Translate this page into:
Reno-protective potential of poncirin against polyethylene microplastics instigated kidney damage in rats via regulating Nrf-2/Keap-1 pathway
⁎Corresponding author. asmaashraf@gcuf.edu.pk (Asma Ashraf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Polyethylene microplastics (PEMPs) are noxious environmental pollutants that are documented to cause organ damage including the kidneys. Poncirin (PON) is a naturally occurring flavonoid which demonstrates a wide range of pharmacological properties. This experiment was conducted to evaluate the palliative potential of PON against PEMPs induced renal toxicity by examining a range of biochemical and physiological parameters. Twenty-four male albino rats were randomly apportioned into four distinct groups including the control, PEMPs (1.5 mgkg−1), PEMPs (1.5 mgkg−1) + PON (20 mgkg−1) and only PON (20 mgkg−1). Our results displayed that PEMPs intoxication escalated the levels of urea, KIM-1, creatinine and NGAL while reducing the creatinine clearance level. Besides reduction in the activities of GPx, GST, HO-1, CAT, GSR & upsurge in the levels of MDA and ROS were detected in PEMPs group. Conversely, the levels of inflammatory markers including COX-2, IL-6, IL-1β, NF-kB and TNF-α were augmented following the PEMPs intoxication. Besides, the results of the current research demonstrated that the expressions of Bax and caspase-3 were esclated whereas the Bcl-2 expression was lowered from its standard value due to PEMPs provision. However, PON treatment significantly restored the PEMPs-induced aforementioned impairments. Therefore, PON could be used as a therapeutic compound to ameliorate PEMPs-induced kidney impairments in rats, possibly due to its tremendous pharmacotherapeutic potential.
Keywords
Poncirin
Polyethylene microplastics
Renal toxicity
Inflammatory
Oxidative stress
1 Introduction
Since decades, the production of plastic products is increasing drastically owing to their household as well as industrial usage (Geyer et al., 2017). It is estimated that on an average 350–400 Mn tonne of non-biodegradable plastics are produced every year in the form of glass, paper, wood and metal (Jambeck et al., 2015). The decomposition of polymers that are plastics in nature results in the formation of various sorts of microplastics (MPs) having diameters less than 5 mm (Ali et al., 2021, Santacruz-Juárez et al., 2021). According to Senathirajah et al. (2021), humans are exposed to 0.1–5 g of microplastics (MPs) each week through inhalation, ingestion, and dermal contact. This exposure occurs as MPs are present in the air we breathe, the food and water we consume, and through direct contact with our skin (Conti et al., 2020).
Numerous investigations have demonstrated that due to their inability to degrade and miniscule sizes, MPs can traverse the body and become lodged in tissues resulting in organ obstruction as well as inflammation (Jin et al., 2021). Naik et al., (2019) elucidated that Previous studies have elucidated that plastic particles can carry and transmit human infections (Naik et al., 2019). Research has shown that PEMPs accumulate in the gonads and exert substantial effects on fertility (Teng et al., 2021). Furthermore, PEMPs exposure instigated gill injury by escalating the body’s OS levels in carp species (Cao et al., 2023). Lee et al. (2022) elucidated that oral administration of PEMPs induces lung injury in rats.
Natural compounds are widely used as adjuvant therapy to treat various disorders (Aboubakr et al., 2023). In recent years, numerous investigations have documented the therapeutic advantages of natural compounds against contemporary disorders. However, flavonoids gained global attention owing to their biological potential (Cao et al., 2022). Poncirin (PON) is a plant-based flavone which demonstrates as anti-tumor, anti-colitic, & anti-Alizheimer properties (Afridi et al., 2019). The study sought to evaluate the effectiveness of PON in mitigating kidney damage induced by PEMPs in albino rats.
2 Materials and methods
2.1 Chemicals
PEMPs (CAS No. 9002-88-4, Purity: HPLC < 98 %) and PON (CAS No. 14941-08-3, Purity: HPLC < 98 %) were acquired from Sigma-Aldrich, Germany.
2.2 Animals
Twenty-four rats were accommodated at Animal Research Station of University of Agriculture Faisalabad, Pakistan. The rats were provided optimum laboratory conditions including 24 ± 1 °C temperature, 12 h of light and dark period as well as 60–65 % humidity. The animals were provided standard rats feed and tap water. Ethical guidelines of institutional Ethical Committee in line with the guidelines of EU Directive 2010/63/EU for animal experiments were followed.
