Translate this page into:
Embryonic development of Indonesian native fish yellow rasbora (Rasbora lateristriata)
⁎Corresponding author. bambang.retnoaji@ugm.ac.id (Bambang Retnoaji),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Yellow rasbora (Rasbora lateristriata) exhibits potential as a novel animal model for research purposes. Nevertheless, the process of embryonic development of this fish species remains inadequately comprehended. This study aimed to characterize the embryonic development stages of yellow rasbora.
Methods
The embryonic development was documented using time-lapse technique to observe the following parameters: the embryonic cleavage, the blastomere, the epiboly area, and the somites. Heart development and morphological changes were also observed, including the number of heartbeats at 24, 30, 48 h post-fertilization. The cartilage structure in the cranium of larvae aged 1 to 3 days post-fertilization were observed as well. Alizarin red and Alcian blue staining were used to stain bone and cartilage structures.
Results
The results of this study describe the important stages of embryogenesis in yellow rasbora, including the zygote, cleavage, blastula, gastrula, segmentation, pharyngula, and hatching stages. The first contraction of the heart muscle was observed at 20 h post-fertilization, with cardiac tube looping occurred at 24–30 h post-fertilization. The cranium cartilage was formed 2 days post-fertilization and completed at 3 days post-fertilization.
Conclusion
In conclusion, this study provided a comprehensive overview of the developmental stages of yellow rasbora embryos.
Keywords
Embryogenesis
Development
Native fish
Indonesia
Cyprinidae
1 Introduction
Fishes, including zebrafish (Danio rerio) and medaka (Oryzias latipes), are important animal models for wide spectrum biomedical research (Choi et al., 2021; Lin et al., 2016). The advantages of using fishes as model organisms include beneficial biological attributes (e.g. transparent embryos, rapid embryological development, year-round breeding availability, and inexpensive maintenance), widely available genetic information, well-developed molecular and genetic biology techniques to create transgenic animal, and conserved genome structure to human (Choi et al., 2021; Howe et al., 2013; Lieschke and Currie, 2007; Lin et al., 2016; Lleras-Forero et al., 2020). Therefore, other fish species that have similar biological attributes with zebrafish and medaka might have potential to be developed as the new generation of animal model in biomedical research.
Yellow rasbora (Rasbora lateristriata) is one of Indonesian native fish that can be developed as an animal model. The yellow rasbora is distributed in Sumatra, Kalimantan, Java, Bali, Sumbawa, and Lombok regions (Djumanto et al., 2008; Kottelat et al., 1993) and occupies freshwater habitats such as lakes, rivers, swamps, and paddy fields (Ahmad and Nofrizal, 2011; Kusuma et al., 2016). In the wild, yellow rasbora spawns once a year from early May to late June (Djumanto et al., 2008). The promising biological attributes of yellow rasbora such as small size, transparent embryo, quick embryonic development, and sustainable of eggs production, are expected to be the supporting factors for developing this fish as a novel animal model, ornamental fish, as well as bioindicator for water quality (Djumanto et al., 2008; Djumanto and Setyawan, 2009; Lailiati et al., 2022).
The embryonic development of animal models is important to be used as references in identifying malformation occurred during experimental studies. The embryonic development of medaka, zebrafish, rainbow trout (Oncorhynchus mykiss), brook trout (Salvelinus fontinalis), and ice goby (Leucopsarion petersii) has been reported previously (Arakawa et al., 1999; Ballard, 1973; Iwamatsu, 2004; Kimmel et al., 1995). Nonetheless, the process of embryonic development in yellow rasbora has yet to be investigated. This study aimed to characterize the stages of embryonic development in yellow rasbora, including the heart and cranium development.
2 Material and methods
2.1 Fish maintenance and spawning
The adult domesticated yellow rasbora were maintained in a water-circulated aquarium system (28–29 °C; pH 7.0–7.5). Photoperiod cycle was established at a ratio of 14:10 light-to-dark hours, with the purpose of replicating the natural condition found in the wild. The fish were fed three times a day. In order to induce spawning, a cohort of 14 mature males and seven mature females were introduced into water-circulated breeding chambers, following a 2:1 male-to-female ratio, during the afternoon hours between 4 and 5 pm. The spawned eggs were collected the next morning.
2.2 Observation of embryonic development
Eggs were transferred to egg water media (1000 mL of water, 1.5 mL of salt stock, 2 drops of methylene blue) using egg pipette and were subsequently sorted out for the viable eggs. The viable eggs were placed in a concave glass containing egg water media. The stages of embryonic development were observed and recorded using a Leica ICC50 microscope camera by referring to the zebrafish stages of embryonic development (Kimmel et al., 1995).
2.3 Observation of heart development
The heart development was observed in 12–48 h post fertilization (hpf) embryos. The embryos were anesthetized with 100 ppm of tricaine and placed in 3% methyl cellulose. The heart was observed by monitoring the first contraction, the number of heartbeats at 24, 30, 48 hpf, and morphological changes during development. Observations were made using a Leica ICC50 microscope.
2.4 Cranium cartilage structure
The cranial cartilage structure was examined in larvae aged 1–3 days post fertilization (dpf) using the Alizarin red and Alcian blue (ARAB) staining method, which is commonly used in whole mount embryo staining protocols (Retnoaji et al., 2014). The larvae were anesthetized using the cold shock method and were subsequently collected in 2 mL microtubes. The larvae were subjected to fixation for 2 days at 28 °C using 96% alcohol. The fixed larvae were then stained with Alizarin red Alcian blue staining solution for 3 days at 28 °C. The staining solution was then discarded and the larvae were washed with distilled water. One milliliter of bleach solution (3% H2O2 and 2% KOH) was added to the microtube containing fish larvae. After 10 min, the bleach solution was then discarded and replaced with 1 mL of 0.05% KOH for 5 min. The larvae were then incubated with 1 mL of Mole solution (20 mL of 20% Glycerin + 1 mL of 1% KOH + 79 mL of distilled water) for 12–24 hrs. The stained cranium was then observed and photographed using a Leica ICC50 microscope camera.
2.5 Histology
Fish embryos were fixed using 10% neutral buffered formalin for 1–2 days, followed by a dehydration process using a series of ethanol solutions (70–100%). Subsequently, the embryos were cleared with xylene overnight and infiltrated with paraffin wax. The embryos were then embedded with freshly melted paraffin wax and longitudinally sectioned using a microtome. The sections were placed onto clean glass slides and proceeded with a Hematoxylin-Eosin staining procedure (Bancroft et al., 1994). Finally, the histological sections were observed and captured using a Leica ICC50 microscope camera.
