Translate this page into:
Integrated physiological and transcriptome analyses of the effects of water-soluble amino acid fertilizer on plant growth
⁎Corresponding authors. mingfeng618@163.com (Mingfeng Yang), liupeng2003@sdau.edu.cn (Peng Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Tobacco is a key component of China’s economy, serving as a major cash crop. With traditional fertilizers reaching their maximum potential in promoting tobacco growth, the exploration of new fertilizers has emerged as a viable solution for advancing experimental sustainable development. Spraying foliar fertilizer is a key measure to improve tobacco yield and quality. This study employed a combination of field and pot experiments to investigate the effects of applying water-soluble amino acid fertilizers on the growth, development, and quality of tobacco. The results of transcriptome analysis and physiological index measurements indicate that the application of water-soluble amino acid fertilizer can enhance the area of tobacco leaves, promote photosynthesis, and improve the chemical composition of the leaves. This research determined that the optimal concentration for spraying water-soluble amino acid fertilizer is diluted 500 times. Transcriptome analysis identified a total of 6,489 differentially expressed genes (DEGs), including 3,843 genes that were up-regulated and 2,646 genes that were down-regulated. Gene Ontology (GO) analysis demonstrated that these DEGs were significantly associated with processes including cell recognition, photosynthesis, thylakoid components, calcium ion binding, and carbohydrate binding. Additionally, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis emphasized that the DEGs were largely found in pathways related to photosynthesis-antenna proteins, interactions between plants and pathogens, photosynthesis itself, phenylpropanoid biosynthesis, and plant hormone signaling. Further research revealed that a significant number of genes involved in the auxin, gibberellin, salicylic acid, and jasmonic acid signal transduction pathways exhibited varying expression patterns following the application of water-soluble amino acid fertilizers. Additionally, the expression levels of bZIP, MYB, WRKY, bHLH, and AP2/ERF transcription factors exhibited significant variations following the application of water-soluble amino acid fertilizer. These results analyze the mechanisms of action of water-soluble amino acid fertilizers, offering new effective strategies to enhance both the yield and quality of tobacco.
Keywords
Fertilizer
Plant hormone
Tobacco
Transcriptome
1 Introduction
Flue-cured tobacco (Nicotiana tabacum L.) is a crucial component of China's economy and plays a vital role in the cigarette manufacturing sector. As the tobacco market continues to evolve, there is a growing demand for higher quality in tobacco production. Consequently, it is imperative to focus on enhancing the quality of flue-cured tobacco throughout the cultivation process.
Tobacco quality is regulated by various agronomic measures, with fertilization being a crucial factor in the production of high-quality leaves (Lisuma et al. 2023). However, due to variations in soil nutrient conditions and the uptake patterns of flue-cured tobacco, relying solely on a single application of chemical fertilizer is insufficient to maximize economic benefits and enhance tobacco quality. Consequently, the use of foliar fertilizer spraying has emerged as an effective strategy to supplement and balance the diverse nutrients required during growth (Tang et al. 2020). Foliar fertilizer application is a rapid, effective, and precise method of fertilization that can be employed alongside soil fertilization to promote plant development (Ishfaq et al. 2022). This approach serves as an effective strategy for improving soil conditions and crop quality, particularly in scenarios where soil nutrient availability is limited and the rate of nutrient loss from the soil is elevated (de Moura et al. 2023). The application of a 0.2 % zinc fertilizer to the foliage can increase the levels of zinc and magnesium, enhance photochemical characteristics, stimulate leaf development, and improve the photosynthetic capacity in sugar beet plants (Zhao et al. 2024). Additionally, applying potash to the foliage improves the nutritional profile of wheat by increasing the levels of micronutrients and reducing phytate concentrations. Furthermore, it enhances flour quality for processing by raising the amount of high-molecular-weight glutenin subunits (HMW-GS) (Gu et al. 2023). The application of molybdenum through foliar spraying elevates the expression levels of CBF/DREB genes, resulting in higher levels of phenolic compounds and free proline. This process assists plants in mitigating the toxic effects associated with elevated cadmium levels (Ali et al. 2023). Amino acid foliar fertilizer, also referred to as nutritional health care fertilizer, is composed of amino acids and various nutrients (Souri, 2016). The application of foliar fertilizers containing amino acids on crops presents several advantages. Certain amino acids serve as growth enhancers, facilitating the uptake of carbon dioxide during photosynthesis. Furthermore, the absorption of amino acid foliar fertilizer by plants results in increased chlorophyll levels, which enhances metabolic activities and improves the photosynthetic efficiency of the leaves. This, in turn, positively influences both the yield of crops and the quality of the harvested produce (Luo et al. 2023). The utilization of amino acids not only increased the quantity but also enhanced the quality of secondary metabolites found in spinach, such as flavonoids and phenolics, while simultaneously promoting growth, improving yield traits, boosting nutrient uptake, and increasing antioxidant activities (Kausar et al. 2023). Research indicates that the foliar application of essential amino acids significantly improved the growth, biochemical properties, antioxidant capacity, and nutritional value of cabbage (Haghighi et al. 2022). However, the effects of amino acid foliar fertilizers on tobacco have yet to be explored.
Advancements in gene sequencing technology have established RNA-seq as a predominant approach in transcriptome studies due to its high throughput, sensitivity, and diverse utility (Cervantes-Pérez et al. 2022). Recently, there has been considerable growth in the field of transcriptomics, which aims to elucidate the molecular mechanisms associated with plant growth. A study conducted by Song et al. (2016) demonstrated that the application of organic fertilizers resulted in a significant increase in starch accumulation in tobacco leaves. Their transcriptomic analysis revealed that the expression levels of AGPase, SS, and SBE were higher in plants treated with organic fertilizer compared to those receiving chemical fertilizer. Notably, genes such as AGPS3, AGPSL, GBSS1, and SS1 are likely crucial for starch synthesis in leaf tissues. The study involved transcriptomic analyses of tobacco plants during the seedling and emergence phases, exposing them to both ambient and low-temperature conditions. They identified multiple genes related to the flowering process and found that the downregulation of NbXTH22 rendered the tobacco plants unresponsive to low-temperature cues, resulting in early flowering (Xu et al. 2022). Transcriptomics plays a vital role in identifying key genes that influence the growth and development of tobacco plants. Consequently, employing transcriptomic analysis is critical for exploring the molecular mechanisms associated with the growth and development of tobacco when utilizing a water-soluble amino acid fertilizer.
In recent years, the availability of various types of foliar fertilizers in China has steadily increased. However, factors such as crop variety, application concentration, and application methods often prevent the achievement of the expected fertilizer efficiency levels. Furthermore, the lack of understanding regarding the mechanisms of action of most foliar fertilizer products has resulted in the widespread issue of irrational fertilization, which has not significantly improved the quality of tobacco leaves. This research utilized a water-soluble amino acid fertilizer to establish a theoretical foundation for the development and application of an innovative foliar fertilizer aimed at enhancing tobacco growth. The study investigated the effects of this fertilizer on tobacco growth, photosynthesis, the quality of post-roasted tobacco, and the underlying mechanisms of its effects through both potting and field experiments.
