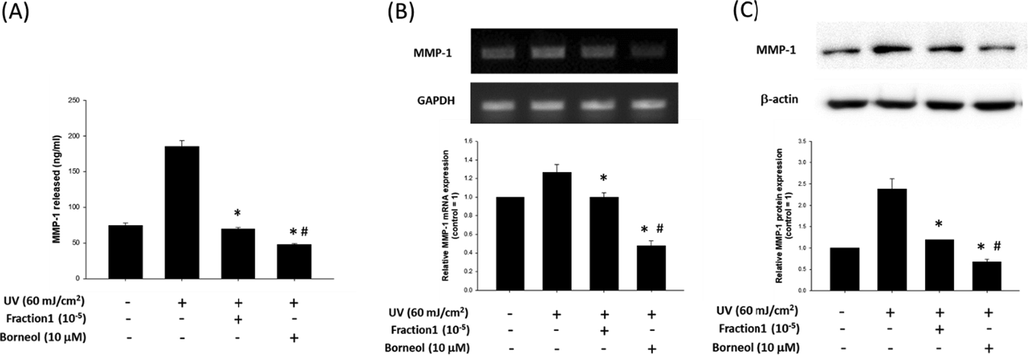
Effect of borneol on MMP-1 production. (A) Effect of 105 fold diluted F1 and borneol (10 μM) on active MMP-1 released from HDFs was evaluated by human active MMP-1 ELISA. Data are represented as a mean ± standard deviation (n = 4). Expression of MMP-1 was evaluated at mRNA level (B) and protein level (C), with or without diluted F1 or borneol treatment. GAPDH and β-actin were used as internal controls. Data are represented as a mean ± standard deviation (n = 3). #p < 0.05 compared to UV treated control and *p < 0.05 compared to untreated control.
Similarly, UV irradiation increased the amount of MMP-1 mRNA, but both diluted F1 and boreol significantly prevented such UV-induced increase of mRNA (Fig. 8B). Such inhibitory effect of both F1 and borneol on MMP-1 synthesis was also observed in protein level (Fig. 8C).
4 Discussion
Excessive production of melanin can cause dermatological problems including, but not limited to, freckles, solar lentigo, melasma, as well as cancer (Brenner and Hearing, 2008). The initial step of the melanogenesis is the oxidation of L-tyrosine to dopaquinone (DQ) by tyrosinase (Hearing and Jimenez, 1987), and thus, targeting tyrosinase may specifically inhibit the melanogenesis. In the present study, the very first fraction (F1) of whole essential oil from A. koreana significantly suppressed tyrosinase activity (Fig. 3). Further analysis of the F1 using GC/MS indicated that borneol and bornyl acetate were unique to the F1, and they might be the key compounds responsible for the observed tyrosinase inhibitory effect of the F1 (Fig. 4). Previous studies also have demonstrated that both borneol and bornyl acetate are present in the essential oil of A. Koreana (Paulauskiene et al., 2021). Since the acetylation of borneol produces bornyl acetate in the plastids of plants (Thiel and Adam, 2002), the effect of borneol was evaluated for its whitening and anti-wrinkle effect to prove the concept.
Borneol is widely used as food and cosmetics additive and known to be presented in the essential oils of plants such as thyme and rosemary (Abdelli et al., 2017; Angioni et al., 2004), and our group also recently reported that it is presented in the essential oils of A. koreana (Song et al., 2018). Known bioactivities of borneol include analgesic, anti-inflammation and antioxidant activity (Granger et al., 2005; Harish et al., 2005; Mihara and Shibamoto, 2015). Interestingly, borneol is also known to enhance the bioavailability of other drugs such as salvianolic acid B, saponin, geniposide, and curcumin (Gao et al., 2019; Lai et al., 2011; Lu et al., 2010; Zhou et al., 2010).
However, to our best knowledge, there are no previous study reported whitening and/or anti-wrinkle effect of borneol to date. According to our data, 10 μM of borneol significantly suppressed both the UV-induced tyrosinase activity and tyrosinase expression in melanoma cells (Fig. 6), suggesting its potential as whitening agent. Pertaining to its effect on either melanoma or melanocytes, although there is one study reported that borneol and curcumin synergistically induced apoptosis of melanoma cells (Chen et al., 2014), the concentration of borneol used in that particular study was 40 μg/ml, which is approximately equivalent to 260 μM of borneol so that it was much higher than the concentration used for the present study. Therefore, it may be possible that borneol can induce apoptosis of melanoma cells at a higher concentration, but it can also suppresses the tyrosinase activity of melanocyte at a lower concentration without causing apoptotic cell death.
One of the typical characteristics of aged skin is the thinning of the skin that impairs the structural integrity and function of the skin (Uitto and Bernstein, 1998). Since dermal collagen type I that is the most abundant extracellular matrix (ECM) protein (90% dry weight) responsible for the skin’s tensile strength and mechanical properties (Uitto, 1989), an ability to maintain an adequate level of collagen is required for an anti-wrinkle agent.
As shown in Fig. 7, both 105 fold diluted F1 and 10 μM of borneol prevented UV-induced loss of collagen type I in HDFs, and especially for 10 μM of boneol, the effects were statistically significant. These data indicated that the essential oil extracted from A. koreana (F1) possesses an anti-wrinkle potential and also borneol is most likely the key ingredient facilitating such anti-wrinkle potential of the F1. Furthermore, our data show that both 105 fold diluted F1 and 10 μM of borneol suppressed both the UV-induced release and synthesis of MMP-1 (Fig. 8). Considering MMPs, especially MMP-1, are responsible for degrading most of the ECM proteins including collagen, fibronectin, elastin, and proteoglycans in the process of photo-aging (Quan et al., 2009; Bae et al., 2008), it can be assumed that borneol not only maintains collagen contents but also protects collagen from degradation by MMP.