2.6 Ultrastructure observation
Ultrastructure observation was performed using neutral buffer formalin fixation for 12 h, followed by dehydration using graded ethanol (70%, 80%, 90%, 96%) with an interval of 2 h in cold condition. After being soaked in 100% alcohol, the alcohol was drained and the larvae were stored directly in the − 18 °C freezer. The mounted sample was prepared by attaching the sample to aluminum stub using double carbon tape and coated with gold. Coating was performed in a voltage of 20 mA for 20 s using the Hitachi MC1000. Ultrastructure observation was performed with Hitachi SU3500 Scanning Electron Microscope (VACC 5 kV, high pressure, Secondary Electron Signal, 20 Spot Intensity). The morphometric data were measured using the built-in SEM measurement software.
2.7 Data analysis
The stages of embryonic development were documented by using a timelapse technique. A total of 15 developmental time-lapses were analyzed to determine the timing of developmental stages (Distel and Köster, 2007). The analysis was carried out by assessing the average of each developmental stage. The parameters for the stages of development are determined by the special characteristics of each stage of development. Some of the specific features were observed to describe the development including the number of cells, the shape and size of the blastomere, the area of the epiboly, the number and shape of the somites, muscle development, heart, blood circulation, body shape, and tail development. Based on these specific features, the observed embryos are grouped into several developmental stages. The development stages include the zygote, cleavage, blastula, gastrula, segmentation, pharyngula, and hatching periods (Kimmel et al., 1995). The observed images were processed using a photo editing application and were displayed based on the stages of development. The morphometric data were analyzed using the Principal Component Analysis (PCA) tool on R software (Team, 2018).
3 Results
3.1 Overview of yellow rasbora embryonic development
Yellow rasbora eggs are spherical in shape and transparent showing similar characteristics to that of zebrafish. Moreover, the developmental stages of yellow rasbora generally resembles that of zebrafish (Kimmel et al., 1995). Therefore, in this study, we categorized the yellow rasbora development using the stages of the zebrafish as a reference, covering the following stages: zygote, cleavage, blastula, gastrula, segmentation, pharyngula, and hatching (Fig. 1; Table 1). The zygote stage occurs from fertilization to early cleavage which lasts up to 45 min post-fertilization (mpf). After the zygote stage, the embryo enters the cleavage stage resulting in more cells with smaller size. These small cells form blastomeres, indicating the start of the blastulation stage which lasted for three hours. The gastrulation stage is indicated by the blastomeres moving toward the yolk (epiboly), which takes place on 4.75 – 7.75 hpf. After the yolk is completely covered, the somites start to develop in the mediodorsal part of the embryo. The somitogenesis is exclusive in the segmentation stage, which takes place on 7.75 – 14.25 hpf. The pharyngula stage is defined by the formation of several organs and occurs on 14.25 – 24 hpf. The yellow rasbora embryos are mostly hatched at 24 hpf, but some slight variation can be still expected among individuals.
Embryonic development stages of yellow rasbora (Rasbora lateristriata) embryo at 0 – 48 hpf.
Stages
Periode (hpf)
Description
Zygote
0 – 0.75
Embryo from fertilization to the early cleavage.
Cleavage
0.75 – 1.75
Cell division produces cells with more numbers and smaller sizes.
Blastula
1.75 – 4.75
Small cells form compact and spherical cells. Two layers of cells are formed: the epiblast and the hypoblast.
Gastrula
4.75 – 7.75
Formation of three germ layers (ectoderm, endoderm, and endoderm). The gastrulation stage is described by the percentage of epiboly.
Segmentation
7.75 – 14.25
Formation of somites.
Pharyngula
14.25 – 24
Several organs are starting to form during the pharyngula stage.
Hatching
24
Several organs such as the heart, gills, jaws, and fins developed rapidly during this period.
3.2 Zygote stage (0–45 min post-fertilization)
The zygote stage starts from fertilization until the early cleavage stage. Upon fertilization, cytoplasmic movements are triggered, causing the non-yolk cytoplasm moving towards the animal poles and is separated from the yolk at the vegetal pole, forming a structure called blastodisc (Fig. 1; Suppl. Fig. 1) (Kimmel et al., 1995). Since one-cell eggs could be observed from the dorsal view of the animal pole, this stage is also named as the one-cell stage (Fig. 1; Suppl. Fig. 2). The transition from zygote to the one-cell stage approximately takes 45 mpf.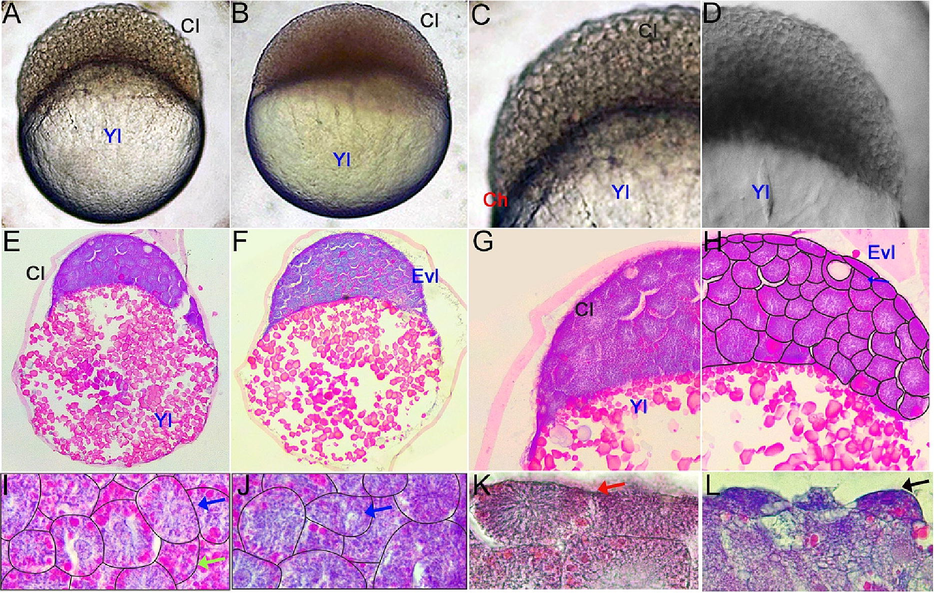
Embryo development of (Rasbora lateristriata) during the blastulation stage. (A) 256 cells. (B) High stages. (C, D) The highlight on the embryonic blastomeres. (E, F) Histological structure of the embryo. (G, H) Histological structure and the cellular reconstruction of the blastomeres showing the development progression of blastomeres cells (Cl), cellular differentiation of blastomeres showed by the present of two different types of cell (EVL = enveloping cell/enveloping layer and internal cell blastomeres). (I, J, K, L) Blastoderm cells and their cellular reconstruction, showing the variation of cytoplasmic vesicular distribution on each blastoderm. Cl (Cell of Blastoderm), Yl (Yolk), EVL (Enveloping layer). Blue arrow: cell with less vesicle, green arrow: cell with dense vesicle, black arrow: differentiated envelope cell. HE staining and 40-400x magnification.