2 Materials and methods
In this study, NC55, one of the key popularized tobacco varieties in Shandong Province, was selected as the material, and the organic water-soluble foliar fertilizer was provided by Shandong Aikang Bio-technology Co. According to the standard of Q/371523SDAK002-2023, and the dosage form was aqueous, with the content of potassium (as K2O) of 168.82 g/L, free amino acid content of 905.55 g/L, organic matter content of 291.09 g/L, total nitrogen content of 50.05 g/L.
2.1 Field experiment
The field study took place in Laiwu, located in Shandong Province (36°20′N, 117°83′E). The fundamental physical and chemical characteristics of the soil are detailed in Table 1. At the start of the peak growing season, foliar application of a water-soluble amino acid fertilizer was performed, targeting the leaves of tobacco plants on both surfaces. The spraying was strategically scheduled for a sunny afternoon, following a period of dry weather. Four distinct concentrations were applied: CK (treated with deionized water), T1 (treated with water-soluble amino acid fertilizer diluted at a ratio of 750:1), T2 (treated with water-soluble amino acid fertilizer at a 500:1 dilution), and T3 (treated with water-soluble amino acid fertilizer diluted to 375:1). Additional field management activities were executed in accordance with established cultivation protocols.
Parameters
Values
Soil pH
6.58
Total nitrogen (N) (mg kg−1)
93.41
Total phosphorus (P) (mg kg−1)
486.37
Total potassium (K) (mg kg−1)
208.66
Organic matter (g kg−1)
18.9
Sand
43.30 %
Silt
43.20 %
Clay
13.50 %
2.2 Pot experiments design
A pot cultivation experiment was carried out in the tobacco laboratory at Shandong Agricultural University, situated in Shandong Province, China, to investigate the effects of water-soluble amino acid fertilizer on tobacco growth.The floating nursery was used to cultivate the tobacco seedlings, and when the seedlings grew 4–5 leaves, the healthy seedlings with uniform size and developed root system were selected to transplant into pots with a diameter of 17 cm, and the pots were placed in-light incubator for cultivation. The seedlings were treated by spraying fertilizers (F) and deionized water (CK) after 7–10 days of seedling slowing down, and the concentration of sprayed fertilizers was 1500 times of liquid solution.
2.3 Agronomic traits
From each plot, five typical tobacco plants were chosen for tagging and labeling. In accordance with the “Tobacco Agronomic Traits Survey and Measurement Methods” (YC/T142-2010), a flexible measuring tape was utilized to assess the stem's circumference. Simultaneously, measurements for plant height, the maximum length of leaves, and the maximum width of leaves were also obtained using the tape. Furthermore, the leaf area was determined using the following formula: Leaf area = Leaf length × Leaf width × 0.6345.
2.4 Photosynthetic parameters
Data was gathered from 9:00 to 11:00 a.m. The central tobacco leaves were measured for net photosynthetic rate, transpiration rate, stomatal conductance, and intercellular CO2 concentration using a LI-6400 photosynthesizer (LI-COR, Lincoln, NE, USA). Furthermore, a SPAD-502 portable chlorophyll meter (Minolta, Ltd., Osaka, Japan) was utilized to evaluate the chlorophyll SPAD value.
2.5 Chemical quality analysis of post-roasting tobacco
A continuous flow analysis system was employed to assess the levels of nicotine, total sugars, reduced sugars, and the concentrations of potassium and chlorine, in accordance with the Tobacco Industry Standard (YC/T468-2013, YC/T159-2019, YC/T 217–2007, and YC/T 162–2011).
2.6 RNA extraction and transcriptome sequencing
RNA extraction and transcriptome sequencing methods were consistent with our previous studies (Liu et al. 2023). Total RNA was isolated from tobacco leaves, and a sequencing library was generated and sequenced on the NovaSeq 6000 platform after multiple steps including mRNA extraction, cDNA synthesis, fragmentation, adapter ligation, size selection, PCR enrichment, and purification.
2.7 Data analysis
Preliminary statistics and data processing were conducted using Microsoft Office Excel 2016; for further analysis of significant differences and correlation, SPSS software was employed; the processed data were visualized with Origin 2021.
3 Results
3.1 Effect of foliar spraying of water-soluble amino acid fertilizer on agronomic traits of tobacco
During the squaring stage, treatments T2 and T3 significantly increased plant height and leaf length compared to the control (CK), whereas T1 exhibited no significant changes. At the topping stage, the observed changes were consistent with those noted during the squaring stage. By the maturing stage, T2 resulted in significant increases in plant height (35.68 %), stem girth (6.17 %), leaf length (15.74 %), leaf width (14.62 %), and leaf area (32.43 %) when compared to CK. Similarly, T3 also produced significant increases in plant height (34.46 %), stem girth (8.82 %), leaf length (16.61 %), leaf width (20.16 %), and leaf area (49.15 %). In contrast, T1 showed no significant changes (Fig. 1). A similar outcome was observed in the pot experiment (Fig. 3).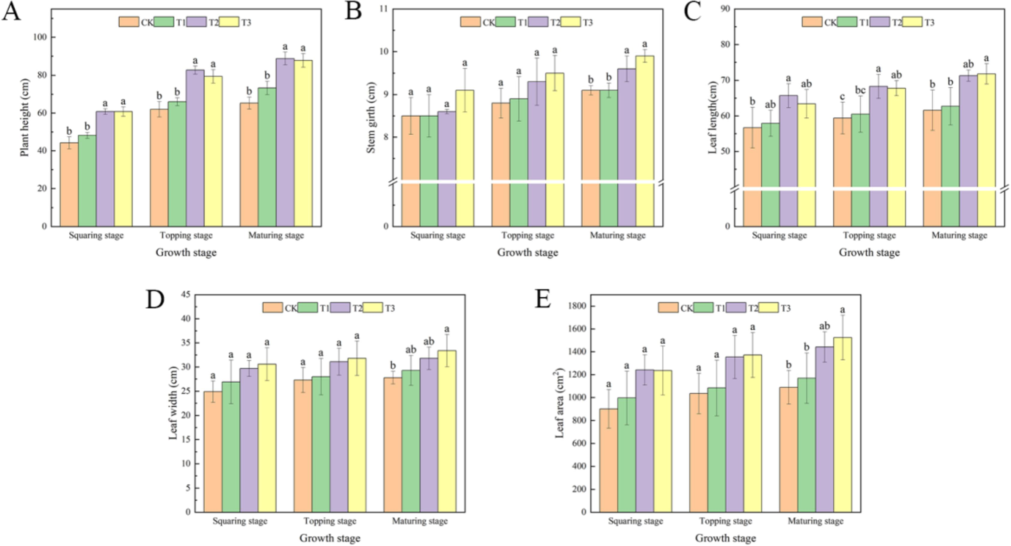
Effects of foliar spraying of different concentrations of water-soluble amino acid fertilizer on plant height (A), stem girth (B), leaf length (C), leaf width (D), leaf area (E).
