Translate this page into:
Comprehensive analysis of antioxidant and antibacterial activities of water and methanol extracts of Hibiscus flower
⁎Corresponding author. hesham@uthm.edu.my (Hesham Hussein Rassem)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This study provides a comprehensive investigation into the antioxidant and antibacterial properties of Hibiscus flower extracts, highlighting their potential for future applications in functional foods. The research addressed key challenges by employing a multi-faceted approach, including four distinct methods for evaluating antioxidant potential: DPPH and FRAP assays, reducing power, and chelating ability of Fe ions. Total phenolic content was quantified using the Folin–Ciocalteu method, while tannins and flavonoids were assessed via vanillin–HCl and aluminum chloride assays. Additional analyses included spectrophotometric measurement of total flavonols and anthocyanins. Antibacterial activity was determined using a modified agar disk diffusion method, with results indicating significant inhibition of Salmonella typhimurium and Staphylococcus aureus by both aqueous and methanolic extracts. Colorimetric analysis revealed a reduction in chroma and hue angles, while FTIR spectroscopy identified key functional groups, such as suberin, lipids, and polysaccharides. Notably, the antioxidant efficacy varied depending on the solvent used, with aqueous extracts showing higher total phenolic content (4325.12 mg GAE/100 g) compared to methanolic extracts (3487.05 mg GAE/100 g). The study concludes that Hibiscus flower extracts, rich in antioxidants like tannins and anthocyanins, possess significant potential for use as natural preservatives and colorants in food products, supporting their role in developing novel functional foods.
Keywords
Hibiscus flowers
Antioxidant activity
FTIR analysis
Antibacterial activity
Tannins
Flavonoids
1 Introduction
There has been a significant surge in research dedicated to studying plants and their derived substances in recent years. In this respect, plants that have been historically used for medicinal purposes are subjected to extensive screening in order to determine their potential as equivalents or superior alternatives to chemical-based food preservatives. Furthermore, plants are recognized as rich sources of natural antioxidants, offering a potential for their application in the food business. They can function as sources for nutritional supplements or serve as natural antioxidants, which help maintain food quality and prolong shelf-life (Voon et al., 2012; Tiwari et al., 2009).
Recent studies have also explored the incorporation of advanced materials into plant extracts to enhance their properties. For example, research has investigated the control of particle growth and enhancement of photoluminescence, adsorption efficiency, and photocatalytic activity in materials like zinc sulfide and cadmium sulfide using CoAl-layered double hydroxide systems (Intachai et al., 2023). Additionally, the easy intercalation and size control of zinc selenide through nanospace modification of montmorillonite and saponite have led to enhanced photoluminescence, further expanding the potential applications of these materials in various industries (Intachai et al., 2021). Such advancements suggest potential improvements in the use of natural substances for both food preservation and functional materials.
“Throughout history, different plants and their derivatives have been used in cooking as herbs or spices.” These organic compounds fulfill functions such as food preservation, augmentation of flavor, and traditional treatments for common ailments. The therapeutic qualities ascribed to these plants are frequently associated with their antibacterial characteristics. Utilizing natural antimicrobials produced from plants might significantly decrease the dependence on antibiotics, decrease the likelihood of antibiotic resistance in foodborne pathogens, and assist in managing cross-contaminations caused by these pathogens (Voon et al., 2012). In addition to their antioxidant and antibacterial properties, plants or their extracts also have the potential to be used as natural food colorants. The natural colorants mentioned in the study (Boo et al., 2012; Rymbai et al., 2011; Gupta and Nair, 2012) are widely regarded as safe and non-toxic to humans. This makes them highly desirable for usage in food products. Recent studies continue to explore these bioactive properties further. For example, Zingiber montanum has been investigated for its potential when combined with ZnAl-LDH-based host materials to enhance its optical, antioxidant, and antibacterial characteristics (Intachai et al., 2024). Such studies further reinforce the potential applications of natural plant extracts in food preservation and as alternatives to synthetic additives.
Recent research has highlighted the antibacterial and antioxidant properties of flowers and their extracts (Jo et al., 2012; Voon et al., 2012; Shyu et al., 2009). Hibiscus, a perennial and ligneous plant from the Malvaceae family, is widely used for ornamental purposes and is renowned for its prolific blooming. Hibiscus rosa-sinensis, commonly found in tropical regions, has been extensively studied for its bioactive properties. According to Obi et al. (1998), this herbal remedy is recommended as an alternative treatment for various health conditions. Hibiscus is a rich source of vitamins, minerals, and bioactive compounds, including organic acids, phytosterols, and polyphenols, many of which exhibit antioxidant properties. Hibiscus flowers contain various bioactive compounds, including suberin, triglycerides, and polysaccharides, which may contribute to their functional properties, such as antioxidant and antimicrobial activity. Its phenolic content primarily consists of anthocyanins such as delphinidin-3-glucoside, sambubioside, and cyanidin-3-sambubioside, alongside other flavonoids like gossypetin, hibiscetin, and their respective glycosides. Additionally, Hibiscus contains protocatechuic acid, eugenol, and sterols like β-sitosterol and ergosterol (Ali-Bradeldin et al., 2005). Moreover, Roselle calyx extract is recognized as a potent source of antioxidants, particularly due to its high anthocyanin content (Ajiboye et al., 2011). Similarly, recent studies have demonstrated the significant influence of extraction solvents on both antioxidant and antibacterial activities of plant extracts. For instance, Zingiber montanum rhizomes have shown varied antioxidant and antibacterial potentials when different solvents were used for extraction (Thepthong et al., 2023).