2.3 Experimental layout
Twenty-four albino rats were apportioned into the following groups i.e., Control, PEMPs 1.5 mgkg−1, PEMPs 1.5 mgkg−1 + PON 20 mgkg−1 and only PON 20 mgkg−1 administrated group. The dose of PEMPs was selected according to the previous investigation of Ijaz et al. (2023a) while the dose of PON was selected as per the previous experiment of Kang and Kim (2016). The doses were administrated for 28 days via oral gavage as per the previous investigation of Hayat et al. (2024). The rats were given ketamine and xylazine and decapitated. Kidneys were removed from the abdominal cavity. The right kidney was kept in 10 % formaldehyde for histology assessment while the left kidney was packed in zipper bag and stored at −20 °C for biochemical assessment.
2.4 RNA isolation and qRT-PCR
Nrf2, Keep1, apoptotic & antioxidative genes were measured with the help of qRT-PCR. RNA separation was undertaken with the help of reagent (TRIzol) and then converted into cDNA. Livak and Schmittgen (2001) protocol were followed to evaluate the variations in the expressions of abovementioned genes against each treatment by using 2-ΔΔCT & β-actin as an internal control. The primer of selected genes are mention in Table 1 as previously reported by Ijaz et al. (2023b).
Gene
Primers 5′ −> 3′
Accession number
Nrf2
F: ACCTTGAACACAGATTTCGGTG
NM_031789.1
R: TGTGTTCAGTGAAATGCCGGA
Keap-1
F: ACCGAACCTTCAGTTACACACT
NM_057152.1
R: ACCACTTTGTGGGCCATGAA
CAT
F: TGCAGATGTGAAGCGCTTCAA
NM_012520.2
R: TGGGAGTTGTACTGGTCCAGAA
SOD
F: AGGAGAAACTGACAGCTGTGTCT
NM_017051.2
R: AAGATAGTAAGCGTGCTCCCAC
GPx
F: TGCTCATTGAGAATGTCGCGTC
NM_030826.4
R: ACCATTCACCTCGCACTTCTCA
GSR
F: ACCAAGTCCCACATCGAAGTC
NM_053906.2
R: ATCACTGGTTATCCCCAGGCT
GST
F: TCGACATGTATGCAGAAGGAGT
NM_031509.2
R: CTAGGTAAACATCAGCCCTGCT
HO-1
F: AGGCTTTAAGCTGGTGATGGC
NM_012580.2
R: ACGCTTTACGTAGTGCTGTGT
Bax
F: GGCCTTTTTGCTACAGGGTT
NM_017059.2
R: AGCTCCATGTTGTTGTCCAG
Bcl-2
F: ACAACATCGCTCTGTGGAT
NM_016993.1
R: TCAGAGACAGCCAGGAGAA
Caspase-3
F: ATCCATGGAAGCAAGTCGAT
NM_012922.2
R: CCTTTTGCTGTGATCTTCCT
β-actin
F: TACAGCTTCACCACCACAGC
NM_031144
R: GGAACCGCTCATTGCCGATA
2.5 Determination of biochemical profile
SOD & CAT’s activity was quantified following Aebi (1984) & Sun et al. (1988)’s approaches. Rotruck et al. (1973) and Jolow et al. (1974)’s methodology was applied to quantify GPx & GSH. Carlberg and Mannervik (1975) along with Younas et al. (2018)’s technique was undertaken to quantify GSR along with GST. Hayashi et al. (2007) and Placer et al. (1966) quantification approach was undertaken to quantify ROS & MDA.
2.6 Determination of renal injury biomarkers
The levels of renal injury biomarkers including creatinine (ab285275), creatinine clearance (ab285275), urea (ab83362), KIM-1 (ab223858) and NGAL (ab119602) were determined by using standard ELISA kits in accordance with the instruction of County Antrim, UK.
2.7 Inflammatory markers analysis
The determination of NF-κB (ab176648), IL-6 (ab234570), IL-1β (ab255730), COX-2 (ab210574) and TNF-α (ab100785) was undertaken using standard kits (ELISA) manufactured by Abcam, International, USA.
2.8 Statistic evaluation
The Data were depicted as Mean ± SE. One way ANOVA followed by Tukey’s test was applied to compare the groups. The statistical calculations were performed by using Minitab (V17) while the normality of data was checked by Shapiro-Wilk test. The level of significance was adjusted as p < 0.05.