3.2 Effect of foliar spraying of water-soluble amino acid fertilizer on photosynthetic characteristics of tobacco
During the squaring phase, there was a notable increase in net photosynthesis, transpiration rates, stomatal conductance, and SPAD measurements for both T2 and T3. Concurrently, these treatments significantly reduced the intercellular CO2 concentration compared to the control group (CK). In contrast, T1 did not exhibit any significant changes. At the topping stage, the trends were similar to those observed during the squaring stage, except that stomatal conductance did not show significant differences across any of the treatments. At the maturing stage, the trends for T2 and T3 remained consistent with those of the squaring stage; however, T1′s transpiration rate was significantly different from CK, while other parameters did not show significant differences from CK (Fig. 2).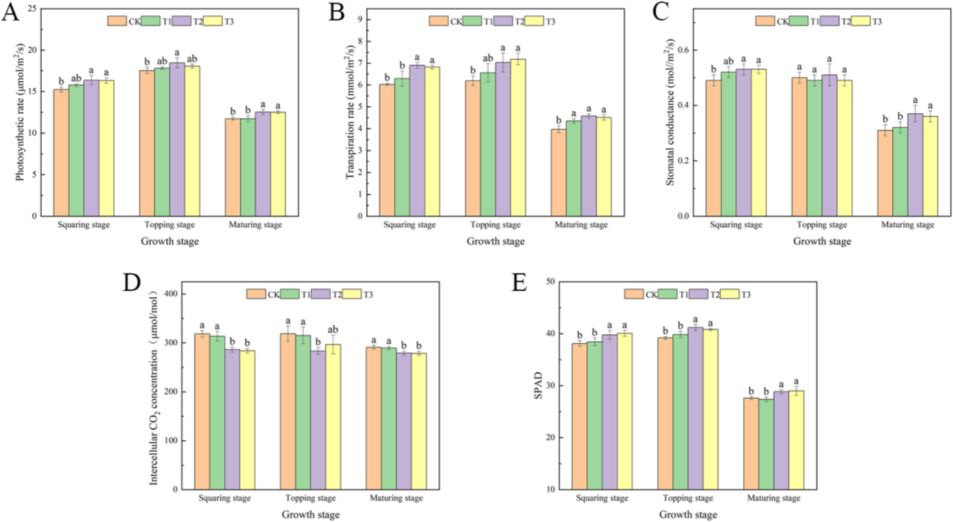
Effect of foliar spraying of different concentrations of water-soluble amino acid fertilizer on Photosynthetic rate(A), Transpiration rate (B), Stomatal conductance (C), Intercellular CO2 concentration (D), and SPAD (E).
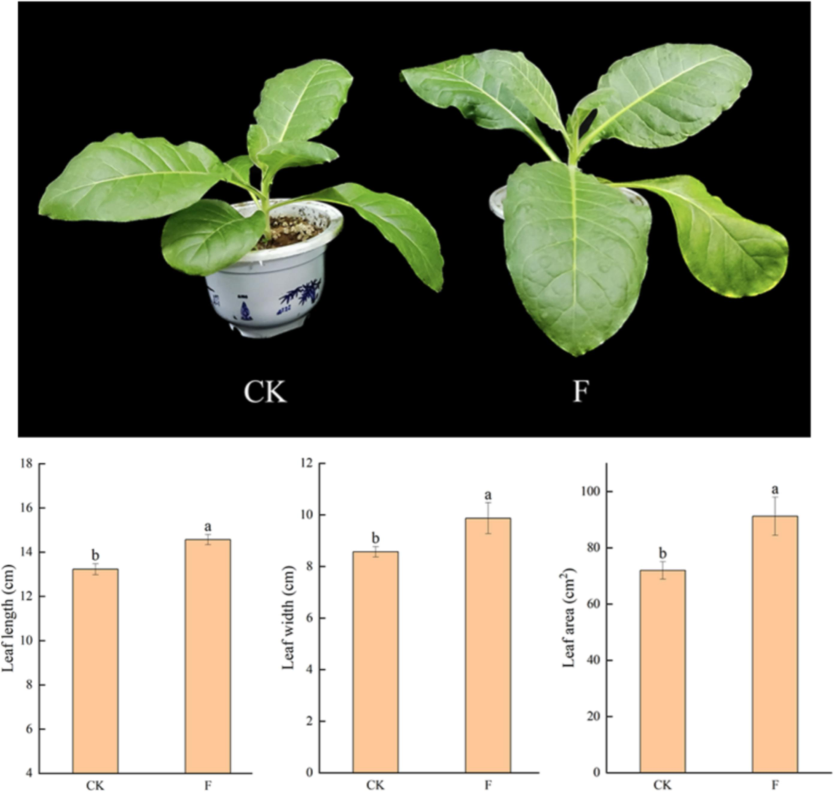
Effect of foliar spraying of water-soluble amino acid fertilizer on phenotype (A), leaf length (B), leaf width (C), and leaf area (D).
3.3 Effect of foliar spraying of water-soluble amino acid fertilizer on chemical composition
The application of water-soluble amino acid fertilizer resulted in enhancements to the chemical composition of tobacco leaves (Table 2). Compared to CK, all experimental treatments demonstrated increased levels of potassium, total sugars, and reducing sugars. Treatment T2 exhibited a significant reduction in chlorine content by 2.31 %, while treatments T1 and T3 showed no notable changes. The nicotine levels in T1, T2, and T3 were significantly decreased by 5.64 %, 7.14 %, and 6.77 %, respectively, with the most substantial reduction occurring in treatment T2. No significant differences were noted in the content of reducing or total sugars among the treatments. The ratio of reducing sugar to nicotine was significantly increased in treatments T1, T2, and T3, with T2 achieving the highest ratio, followed by treatment T3. The same alphabets in right side of the same list show no significance (P > 0.05), on the contrary, having significance (P < 0.05).
Treatment
Potassium/%
Chlorine/%
Nicotine/%
Total
sugar/%Reducing
sugar/%Reducing sugar/ Total sugar
Reducing sugar/
Nicotine
CK
1.77a
0.260a
2.66a
25.11a
22.03a
0.88a
8.27b
T1
1.80a
0.259a
2.51b
25.14a
22.32a
0.89a
8.89a
T2
1.83a
0.254b
2.47b
25.28a
22.58a
0.89a
9.3a
T3
1.79a
0.260a
2.48b
25.28a
22.34a
0.88a
9.02a
3.4 Differentially expressed genes (DEGs)
Principal component analysis (PCA) and Pearson's correlation coefficient (PCC) are effective tools for uncovering the degree of similarity among various samples. A closer PCA distance indicates greater similarity between samples. In this study, the relationships among different samples were assessed by analyzing the FPKM values corresponding to each gene across all samples, leading to the development of a three-dimensional scatterplot model based on PCA (Fig. 4A). The experimental results revealed that the three sets of replicates for the six samples in the CK treatment and the F treatment were distinctly separated into two groups. In contrast, the three sets of replicates for the two treatment groups were predominantly clustered together, thereby reinforcing the credibility of the test results. Fig. 4B illustrates that the coefficient of correlation (R2) among biological replicates ranged from 0.86 to 0.99, indicating strong reproducibility within the treatment sets. In leaf samples treated with a fertilizer composed of water-soluble amino acids, a total of 6,489 genes exhibited differential expression, with 3,843 genes being up-regulated and 2,646 down-regulated relative to the control group (Fig. 5).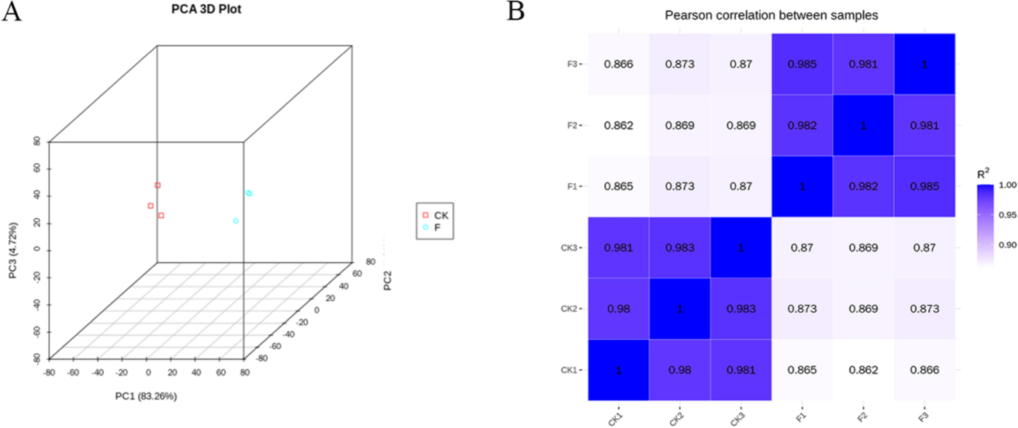
Control analysis of differentially expressed genes in water-soluble amino acid fertilizer treatment (F) versus deionized water treatment (CK). (A) PCA analysis of differentially expressed genes (DEMs) (B) PCC analysis.