Altogether, the results of our study demonstrated that the essential oils from A. koreana has whitening and anti-wrinkle potential and identified borneol as the key compound responsible for the observed effects. However, the whitening and anti-wrinkle efficacy of the A. koreana essential oils still needs to be further scrutinized in future studies to better understand the underlying mechanisms.
5 Conclusions
In the present study, borneol was identified as a key component that may facilitates the skin-improving activity of the essential oil from A. koreana based on its inhibitory effect on tyrosinase and MMP-1 activity as well as on its ability to maintain collagen contents.
Acknowledgement
This study was carried out with the support of R&D Program for Forest Science Technology (Project No. 2020169C10-2022-AD03) provided by Korea Forest Service.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chemical composition and anti-inflammatory activity of algerian thymus vulgaris essential oil. Nat. Prod. Commun.. 2017;12:611-614.
- [Google Scholar]
- chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus officinalis L. J. Agric. Food Chem.. 2004;52:3530-3535.
- [Google Scholar]
- Techniques for extraction of bioactive compounds from plant materials: a review. J. Food Eng.. 2013;117:426-436.
- [Google Scholar]
- (-)Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: involvement of mitogen-activated protein kinase. Food Chem. Toxicol.. 2008;46:1298-1307.
- [Google Scholar]
- The protective role of melanin against UV damage in human skin. Photochem. Photobiol.. 2008;84:539-549.
- [Google Scholar]
- Synergistic apoptosis-inducing effects on A375 human melanoma cells of natural borneol and curcumin. PLoS One. 2014;9:e101277.
- [Google Scholar]
- Improved oral absorption of poorly soluble curcumin via the concomitant use of borneol. AAPS PharmSciTech. 2019;20:150.
- [Google Scholar]
- (+)- And (-)-borneol: efficacious positive modulators of GABA action at human recombinant alpha1beta2gamma2L GABA(A) receptors. Biochem. Pharmacol.. 2005;69:1101-1111.
- [Google Scholar]
- Isolation of antioxidant compounds from the methanolic extract of the roots of Decalepis hamiltonii (Wight and Arn.) J. Agric. Food Chem.. 2005;53:7709-7714.
- [Google Scholar]
- Mammalian tyrosinase – the critical regulatory control point in melanocyte pigmentation. Int. J. Biochem.. 1987;19:1141-1147.
- [Google Scholar]
- Chemical composition and antibacterial activities of the essential oil from Abies koreana. Phytother. Res.. 2007;21:1246-1250.
- [Google Scholar]
- Transforming growth factor-beta1 decreases melanin synthesis via delayed extracellular signal-regulated kinase activation. Int. J. Biochem. Cell Biol.. 2004;36:1482-1491.
- [Google Scholar]
- Comparative pharmacokinetic and bioavailability studies of three salvianolic acids after the administration of Salviae miltiorrhizae alone or with synthetical borneol in rats. Fitoterapia. 2011;82:883-888.
- [Google Scholar]
- Comparative analysis of chemical compositions and antimicrobial activities of essential oils from Abies holophylla and Abies koreana activities of essential oils from Abies holophylla and Abies koreana. J. Microbiol. Biotechnol.. 2009;19:372-377.
- [Google Scholar]
- The in situ and in vivo study on enhancing effect of borneol in nasal absorption of Geniposide in rats. Arch. Pharm. Res.. 2010;33:691-696.
- [Google Scholar]
- The role of flavor and fragrance chemicals in TRPA1 (transient receptor potential cation channel, member A1) activity associated with allergies. Allergy Asthma Clin. Immunol.. 2015;11:11.
- [Google Scholar]
- Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica dioica L.) Plants (Basel). 2021;10
- [Google Scholar]
- Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc.. 2009;14:20-24.
- [Google Scholar]
- Ascaris lumbricoides found in ashore corpses from Korean peninsula to Japan. Parasitol. Int.. 2019;70:1-4.
- [Google Scholar]
- Anti-wrinkle and Whitening Effects of Essential Oil from Abies koreana. J Life Sci. 2018;28:524-531.
- [Google Scholar]
- Incorporation of [1-(13)C]1-deoxy-D-xylulose into isoprenoids of the liverwort Conocephalum conicum. Phytochemistry. 2002;59:269-274.
- [Google Scholar]
- Inhibition of tyrosinase activity and melanine pigmentation by 2-hydroxytyrosol. Acta Pharm. Sin. B. 2014;4:141-145.
- [Google Scholar]
- Connective tissue biochemistry of the aging dermis. Age-associated alterations in collagen and elastin. Clin. Geriatr. Med.. 1989;5:127-147.
- [Google Scholar]
- Molecular mechanisms of cutaneous aging: connective tissue alterations in the dermis. J. Investig. Dermatol. Symp. Proc.. 1998;3:41-44.
- [Google Scholar]
- Composition of essential oils from seeds of Abies koreana. Nat. Prod. Commun.. 2013;8:227-230.
- [Google Scholar]
- Abies koreana essential oil inhibits drug-resistant skin pathogen growth and LPS-induced inflammatory effects of murine macrophage. Lipids. 2009;44:471-476.
- [Google Scholar]
- Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med.. 2018;13:20.
- [Google Scholar]
- Enhancement of intestinal absorption of akebia saponin D by borneol and probenecid in situ and in vitro. Environ. Toxicol. Pharmacol.. 2010;29:229-234.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102886.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
These supplementary table contains GC/MS results.