3 3.Results
3.1 Results of PEMPs and PON on Nrf2/keap1 pathway
Exposure to PEMPs led to a significant (p < 0.05) increase in Keap1, while concurrently causing a marked reduction in Nrf-2 and antioxidant gene expressions as compared to the control group. However, the administration of both PEMPs and PON together effectively (p < 0.05) upregulated the gene expression of antioxidant genes and Nrf-2 while reducing the gene expression of Keap-1. Nonetheless, the expressions of the biomarkers in the PON-dosed group remained close to those in the control group, as shown in Fig. 1.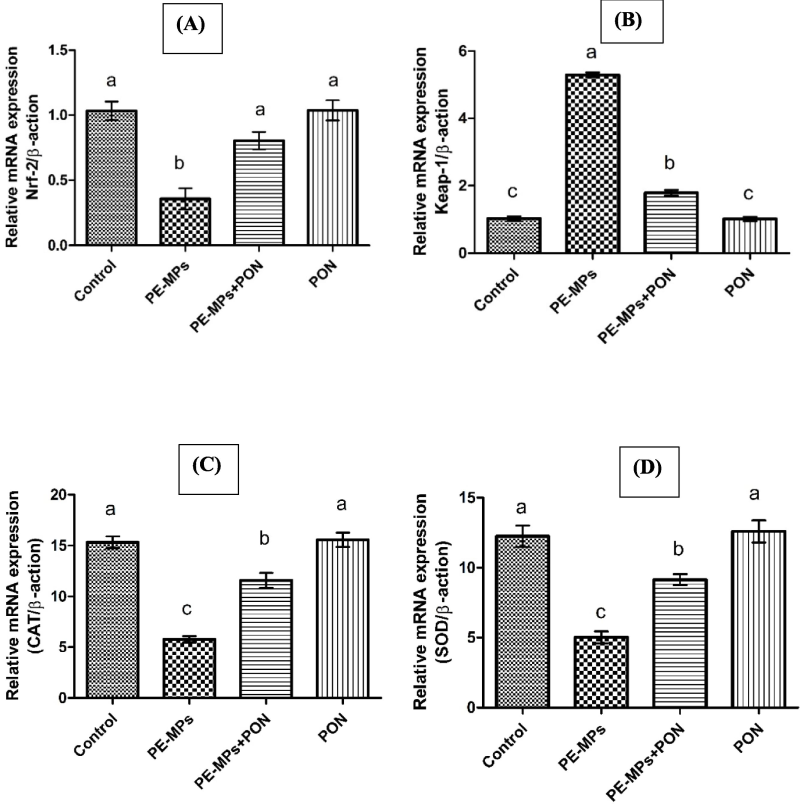
This figure visualizes the bar graph of different genes and their comparative analysis with other groups. (A) Nrf-2 (B) Keap-1 (C) CAT (D) SOD (E) GPx (F) GSR (G) GST (H) HO-1. Dissimilar superscripts show significant difference among different groups (p < 0.05).
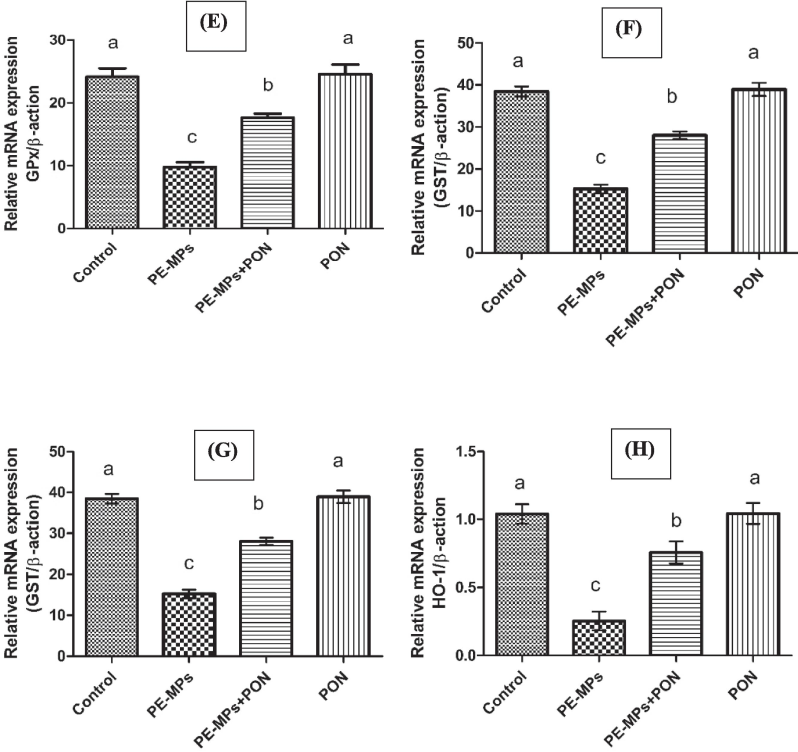
This figure visualizes the bar graph of different genes and their comparative analysis with other groups. (A) Nrf-2 (B) Keap-1 (C) CAT (D) SOD (E) GPx (F) GSR (G) GST (H) HO-1. Dissimilar superscripts show significant difference among different groups (p < 0.05).