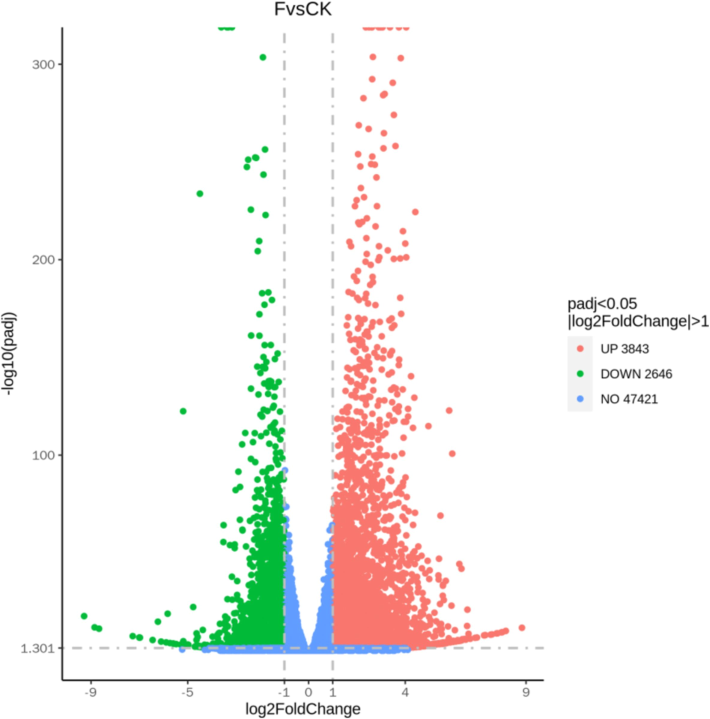
Volcano plot of DEGs under water-soluble amino acid fertilizer treatment in tobacco leaves. Red and green spots show the up-regulated and down-regulated DEGs, respectively.
3.5 KEGG pathway analysis and GO analysis
The analysis of KEGG metabolic pathways for functional enrichment revealed that 1,135 differentially expressed genes (DEGs) were significantly associated with 122 pathways. Notable pathways exhibiting significant enrichment included those related to antenna proteins in photosynthesis, interactions between plants and pathogens, the process of photosynthesis itself, the biosynthesis of phenylpropanoids, the metabolism of glyoxylate and dicarboxylate, the MAPK signaling pathway in plants, and pathways involved in hormone signal transduction (Fig. 6A). Gene Ontology (GO) analysis of the aforementioned DEGs indicated a substantial presence of both significantly up-regulated and down-regulated DEGs across various functional classifications in tobacco seedlings treated with a water-soluble amino acid fertilizer. An examination of the top 20 GO terms that were significantly enriched in the modules of up- and down-regulated genes revealed a focus on processes such as cell recognition, pollination, multicellular organism processes, photosynthesis, components of thylakoids, calcium ion binding, carbohydrate binding, and iron ion binding, among other biological functions (Fig. 6B).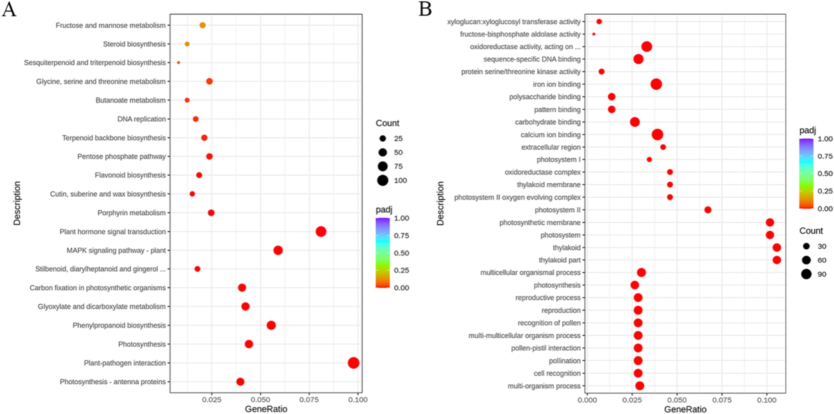
Identification of differentially expressed genes under water-soluble amino acid fertilizer treatment. (A) Kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis of differentially expressed genes. (B) Gene ontology (GO) enrichment analysis of the differentially expressed genes. The color of the dot represents P-value, and the size of the dot represents the number of DEGs mapped to the referent pathway.
3.6 Analysis of DGEs for plant hormone signal transduction
The influence of fertilizer containing water-soluble amino acids on the expression of genes associated with hormone signaling molecules was substantial. This fertilizer significantly promoted the levels of four types of phytohormones: auxin (IAA), gibberellin (GA), jasmonic acid (JA), and salicylic acid (SA) (Table 3, Fig. 7, and Fig. 8). Within the IAA signaling pathway, nine differentially expressed genes were identified, including the early growth hormone-responsive genes SAUR and AUX/IAA, the growth hormone-responsive factor ARF, and the gene encoding a growth hormone-inducible protein 15A. In the GA signaling pathway, three genes encoding the soluble GA receptor protein GID1 were discovered. In the JA signaling pathway, the gene for the transcriptional activator MYC2, along with the gene for the TIFY protein, as well as the jasmonate-amide synthetase JAR1 gene, were identified as exhibiting significantly different expression trends. Additionally, one gene involved in the SA signaling pathway was identified, which displayed significantly different expression trends induced by the water-soluble amino acid fertilizer.