3.3 Cleavage period (45 min – 1 h 45 min post-fertilization)
The cleavage type of yellow rasbora embryo is meroblastic. Meroblastic cleavage is a distinctive phenomenon that exclusively takes place in the blastodisc region of the embryo, while no segmentation process is discernible in the yolk area (Gilbert, 2000). This mode of cleavage is characterized by a vertical division from the animal to the vegetal poles, culminating at the outer edge of the yolk. Consequently, an increase in the number of cells with smaller size is achieved (Suppl. Fig. 2).
The first five cleavages of yellow rasbora embryos generate odd-numbered cleavages crossing over the short axis of the blastodisc and even-numbered ones crossing over the long axis (Fig. 1; Suppl. Fig. 2 H–L) which are also established in zebrafish and medaka embryos (Iwamatsu, 2004; Kimmel et al., 1995). Moreover, the 16 cells stage in the yellow rasbora embryo display four blastomeres in the center and surrounded by 12 other blastomeres (Suppl. Fig. 2K), which are called marginal blastomeres in medaka embryos (Iwamatsu, 2004). In addition, two layers of marginal blastomeres are also found in the 64-cell stage of yellow rasbora embryo covering the other inner blastomeres. Cellular morphological differentiation of blastomeres start to occur in parallel with the cells cleavage progression. Cell morphological differentiation were characterized by transformation of blastomeres cell shape. The shape of outer covering blastomeres become more flattened, which is labeled as the Enveloping Layer (EVL), in contrast to polygonal or cuboidal shape of inner blastomeres. The EVL is still visible at the 256 cell stage (Kimmel et al., 1995).
3.4 Blastula stage (1 h 57 min – 4 h 48 min post-fertilization)
The blastula stage of yellow rasbora embryo is started when the blastomeres reach 256 cells and form a solid semicircular mound of cells on the yolk (Fig. 2A, C). There are more than 11 piles of enveloping layers (EVL) at the high stage (Fig. 2D, F, H). Subsequently, the blastomere becomes more rigid and compact at the oblong stage. The blastomere compresses the yolk, thus increasing the surface area and forming an ellipse. The transition from the high to the oblong stage (Fig. 1) takes place at 3 h 8 min post-fertilization. During the embryonic development, the animal-vegetal axis undergoes a notable transformation whereby it becomes markedly shortened, taking on an almost circular shape during the sphere stage (Fig. 2B). At the dome stage, the yolk moves toward the animal pole, assuming a dome shape. The start of epiboly is indicated by a sudden and rapid shift in the distribution of yolk and blastodisc cells (Kimmel et al., 1995). During the epibolic stage, the level of blastoderm covering the yolk area is used to quantify embryonic development. Specifically, the 30% epiboly stage is characterized by the proportion of blastoderm that encloses 30% of the yolk surface (Kimmel et al., 1995).
The blastula stage in yellow rasbora is a stereoblastula which is characterized by the absence of a blastocoel cavity (Fig. 2A, B; Supl. Fig. 3A, B). The blastomeres, cells constructing blastoderm, can be distinguished into enveloping cells/layers spread along the superficial part of blastoderm, and internal blastomeres located underneath the enveloping cells (Fig. 2E–H). Moreover, various morphological features of blastomeres are clearly visible, such as cytoplasmic vesicular distribution of internal blastomeres (Fig. 2I–L) and shapes and cytoplasmic changes of enveloping cells (Fig. 2K, L). These variations indicate the rapid cell development and differentiation occurred in this stage.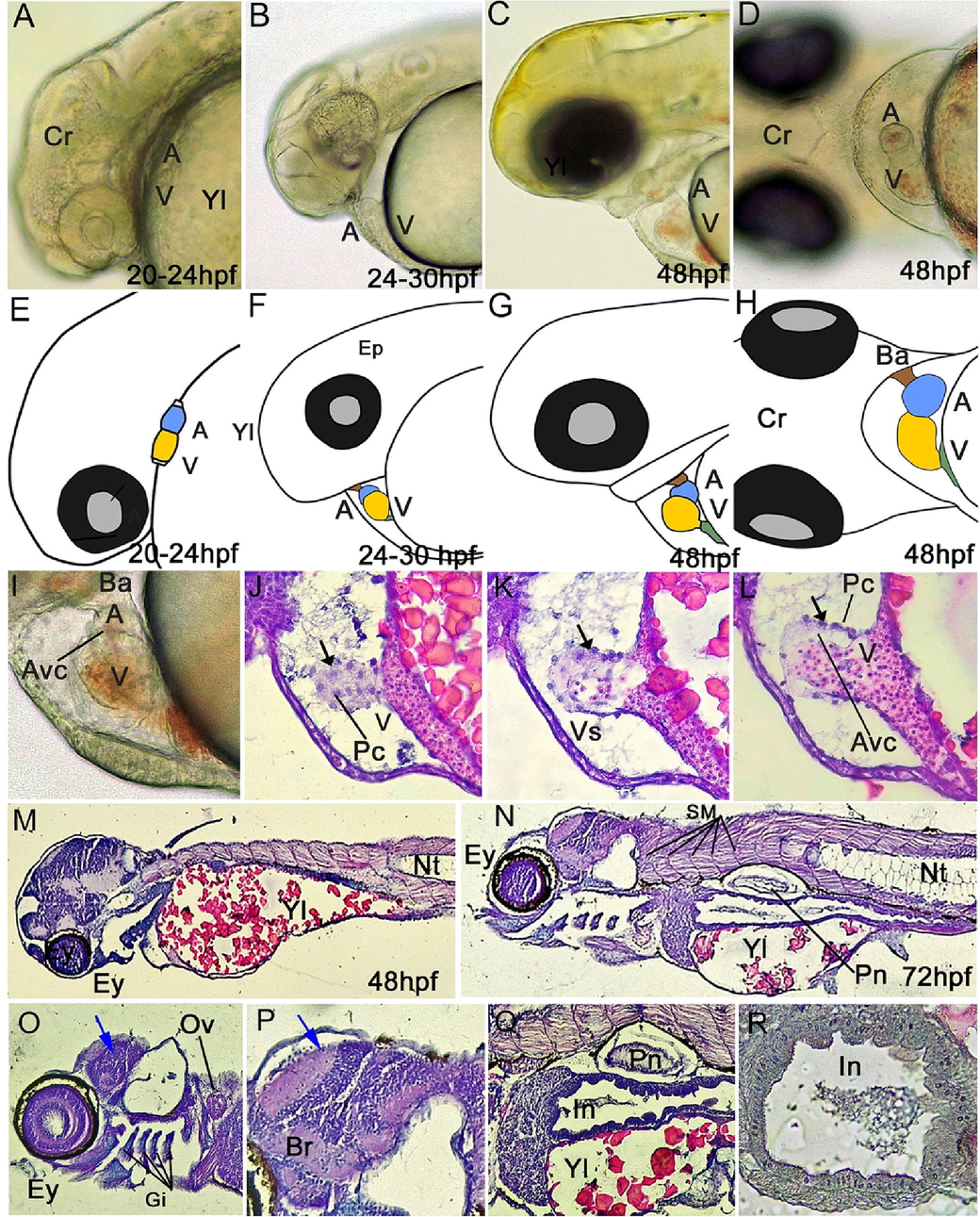
