Translate this page into:
Cardioprotective potential of sakuranetin to counteract polyethylene microplastics induced cardiotoxicity
⁎Corresponding author. nazia.ehsan@uaf.edu.pk (Nazia Ehsan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Polyethylene microplastics (PEMPs) are toxic environmental contaminants which can impair multiple organs including heart. Sakuranetin (SKN) is a potential flavonoid with diverse pharmacological benefits. This research was undertaken to analyze the defensive impact of SKN to avert PEMPs-induced cardiotoxicity. 24 male albino rats were randomly allocated into 4 separate groups: control, PEMPs (1.5 mg kg−1), PEMPs + SKN (1.5 mg kg−1 + 10 mg kg−1) and only SKN (10 mg kg−1) treated group. After 30 days of treatment, our results revealed that PEMPs exposure reduced the expressions of Nrf2 and antioxidant genes while increasing Keap1 expression. Besides, PEMPs intoxication escalated the levels of cardiac markers (CPK, LDH, Troponin I & CK-MB). Additionally, it lessened the activities of GSH, GST, SOD, HO-1, CAT, GSR, GPx whereas the levels of MDA and ROS were increased. Conversely, the levels of inflammatory markers i.e., COX-2 activity IL-1β, TNF-α, NF-kB & IL-6 were augmented. Moreover, the expressions of apoptotic markers i.e., Bax and caspase-3 were elevated while the Bcl-2 expression was decreased. However, SKN treatment significantly restored the PEMPs-induced biochemical dysregulations. Therefore, SKN could be used as a therapeutic compound to ameliorate PEMPs-induced cardiac impairments in rats, possibly due to its tremendous pharmacotherapeutic potential.
Keywords
Sakuranetin
Polyethylene microplastics
Cardiotoxicity
Oxidative stress
Apoptosis
1 Introduction
The growing environmental pollution caused by plastics has attracted a significant global attention (Laskar and Kumar, 2019). Plastic materials are widely adopted as substitutes for various traditional materials, including paper, glass, wood and metals (Raheem, 2013). It is reported that global plastic production has reached up to 0.368 billion MT and is expected to upsurge twofold in upcoming few days (Yao et al., 2022). When plastics enter the environment, they undergo continuous fragmentation into small particles known as microplastics (MPs). Smaller MPs with pointed structure are more susceptible to pass through membrane barriers present in the body of animals (Sharma and Sharma, 2007). MPs can get access to human body via the consumption of various edible products i.e., sugar (7-32particles/kg), honey (40–60 particles/kg), table salt (7–681 particles/kg) & beer (12–109 particles/L) (Bouwmeester et al., 2015).
The most frequently recognized polymer among microplastics in terrestrial environment is polyethylene microplastics (PEMPs) (de Souza Machado et al., 2018). Recent studies have discovered that PEMPs can induce various adverse effects including cytotoxicity, developmental toxicity & hematological disturbances in the body (Ge et al., 2021). Recent evidences have revealed that, PEMPs administration leads to reproductive abnormalities and disrupts the process of gametogenesis (Mak et al. 2019). Beyond that, exposure to PEMPs has been reported to elicit oxidative stress (OS), which can have potential harmful effects on the normal function of the cells and disrupt the redox status (Silva et al., 2021). Moreover, PE-MP exposure may also alter signalling pathways leading to autophagy and apoptosis (Zhao et al., 2020). Furthermore, it has been reported that exposure to polyethylene can reduce the heart rate and instigate pericardial edema in the living organisms (Sun et al., 2021).
Flavonoids are recognized as valuable compounds in the field of traditional medicine owing to their remarkable pharmacological properties (Ullah et al., 2020). Sakuranetin (SKN) is a flavonoid with bioactive potential which is sourced from different plants as well as honey derived from several floral sources (Shen et al., 2019). SKN has long been utilized in traditional medicine for their remedial impacts, particularly for the mitigation of diabetes, inflammatory ailments and cancer (Stompor, 2020). However, no research has been performed to assess the cardioprotective effects of SKN. Therefore, this study was commenced to measure the amelioratory role of SKN to cure PEMPs-induced cardiac toxicity.
2 Materials & methods
2.1 Chemicals
PEMPs and SKN were procured from Sigma-Aldrich, Germany.