Gene ID
Gene description
FPKM
up/ down
Fold change
CK
F
LOC107774052
auxin-responsive protein SAUR32
2.16
29.27
up
3.71
LOC107760702
auxin-responsive protein SAUR36
7.93
19.46
up
1.28
LOC107795057
auxin-responsive protein SAUR36
0.42
4.05
up
3.26
LOC107794246
auxin-responsive protein SAUR36
0.59
4.02
up
2.74
LOC107762212
auxin-responsive protein SAUR36
0.32
2.69
up
3.09
LOC107762212
auxin-responsive protein SAUR36
0.54
3.92
up
2.80
LOC107830067
auxin-responsive protein IAA4
5.25
10.67
up
1.02
LOC107822108
auxin response factor 19
3.07
6.28
up
1.04
LOC107794676
auxin-induced protein 15A
0.08
1.78
up
4.27
LOC107784593
transcription factor PIF3
11.78
4.83
down
−1.29
LOC107798341
gibberellin receptor GID1B
1.48
6.84
up
2.21
LOC107809791
gibberellin receptor GID1B
1.67
5.56
up
1.74
LOC107799015
gibberellin receptor GID1B
4.07
9.56
up
1.22
LOC107783750
protein TIFY 10A
6.04
26.23
up
2.10
LOC107815661
protein TIFY 10A
0.91
11.43
up
3.67
LOC107801671
protein TIFY 10A
0.42
7.54
up
4.16
LOC107772988
jasmonic acid-amido synthetase JAR1
2.98
8.00
up
1.41
LOC107767049
transcription factor MYC2
9.03
32.30
up
1.84
LOC107817883
transcription factor MYC2
21.06
42.66
up
1.02
LOC107792106
transcription factor TGA2
0.12
0.41
up
1.88
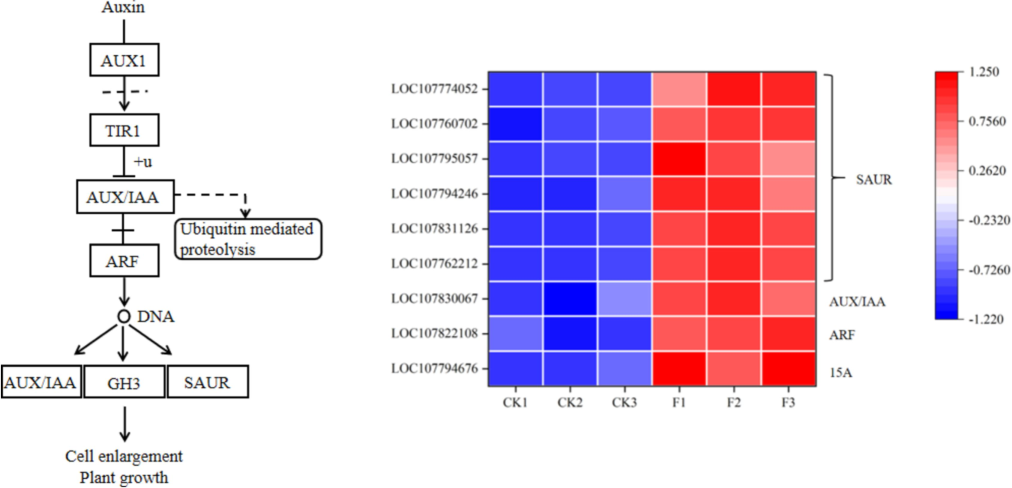
Identification of differentially expressed genes under water-soluble amino acid fertilizer treatment. Expression of genes related to IAA biosynthesis and signaling pathways. The expression levels of each DEG were averaged by the three biological samples and then normalized to a Z-score.
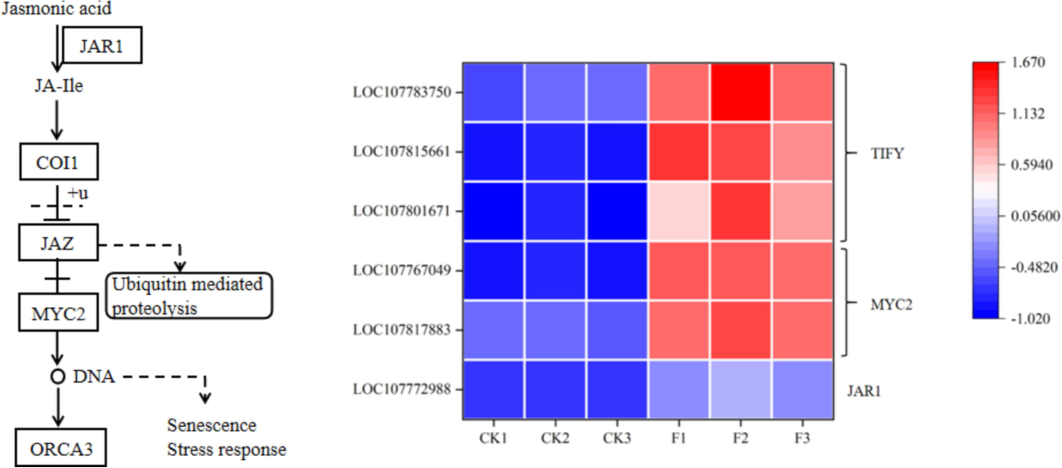
Identification of differentially expressed genes under water-soluble amino acid fertilizer treatment. Expression of genes related to JA biosynthesis and signaling pathways. The expression levels of each DEG were averaged by the three biological samples and then normalized to a Z-score.
3.7 Analysis of transcription factors
Application of water-soluble amino acid fertilizer significantly affected the expression of transcription factors (Fig. 9). Genes encoding the WRKY family and the AP2/ERF family were significantly up-regulated in response to water-soluble amino acid fertilizer induction. LOC107781073, LOC107782356 encoding bZIP family were significantly up-regulated, LOC107777300, LOC107800850, LOC107824387, LOC107827402 were significantly down-regulated. LOC107822143, LOC107810011, LOC107782691, LOC107810761, LOC107819472, and LOC107812738 encoding the MYB family were significantly up-regulated, LOC107813082, LOC107767321, LOC107807713, and LOC107812656 significantly downgraded. LOC107783787, LOC107808991 encoding the bHLH family were significantly up-regulated and LOC107769162, LOC107760028, LOC107780395, LOC107811315 were significantly down-regulated.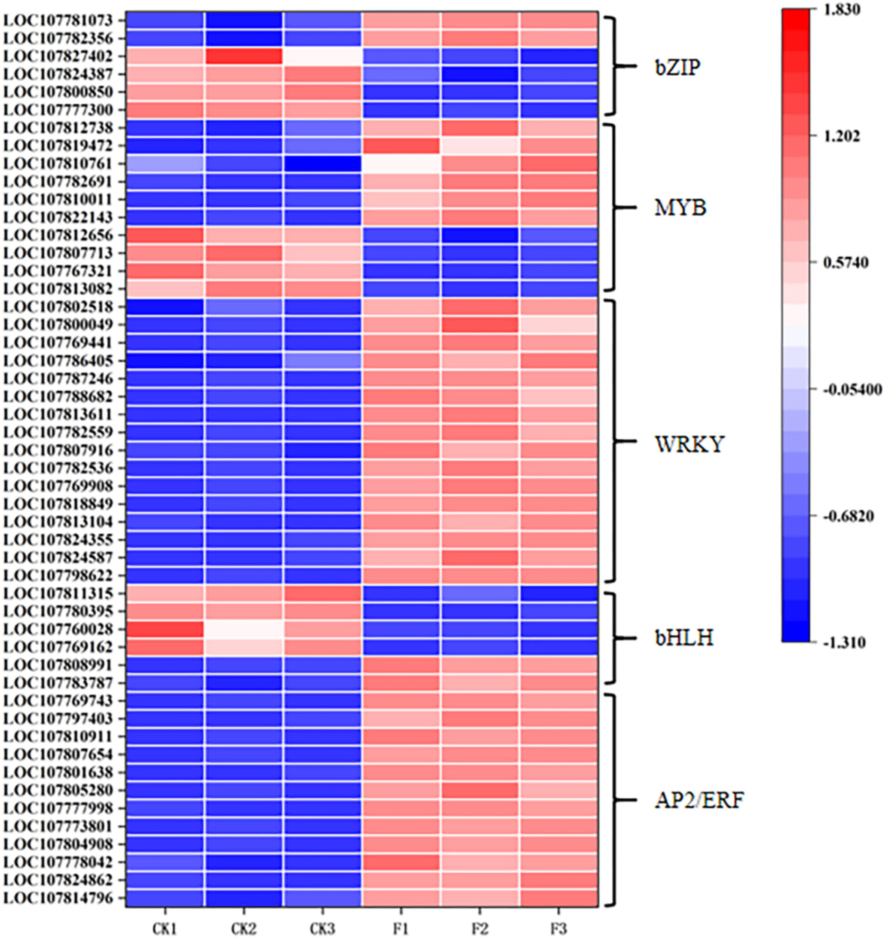
Expression of transcription factors AP2/ERF, bHLH, WRKY, MYB and bZIP under water-soluble amino acid fertilizer treatment. The expression levels of each DEG were averaged by the three biological samples and then normalized to a Z-score.