Anthocyanin, a type of flavonoid found in Roselle calyces, has been identified as a significant contributor to the antioxidant capacity in Roselle petal extract (Tsai et al., 2002). Tsai et al. (2002) suggest that anthocyanin is the primary source of antioxidant activity in Roselle extracts. Previous studies have reported that the aqueous extract of this plant can inhibit several nosocomial infectious bacteria, including methicillin-resistant Staphylococcus aureus and Klebsiella pneumoniae (Liu et al., 2005). However, the efficacy of Roselle calyx aqueous extract against other pathogens such as Salmonella typhimurium DT104, E. coli O157, Listeria monocytogenes, Staphylococcus aureus, and Bacillus cereus remains uncertain. Additionally, protocatechuic acid, a polyphenol with a 3,4-dihydroxy substructure, naturally occurs in Roselle calyx and has been shown in several in vitro studies to inhibit the growth of E. coli and fungi (Fernandez et al., 1996; Aziz et al., 1998). The aqueous-methanolic extract of Hibiscus sabdariffa L. calyces has demonstrated antibacterial activity against S. aureus, Bacillus stearothermophilus, Micrococcus luteus, Serratia marcescens, Clostridium sporogenes, E. coli, K. pneumoniae, B. cereus, and Pseudomonas fluorescens (Olaleye, 2007). The antibacterial effects of this plant extract against E. coli, P. aeruginosa, and S. aureus suggest potential therapeutic applications in treating gastrointestinal infections, diarrhea, and skin diseases in humans (Rogger et al., 1990).
The present study aims to evaluate the antioxidant capacities, antimicrobial properties against foodborne pathogens diseases, color attributes, and identification of functional groups using FTIR spectra in Hibiscus flowers. This plant is widely known for its red-colored flowers and is commonly used as an ornamental flowering plant. The expectation is that the results of this study will build a strong basis for utilizing these flowers as natural additions or for enhancing the nutritional value in the development of novel functional meals. Fig. 1 depicts a concise graphic illustrating the investigation that was undertaken.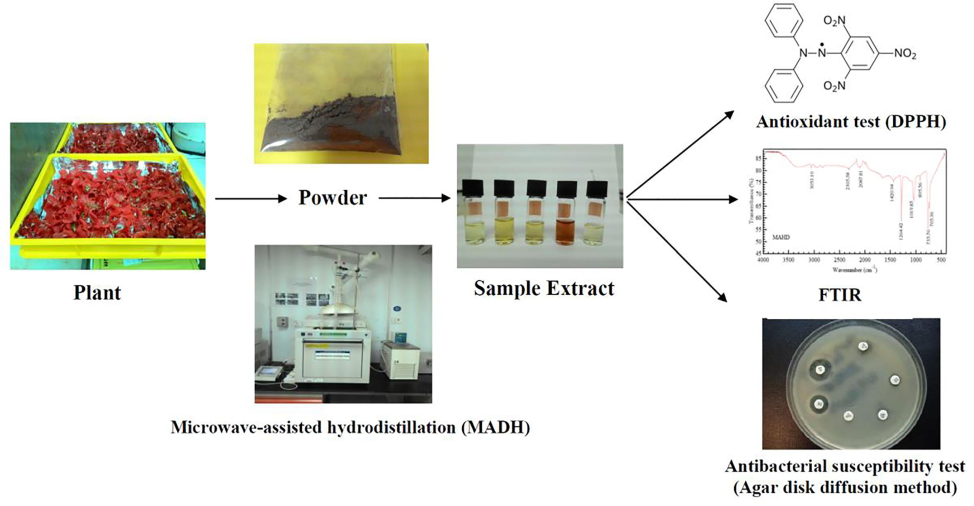
Diagram showing a summary of the study conducted.
2 Materials and methods
2.1 Plant
Hibiscus blooms in pristine condition, free from any visible physical, microbial, or insect harm, were gathered from the garden at Universiti Malaysia Pahang, Pahang. The flowers were grown in a climate-resilient habitat, flourishing continuously year-round, regardless of seasonal changes. The plant exhibits intolerance towards temperatures below 10 °C, a threshold that remains unattained in Malaysia's winter environment. The essential oil extraction in our study utilized only analytical-grade chemicals to guarantee environmental safety. The chemicals, namely dimethyl sulfoxide (DMSO), anhydrous sodium sulfate (Na2SO4), and potassium dichromate (K2Cr2O7), were obtained from Sigma-Aldrich (USA).
2.2 Cleaning and preparation of Hibiscus flowers
The Hibiscus flowers were painstakingly purified to eliminate impurities such as minuscule sand particles and adhesive substances, in order to reduce the risk of exposure to hazardous chemicals. The cleaning procedure entailed using newly harvested Hibiscus flower specimens, which were rinsed in distilled water for a duration of 60 min and thereafter let to dry naturally for 24 h at a temperature ranging from 60 to 70 °C. After completing the initial stage, around 70 g of dehydrated Hibiscus flower petals were crushed to decrease the particle size, as outlined by Hesham et al. (2022). Achieving a particle size of 80 μm, a Hibiscus flower powder was created by grinding and sifting using a mechanical sieve shaker. In order to maintain a consistent weight, the flower powder was exposed to a desiccation procedure at a specific temperature of 105 ± 5 °C. The moisture content of the floral powder was subsequently determined using Equation No. 1.
The completely desiccated sample of Hibiscus flower powder was kept in a regulated condition. Prior to extraction, a precise measurement of 35 g of powdered Hibiscus flowers was taken. The powder was then steeped in distilled methanol for 1 h, using a ratio of 8:1 w/w of methanol to the entire dried Hibiscus flower powder. The same steps were followed for the aqueous extraction of hibiscus flowers. This procedure follows the approach described by Hesham et al. (2017) using microwave-assisted hydrodistillation (MADH) to obtain aqueous and methanolic extracts. Subsequent measurements were derived from the weight of the dehydrated samples. The dehydrated raw extracts were then precisely measured and stored in a refrigerator at −4°C. The percentage yield of the extracts was calculated using Equation No. 2.