2.2 Animals
The research trial was conducted on male albino rats (n = 24) in the animal research center of the University of Agriculture, Faisalabad. The rats were kept in cages and maintained under uniform conditions (temperature, 24 ± 1 °C; 12 h day/night cycle) and treated with standard diet and water. Rats were acclimatized for 1 week before the initiation of the trial. The animals were treated and handled according to the guidelines of EU Directive 2010/63/EU for animal experiments.
2.3 Experimental plan
24 rats were randomly distributed in 4 different groups (n = 6) i.e., Control, PEMPs (1.5 mgkg−1), PEMPs (1.5 mgkg−1 + SKN 10 mgkg−1) and only SKN (10mgkg−1) administrated group. Following the completion of experiment, the animals were anesthetized, decapitated and heart was excised. The heart was homogenized, centrifuged and the resulting supernatant was kept at −20 °C which was used for further analysis
2.4 Estimation of cardiac markers
The levels of LDH, CPK, CK-MB and Troponin-I were measured by following the protocols of Bais & Philcox (1994), Tietz et al. (1983) and Panteghini et al. (2004), respectively.
2.5 Evaluation of biochemical parameters
The CAT activity was measured through the technique of Aebi (1974). The SOD activity was calculated by following the technique documented by Kakkar et al. (1984). For the quantification of GPx Rotruck et al. (1973) technique was employed. Carlberg and Mannervik (1975) and Jollow et al. (1974) protocol was followed for the estimation of GSR and GSH. The Younis et al. (2018) protocol was followed for the measurement of GST. The levels of ROS and MDA were ascertained by using Hayashi et al. (2007) and Ohkawa et al. (1978) approaches, respectively.
2.6 RNA isolation & qRT-PCR
qRT-PCR was employed to estimate the expressions of Nrf2/Keap1, apoptotic markers (Bcl-2, caspase-3, and Bax) and antioxidative genes. Total RNA isolation was accomplished using the TRIzol reagent, followed by reverse transcription to produce cDNA. The evaluation of changes in the gene expression was carried out following 2−ΔΔCT method and β-actin served as an internal control, as outlined by Livak & Schmittgen (2001). Table 1 demonstrates the primer sequence of β-actin as well as apoptotic markers and Nrf-2/Keap1 and its target genes, as previously reported by Ijaz et al. (2023).
Gene
Primers 5′ → 3′
Accession number
Nrf-2
ACCTTGAACACAGATTTCGGTG
R: TGTGTTCAGTGAAATGCCGGANM_031789.1
Keap-1
F: ACCGAACCTTCAGTTACACACT
R: ACCACTTTGTGGGCCATGAANM_057152.1
CAT
F: TGCAGATGTGAAGCGCTTCAA
R: TGGGAGTTGTACTGGTCCAGAANM_012520.2
SOD
F: AGGAGAAACTGACAGCTGTGTCT
R: AAGATAGTAAGCGTGCTCCCACNM_017051.2
GPx
F: TGCTCATTGAGAATGTCGCGTC
R: ACCATTCACCTCGCACTTCTCANM_030826.4
GSR
F: ACCAAGTCCCACATCGAAGTC
R: ATCACTGGTTATCCCCAGGCTNM_053906.2
GST
F: TCGACATGTATGCAGAAGGAGT
R: CTAGGTAAACATCAGCCCTGCTNM_031509.2
HO-1
F: AGGCTTTAAGCTGGTGATGGC
R: ACGCTTTACGTAGTGCTGTGTNM_012580.2
Bax
F: GGCCTTTTTGCTACAGGGTT
R: AGCTCCATGTTGTTGTCCAGNM_017059.2
Bcl-2
F: ACAACATCGCTCTGTGGAT
R: TCAGAGACAGCCAGGAGAANM_016993.1
Caspase-3
F: ATCCATGGAAGCAAGTCGAT
R: CCTTTTGCTGTGATCTTCCTNM_012922.2
β-actin
F: TACAGCTTCACCACCACAGC
R: GGAACCGCTCATTGCCGATANM_031144
2.7 Estimation of inflammatory markers
The analysis of inflammatory markers (TNF-α, Nf-kB, IL-6, IL-1β, and Cox-2) was executed using ELISA kits. The analysis was accomplished following the recommended protocol of the manufacturer.
2.8 Statistical analysis
Data were shown as Mean ± SEM. One-way analysis of variance (ANOVA) & Tukey’s test was carried out to compare different groups using Minitab (V17) Software. P < 0.05 was set as level of significance.