4 Discussion
4.1 Water-soluble amino acid fertilizer promoted the growth of tobacco
The traits of leaves, including their length, width, and surface area, influence not only the yield of tobacco but also the quality and market appeal of tobacco products (Ikram et al. 2022). The use of foliar fertilizers in the cultivation of flue-cured tobacco is a critical strategy for enhancing both the quality and yield of tobacco leaves. Research indicates that a 0.25 % solution of water-soluble foliar fertilizer significantly improved root activity, leaf area, dry weight of shoots, root length, and root mass in rapeseed plants (Wang et al. 2014). Additionally, studies have shown that the application of glycine to the leaves markedly increased leaf surface area, dry weight of stems, yield, and quality of cucumbers (Zargar Shooshtari et al. 2020). This research further demonstrated that the application of water-soluble amino acid fertilizer through foliar spraying significantly enhanced the length, width, and surface area of flue-cured tobacco leaves, thereby promoting the growth and development of the plants (Fig. 1). This approach has shown beneficial effects on the development of tobacco plants and improved the quality of tobacco production at various stages, including seedling, squaring, topping, and maturation (Wang et al. 2023).
4.2 Water-soluble amino acid fertilizer improved photosynthetic characteristics of tobacco
Enhancing the photosynthetic efficiency of tobacco is essential for improving both its yield and quality (Chen et al. 2023). The SPAD value of chlorophyll serves as a comparative metric indicating the chlorophyll levels in a crop and demonstrates a positive correlation with leaf photosynthetic activity (Castelli and Contillo 2009). Treatments T2 and T3 significantly improved the photosynthetic characteristics and chlorophyll SPAD values of flue-cured tobacco leaves throughout all reproductive phases (Fig. 2). Research indicates that amino acid fertilizers promote crop growth and enhance photosynthetic features when compared to conventional fertilizers. Furthermore, these fertilizers facilitate the synthesis and distribution of photosynthetic compounds throughout the entire plant (Masclaux-Daubresse et al. 2010). Studies have shown that the application of foliar sprays containing amino acids markedly boosts plant growth, resulting in increased levels of dry matter and chlorophyll in soybean crops (El-Aal 2018).
4.3 Water-soluble amino acid fertilizer improved tobacco leaf chemistry composition
In the tobacco quality assessment framework, the uniformity of inherent tobacco quality is a significant evaluation parameter that receives considerable attention from tobacco processing companies. The fundamental traits include total sugar, reducing sugar, total nitrogen, total alkali, chlorine, and potassium. Optimal levels of these components play a crucial role in influencing the characteristics of flue-cured tobacco smoke and the overall smoking experience (Hu et al., 2022). The morphological development of the tobacco plant greatly affects the chemical characteristics of the tobacco leaf, which in turn impacts the quality and availability of the leaf. Hu et al. (2023) demonstrated that applying amino acids through foliar spraying significantly increased the concentrations of glycine, methionine, and phenylalanine in potato tubers. Consequently, the concentrations of 2,3-dimethylpyrazine and 2-ethyl-3-methylpyrazine in the potatoes increased, leading to an improved aroma profile for baking in the tubers. This study suggested that the application of water-soluble amino acid fertilizer through foliar treatment might reduce nicotine levels while enhancing the ratio of reducing sugars to nicotine, thereby favorably influencing the inherent chemical composition of the upper leaves (Table 2).
4.4 Molecular mechanism of water-soluble amino acid fertilizer in regulating tobacco growth
Transcriptome sequencing technology can be applied to the analysis of plant metabolic pathways, refinement of genome annotation, and screening of specific functional genes. This research utilized Illumina high-throughput sequencing techniques to analyze the transcriptome of tobacco leaves, which were either treated with water-soluble amino acid fertilizer or left untreated. A comparison between the fertilizer-treated and CK groups revealed a total of 6,489 DEGs, comprising 3,843 that were up-regulated and 2,646 that were down-regulated (Fig. 5). GO analysis indicated that the DEGs were closely associated with processes related to cell recognition, pollination, multicellular organism processes, photosynthesis, thylakoid components, calcium ion binding, carbohydrate binding, and iron ion binding (Fig. 6B). Enrichment analysis of KEGG pathways showed that the DEGs were predominantly enriched in several pathways, including photosynthesis-antenna proteins, interactions with plant pathogens, photosynthesis, biosynthesis of phenylpropanoids, metabolism of glyoxylate and dicarboxylate, signaling through the MAPK pathway, and transduction of plant hormone signals (Fig. 6A). These pathways likely play a crucial role in regulating tobacco growth and development through the application of water-soluble amino acid fertilizers.