In the present study, extracts for antimicrobial and antioxidant analyses were prepared using different methodologies to optimize their respective bioactive properties. For antimicrobial testing, aqueous and methanolic extracts were filtered through sterile 0.22 µm filters to prevent contamination, while the extracts for antioxidant assays were prepared without sterilization.
2.3 Antioxidant analysis
2.3.1 DPPH Radical scavenging activity and ferric reducing antioxidant power (FRAP) assay
The effectiveness of Hibiscus extracts as antioxidants was evaluated based on their capacity to deactivate 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals. Following Bozin et al. (2006)'s methodology, the extracts were mixed with 1 mL of 90 μM DPPH solution and then diluted with 95 % methanol for a final volume of 4 mL. The solutions, along with a blank sample, were left undisturbed for an hour at room temperature before measuring their absorbance. Butylated hydroxytoluene (BHT) served as a benchmark. The absorbance was recorded at 515 nm using a UV–visible spectrophotometer, and the reduction in DPPH radicals was calculated using a specific formula No3.
“Acontrol” represents the absorbance value of the DPPH solution without the addition of any sample extract. “Asample,” on the other hand, refers to the absorbance level demonstrating the extent to which the sample extract has interacted with the DPPH solution.
To assess the extracts' capacity to decrease ferric ions, a modified FRAP test, as outlined by Benzie and Strain (1996), was employed. Calibration curves were generated using ferrous sulfate solutions with concentrations varying from 0.1 to 1 µM. The FRAP activity was quantified and reported as micromoles of Fe (II) per 100 g dry weight for each material.
2.3.2 Quantification of overall phenolic content, tannins, flavonoids, and flavonols
The phenolic content in the flower extracts was measured using the Folin–Ciocalteu method as described by Singleton and Rossi (1965). This process involved mixing 400 µL of extract with 2 mL of a tenfold diluted Folin–Ciocalteu reagent and allowing it to stand for 3 min. Then, 3 mL of 7.5 % sodium carbonate was added, the mixture was thoroughly shaken, and left to sit for 60 min at room temperature. The absorbance was then recorded at 760 nm using a UV–visible spectrophotometer. Gallic acid was used to create a standard curve, and the phenolic content was expressed in milligrams of Gallic acid equivalent per gram of the extract.
The tannin content in the samples was determined using the vanillin-HCl method, as detailed by Bhat et al. (2007). Briefly, 2 mL of each extract was mixed with an equal volume of a reagent made from 4 % vanillin in methanol and 8 % HCl in methanol, in a 1:1 ratio. The mixture's color intensity was measured at 550 nm with a UV–visible Spectrophotometer after 30 min of incubation. The tannin content was calibrated against a catechin standard, with a range from 10 to 500 µg/mL, and quantified as milligrams of Catechin Equivalent per 100 g of dry weight of the samples.
The flavonoid levels in extract samples were analyzed using the aluminum chloride method as per Liu et al. (2008). Initially, a solution was prepared by mixing the extract with distilled water, sodium nitrite, and aluminum chloride, followed by sodium hydroxide. After specific incubation times, the solution was diluted and its absorbance was measured at 650 nm. Flavonoid concentrations were then expressed as Catechin Equivalents per 100 g of the sample's dry weight.
Miliauskas et al. (2004) developed a method, with some modifications, for determining total flavonols in extracts using a quercetin calibration curve. This involved preparing solutions of quercetin in methanol at varying concentrations, mixed with aluminum trichloride and sodium acetate. After incubating at 20 °C for 120 min, absorbance was measured at 540 nm. This method was then applied to sample extracts, and flavonol content was expressed in Quercetin Equivalents per 100 g of dry weight.
Abdel-Aal and Hucl (1999) developed a spectrophotometric technique for estimating total anthocyanin content. In brief, the process involved acidifying a methanol and hydrochloric acid mixture to extract anthocyanins. The mixture, with a solvent to extract ratio of 10:1, was centrifuged, and absorbance was measured at 550 nm. A calibration curve was created using Cyanidin-3-Glucoside, showing a high correlation (r2 = 0.9982). The anthocyanin content in flower extracts was then quantified as Cyanidin-3-Glucoside Equivalents per 100 g of dry sample weight.
2.4 Determination of reducing power
The method for assessing the antioxidant capacity of Hibiscus extract, as described by Oyaizu (1986), involves treating different concentrations of the extract with phosphate buffer, potassium ferricyanide, trichloroacetic acid, and ferric chloride. After incubation and centrifugation, the absorbance of the supernatant is measured. The reducing ability value is indicative of the extract's ability to reduce substances, with a higher value signifying greater antioxidant capacity.
2.5 Chelating of ferrous ion
The method by Decker and Welch (1990) was used to assess the iron-binding capacity of different fractions from Hibiscus flowers. In this process, aliquots of the sample were treated with ferrous chloride and ferrozine solutions. The mixture's absorbance was measured at 560 nm post-incubation, a wavelength where the ferrous/ferrozine complex shows significant absorption. This procedure helps determine the sample's ability to bind ferrous ions. The percentage of chelated ferrous ions was calculated using the following equation.
2.6 Test for sensitivity to antibacterial agents using the agar disk diffusion technique
2.6.1 Microorganisms and their respective conditions for growth
The antibacterial activity of Hibiscus flower extracts was tested against eight food-borne pathogens, including four Gram-positive and four Gram-negative bacteria, using the agar disk diffusion method. This method, detailed by Adeniyi et al. (1996), involved inoculating the bacteria into a culture medium and standardizing their concentration for the antibacterial test. The method is a reliable approach to evaluate the extracts' effectiveness against various bacterial strains.
2.6.2 Preparing extracts for microbiological analysis
The extraction process for the plant material involved two solvents: distilled water and 80 % methanol, following a modified version of the method by Alade and Irobi (1993). In brief, 2 g of powdered sample were soaked in 100 mL of the selected solvent and stirred for 4 h. After filtering, a second extraction was performed for 10 h. The filtrates were then concentrated and purified. For methanol extracts, a rotary vacuum evaporator was used, while water extracts were condensed under pressure. The concentrated extracts were dissolved to form a 100 mg/mL solution and stored at −20 °C for future use in disk diffusion tests.