3 Results
3.1 Results of PEMPs & SKN on Nrf2/Keap1 pathway
Exposure to PEMPs substantially (P < 0.05) decreased the expressions of Nrf2 and antioxidative genes while escalating the Keap1 expression, contrary to the control. Combined treatment with SKN + PEMPssubstantially (P < 0.05) increased the expressions of Nrf2 and antioxidant genes while decreasing the expression of keep1 in comparison to PEMPs-group. No substantial alterations were detected in Nrf2/Keap1 expressions among the SKN-supplemented rats and the control (Fig. 1).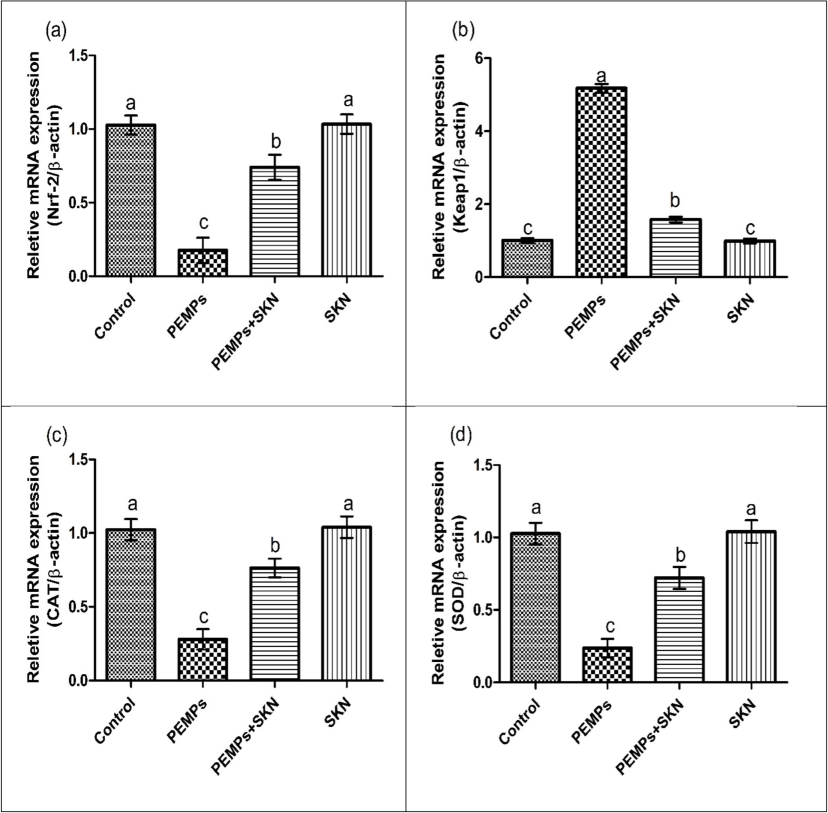
Effects of PEMPs and SKN on the expression of (a) Nrf2, (b) Keap1, (c) CAT, (d) SOD, (e) GPx, (f) GSR and (g) HO-1, (h) GST. Dissimilar superscripts show significant difference among different groups (p < 0.05).
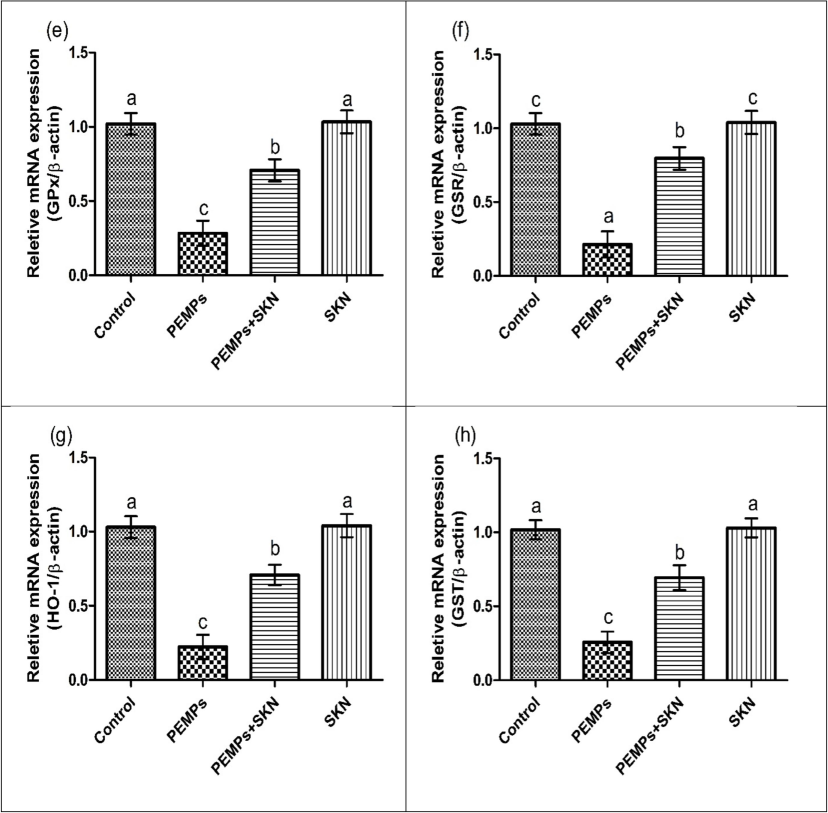
Effects of PEMPs and SKN on the expression of (a) Nrf2, (b) Keap1, (c) CAT, (d) SOD, (e) GPx, (f) GSR and (g) HO-1, (h) GST. Dissimilar superscripts show significant difference among different groups (p < 0.05).