Developmental progression of the heart and organs in the larval stage of yellow rasbora (Rasbora lateristriata). (A–F) Lateral and ventral view. (A, D) Hearts on 20–24 hpf are linear tubular. (B, E) The looping process occurs in larvae of 24–30 hpf, in which the heart begins to form an arc. (C, F) Heart on 48 hpf. (G, H) Ventral view of the heart on 48 hpf, (I, J, K, L) heart morphology and histology, (M, N) 48 and 72 hpf mid saction histological structure of embryo (O) head region of 72 hpf embryo, (P) brain layer, (Q) body organ, (R) intestine of 72 hpf embryo. Abbreviations: (a) atrium, (v) ventricle, (sv) sinus venosus, (ba) bulbus arteriosus, (avc) atrioventricular canal, (Yl) Yolk, (Evl) Enveloping layer, (Ey) eyes, (Br) brain, (Ov) Otic Vesicle, (Hr) heart, (Nt) notochord, (Pn) Pneumatocyst, (In) Intestine. Color captions: (yellow) atrium, (blue) ventricle, (green) sinus venosus, (brown) bulbus arteriosus, Blue arrow: brain with brain layer, black arrow: endothelial cell. HE staining and 40-400x magnification.
3.5 Gastrula stage (4.75 – 7.75 hpf)
The gastrula stage in yellow rasbora embryo is indicated by 50% of the blastoderm covering the yolk (50% epibolic stage) (Fig. 1; Suppl. Fig. 3). A few minutes after reaching 50% epiboly, the marginal part of the blastoderm thickens and forms a germ ring (Fig. 1). Afterward, the yellow rasbora embryo develops into a shield stage (Fig. 1). The shield stage is characterized by accumulation of cells at one location within the germ ring called embryonic shields (Kimmel et al., 1995). At the 70% epibolic stage, the blastoderm covers the almost 3/4 of yolk area and the prechordal plate is visible reaching in the axial part of blastoderm (Fig. 1; Suppl. Fig. 2). The blastoderm of yellow rasbora embryo continues to grow covering the yolk and leave the yolk plug visible at the 90% epibolic stage (Fig. 1). Finally, the blastoderm covers the whole yolk at the bud stage, which completes the epibolic stage. During this stage, the formation of an anterior bud, which will ultimately develop into the head, and a posterior bud, which will give rise to the tail, takes place (Suppl. Fig. 3C–E) (Iwamatsu, 2004; Kimmel et al., 1995).
During the germ-ring stage of embryonic development in zebrafish, the blastoderm is composed of two layers, which include the epiblast and the hypoblast (Kimmel et al., 1995). These layers are also visible in yellow rasbora embryos at the same stage (Suppl. Fig. 3G–J). During the epibolic movement, the cells of these two layers undergo proliferation and differentiation (Suppl. Fig. 3K–N). The complete epibolic stage and the formation of the tailbud indicate the end of the gastrulation stage in the yellow rasbora embryo.
3.6 Segmentation stage (7.75 – 14.25 hpf)
The segmentation stage is characterized by the formation of segments called somites. In vertebrate development, the primary body axis undergoes a process of elongation directed towards the posterior section. This elongation process is marked by periodic division of the axis into somites, which are crucial in the generation of various tissue types, including vertebrae, skeletal muscles, and dermis (Kimmel et al., 1995). Somite formation proceeds in a predictable pattern, whereby the somites located in the anterior region exhibit a higher rate of development compared to those situated in the posterior region (Retnoaji et al., 2014). At the segmentation stage, a linear expansion of the embryo length occurs in response to an augmented number of somites, leading to the determination of the Embryo Length (EL) measurement (Kimmel et al., 1995).