2.6.3 Preparation of culture
To assess the antibacterial activity of the extracts, a sterile glass hockey stick was used for even distribution of a pathogen suspension on agar plates. After applying the extracts on sterile paper disks, the plates were incubated to observe zones of inhibition. This procedure, based on the modified agar disk diffusion method, allowed for the evaluation of the extracts' antibacterial efficacy against specific pathogens, using chloramphenicol as a positive control and distilled water and methanol as negative controls. The effectiveness was determined by measuring the clear zones around the disks after incubation.
2.7 Color analysis
The color properties of the powdered flower samples were measured using a colorimeter. The ground sample was placed in the measurement chamber, and the color was quantified using the Minolta color scale. This scale measures luminance (L* value), with 0 representing black and 100 white, and chromaticity with a* values indicating a shift from bluish-green to purplish-red, and b* values from blue to yellow.
2.8 (FTIR) analysis
FTIR spectral analysis was performed on the flower samples by creating KBr pellets mixed with the powdered material. The spectra were captured using a Thermo Scientific Nicolet iS5 FTIR Spectrometer within a specific frequency range, with precise data collection settings to ensure accurate results. This method allowed for the detailed analysis of the samples' molecular composition.
2.9 Analysis of data using statistical methods
In this study, data analysis was conducted using SPSS Statistics Version 17.0. Differences among sample means were assessed using one-way ANOVA, with a significance threshold of P < 0.05. Duncan's test was applied for mean comparisons, and results were expressed as means ± standard deviations. The data represent the average of three independent experiments, reported as triplicates, unless otherwise specified.
3 Results and discussion
3.1 Evaluation of extraction yields
The extraction of bioactive compounds was carried out using MADH with both aqueous and methanolic solvents. Methanol demonstrated a higher extraction yield (19.32 ± 4.78 %) compared to the aqueous solvent (15.47 ± 3.19 %). The extraction yield, along with the results of antioxidant assays and chemical tests, are presented based on the dried weight of the sample, as detailed in Table 1. Both methanolic and aqueous extractions produced a visually red Hibiscus extract. The provided findings correspond to the average of three distinct duplicates (n = 3 ± S.D.), calculated using dry weight measurements. Mean values denoted by distinct letters within a row exhibit statistical significance (p < 0.05) when compared to one another.
Parameter
Hibiscus flower extracts
Methanol
Aqueous
Yield (g/100 g dry extract)
19.32 ± 4.78 %
15.47 ± 3.19 %
% DPPH inhibition
72.97 ± 0.1a
86.24 ± 0.5c
FRAP Values (µmoles Fe (II)/100 g)
2451.17 ± 228.3ab
2974.34 ± 218.7c
Total Phenolics (mg GAE/100 g)
3487.05 ± 115.7a
4325.12 ± 158.5b
Total Tannins (mg CE/100 g)
3950.54 ± 132.1c
5431.97 ± 121.8d
Total Flavonoids (mg CE/100 g)
3266.48 ± 123.6b
3879.17 ± 197.3c
Total Flavonols (mg QE/100 g)
683.11 ± 1.4c
431.74 ± 2.3b
Total Anthocyanins (mg c-3-QE/100 g)
265.37 ± 4.5c
316.85 ± 2.5d
3.1.1 Measurement of DPPH radical inhibition and FRAP test-significant
Both methanolic and aqueous extracts from Hibiscus flowers shown substantial antioxidant activity against DPPH in the ongoing experiment. The increased effectiveness in scavenging radicals could be ascribed to elevated concentrations of flavonols, phenolics, or tannins in the extracted samples.
The FRAP test, like the DPPH assay, is regarded as both fast and sensitive, providing a semi-quantitative evaluation. The FRAP test measures the antioxidant potential by assessing the sample extracts' capability to convert Fe (III)-TPTZ complexes to ferrous ion-2,4,6-tripyridyl-s-triazine. This test uses a newly made FRAP solution that contains 2,4,6-tris(1-pyridyl)-5-triazine at a PH of 3.6. The FRAP reagent undergoes a reduction of ferric iron, leading to the creation of a blue product known as the ferrous-TPTZ complex (Benzie and Strain, 1996). Greater antioxidant activity in sample extracts is indicated by higher FRAP values. The research findings indicated that the aqueous extracts of Hibiscus shown higher reducing power compared to its methanolic extracts. The methanol and aqueous extracts of Hibiscus were quantified for their reducing capacities, resulting in values of 2451.17 and 2974.34 µmoles Fe(II) per 100 g of materials, respectively. The results are consistent with other studies on different floral extracts, which emphasize their significant antiradical properties (Wijekoon et al., 2011; O¨zkan et al., 2004).
3.1.2 Measurement of the overall amount of phenolic compounds, tannins, flavonoids, flavonols, and anthocyanins
Phenolic substances obtained from plants exhibit strong antioxidant activity by counteracting free radicals produced during normal metabolic activities. This group includes a wide range of compounds, mostly consisting of proanthocyanidins and flavonoids (condensed tannins) (Shahidi and Naczk, 2004). The ongoing analysis has revealed significant differences in the total phenolic content between the two floral extracts, which can likely be linked to the choice of solvents used for extraction. The total phenols varied between 3487.05 and 4325.12 mg GAE/100 g, as specified in Table 1. Both the methanolic and aqueous extracts of Hibiscus exhibited a substantial concentration of phenolic chemicals, with the aqueous extract showing a greater phenolic content in comparison to the methanol extract of Hibiscus flower.