3.2 Impact of PEMPs & SKN on biochemical parameters
PEMPs exposure remarkably (P < 0.05) lowered the activities of GSH, GST, SOD, HO-1, CAT, GSR, GPx and escalated ROS and MDA levels, contrary to the control rats. Conversely, the concurrent treatment of PEMPs + SKN significantly (P < 0.05) recovered the PEMPs-induced dysregulations in contrast to the PEMPs- group. Only SKN administration showed insignificant differences contrary to the control rats as depicted in Table 2. Dissimilar superscripts show significant difference among different groups (p < 0.05).
Parameters
Groups
Control
PEMPs
PEMPs + SKN
SKN
CAT (U/mg protein)
15.153 ± 1.49a
5.273 ± 0.23c
11.563 ± 1.13b
15.290 ± 1.46a
SOD (U/mg protein)
10.397 ± 1.01a
4.430 ± 0.45b
8.463 ± 0.59a
10.507 ± 1.13a
GSR (nM NADPH oxidized/min/mg tissue
7.590 ± 0.63a
3.703 ± 0.22c
5.513 ± 0.43b
7.543 ± 0.58a
GPx (U/mg protein)
19.943 ± 1.52a
7.960 ± 0.69c
15.607 ± 1.16b
20.87 ± 2.19a
GSH (U/mg protein)
26.35 ± 2.04a
9.810 ± 0.64c
21.940 ± 1.23b
27.06 ± 2.45a
GST (U/mg protein)
35.263 ± 1.73a
11.480 ± 0.97c
27.820 ± 1.35b
36.08 ± 2.21a
HO-1 (pmoles bilirubin/ mg protein/h)
263.5 ± 21.20a
66.03 ± 9.96c
188.79 ± 14.13b
269.5 ± 25.91a
MDA (nmol/g)
0.433 ± 0.21c
2.570 ± 0.28a
1.070 ± 0.36b
0.370 ± 0.25c
ROS (nmol/g)
1.270 ± 0.34c
6.420 ± 0.41a
2.297 ± 0.26b
1.217 ± 0.37c
3.3 Impact of PEMPs & SKN on cardiac function markers
Analysis of cardiac function markers showed that PEMPs treatment instigated cardiac disturbances as confirmed by a notable (P < 0.05) elevation in CPK, LDH, CK-MB & troponin I levels, as compared to the control. However, concurrent treatment of SKN & PEMPs markedly (P < 0.05) restored their levels, in comparison to the PEMPs-exposed animals. SKN (only) exposed group displayed mean values of cardiac markers approximately similar to the control as depicted in Table 3. Dissimilar superscripts show significant difference among different groups (p < 0.05).
Parameters
Groups
Control
PEMPs
PEMPs + SKN
SKN
CK-MB (ng/mL)
17.60 ± 1.85c
84.70 ± 2.57a
31.30 ± 2.50b
16.79 ± 2.28c
CPK (mcg/L)
113.27 ± 11.18c
520.46 ± 16.51a
199.20 ± 14.73b
108.96 ± 10.50c
Troponin-I (pg/ml)
0.540 ± 0.29b
12.227 ± 1.68a
2.407 ± 0.37b
0.483 ± 0.35b
LDH (mg/dl)
9.900 ± 1.62c
52.67 ± 2.80a
19.74 ± 2.52b
9.47 ± 1.80c
3.4 Results of PEMPs & SKN on inflammatory indices
PEMPs treatment considerably (P < 0.05) upregulated COX-2 activity and IL-1β, TNF-α, NF-kB & IL-6 levels, contrary to the control. The co-administration of SKN and PEMPs decreased the levels of inflammatory markers, in contrast PEMPs administrated rats. Only SKN supplementation displayed normal level of these markers almost near to the control as demonstrated in Table 4. Dissimilar superscripts show significant difference among different groups (p < 0.05).