The first somite pair on the yellow rasbora embryo is formed in the anterior region (Fig. 1; Suppl. Fig. 4). Somite formation progresses in a caudal direction, with a new pair of somites being generated every 15 min following the formation of the preceding pair (Suppl. Fig. 4A–K). Kupffer's vesicle is visible in the tailbud area of yellow rasbora embryo at 14 somites stage (Suppl. Fig. 4L). The Kupffer's vesicle is needed to determine the right and left orientation of the organ formation. Upon reaching 17 somites, the separation of the tailbud from the yolk occurs (Suppl. Fig. 4F). Muscle initial contraction can also be observed in this stage. The muscle contraction is produced by individual (unilateral) myotome activity (Kimmel et al., 1995). The tail of the yellow rasbora embryo is elongated at the 20 and 25 somites stages (Suppl. Fig. 4G, H) and muscle contraction become more complex and frequent.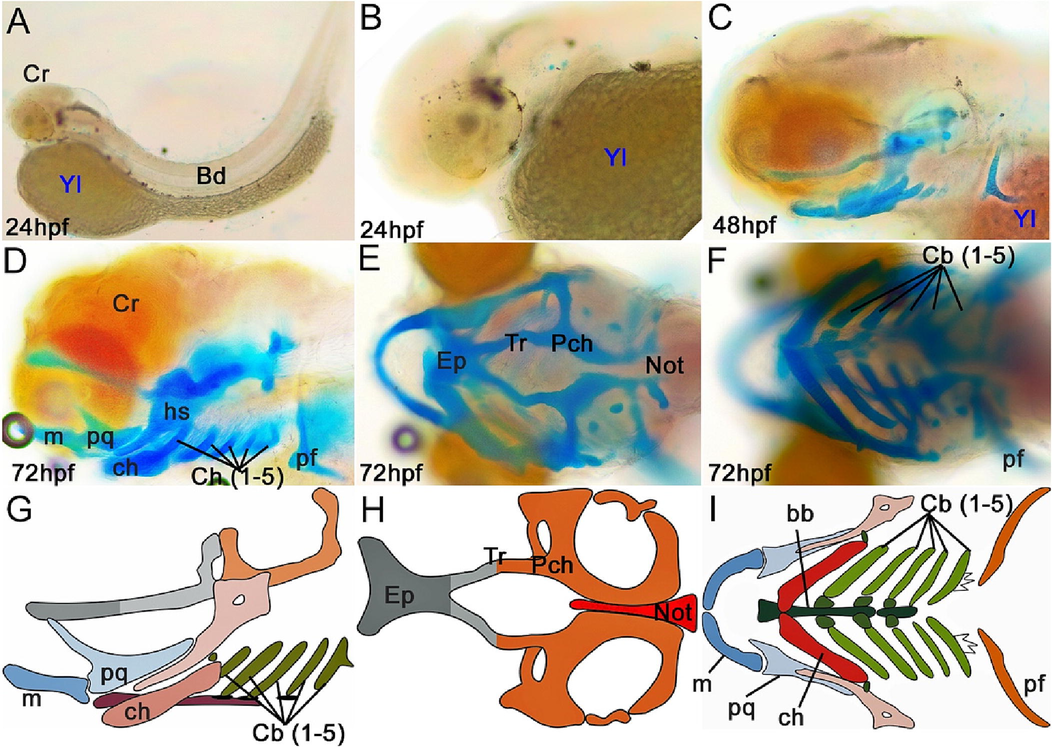
Cranial cartilage structure of yellow rasbora (Rasbora lateristriata) larvae at 24, 48, and 72 hpf with ARAB staining (A–F) and cranial cartilage illustration of 72 hpf larva (G–I). (D, G) Lateral view. (E, H) Dorsal view. (F, I) Ventral view. Abbreviations: (bb) basibranchial, (cb) ceratobranchial, (ch) ceratohyal, (ep) ethmoid plate, (hs) hyosymplectic, (m) Meckel's cartilage, (n) notochord, (pch) parachordal, (pf) pectoral fin, (pq) palatoquadrate, (tr) trabecula.
3.7 Pharyngeal stage (14 h 18 min – 24 hpf)
The pharyngeal stage is a period in embryonic development during which the germ layer undertakes migration to establish various organ linings, including the skin and pigments. During the primordial stage, multiple anatomical structures are formed, which include the aortic arches, eyes, fins, vessels, pericardial cavity, and brain parts. By the time the embryo reaches the prim-6 stage, it will have formed approximately 30 somites, with 15 of them near the tail. At this stage, pigmentation becomes visible in the epithelial layer of the retina and the dorsal (anterior-posterior) skin, and the organs of the heart and brain can be observed. During the prim-16 and prim-22 stages, retinal pigmentation occurs, and the heart remains in the form of a straight tube with developing chambers. Moreover, there is folding of the median fins, and the pectoral fins continue to undergo development. Hatching period of yellow rasbora embryo starts after 24 h post-fertilization (Fig. 1; Suppl. Fig. 5). However, the hatching time may be slightly varied among individuals depending on the environmental conditions.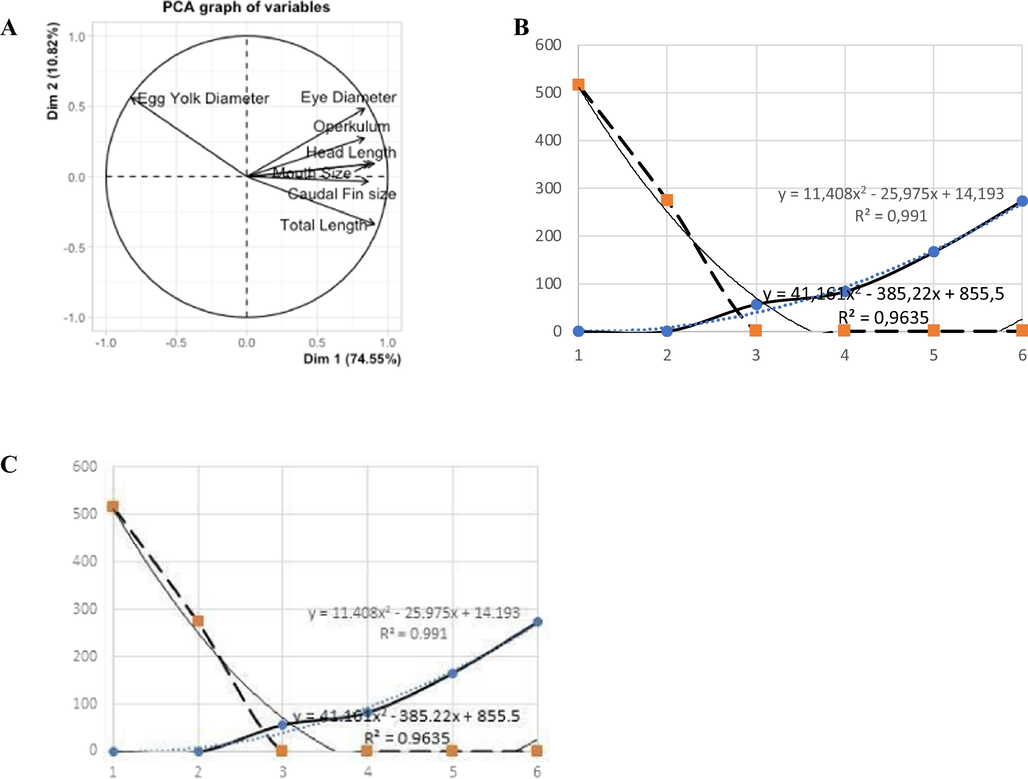
The PCA analysis of the overall rate of growth of (Rasbora lateristriata) (A). Details on mouth development versus egg yolk rate chart (B), and graph of the growth rate of the total length of the larvae (C).