3.2 Results of PEMPs and PON on biochemical parameters
Exposure to PEMPs led to a significant (p < 0.05) increase in MDA and ROS, while reducing the activities of CAT, SOD, GPx, GSR and GSH. However, the administration of both PEMPs and PON together effectively (p < 0.05) escalated the activities of antioxidant enzymes while reducing the levels of ROS and MDA. Nonetheless, insignificant changes were observed among the values of these parameters in the control and PON alone treated group, as shown in Table 2. Dissimilar superscripts show significant difference among different groups (p < 0.05).
Parameters
Groups
Control
PEMPs
PEMPs + PON
PON
CAT (U/mg protein)
15.33 ± 0.98a
5.78 ± 0.53c
11.59 ± 1.27b
15.57 ± 1.21a
SOD (U/mg protein)
12.25 ± 1.31a
5.01 ± 0.73c
9.14 ± 0.67b
12.60 ± 1.37a
GPx (U/mg protein)
24.14 ± 2.32a
9.78 ± 1.33c
17.66 ± 1.09b
24.56 ± 2.62a
GSH (U/mg protein)
18.95 ± 1.39a
7.17 ± 0.41c
14.52 ± 0.95b
19.59 ± 1.75a
GSR (nm NADPH oxidized/ min/mg tissue
9.38 ± 1.21ab
3.10 ± 0.48c
7.27 ± 0.56b
9.68 ± 1.11a
GST (U/mg protein)
38.48 ± 2.01a
15.25 ± 1.79b
28.02 ± 1.54c
38.95 ± 2.66a
HO-1 (U/mg protein)
348.31 ± 16.03 a
50.33 ± 7.89c
184.46 ± 12.84b
353.5 ± 18.3a
ROS (U/mg tissue)
1.10 ± 0.28c
7.82 ± 0.86a
2.63 ± 0.31b
0.92 ± 0.43c
MDA (nmol/mg protein)
0.55 ± 0.31b
5.58 ± 0.48a
1.39 ± 0.36b
0.45 ± 0.40b
3.3 Results of PEMPs and PON administration on renal parameters
PEMPs intoxication substantially (p < 0.05) increased the levels of urea, KIM-1, creatinine, NGAL, while reducing the levels of creatinine clearance as compared to the control group. However, the administration of both PEMPs and PON together notably (p < 0.05) reduced the levels of KIM-1, urea, creatinine and NGAL while enhancing the levels of creatinine clearance. The levels of these biomarkers remained closed to each other in the control and PON alone treated group as illustrated in Table 3. Dissimilar superscripts show significant difference among different groups (p < 0.05).
Parameters
Groups
Control
PEMPs
PEMPs + PON
PON
Urea (mg/dl)
18.60 ± 1.95c
63.43 ± 4.10a
27.89 ± 1.50b
17.75 ± 1.63c
Creatinine (mg/dl)
1.01 ± 0.23c
7.04 ± 0.51a
2.71 ± 0.30b
0.97 ± 0.26c
Creatinine Clearance (ml/min)
1.67 ± 0.16a
0.28 ± 0.28b
1.37 ± 0.24a
1.76 ± 0.19a
KIM-1 (mg/ml)
0.23 ± 0.21c
3.40 ± 0.46a
1.18 ± 0.17b
0.17 ± 0.15c
NGAL (ng/day)
0.48 ± 0.29c
4.32 ± 0.40a
1.70 ± 0.56b
0.43 ± 0.30c
3.4 Results of PEMPs and PON administration on parameters of inflammation
PEMPs administration markedly (p < 0.05) upregulated the levels of inflammatory markers including NF-κB, TNF-α, IL-1β, IL-6 and COX-2 as compared to the control group. Moreover, PEMPs + PON administration remarkably (p < 0.05) reduced the levels of abovementioned inflammatory cytokines. The levels of these markers were almost similar in PON alone and the control group as displayed in Table 4. Dissimilar superscripts show significant difference among different groups (p < 0.05).