Parameters
Groups
Control
PEMPs
PEMPs + SKN
SKN
NF-kB (ng/g tissue)
27.41 ± 2.22c
83.54 ± 1.98a
38.92 ± 2.13b
27.02 ± 2.38c
TNFα (ng/g tissue)
13.147 ± 1.66c
63.26 ± 1.91a
21.63 ± 2.22b
12.773 ± 1.59c
IL-1ß (ng/g tissue)
28.07 ± 2.20c
83.80 ± 2.32a
37.10 ± 2.70b
26.98 ± 2.26c
IL-6 (ng/g tissue)
5.743 ± 1.58b
37.58 ± 2.64a
9.853 ± 1.48b
5.663 ± 1.64b
COX-2 (ng/g tissue)
11.263 ± 1.16c
78.99 ± 2.05a
19.48 ± 2.11b
10.900 ± 1.14c
3.5 Impact of PEMPs & SKN on apoptotic markers
PEMPs treatment notably (P < 0.05) elevated the expressions of Bax and caspase-3 while lowering the expression of Bcl-2, in comparison to the control. SKN and PEMPs co-adminisration markedly (P < 0.05) restored the expressions of these markers, contrary to PEMPs animals. No considerable alterations were examined in SKN only treated and control group (Fig. 2).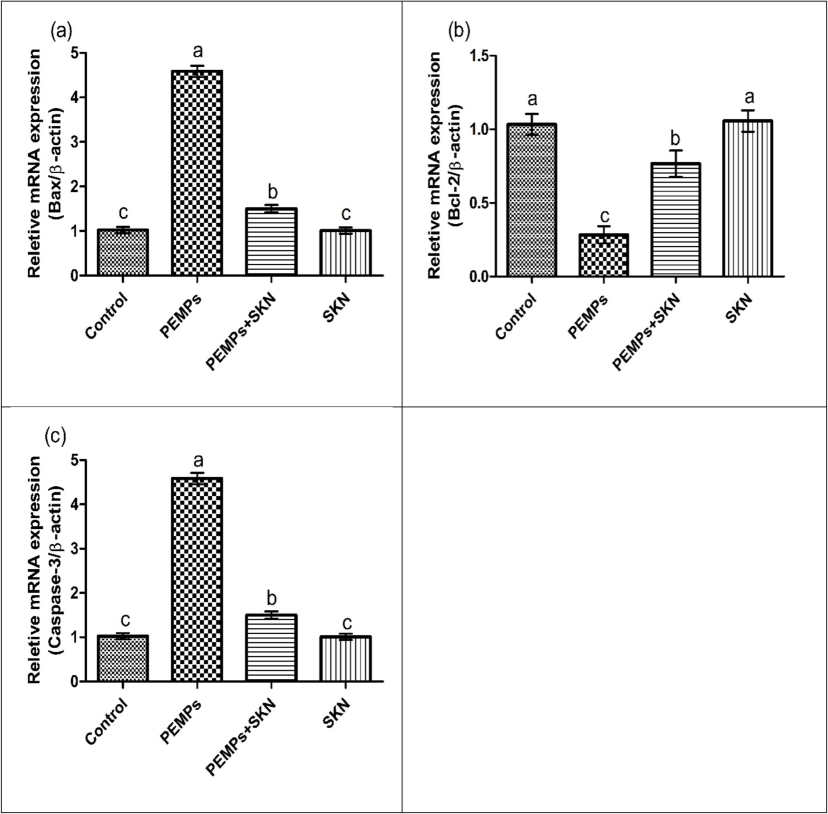
Effects of PEMPs and SKN on (a) Bax, (b) Bcl-2 and (c) Caspase-3. Dissimilar superscripts show significant difference among different groups (p < 0.05).