3.8 Larval stage (48–72 hpf)
After hatching, the yellow rasbora embryo enters the larval phase. Several organs such as the heart, gills, jaws, and fins develop rapidly during this stage (Fig. 3; Suppl. Fig. 5).
3.9 Heart development
The heart is the first organ to form in the development of the vertebrate embryo. Cardiac progenitor cells (CPCs) are located in the epiblast at the end of the primitive streak. In fish, these progenitor cells differentiate into three types of cells, the atrial cardiomyocytes (CMs), ventricular CMs, and endocardial cells (Brown et al., 2016). These cells play a role in the first contraction of the heart muscle. The first contraction of the heart muscle occurs in 20 hpf of yellow rasbora embryo (Table 2) with heart rate of 52 beats/minute. The heart rate keeps increasing and reaches 165 beats/minute at 48 hpf.
Periode (hpf)
Description
Average Heart Rate (beats/minute)
20
The first contraction of the heart muscle
52
20–24
The heart is a linear tubular
106
24–30
Cardiac tube looping
150
48
The main components of the heart are formed
165
In the period of 20–24 hpf, the heart of the yellow rasbora embryo is in linear tubular form (Fig. 3). During this period, the heart is comprised of two distinct chambers, with the atrium situated in the anterior portion and the ventricle located in the posterior region (Fig. 3A, E). Cardiac tube looping of yellow rasbora larvae develop at 24–30 hpf on which the cardiac tube rotates and migrates to the leftward (Fig. 3B, F) (Rohr et al., 2008). The linear tube turns into a looped heart. This pattern is the basic pattern of a mature heart (Ramasubramanian et al., 2008). The atrium and ventricle are distinguishable at 24–30 hpf larvae (Fig. 3B, F). The main components of the yellow rasbora heart are finally formed completely at 48 hpf (Fig. 3C, G). These components include the sinus venosus (sv), atrium (a), ventricle (v), and bulbus arteriosus (ba) (Fig. 3D, H). These are located in the pericardial cavity forming an S shape confirming a mature structure of heart with the atrium located posteroventral to the ventricle (Fig. 3I–L).
3.10 Cranial cartilage development
The cranial cartilage of yellow rasbora larvae begin to form at 48 hpf with incomplete structure (Fig. 4A–C). The cranial cartilage reaches a complete structure at 72 hpf (Fig. 4D–F). The cranial structure completion of yellow rasbora is faster than that of zebrafish, which reach completion at 5 dpf (Mork and Crump, 2015). The 72 hpf yellow rasbora cranial cartilage consists of cartilages constructing neurocranium and splanchnocranium (Fig. 4G–I). Several cartilages, such as ethmoid plate, trabeculae, and parachordals, can be identified forming the neurocranium. The neocranium serves to protect the brain and sensory organs. Moreover, Meckel's cartilage, palatoquadrate, ceratohyal, basibranchial, hyosymplectic, and ceratobranchial are visible to compose the splanchnocranium. In addition, a pair of pectoral fin cartilage is visible in the posterior part of these cranial cartilages.
3.11 Larvae growth morphometry
Seven morphometrics parameters of yellow rasbora were measured during the first six days after hatching (Table 3). Most of the measured parameters showed a growth trend from the first to the sixth day post-hatching. However, the egg yolk diameter exhibited a sharp reduction on the second day post-hatching and vanished entirely by the third day post-hatching. On the other hand, the diameter of the yellow rasbora eye displayed an increment with each passing day following hatching (Table 3, Suppl. Fig. 7, 8). The eye of the yellow rasbora experiences the fastest growth rate on the second day following hatching, increasing by almost twice its previous size. The mouth of the yellow rasbora appears in a dot-like form and with no mouth opening on the second day after hatching. The mouth opening is clearly visible on the third day after hatching, but the maxilla and mandible are not clearly visible. The maxilla and mandible can be distinguished on the fifth day after hatching. The operculum of the yellow rasbora shows a significant growth on the third and fifth days after hatching. The width of the caudal fin, the head length, and the total length were constantly increasing. In addition, the pigmentation also begins to occur on the third day after hatching indicated by the presence of a slight scale pattern and develops fully on the fourth day after hatching. Before the third day after hatching, the body of the fish larvae is transparent.
Morphometric Parameter
Morphometric measurements (μm) per day
1
2
3
4
5
6
Eye Diameter
47.43 ± 3.52
90.37 ± 6.70
96.70 ± 3.96
98.07 ± 2.59
125.40 ± 65.14
172.67 ± 14.57
Egg Yolk Diameter
515.67 ± 16.80
273.33 ± 15.95
0
0
0
0
Operculum length
n/a
114 ± 16,64
140 ± 6.64
142.67 ± 5.03
194.00 ± 7.07
200 ± 60.77
Mouth Length
0
0
60 ± 12.22
152.33 ± 37.87
151.67 ± 17.95
190 ± 21.28
Width of Caudal Fin
301.33 ± 47.82
306 ± 61.49
396 ± 71.02
421.33 ± 32.01
429.67 ± 66.43
465.67 ± 37.07
Head Length
250.33 ± 10.26
331 ± 17.09
386.33 ± 21.22
390 ± 27.87
431.67 ± 95.17
530 ± 83.37
Total Length
2053.33 ± 11.50
3106 ± 184.48
3546.67 ± 90.74
3560 ± 87.18
3706.67 ± 370.72
3917 ± 174.58
The growth rate of the larvae total length is inversely proportional to the diameter of the larvae egg yolk (Fig. 5). The development pattern of the total length is a logarithmic rate with a value of r2 = 0.9345. Moreover, the rate of oral development is a positive polynomial with a value of r2 = 0.991 while the absorption rate of egg yolk is a negative polynomial rate with a value of r2 = 0.9635. Furthermore the growth ratio of yellow rasbora body length is decreasing along with the increase of development days. This decrease is related to the reducing size of egg yolk due to absorption up until the yolk completely disappeared at 3 days after hatching. Hence, the decreasing egg yolk in yellow rasbora occurs along with the readiness to do foraging activity, such as wide mouth opening, well developed eyes, proportional body, and well developed fins.