The tannin concentration in both floral extracts showed significant variance, ranging from 3950.54 to 5431.97 mg CE/100 g (see Table 1). The aqueous extract of Hibiscus exhibited a significantly elevated tannin content. Tannins, known for their large molecular size, provide advantageous properties due to their exceptional capacity to neutralize free radicals. “The efficacy of tannins in this context is influenced by factors such as the quantity of aromatic rings, molecular weight, and the type of hydroxyl group substitution (Cai et al., 2006).”.
The aqueous extracts of Hibiscus displayed a higher total flavonoid or bioflavonoid content (3879.17 mg CE/100 g) compared to its methanolic extracts (3266.48 mg CE/100 g). Flavonoids, a class of natural secondary metabolites abundant in plants, are renowned for their robust antioxidant effects. This category encompasses subcategories like isoflavones, flavonols, flavones, and anthocyanins, each featuring distinct structural properties. Flavonoids have demonstrated effective interactions with and elimination of free radicals, which pose a potential threat to cellular membranes and biomolecules (Rice-Evans and Miller, 1997).
The total flavonols, the predominant type of flavonoids in plant-based foods, exhibited significant variability among the flowers and their extracts. The methanolic extract of Hibiscus flowers had a much higher flavonol level (683.11 mg QE/100 g), while the aqueous extract had a lower level (431.74 mg QE/100 g). These results suggest that methanol is a more effective solvent for extracting flavonols than distilled water.
In the study, the water-based extract of Hibiscus showed higher total anthocyanin content (316.85 mg c-3-gE/100 g) compared to the methanol extract (265.37 mg c-3-gE/100 g). Anthocyanins, known for their antioxidant properties, antibacterial effects, and use as natural food colorants, have gained significant interest in recent research.
This conclusion is supported by previous research that has confirmed the existence of significant quantities of total phenols, flavonols, flavonoids, and anthocyanins in various flowers and their extracts (Wijekoon et al., 2011; Cai et al., 2004; Yang et al., 2012; Gouveia et al., 2013). Moreover, scientific studies have confirmed a clear link between antioxidant chemicals and their ability to produce antioxidative effects in many parts of plants, including flowers, fruits, leaves, and seeds, as well as in their extracts (Voon et al., 2012; Wijekoon et al., 2011; Shyu et al., 2009). The current investigation confirms that the antioxidant activity of extracts might vary greatly based on the solvent extraction procedures used, which is consistent with prior research findings (Wijekoon et al., 2011; Tian et al., 2009). The results strongly indicate that the antioxidant properties reported in both extracts of Hibiscus flowers are mostly attributed to phenolic components, such as flavonoids, tannins, flavonols, and anthocyanins.
3.2 Estimation of reducing power
Table 2 presents a concise overview of the capacity for lowering power demonstrated by the methanolic and water extracts derived from Hibiscus flowers. The reducing power exhibited a significant increase (P < 0.05) with rising extract concentrations. The results indicate that the reducing ability of all samples heightened proportionally with increasing extract concentration. Specifically, the methanolic extract exhibited a noteworthy capacity for reduction, achieving a value of 233.71 % at a concentration of 5 mg/ml, which nearly approached the reducing power reported with ascorbic acid (229.32 %). Following closely were citric acid (197.92 %), the aqueous extract (171.94 %), surpassing α-tocopherol (94.21 %), and BHT (167.36 %) at the equivalent concentration.
Concentratemg/ml
Reducing power (%)
Aqueous extract
Methanol extract
BHT
α −Tocopherol
Ascorbic acid
Citric acid
20
51.76 ± 3.0.00
82.41 ± 0.01
56.33 ± 0.01
38.78 ± 0.01
61.43 ± 0.01
34.13 ± 0.01
40
76.84 ± 0.01
120.45 ± 0.00
82.36 ± 0.00
47.47 ± 0.00
159.48 ± 0.00
78.87 ± 0.00
60
120.29 ± 0.001
198.93 ± 0.01
86.95 ± 0.01
56.71 ± 0.01
184.86 ± 0.001
151.87 ± 0.01
80
152.16 ± 0.02
206.76 ± 0.00
156.75 ± 0.00
84.14 ± 0.001
191.91 ± 0.00
183.47 ± 0.001
100
171.94 ± 0.01
233.71 ± 0.01
167.36 ± 0.02
94.21 ± 0.00
229.32 ± 0.00
197.92 ± 0.00
3.3 Chelating of ferrous ion
The ferrous ion chelating capacity of Hibiscus flowers calyx alcoholic and aqueous extracts is presented in Table 3. Notably, At a concentration of 5 mg/ml, EDTA demonstrated a significant capacity to chelate ferrous ions, with a rate of 97.15 %. The aqueous extract demonstrated superior ferrous ion chelating efficacy, reaching 86.18 %, followed by citric acid (54.13 %). In contrast, the alcoholic extracts exhibited a decrease in their capacity to chelate ferrous ions, with a reduction of 43.31 %.
Concentratemg/ml
Chelating ferrous (%)
Aqueous extract
Methanol extract
EDTA
Citric acid
20
28.96 ± 0.001
36.86 ± 0.01
34.29 ± 0.00
10.23 ± 0.01
40
49.24 ± 0.01
37.27 ± 0.001
45.12 ± 0.01
18.13 ± 0.00
60
62.81 ± 0.00
39.86 ± 0.00
95.94 ± 0.00
28.68 ± 0.001
80
82.14 ± 0.001
42.65 ± 0.001
96.24 ± 0.001
35.94 ± 0.00
100
86.18 ± 0.00
43.31 ± 0.00
97.15 ± 0.01
54.13 ± 0.01
3.4 Antibacterial activity assay
The antibacterial activity experiment demonstrated that both aqueous and alcoholic extracts of Hibiscus displayed inhibitory effects on a range of investigated microorganisms. The findings, presented in Table 4, demonstrate the antimicrobial efficacy of aqueous and methanolic extracts of Hibiscus flowers against several Gram-positive and Gram-negative pathogenic bacteria at a concentration of 100 mg/mL. Both extracts exhibited extensive inhibitory zones against Bacillus cereus, Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, and Klebsiella. pneumonia, as specified in Table 4. The aqueous extract of Hibiscus exhibited notable inhibitory effects on Escherichia coli and Staphylococcus aureus, with measurements of 14 mm and 15 mm, respectively. These measurements surpassed the inhibition seen against Salmonella typhimurium, which measured 9 mm. Conversely, the alcoholic extract shown greater inhibition against Escherichia coli (16 mm) in comparison to Bacillus cereus (11 mm), Staphylococcus aureus (10 mm), and Klebsiella pneumonia (12.50 mm). The diameter of the disc is − 6 mm. –: There is no zone of inhibition.