4 Discussion
Over the last few years, MPs have garnered great interest due to their ubiquitous prevalence and harmful effects on ecosystem as well as living organisms (Laskar and Kumar, 2019). Previous investigations have demonstrated that MPs intoxication causes nephrotoxicity (Ahmad et al., 2023; Ehsan et al., 2023), neurotoxicity (Yang et al., 2019), gastrointestinal toxicity (Dong et al., 2020), testicular toxicity (Alvi et al., 2024) and cardiotoxicity (Umamaheswari et al., 2021) in the living system. Moreover, bioactive compounds with effective antioxidative abilities may impede the formation of oxidative radicles (Erdemli et al., 2018). SKN is a natural compound with significant therapeutic properties. However, the existing research was intended to explore the impact of SKN to avert PEMPs prompted cardiac impairments in rats.
In our current investigation, PEMPs exposure resulted in a downregulation in Nrf2 expression and upregulation in Keap1 expression which decreased the expressions of cytoprotective genes including GSR, SOD, GPx, CAT & HO-1. It has been documented that regulation of cytoprotectant gene expression through the Nrf2/Keap1 pathway serves as an inducible defense mechanism to mitigate OS (Yamamoto et al., 2018). Nrf2 functions as the central element to coordinate the cellular antioxidative immune response that can effectively neutralize ROS. Conversely, Keap1 acts as an inhibitor of Nrf2 that facilitates Nrf2 degradation (Bellezza et al., 2018). Notably, supplementation of SKN significantly recovered the expression of the aforementioned cytoprotective genes by modulating the Nrf2/Keap1 pathway. Our results were in line with the study of Akbar et al. (2024) who reported the Nrf2/Keap1 modulating property of SKN.
Cardiac muscle injury results in the release of various cardiac damage markers in the blood. CK-MB is particularly recognized as cardiac injury marker which is used to detect certain coronary disorders including critical myocardial damage (Christenson et al., 1997). As per the investigation of Ibrahim and Abdel-Daim, (2015), the extent of the cardiac injury markers in the blood stream indicates the myocardial injury, suggesting the leakage of these enzymes from damaged heart cells. Our investigation explored that the level cardiac damage markers were augmented owing to PEMPs dosage. Nonetheless, SKN supplementation exhibited tremendous restoration in the levels of these markers due to its cardioprotective abilities.
PEMPs administration reduced the activities SOD, GPx, CAT, HO-1 and escalated the levels of ROS and MDA. The aforementioned endogenous enzymes play a crucial role in regulating the levels of ROS and OS, ultimately preventing cellular damage in the body (Adejuwon et al., 2015). The incidence of lipid peroxidases is parallel to the creation of free radicals and can be diagnosed by the level of its end product i.e., level of MDA (Adejuwon et al., 2015). In addition to the body’s endogenous defensive antioxidant system, these antioxidants from various plant sources can be supplemented in order to suppress OS (Nahid et al., 2017). Therefore, SKN dosage regulated PEMPs induced imbalance in pro-oxidants and antioxidants by reducing OS and elevating antioxidants in the cardiac tissues. The antioxidant characteristics of these bioactive compounds mainly exist on account of their multiple OH configuration, that enable them to avert OS (Teixeira et al. 2005).
PEMPS administration elevated the levels of inflammatory markers. NF-κB serves as a major element that activates the levels of pro-inflammatory cytokines which induce acute inflammation and ROS-associated damage in the body (Khan et al., 2020). Moreover, COX-2 is fundamental inflammatory mediator that serves a key element in inducing cardiac inflammation (Agarwal et al., 2009). However, SKN treatment not only suppressed the activation of NF-κB, a major culprit underlying cardiac inflammation but also regulated the other inflammatory markers concentration. These outcomes are in line with the research of Ali et al. (2024) who reported that SKN protects from gastric ulcers due to its anti-inflammatory and antioxidant properties.
PEMPs treatment increased the expressions of Bax and Caspase-3 and lowered the expressions of Bcl-2. Apoptosis occurs on account of an imbalance in pro- and anti-apoptotic proteins. The downregulation of Bcl-2 and upregulation in Bax impairs the translocation between mitochondrial inner and outer layer (Gu et al., 2017). Their imbalance also mediates the discharge of cytochrome-C from mitochondria, initiating the death response in cells (Caglayan et al., 2019). Caspases particularly caspase-3 is reported as a fundamental mediator of apoptotic response as it triggers the death mechanism of cell by accelerating other enzymes (Eldutar et al., 2017). However, SKN treatment restored the expressions of these markers due to its anti-apoptotic properties.