4 Discussion
The embryonic development stage of yellow rasbora demonstrates similarities with the developmental stages of zebrafish and medaka (Iwamatsu, 2004; Kimmel et al., 1995). Specifically, the cell cleavage process of yellow rasbora resembles that of zebrafish and medaka, despite the cleavage period being comparatively shorter. Yellow rasbora, medaka dan zebrafish have similar cleavage type which is meroblastic. Moreover, the cleavage planes and cell arrangement of yellow rasbora embryos are also similar with that of zebrafish and medaka. The general morphological features of embryos during the embryonic development of these three fish species are also similar. Some structures, namely marginal blastomere and Enveloping Layer (EVL) that are found in zebrafish and medaka embryo in cleavage stage, are also found in yellow rasbora embryo (Iwamatsu, 2004; Kimmel et al., 1995). The embryonic shields are also found on the embryo of these three fish species in the late gastrulation. The comparable embryonic development observed between yellow rasbora and zebrafish is not surprising, given that they are both members of the same infraclass (Teleostei). Moreover, yellow rasbora and zebrafish share the same subfamily, Danioninae (Betancur-R et al., 2017). Thus, the yellow rasbora's similar embryonic development and close relation to existing fish models make it a strong candidate as an alternative fish model for biomedical and toxicological studies.
Despite the similarity in embryonic development stages and characteristics of yellow rasbora, zebrafish, and medaka embryos, a distinct difference exists in the duration of their embryonic development. Yellow rasbora embryos exhibit a shorter duration of embryonic development compared to zebrafish and medaka, taking only 24 h to complete the embryonic stage and reach hatching stage, while zebrafish and medaka embryos take 48 h and 9 days, respectively. The rapid embryonic development might be beneficial characteristics to support yellow rasbora as an animal model in experimental research, as ornamental fish, as well as environmental toxicity indicator. The results of the experiment can be obtained faster and more replications can be performed in relatively shorter time periods. In addition, similar to several fish used as animal models, yellow rasbora also has transparent larvae. The transparent larvae of yellow rasbora allows the observation of organts development and also for molecular markers clear detection within the larvae.
The larva growth morphometry of yellow rasbora was similar to that of Rasbora daniconius and Rasbora argyrotenia (Mahapatra and Krishna, 2016). The development of the yellow rasbora intestine is noticeable at 48 hpf, and by 72 hpf, the intestinal crypt has formed and covered with epithelial cells on the surface. It was reported that on day two or 48 h after hatching of R. daniconius, the alimentary canal began to form. In R. argyrotenia, it was found that the average egg yolk was depleted at 78 – 84 h after hatching and pigmentation began to occur at 42 h after hatching. Several digestive enzymes have been activated during the larval period of fish (Buckley and Dillmann, 1982). Some fish larvae were also found to have zooplankton or artemia in their alimentary canal on the second to eight days after hatching (Govoni et al., 1986).
The morphology of cranial cartilage can serve as an indicator of embryonic development. The cranial cartilage development is a highly conserved process among vertebrates, mediated by cells derived from neural crest, endoderm, mesoderm, and ectoderm (Kaucka and Adameyko, 2019; Mork and Crump, 2015). The cranial cartilage development also helps to understand the evolutionary aspect of fish within the vertebrates. Moreover, since it involves complex morphogenetic events, a fine description of cranial cartilage development is important for toxicological study on fish.
The cranial bone and operculum of yellow rasbora were already established at 72 hpf. In teleostei fish, the operculum is an outgrowth of the dermal bone structure which also forms scales (Kimmel et al., 2003). The significant growth of operculum on the third day after hatching might be related to respiration and foraging behavior. The larvae of yellow rasbora are already actively swimming on the third day after hatching and start to consume the zooplankton on the fifth day after hatching. Greater opercular width corresponds to increased gill surface area and stronger muscles connecting the operculum and lower jaws. These muscles will support the eating behavior of the fish when the digestive system, as well as the maxilla and mandible, are fully formed (Herbing, 2001; Verraes, 1977). In addition, the operculum is known to have neuromasts as hydrodynamic receptors that also develop in the larval stage (Pichon and Ghysen, 2004; Sapède et al., 2002). The development of this neuromast differs in each individual depending on the body region and also differs in each species (Coombs et al., 1988; Dijkgraaf, 1963; Wada et al., 2008; Webb, 1989). Hence, the operculum needs to grow significantly to facilitate optimum respiration and to support foraging behavior in terms of digestive system readiness and foraging movement.
The sensory organs of yellow rasbora, including the eyes, otic vesicle, and brain, begin to develop as early as 20–24 h after fertilization. Eye pigmentation and layer formation as well as brain layer development were histologically observed at 72 hpf. Larger eyes have a higher sensitivity to light (Land and Nilsson, 2012; Warrant, 2004). A significant increase in eye diameter on day two might be related to swimming patterns, ecological behavior, and foraging behavior. At three days after hatching, pigmentation begins to appear which is indicated by a black color and on the fifth day or 119 h after hatching, pigmentation has occurred completely with the appearance of yellow color on the body of the fish larvae (Buckley and Dillmann, 1982).
5 Conclusion
The yellow rasbora displays potential as a promising animal model for scientific research. However, our understanding of the process of embryonic development in this fish species is currently incomplete. This study presentes the embryonic developmental stages of yellow rasbora through the use of time-lapse imaging to observe key parameters such as embryonic cleavage, blastomere development, epiboly area, and somite formation. Additionally, heart development and morphological changes were monitored by measuring the number of heartbeats at 24, 30, and 48 h post-fertilization. The study also examined the cartilage structure of the cranium in larvae aged 1–3 days post-fertilization through the use of Alizarin red and Alcian blue staining to visualize bone and cartilage structures. The results of this investigation provide a detailed characterization of the important stages of embryogenesis in yellow rasbora, including the zygote, cleavage, blastula, gastrula, segmentation, pharyngula, and hatching stages. The first contraction of the heart muscle was observed at 20 h post-fertilization, with cardiac tube looping occurring at 24–30 h post-fertilization. The formation of cranium cartilage was observed at 2 days post-fertilization, and was completed by 3 days post-fertilization. In summary, this study offers a comprehensive overview of the embryonic developmental stages of yellow rasbora, which can aid in the use of this species as a model for future research endeavors.
Acknowledgment
The authors would like to thank Dr. Ryutaro Akiyama for critically reading the manuscript and helpful discussion during the manuscript preparation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Pemijahan dan penjinakan ikan pantau (Rasbora latestriata) Jurnal Perikanan dan Kelautan. 2011;16:71-78.
- [CrossRef] [Google Scholar]
- Stages of embryonic development of the ice goby (Shiro-uo), Leucopsarion petersii. Zoolog. Sci.. 1999;16:761-773.
- [CrossRef] [Google Scholar]
- Normal embryonic stages for salmonid fishes, based on Salmo gairdneri Richardson and Salvelinus fontinalis (Mitchill) J. Exp. Zool.. 1973;184:7-25.