Parameters
Groups
Control
PEMPs
PEMPs + PON
PON
NF-κB (ng/g tissue)
21.02 ± 1.95c
81.77 ± 2.56a
38.82 ± 3.17b
20.41 ± 1.90c
TNF-α (ng/g tissue)
10.00 ± 1.69c
30.86 ± 3.01a
18.23 ± 1.40b
9.89 ± 1.75c
IL-1β (ng/g tissue)
14.30 ± 1.77c
70.09 ± 1.49a
30.08 ± 2.20b
14.15 ± 1.85c
IL-6 (ng/g tissue)
3.73 ± 1.02c
47.28 ± 2.06a
9.83 ± 1.64b
3.54 ± 1.16c
COX-2 (ng/g tissue)
22.15 ± 1.49c
88.49 ± 2.76a
34.26 ± 1.98b
21.88 ± 1.33c
3.5 Impact of PEMPs and PON administration on apoptotic biomarkers
Exposure to PEMPs led to a significant (p < 0.05) increase in the expression of Bax and Caspase-3 while downregulating the expression of Bcl-2. However, the administration of both PEMPs and PON together notably (p < 0.05) promoted the gene expression of Bcl-2 while reducing the gene expression of Bax and Caspase-3. Nevertheless, the gene expression of these biomarkers had remained close to each other in the control and PON alone treated group as shown in Fig. 2.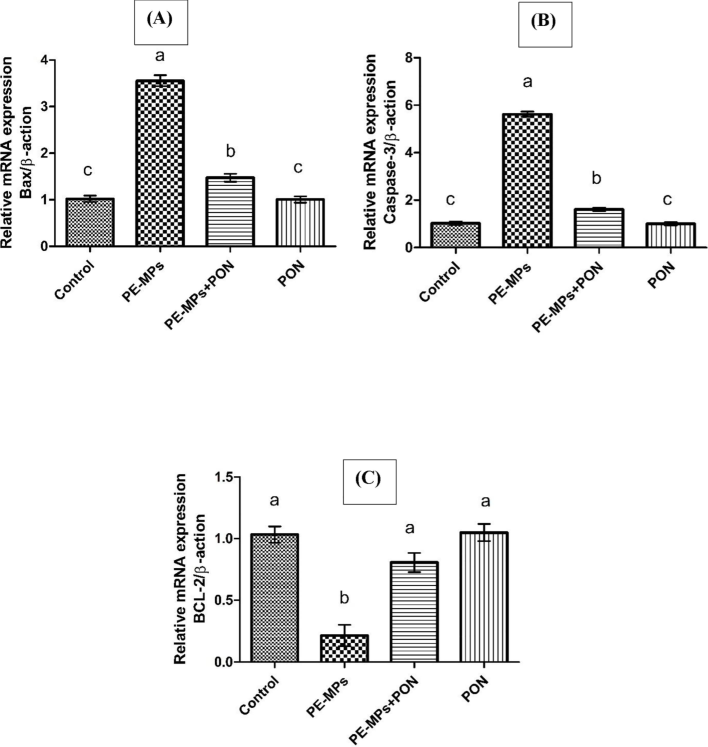
This figure visualizes the bar graph of different genes and their comparative analysis with other groups. (A) BAX (B) CASPASE-3 (C) BCL-2. Dissimilar superscripts show significant difference among different groups (p < 0.05).
4 Discussion
Concentration of plastics, particularly PEMPs is continuously increasing in the environment which poses an inevitable hazard to the health of humans as well as other animals (Geyer et al., 2017). Kidneys are essential organs which are responsible for eliminating metabolic waste from the body. Acute kidney injury is one of the several fatal diseases which is caused by escalated the intensity of oxidative burden in the tissues of renal system. Due to these findings, we have conducted our research to quantify the palliative power of PON to defend against PEMPs promoted renal impairment via regulating various biochemical and histological indices.