5 Conclusion
In conclusion, PEMPs exposure induced cardiac impairments in rats by increasing the expressions of Keap1 while downregulating Nrf2 and its antioxidant genes. Moreover, PEMPs inebriation also elevated cardiac injury markers, inflammatory and pro-apoptotic mediators as well as OS. Additionally, PEMPs lessened the levels of cardiac anti-apoptotic markers and antioxidative genes Nevertheless, SKN supplementation restored all the impairments that were induced by PEMPs intoxication due to its antioxidant, anti-inflammatory and anti-apoptotic properties. However, the current study was performed on model animals, therefore, we recommend clinical trials of this compound to check its effectiveness on human beings.
CRediT authorship contribution statement
Nazia Ehsan: Writing – original draft, Methodology, Investigation, Conceptualization. Muhammad Gulfam: Writing – original draft, Methodology, Investigation, Conceptualization. Ali Akbar: Writing – review & editing, Writing – original draft, Methodology. Moazama Batool: Visualization, Validation, Software, Data curation. Mohammad Z. Ahmed: Writing – review & editing, Resources, Funding acquisition. Mian Nadeem Riaz: Writing – review & editing, Visualization, Data curation.
Acknowledgements
The authors are thankful to the Researchers Supporting Project number (RSPD2024R728), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cisplatin-induced testicular dysfunction and its amelioration by L aunaea taraxacifolia leaf extract. Andrologia. 2015;47:553-559.
- [Google Scholar]
- Aebi, H., 1974. Catalase. In Methods of enzymatic analysis, Academic press. 673-684.
- Eicosanoids in inflammation and cancer: the role of COX-2. Expert Rev. Clin. Immunol.. 2009;5:145-165.
- [Google Scholar]
- Ameliorative Effects of Rhamnetin against Polystyrene Microplastics-Induced Nephrotoxicity in Rats. Pakistan Veterinary Journal. 2023;43(3):623-627.
- [Google Scholar]
- Sakuranetin counteracts polyethylene microplastics induced nephrotoxic effects via modulation of Nrf2/Keap1 pathway. J. King Saud Univ.-Sci.. 2024;36(8):103343
- [Google Scholar]
- In vivo anti-gastric ulcer activity of 7-O-methyl aromadendrin and sakuranetin via mitigating inflammatory and oxidative stress trails. J. Ethnopharmacol.. 2024;335:118617
- [Google Scholar]
- Juglanin cures polyethylene microplastics-induced testicular damage in rats. Journal of King Saud University-Science. 2024;36(9):103394.
- [Google Scholar]
- IFCC methods for the measurement of catalytic concentration of enzymes. Part 8. IFCC method for lactate dehydrogenase (L-lactate: NAD+ oxidoreductase, EC 1.1.1.27) Journal of Analytical Methods in Chemistry. 1994;16:597912
- [Google Scholar]
- Nrf2-Keap1 signaling in oxidative and reductive stress. Biochimica et Biophysica Acta (BBA)-Molecular Cell Res.. 2018;1865(5):721-733.
- [Google Scholar]
- Potential health impact of environmentally released micro-and nanoplastics in the human food production chain: experiences from nanotoxicology. Environ. Sci. Tech.. 2015;49:8932-8947.
- [Google Scholar]
- Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J. Trace. Elem. Med. Biol.. 2019;54:69-78.
- [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Bio Chem.. 1975;250:5475-5480.
- [Google Scholar]
- Cardiac markers in the assessment of acute coronary syndromes. Md. Med. J. 1997:18-24.
- [Google Scholar]
- Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol.. 2018;24:1405-1416.
- [Google Scholar]
- Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater.. 2020;385:121575
- [Google Scholar]
- Attenuative Effects of Ginkgetin Against Polystyrene Microplastics-Induced Renal Toxicity in Rats. Pakistan Veterinary Journal. 2023;43(4):819-823.
- [Google Scholar]
- Restorative effects of Chrysin pretreatment on oxidant–antioxidant status, inflammatory cytokine production, and apoptotic and autophagic markers in acute paracetamol-induced hepatotoxicity in rats: an experimental and biochemical study. J. Biochem. Mol. Toxicol.. 2017;31:21960.
- [Google Scholar]
- Biochemical changes induced by grape seed extract and low level laser therapy administration during intraoral wound healing in rat liver: an experimental and in silico study. J. Biomol. Struct. Dyn.. 2018;36:993-1008.
- [Google Scholar]
- Review of the toxic effect of microplastics on terrestrial and aquatic plants. Sci. Total Environ.. 2021;791:148333
- [Google Scholar]
- Inhibition of chemotherapy–induced apoptosis of testicular cells by squid ink polysaccharide. Exp. Ther. Med.. 2017;14:5889-5895.