- [CrossRef] [Google Scholar]
- Bancroft, J.D., Cook, H.C., Stirling, R.W., 1994. Manual of histological techniques and their diagnostic application, in: Manual of histological techniques and their diagnostic application. pp. 457–457.
- Advances in the study of heart development and disease using zebrafish. JCDD. 2016;3:13.
- [CrossRef] [Google Scholar]
- Nitrogen utilization by larval summer flounder, Paralichthys dentatus (Linnaeus) J. Exp. Mar. Biol. Ecol.. 1982;59:243-256.
- [CrossRef] [Google Scholar]
- Zebrafish as an animal model for biomedical research. Exp Mol Med. 2021;53:310-317.
- [CrossRef] [Google Scholar]
- Diversity of lateral line systems: evolutionary and functional considerations. In: Sensory Biology of Aquatic Animals. New York: Springer; 1988. p. :553-593.
- [Google Scholar]
- The functioning and significance of the lateral-line organs. Biol. Rev.. 1963;38:51-105.
- [CrossRef] [Google Scholar]
- In vivo time-lapse imaging of zebrafish embryonic development. CSH protocols. 2007;2007
- [CrossRef] [Google Scholar]
- Food habits of the yellow rasbora, Rasbora lateristriata, (family: Cyprinidae) broodfish during moving to spawning ground. Jurnal Perikanan Universitas Gadjah Mada. 2009;11:107-116.
- [CrossRef] [Google Scholar]
- Reproductive biology of the yellow rasbora (Rasbora Lateristriata) inhabitat of the Ngrancah river, Kulon Progo regency. Jurnal Perikanan Universitas Gadjah Mada. 2008;10:261-275.
- [CrossRef] [Google Scholar]
- Gilbert, S.F., 2000. An introduction to early developmental processes, in: Developmental Biology. Sinauer Associates.
- The physiology of digestion in fish larvae. Environ Biol Fish. 1986;16:59-77.
- [CrossRef] [Google Scholar]
- The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498-503.
- [CrossRef] [Google Scholar]
- Stages of normal development in the medaka Oryzias latipes. Mech. Dev.. 2004;121:605-618.
- [CrossRef] [Google Scholar]
- Evolution and development of the cartilaginous skull: From a lancelet towards a human face. Semin. Cell Dev. Biol.. 2019;91:2-12.
- [CrossRef] [Google Scholar]
- Stages of embryonic development of the zebrafish. Dev. Dyn.. 1995;203:253-310.
- [CrossRef] [Google Scholar]
- Endothelin 1-mediated regulation of pharyngeal bone development in zebrafish. Development. 2003;130:1339-1351.
- [CrossRef] [Google Scholar]
- Freshwater fishes of Western Indonesia and Sulawesi (Ikan Air Tawar Bagian Barat Indonesia dan Sulawesi). Jakarta: Periplus Edition; 1993.
- Molecular phylogeny and historical biogeography of the Indonesian freshwater fish Rasbora lateristriata species complex (Actinopterygii: Cyprinidae): Cryptic species and west-to-east divergences. Mol. Phylogenet. Evol.. 2016;105:212-223.
- [CrossRef] [Google Scholar]
- Lailiati, I.R., Suci, D.A., Rosa, A.A., Fernanda, V.A., Retnoaji, B., 2022. Reproductive aspect and embryonic development of wader fish (Rasbora lateristriata Bleeker, 1854) from Purworejo, Central Java:, in: Proceedings of the 7th International Conference on Biological Science (ICBS 2021). Presented at the 7th International Conference on Biological Science (ICBS 2021), Atlantis Press., Yogyakarta, Indonesia, pp. 540–544.
- Land, M.F., Nilsson, D.E., 2012. Animal eyes. OUP Oxford.
- Lieschke, G.J., Currie, P.D., 2007. Animal models of human disease: zebrafish swim into view 15.
- Lin, C.Y., Chiang, C.Y., Tsai, H.J., 2016. Zebrafish and medaka: new model organisms for modern biomedical research. Journal of biomedical science 23, 1–11. Doi: 10.1186/s12929-016-0236-5.
- Zebrafish and medaka as models for biomedical research of bone diseases. Dev. Biol.. 2020;457:191-205.
- [CrossRef] [Google Scholar]
- Embryonic and larval development of Rasbora daniconius (Hamilton): A potential indigenous ornamental fish of north-east India. International Journal of Fisheries and Aquatic Studies. 2016;4:187-190.
- [Google Scholar]
- Mork, L., Crump, G., 2015. Zebrafish craniofacial development, in: Current topics in developmental biology. Elsevier, pp. 235–269. Doi: 10.1016/bs.ctdb.2015.07.001.
- Evolution of posterior lateral line development in fish and amphibians. Evol Dev. 2004;6:187-193.
- [CrossRef] [Google Scholar]
- On modeling morphogenesis of the looping heart following mechanical perturbations. J. Biomech. Eng.. 2008;130:061018
- [CrossRef] [Google Scholar]
- Retinoic acid controls proper head-to-trunk linkage in zebrafish by regulating an anteroposterior somitogenetic rate difference. Development. 2014;141:158-165.
- [CrossRef] [Google Scholar]
- Asymmetric involution of the myocardial field drives heart tube formation in zebrafish. Circ. Res.. 2008;102
- [CrossRef] [Google Scholar]
- Cell migration in the postembryonic development of the fish lateral line. Development. 2002;129:605-615.
- [CrossRef] [Google Scholar]
- Team, R.C., 2018. R: A language and environment for statistical computing. 2014.
- Postembryonic ontogeny and functional anatomy of the ligamentum mandibulo-hyoideum and the ligamentum interoperculo-mandibulare, with notes on the opercular bones and some other cranial elements in Salmo gairdneri Richardson, 1836 (Teleostei: Salmonidae) J. Morphol.. 1977;151:111-119.
- [CrossRef] [Google Scholar]
- Development of feeding structures in larval fish with different life histories: winter flounder and Atlantic cod. J. Fish Biol.. 2001;59:767-782.
- [CrossRef] [Google Scholar]
- Development of diverse lateral line patterns on the teleost caudal fin. Dev. Dyn.. 2008;237:2889-2902.
- [CrossRef] [Google Scholar]
- Vision in the dimmest habitats on Earth. J Comp Physiol A. 2004;190:765-789.
- [CrossRef] [Google Scholar]
- Neuromast morphology and lateral line trunk canal ontogeny in two species of cichlids: An SEM study. J. Morphol.. 1989;202:53-68.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102810.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