Microorganisms
Zone of inhibition (mm)
Antimicrobial disk
Aqueous extract Methanolic extract
Chloramphenicol
10 µg/disk
Gram-positive
B. cereus
−
11
14
B. subtilis
−
−
23
L. monocytogenes
−
−
16
S. aureus
15
10.0
13
Gram-negative
E. coli
14
16
29
S. enteritidis
−
−
22
S. typhimurium
9.0
−
21
K. pneumonia
−
12.5
20
Research has shown that crude plant extracts typically have more potent antibacterial effects against Gram-positive bacteria than Gram-negative bacteria, a distinction attributed to structural differences in their cell envelopes (Tian et al., 2009; Kabuki et al., 2000). These differences include variations in cell wall components and cytoplasmic membranes (Silhavy et al., 2010).
The antibacterial effectiveness observed in the study is likely due to the presence of bioactive compounds such as polyphenols, flavonoids, and tannins in the extracts. These substances are known for their potent antimicrobial properties. Plants often produce these compounds as a means of protecting themselves against microbial infections. Prior scientific research has emphasized the efficacy of tannins in controlling bacteria, yeasts, and fungus (Scalbert, 1991). The effectiveness of this process is credited to the creation of compounds formed by the interaction of tannins, microbial enzymes, and cell membrane transport proteins. These complexes are thought to result in protein inactivation, which hinders the development of microorganisms (Haslam, 1996). Furthermore, the antibacterial activity of the samples may be greatly enhanced by the presence of essential oils (Voon et al., 2012).
Prior research has indicated that methanol extracts exhibit significant extraction efficiency and potent antimicrobial properties (Jo et al., 2012; Mann et al., 2011; Quarenghi et al., 2000). Nevertheless, considering the significant toxicity of methanol and its inappropriateness for human or cattle ingestion due to its non-food-grade quality, it is crucial to assess the actions in aqueous extracts. Aqueous extracts, while retaining notable antibacterial activity, are regarded as far safer for ingestion. The water extracts may yield more advantageous results for culinary and medicinal applications in comparison to methanol or other solvents.
3.5 Color analysis
The analysis determined that the lightness (L*) value of Hibiscus is 35.06. The significantly reduced L* value of Hibiscus may be associated with the deeper shade of its flower petals compared to other types of flowers (see Table 5). Additionally, for Hibiscus, the chromatic component values were measured at 13.84 for a* and 4.62 for b*. “a* and b*: These parameters indicate chromatic components, with a* representing lightness and b* representing chroma. Chroma can be calculated using the formula (a*^2 + b*^2)^(1/2). The hue angle, denoted as c, can be determined by taking the arctangent of b*/a*. ”The presented results represent the average of three independent replicates (n = 3 ± S.D.) based on dry weight measurements. Mean values labeled with different letters in sequence are statistically significant (p < 0.05) and differ from each other.“.
Analysis
Hibiscus
L*
35.06 ± 0.01x
a*
13.84 ± 0.05x
b*
4.62 ± 0.04x
C*
12.36 ± 0.02x
Δh
15.74 ± 0.03x
The chroma (C*) and hue angle (Δh) values derived from the a* and b* components were obtained measurements for Hibiscus were found to be 12.36 and 15.74, respectively. The chroma of a color indicates its intensity, whereas the hue angle values are measured in a counterclockwise direction from red to purple (Δh = 0) around a color circle that gradually transitions between colors. The circle undergoes rotations of 90° (yellow), 180° (bluish-green), and 270° (blue). Upon investigation, it was observed that Hibiscus displayed reduced chroma and hue angle values, indicating that its flower petals possess fewer vibrant colours. In general, the color of Hibiscus was described as having a low L* value, indicating a dark shade, but with a low intensity, making it less bright.
The vivid hues in flowers result from the combined impact of several pigments present in nature, including flavonoids, anthocyanins, chlorophyll, xanthones, and betalains (Brouillard and Dangles, 1993). The variety of flower colors is influenced by several elements, including the chemical composition of pigments, their methylation and acylation states, the acidity of the vacuole, the presence of cyanidins or genetic inheritance, and pelargonidin derivatives (Gettys and Wofford, 2007).
3.6 FTIR analysis
Fig. 2 depicts the FTIR spectra of Hibiscus flower oils obtained through MAHD. These spectra reveal overlapping absorption spectra of the diverse oil components, reflecting the intricate nature of volatile oils. The informative region for Hibiscus flower oil's distinctive fingerprint is between 4000 and 400 cm−1, making this spectrum highly elucidative.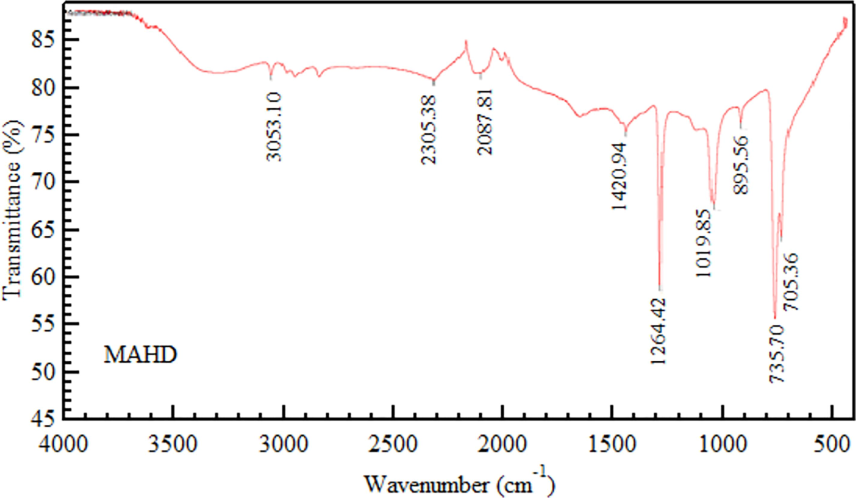
Fourier Transform Infrared (FTIR) spectra of powdered Hibiscus flower.