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007;631:55-61.
- [Google Scholar]
- Modulating effects of Spirulina platensis against tilmicosin-induced cardiotoxicity in mice. Cell Journal (yakhteh). 2015;17:137.
- [Google Scholar]
- Sciadopitysin attenuates paraquat induced renal toxicity by modulating Nrf-2/Keap-1 pathway in male albino rats. Asian Journal of Agriculture and Biology 20232023110
- [Google Scholar]
- Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151-169.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:30-32.
- [Google Scholar]
- Naringenin prevents doxorubicin-induced toxicity in kidney tissues by regulating the oxidative and inflammatory insult in Wistar rats. Arch. Physiol. Biochem.. 2020;126:300-307.
- [Google Scholar]
- Plastics and microplastics: A threat to environment. Environ. Technol. Innov.. 2019;14:100352
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402-408.
- [Google Scholar]
- Acute toxic effects of polyethylene microplastic on adult zebrafish. Ecotoxicol. Environ. Saf.. 2019;182:109442
- [Google Scholar]
- Antioxidant and antimicrobial potentials of Artemisia Indica collected from the Nepal region. J. Pharm. Sci. Rev.. 2017;9:1822-1826.
- [Google Scholar]
- Reaction of linoleic acid hydroperoxide with thiobarbutiric acid. J. Lipid Res.. 1978;19:1053-1057.
- [Google Scholar]
- Standardization of immunoassays for measurement of myoglobin in serum. Phase I: evaluation of candidate secondary reference materials. Clinica Chimica Acta. 2004;341:65-72.
- [Google Scholar]
- Application of plastics and paper as food packaging materials-An overview. Emir. J. Food Agric.. 2013;25:177.
- [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588-590.
- [Google Scholar]
- Nanoparticles aggravate heat stress induced cognitive deficits, blood–brain barrier disruption, edema formation and brain pathology. Prog. Brain Res.. 2007;182:245-273.
- [Google Scholar]
- Evaluation of cellular antioxidant components of honeys using UPLC-MS/MS and HPLC-FLD based on the quantitative composition-activity relationship. Food Chem.. 2019;293:169-177.
- [Google Scholar]
- Immune response triggered by the ingestion of polyethylene microplastics in the dipteran larvae Chironomus riparius. J. Hazard. Mater.. 2021;414:125401
- [Google Scholar]
- A review on sources and pharmacological aspects of sakuranetin. Nutrients. 2020;12:513.
- [Google Scholar]
- Cardiovascular toxicity assessment of polyethylene nanoplastics on developing zebrafish embryos. Chemosphere. 2021;282:131124
- [Google Scholar]
- Structure–property studies on the antioxidant activity of flavonoids present in diet. Free Radic. Biol. Med.. 2005;39:1099-1108.
- [Google Scholar]
- IFCC methods for the measurement of catalytic concentration of enzymes Part 5. IFCC method for alkaline phosphatase (orthophosphoric-monoester phosphohydrolase, alkaline optimum, EC 3.1. 3.1). Journal of clinical chemistry and clinical biochemistry. Zeitschrift Fur Klinische Chemie Und Klinische Biochemie. 1983;21:731-748.
- [Google Scholar]
- Important flavonoids and their role as a therapeutic agent. Molecules. 2020;25:5243.
- [Google Scholar]
- Exposure to polystyrene microplastics induced gene modulated biological responses in zebrafish (Danio rerio) Chemosphere. 2021;281:128592
- [Google Scholar]
- The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev.. 2018;98:1169-1203.
- [Google Scholar]
- Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. J. Hazard. Mater.. 2019;366:703-713.
- [Google Scholar]
- Conversion of waste plastic packings to carbon nanomaterials: investigation into catalyst material, waste type, and product applications. ACS Sustain. Chem.. 2022;10:1125-1136.
- [Google Scholar]
- Ameliorating role of methanolic leaves extract of Fraxinus xanthoxyloides against CCl 4-challanged nephrotoxicity in rats. Pak. J. Pharm. Sci.. 2018;31:1475-1484.
- [Google Scholar]
- Polystyrene microplastic exposure disturbs hepatic glycolipid metabolism at the physiological, biochemical, and transcriptomic levels in adult zebrafish. Sci. Total Environ.. 2020;710:136279
- [Google Scholar]