The growth and development of plants are significantly influenced by phytohormones, which are essential in overseeing leaf differentiation, growth, and overall development. These hormones are crucial for controlling numerous facets of plant growth, such as cell elongation, division, orientation, phototropism, as well as the development of primary and lateral roots, vascular tissues, root hairs, and flowers (Vanneste and Friml 2009). The regulation of hundreds of genes is influenced by growth hormone. The primary families of early response genes associated with this hormone include auxin/IAA, GH3, and SAUR. AUX/IAA functions as a transcriptional repressor consisting of four conserved domains. In conditions where growth hormone levels decrease, free AUX/IAA binds with ARF to create a heterodimer, subsequently preventing the expression of genes responsive to growth hormone. Conversely, as the concentration of growth hormone increases, it binds to the growth hormone receptor transporter inhibitory factor 1 (TIR1/AFB) protein. This binding leads to the ubiquitylation of AUX/IAA and subsequent degradation, releasing ARF and ultimately facilitating the expression of growth hormone-responsive genes (Tan et al. 2007). The SAUR gene family is the largest group of plant-specific growth hormone response factors, with SAUR being a key regulator of cell growth by responding to auxin. Overexpression of SAUR protein has been shown to significantly enhance cell growth (Ren and Gray 2015). In this study, 9 differentially expressed genes related to hormone signaling were identified, including early growth hormone-responsive genes such as SAUR and AUX/IAA, the growth hormone-responsive factor ARF, and the gene encoding a growth hormone-inducible protein 15A (Table 3 and Fig. 7). These findings suggest that the growth hormone signaling pathway in leaves is influenced by water-soluble amino acid fertilizer. GA is a crucial phytohormone that plays a significant role in plant growth and development processes, such as seed germination, as well as the development of stems, leaves, flowers, and fruits. Gibberellin influences these growth and developmental processes via biosynthesis and signaling pathways, leading to enhanced cell elongation, increased biomass, and stimulation of fruit formation (Ueguchi-Tanaka et al. 2007). In this experiment, three soluble GA receptor GID1 protein genes were identified in the gibberellin signaling pathway (Table 3). JA is a class of fatty acid derivatives that encompasses jasmonic acid and its free state derivatives, as well as the active precursor substance 12-oxo phytodienoic acid (OPDA). According to the findings of Wasternack et al. (2013), jasmonic acid (JA) and its derivatives are crucial for plant development as well as their reactions to both biotic and abiotic stresses. The activation of the JA pathway is controlled by a negative feedback loop that includes MYC2 and JAZ proteins. The TIFY family of transcription factors, which is exclusive to plants and previously known as ZIM, plays a key role in mediating interactions between JA and several other hormone signaling pathways including IAA, GA, ABA, SA, and ethylene (ETH) (Singh and Mukhopadhyay 2021). The TIFY family, prevalent in plants, holds significance in regulating stem, leaf, and flower development through its four subfamilies. Overexpression of OsTIFY11b in rice leads to longer leaves during the tasseling stage, higher levels of starch and sucrose in leaf sheaths and stems, and improved grain traits such as increased length, width, thickness, and a 9 %-21 % increase in grain weight (Hakata et al. 2012). In this experiment, the transcriptional activator MYC2 gene, the TIFY protein gene, and the JA synthase JAR1 gene were identified in the JA signaling pathway as showing significantly different expression trends. Salicylic acid (SA), chemically referred to as o-hydroxybenzoic acid (OHA), is a small, basic phenolic compound commonly present in plants and derived from cinnamic acid. Research indicates that SA serves as a crucial endogenous signaling molecule that can trigger allergic reactions and induce systemic acquired resistance within plant organisms (Peng et al. 2021). Moreover, the diverse physiological roles of salicylic acid (SA) in plants are clearly evident in its regulation of physiological processes, which encompass plant growth, development, maturation, and aging. Additionally, it enhances anti-stress responses, facilitating resistance to various stressors such as salinity, drought, low temperatures, ultraviolet radiation, heavy metal exposure, and others (Kaya et al. 2023).In this study, a specific gene within the salicylic acid signaling pathway was discovered to exhibit notably varied expression patterns triggered by an amino acid fertilizer that is water-soluble. These genes, which were expressed differently, working together influenced the growth of tobacco plants.
Transcription factors play crucial regulatory roles in various processes, including plant growth, development, and stress response. In our investigation, we discovered that the transcription factor families bZIP, MYB, WRKY, bHLH, and AP2/ERF exhibited significant differential expression when induced by water-soluble amino acid fertilizers. The AP2/ERF transcription factor family is prevalent throughout the plant kingdom. These regulatory proteins participate in regulating primary and secondary metabolism, as well as growth and development programs, and they respond to environmental stimuli (Licausi et al. 2013). Arabidopsis thaliana AtbZIP18 interacted with AtbZIP34, AtbZIP52 and AtbZIP61 in yeast and is involved together in pollen development (Gibalová et al. 2009). Research has indicated that OsMYB1R could serve as a promising gene for improving resistance in rice while maintaining yield levels (Zhang et al. 2024). In this study, the application of water-soluble amino acid fertilizer positively influenced the expression of several transcription factors, including bZIP, MYB, WRKY, bHLH, and AP2/ERF, while it had a negative impact on the expression of certain others, such as bZIP, MYB, and bHLH (Fig. 9). These findings indicate that the use of water-soluble amino acid fertilizer plays a role in directing the growth of roasted tobacco plants through the modulation of transcription factor expression.
5 Conclusion
Increasing the production and enhancing the quality of tobacco leaves are crucial for achieving rapid and sustainable development within the tobacco industry. This study integrates transcriptional and physiological experiments to demonstrate that the application of water-soluble amino acid fertilizer is an effective strategy for increasing tobacco leaf yield and improving leaf quality. It was determined that a dilution of 500 times represents the most effective spray concentration in field applications. Compared to the control group, the application of water-soluble amino acid fertilizer resulted in the up-regulation of most genes associated with the plant hormone signal transduction pathway in tobacco leaves, significantly improved photosynthetic gas exchange parameters, and increased tobacco leaf yield. Additionally, the application of the 500-fold diluted water-soluble amino acid fertilizer significantly reduced the chlorine and nicotine content in the tobacco leaves, thereby enhancing their chemical composition. These findings provide a theoretical basis for the use of novel water-soluble amino acid foliar fertilizers in flue-cured tobacco cultivation.
CRediT authorship contribution statement
Tong Li: Writing – original draft, Methodology, Investigation, Formal analysis. Xiuzhai Chen: Writing – original draft, Software, Methodology, Investigation, Conceptualization. Shoutao Cao: Writing – original draft, Formal analysis, Conceptualization. Zhongqing Liu: Resources, Conceptualization. Lei Tian: Software, Investigation, Formal analysis. Zhengxu Gao: Project administration, Formal analysis. Mingming Sun: Validation, Resources, Formal analysis. Hao Zong: Visualization, Software. Dequan Wang: Resources, Methodology, Data curation. Mohamed A. El-Sheikh: Writing – original draft, Conceptualization. Mingfeng Yang: Writing – review & editing, Supervision, Methodology, Conceptualization. Peng Liu: Writing – review & editing, Supervision, Funding acquisition.
Funding
This work was supported by the Demonstration and promotion of technology to suppress potassium reflux in upper tobacco leaves after topping (202210); The foundation of Taishan brand cigarette high-quality core raw material development and application in Shandong (202102004); Foundation of Modern Agricultural Technology Industry System of Shandong Province (SDAIT-25–07); Construction and Application of Quality Control Model for Tobacco Production and Acquisition in Shandong Province (202302001); Analysis of the characteristic styles and mellowing characteristics of American functional Roubaix tobaccos (202302004).
Acknowledgments
The authors would also like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2025R182) King Saud University, Riyadh, Saudi Arabia.