The FT-IR spectrophotometer analysis of Hibiscus flower essential oil revealed specific functional groups by comparing the sample's vibration frequencies with an IR correlation chart. The spectrum indicated the presence of alcohol and phenol O–H stretch at 3053 cm-1, and an absorbance band at 1629 cm-1 suggested the presence of aldehyde. This method allows for the identification of various chemical groups in the oil. The C = C skeletal vibration of aromatic components peaked at 1420 cm−1. Ring stretching specifically contributed to the 1264 cm−1 peak. The C–H stretching vibration was represented by the peak at 1019 cm−1. Vibrational bending absorption of C-N groups led to the 895 cm−1 peaks, while the nitro group's vibrations were reflected in the absorption peak at 735 cm−1. The presence of suberin, triglycerides, and polysaccharides in hibiscus extracts was confirmed by FTIR analysis, suggesting potential roles in the bioactive properties of the extracts. Table 6 visually summarizes these significant peaks. –: no peak wavenumber. (Source: Hesham et al., 2022).
Functional group representation
Vibration assignment (cm−1)
MAHD
O–H
3053.10
C = O stretching
1629.29
C = C stretching
1420.94
Ring stretching
1264.42
C–H wagging
1019.85
C-N bending
895.56
NO2 wagging
735.70
4 Conclusion
This study demonstrated that Hibiscus flower calyxes are rich in natural phenols, flavonols, and tannins, exhibiting significant antioxidant and antibacterial properties in both methanolic and aqueous extracts. Methanolic extracts showed a higher extraction yield and greater antibacterial efficacy, particularly against Bacillus cereus, Escherichia coli, and Staphylococcus aureus. Both extracts demonstrated notable reducing power, effective chelation of ferrous ions, and antioxidant activity, outperforming synthetic antioxidants like BHT and α-tocopherol.
The presence of bioactive compounds such as anthocyanins, suberin, triglycerides, and polysaccharides further enhances their potential application. These findings suggest that Hibiscus extracts hold promise as natural preservatives, food colorants, and antimicrobial agents. Their potential use extends to functional foods, pharmaceuticals, and cosmetics, offering sustainable, eco-friendly alternatives to synthetic additives. Future research is recommended to explore the economic viability of Hibiscus blossoms in these applications, promoting their use in developing novel nutraceuticals and other health-promoting products.
CRediT authorship contribution statement
Hesham Hussein Rassem: . Mohd Hairul Bin Khamidun: Writing – review & editing, Writing – original draft, Investigation, Funding acquisition, Formal analysis, Conceptualization. Umi Fazara Md Ali: Writing – review & editing, Validation, Supervision, Software, Investigation, Formal analysis. Tony Hadibarata: Writing – review & editing, Visualization, Resources, Investigation, Data curation, Conceptualization. Nabeel Abdullah Alrabie: Writing – review & editing, Visualization, Supervision, Software, Investigation, Conceptualization.
Acknowledgement
The authors would like to thank Universiti Tun Hussein Onn Malaysia for assisting with this study. Communication of this research is made possible through monetary assistance by Universiti Tun Hussein Onn Malaysia and the UTHM Publisher’s Office via publication fund E15216.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chem.. 1999;76(3):350-354.
- [Google Scholar]
- Antimicrobial potentials of Diospyros mespiliformis (Ebenaceae) Afr. J. Med. Med. Sci.. 1996;25:179-184.
- [Google Scholar]
- Antioxidant and drug detoxification potentials of Hibiscus sabdariffa anthocyanin extract. Drug Chem. Toxicol.. 2011;34(2):109-115.
- [Google Scholar]
- Antimicrobial activities of crude leaf extracts of Acalypha wilkesiana. J. Ethnopharmacol.. 1993;39(3):171-174.
- [Google Scholar]
- Phytochemical, pharmacological, and toxicological aspects of Hibiscus sabdariffa: A review. Phytother. Res.. 2005;19(5):369-375.
- [Google Scholar]
- Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios. 1998;93(371):43-54.
- [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem.. 1996;239(1):70-76.
- [Google Scholar]
- Effect of ionizing radiation on antinutritional features of velvet bean seeds (Mucuna pruriens) Food Chem.. 2007;103(3):860-866.
- [Google Scholar]
- Extraction and characterization of some natural plant pigments. Ind. Crop. Prod.. 2012;40:129-135.
- [Google Scholar]
- Flavonoids and flower colour. In: Harborne J.B., ed. The Flavonoids: Advances in Research since 1986. London, UK: Chapman and Hall; 1993. p. :565-588.
- [Google Scholar]
- Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci.. 2004;74(17):2157-2184.
- [Google Scholar]
- Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Science. 2006;78(25):2872-2888.
- [Google Scholar]
- Antibacterial activity of the phenolic acids fractions of Scrophularia frutescens and Scrophularia sambucifolia. J. Ethnopharmacol.. 1996;26(1):11-14.
- [Google Scholar]
- Inheritance of flower color in pickerelweed (Pontederia cordata L.) J. Hered.. 2007;98(6):629-632.