Author contribution
Conceptualization, Shoutao Cao, Mohamed A. El-Sheikh and Zhongqing Liu; Data curation, Zhongqing Liu and Dequan Wang; Formal analysis, Tong Li, Shoutao Cao, Lei Tian, Zhengxu Gao and Mingming Sun; Funding acquisition, Peng Liu; Investigation, Tong Li, Xiuzhai Chen, Shoutao Cao and Lei Tian; Methodology, Tong Li, Xiuzhai Chen, Dequan Wang and Mingfeng Yang; Project administration, Peng Liu; Resources, Zhongqing Liu, Zhengxu Gao, Mingming Sun, Hao Zong and Dequan Wang; Software, Xiuzhai Chen, Lei Tian, Zhengxu Gao and Hao Zong; Supervision, Mingfeng Yang and Peng Liu; Validation, Mingming Sun; Visualization, Hao Zong; Writing – original draft, Tong Li, Xiuzhai Chen, Shoutao Cao and Mohamed A. El-Sheikh; Writing – review & editing, Mingfeng Yang and Peng Liu.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Expression analysis of transcription factor (CBF/DREB) gene and biochemical response of Cannabis sativa to cadmium toxicity and foliar application of molybdenum. S. Afr. J. Bot.. 2023;162:160-170.
- [Google Scholar]
- Using a chlorophyll meter to evaluate the nitrogen leaf content in flue-cured tobacco (Nicotiana tabacum L.) Ital. J. Agron.. 2009;4:3-12.
- [Google Scholar]
- Challenges and perspectives in applying single nuclei RNA-seq technology in plant biology. Plant Sci.. 2022;325:111486
- [Google Scholar]
- Producing fast and active Rubisco in tobacco to enhance photosynthesis. Plant Cell. 2023;35:795-807.
- [Google Scholar]
- Humic foliar application as sustainable technology for improving the growth, yield, and abiotic stress protection of agricultural crops. A review. J. Saudi Soc. Agric. Sci.. 2023;22:493-513.
- [Google Scholar]
- Effect of foliar spray with lithovit and amino acids on growth, bioconstituents, anatomical and yield features of soybean plant. Annals of Agricultural Science, Moshtohor. 2018;56:187-202.
- [Google Scholar]
- Gibalová A, Reňák D, Matczuk K, Dupl’áková N, Cháb D, Twell D, Honys D (2009) AtbZIP34 is required for Arabidopsis pollen wall patterning and the control of several metabolic pathways in developing pollen. Plant Molecular Biology 70:581-601.
- Effects of foliar spraying of potassium fertilizer on the contents of microelement, phytic acid and HMW-GS in wheat flour. J. Cereal Sci.. 2023;110:103621
- [Google Scholar]
- Effect of exogenous amino acids application on the biochemical, antioxidant, and nutritional value of some leafy cabbage cultivars. Sci. Rep.. 2022;12:17720.
- [Google Scholar]
- Overexpression of a rice TIFY gene increases grain size through enhanced accumulation of carbohydrates in the stem. Biosci. Biotech. Bioch.. 2012;76:2129-2134.
- [Google Scholar]
- Study on the chemical compositions and microbial communities of cigar tobacco leaves fermented with exogenous additive. Sci. Rep.. 2022;12:19182.
- [Google Scholar]
- The Impact of the Foliar Application of Amino Acid Aqueous Fertilizer on the Flavor of Potato Tubers. Foods. 2023;12:3951.
- [Google Scholar]
- Identification of superior haplotypes and candidate genes for yield-related traits in tobacco (Nicotiana tabacum L.) using association mapping. Ind. Crop. Prod.. 2022;189:115886
- [Google Scholar]
- Foliar nutrition: Potential and challenges under multifaceted agriculture. Environ. Exp. Bot.. 2022;200:104909
- [Google Scholar]
- Modulation of growth and biochemical responses in spinach (Spinacia oleracea L.) through foliar application of some amino acids under drought conditions. S. Afr. J. Bot.. 2023;158:243-253.
- [Google Scholar]
- Salicylic acid interacts with other plant growth regulators and signal molecules in response to stressful environments in plants. Plant Physiol. Biochem.. 2023;196:431-443.
- [Google Scholar]
- APETALA 2/Ethylene Responsive Factor (AP 2/ERF) transcription factors: Mediators of stress responses and developmental programs. New Phytol.. 2013;199:639-649.
- [Google Scholar]
- Effect of Timing Fertilizer Application on leaf yield and quality of tobacco. Heliyon. 2023;9
- [Google Scholar]
- Effects of cadmium on transcription, physiology, and ultrastructure of two tobacco cultivars. Sci. Total Environ.. 2023;869:161751
- [Google Scholar]
- Foliar application of glutamate and phenylalanine induced regulation in yield, protein components, aroma, and metabolites in fragrant rice. Eur. J. Agron.. 2023;149:126899
- [Google Scholar]
- Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann. Bot.. 2010;105:1141-1157.
- [Google Scholar]
- Salicylic acid: biosynthesis and signaling. Annu. Rev. Plant Biol.. 2021;72:761-791.
- [Google Scholar]
- SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol. Plant. 2015;8:1153-1164.
- [Google Scholar]
- Comprehensive molecular dissection of TIFY Transcription factors reveal their dynamic responses to biotic and abiotic stress in wheat (Triticum aestivum L.) Sci. Rep.. 2021;11:9739.
- [Google Scholar]
- Effects of organic fertilizer applications on starch changes in tobacco (Nicotiana tabacum L.) leaves during maturation. Soil Sci. Plant Nutr.. 2016;62(2):173-179.
- [Google Scholar]
- Aminochelate fertilizers: the new approach to the old problem; a review. Open Agric.. 2016;1:118-123.
- Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640-645.
- [Google Scholar]
- Climatic factors determine the yield and quality of Honghe flue-cured tobacco. Sci. Rep.. 2020;10:19868.
- [Google Scholar]
- Gibberellin receptor and its role in gibberellin signaling in plants. Annu Rev Plant Biol. 2007;58:183-198.
- [Google Scholar]
- Amino acid fertilizer strengthens its effect on crop yield and quality by recruiting beneficial rhizosphere microbes. J. Sci. Food Agric.. 2023;103:5970-5980.
- [Google Scholar]
- Production of a water-soluble fertilizer containing amino acids by solid-state fermentation of soybean meal and evaluation of its efficacy on the rapeseed growth. J. Biotechnol.. 2014;187:34-42.
- [Google Scholar]
- Transcriptomic insights into the regulatory networks of chilling-induced early flower in tobacco (Nicotiana tabacum L.) J. Plant Interact.. 2022;17:496-506.
- [Google Scholar]
- Glycine mitigates fertilizer requirements of agricultural crops: case study with cucumber as a high fertilizer demanding crop. Chem. Biol. Technol. Agric.. 2020;7:1-10.
- [Google Scholar]
- Single-repeat MYB transcription factor, OsMYB1R, enhanced phytoalexin sakuranetin accumulation and Magnaporthe oryzae resistance. Curr. Plant Biol.. 2024;38:100351
- [Google Scholar]
- Foliar zinc spraying improves assimilative capacity of sugar beet leaves by promoting magnesium and calcium uptake and enhancing photochemical performance. Plant Physiol. Biochem.. 2024;206:108277
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103504.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