- [Google Scholar]
- Gouveia, S., Gonc¸alves, J., & Castilho, P. C. (2013). Characterization of phenolic compounds and antioxidant activity of ethanolic extracts from flowers of Andryala glandulosa ssp. varia (Lowe ex DC.) R.Fern., an endemic species of Macaronesia region. Industrial Crops and Products, 42, 573–582.
- Application of botanicals as natural preservatives in food. In: Bhat R., Karim A.A., Paliyath G., eds. Progress in Food Preservation. UK: Wiley Blackwell Publishers; 2012. p. :513-524.
- [Google Scholar]
- Natural polyphenols (vegetable tannins) as drugs: possible modes of action. J. Nat. Prod.. 1996;59(2):205-215.
- [Google Scholar]
- GC-MS analysis of bioactive constituents of Hibiscus flower. Aust. J. Basic Appl. Sci.. 2017;11:91-97.
- [Google Scholar]
- Essential oil from Hibiscus flowers through advanced microwave-assisted hydrodistillation and conventional hydrodistillation. J. Chem.. 2022;22:1-10.
- [Google Scholar]
- Easy intercalation and size control of zinc selenide by nanospace modification of montmorillonite and saponite with enhanced photoluminescence. Opt. Mater.. 2021;122(Part A):111655.
- [Google Scholar]
- Control of particle growth and enhancement of photoluminescence, adsorption efficiency, and photocatalytic activity for zinc sulfide and cadmium sulfide using CoAl-layered double hydroxide system. Environ. Sci. Pollut. Res.. 2023;30:63215-63229.
- [CrossRef] [Google Scholar]
- The effect of ZnAl-LDH-based host material on optical, antioxidant and antibacterial characteristics of Zingiber montanum (Koenig) Link ex Dietr. extract. Chem. Pap.. 2024;78:4119-4128.
- [CrossRef] [Google Scholar]
- Antioxidant and tyrosinase inhibitory activities of methanol extracts from Magnolia denudata and Magnolia denudata var. purpurascens flowers. Food Res. Int.. 2012;47(2):197-200.
- [Google Scholar]
- Characterization of novel antimicrobial compounds from mango (Mangifera indica L.) kernel seeds. Food Chem.. 2000;71(1):61-66.
- [Google Scholar]
- In vitro antibacterial activity of Roselle calyx and protocatechuic acid. Phytother. Res.. 2005;19(10):942-945.
- [Google Scholar]
- Antioxidant activity of methanolic extract of emblica fruit (Phyllanthus emblica L.) from six regions in China. J. Food Compos. Anal.. 2008;21(3):219-228.
- [Google Scholar]
- Antimicrobial activity of Bombax buonopozense P. Beauv. (Bombacaceae) edible floral extracts. European Journal of Science. 2011;48:627-630.
- [Google Scholar]
- Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem.. 2004;85(2):231-237.
- [Google Scholar]
- O¨zkan, G., Sagdic, O., Baydar, N. G., & Baydar, H. (2004). Antioxidant and antibacterial activities of Rosa damascena flower extracts. Food Science and Technology, 10, 277–281.
- Prevention of carbon tetrachloride-induced hepatotoxicity in the rat by Hibiscus rosa-sinensis anthocyanin extract administered in ethanol. Toxicology. 1998;131(2–3):93-98.
- [Google Scholar]
- Cytotoxicity and antibacterial activity of methanolic extract of Hibiscus sabdariffa. J. Med. Plants Res.. 2007;1(1):9-13.
- [Google Scholar]
- Antimicrobial activity of flowers from Anthemis cotula. Fitoterapia. 2000;71(6):710-712.
- [Google Scholar]
- Structure-antioxidant activity relationships of flavonoids and isoflavonoids. In: Rice-Evans C.A., Packer L., eds. Flavonoids in Health and Disease. New York: Marcel Dekker; 1997.
- [Google Scholar]
- General Microbiology ((5th ed.).). London: Macmillan Education Ltd; 1990. p. :626-642.
- Bio-colorants and its implications in health and food industry – a review. Int. J. PharmaTech Res.. 2011;3(4):2228-2244.
- [Google Scholar]
- Phenolics in Food and Nutraceuticals. Boca Raton, FL: CRC Press; 2004. p. :1-576.
- Evaluation of antioxidant ability of ethanolic extract from dill (Anethum graveolens L.) flower. Food Chem.. 2009;115(2):515-521.
- [Google Scholar]
- Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic.. 1965;16(3):144-158.
- [Google Scholar]
- Effect of extraction solvents on antioxidant and antibacterial activity of Zingiber montanum rhizomes. J. Plant Stud.. 2023;26(3):1-9.
- [Google Scholar]
- Antioxidant and antimicrobial activities of consecutive extracts from Galla chinensis: The polarity affects the bioactivities. Food Chem.. 2009;113(1):173-179.
- [Google Scholar]
- Application of natural antimicrobials for food preservation. J. Agric. Food Chem.. 2009;57(14):5987-6000.
- [Google Scholar]
- Anthocyanin and antioxidant capacity in Roselle (Hibiscus sabdariffa L.) extract. Journal of the Canadian Institute of. Food Sci. Technol. (. 2002;35(4):351-356.
- [Google Scholar]
- Flower extracts and their essential oils as potential antimicrobial agents. Compr. Rev. Food Sci. Food Saf.. 2012;11(1):34-55.
- [Google Scholar]
- Effect of extraction solvents on the phenolic compounds and antioxidant activities of bungakantan (Etlingera elatior Jack.) inflorescence. J. Food Compos. Anal.. 2011;24(4–5):615-619.
- [Google Scholar]
- Antioxidant effect and active components of litchi (Litchi chinensis Sonn.) flower. Food Chem. Toxicol.. 2012;50(9):3056-3061.
- [Google Scholar]