Nrf2 is a transcriptional factor for various cytoprotective genes whereas Keap1 is the negative regulator of Nrf2 (Sahu et al., 2020). The findings of our trial revealed that PEMPs provision reduced Nrf2 expression while increasing the transcription of keap1. The excessive generation of ROS dissociates the Nrf2-keap1 complex which subsequently degenerates Nrf2 owing to various conformational changes in keap1 domain. However, under normal cellular processes, Nrf2 enters the nucleus and binds with specific genomic factors to regulate the transcription of aforementioned cytoprotective genes to counteract the cellular oxidative stress (Huang et al., 2015). Nevertheless, co-administration of PEMPs + PON upregulated the expression of Nrf2 while downregulating the expression of keap1 owing to the electron donating potential of PON to counteract oxidative stress.
In the current investigation, PEMPs exposure reduced enzymatic activities of antioxidants while escalating the intensity of oxidants (ROS and MDA). A dynamic state among the concentrations of oxidative species and antioxidants triggers the cellular phenomena that is called oxidative stress. Various sorts of enzymes (antioxidants) perform an important action in countering the cellular burden of oxidants. SOD is the first & foremost enzyme which is involved in the detoxification processes (Saxena et al., 2022). Moreover, excessive production of free radicals decreases the activities of antioxidant enzymes which ultimately impairs the endogenous cellular defense system (Ahmad et al., 2023). Oxidative species in cell disrupts the cellular redox balance therefore considered as the major culprit underlying renal toxicity (González et al., 2022). However, the supplementation of PON escalated the activities of antioxidant enzymes while reducing the levels of ROS and MDA owing to its polyphenolic nature.
The efficiency of renal functions depends upon the rate of elimination of urea and creatinine (Sahu et al., 2020). Creatinine and urea are considered as primary excretory compounds in glomerular filtration (Higgins, 2016). Furthermore, excessive levels of urea can cause tissue injury, inappropriate excretion, and renal failure (Yousef et al., 2006). Any dysregulation in the function as well as various sorts of injury to renal tubules can cause a significant rise in creatinine concentration (Mansour and Mossa, 2010). Our results demonstrated that administration of PEMPs considerably promoted the levels of creatinine and urea while substantially reducing the levels of creatinine clearance. However, PEMPs + PON provision escalated the levels of creatinine clearance while downregulating the levels of urea and creatine in renal tissues.
KIM-1 and NGAL hold a significant importance in the quantification of acute renal failure. During the basic phases of renal toxicity, the concentrations of these biomarkers show a spectrum of variations to assess the stage of kidney damage (Song et al., 2019). Ichimura et al. (2008) elucidated that exposure to nephrotoxic substances escalated the levels of KIM-1 in proximal tubule of kidneys. Our investigation revealed that PEMPs exposure remarkably escalated the levels of KIM-1 and NGAL. It is documented that levels of KIM and NGAL increase after few hours of acute kidney injury owing to various etiologies such as transplant rejection or drug induced nephrotoxicity (Zhang et al., 2008). However, PON provision markedly subsided the levels of aforementioned kidney injury biomarkers which is credited to its nephroprotective potential.
This experiment demonstrated that the administration of PEMPs upregulated the transcription of Caspase-3 & Bax while subsiding the transcription of Bcl-2 gene. Cell death is a ubiquitously conserved phenomenon in both eukaryotic and prokaryotic cells which takes place in response to physical damage or various programmed pathways (Galluzzi et al., 2018). It is observed that reduced levels of Bcl-2 while escalated levels of Caspase-3 and Bax provokes cellular apoptosis. Besides, Bcl-2 blocks the liberation of cytochrome C from mitochondrial membrane thereby prevent the cell death. It is found that excessive generation of ROS suppress the intensity of Bcl-2 while elevating the intensity of Caspse-3 and Bax which ultimately triggers the mitochondrial apoptotic pathways (Herrera et al., 2001). However, PEMPs + PON provision prevents cellular apoptosis via regulating the expressions of aforementioned apoptotic markers.
This experimental trial indicated that PEMPs provision substantially increased the levels of inflammatory markers. It is reported that NF-κB stimulation modulates the activation of a wide range of pro-inflammatory parameters (Kandemir et al., 2018). NF-κB is an important transcriptional regulator which contributes to various biological processes and acts as intermediate hub for intracellular signal transduction (Takayanagi et al., 2000). Inflammation is a crucial mechanism underlying the onset of acute kidney injury therefore targeting NF-κB pathway may be an effective approach to ameliorate the inflammatory responses (Korrapati et al., 2012). Our findings revealed that supplementation of PON downregulated the levels of aforementioned inflammatory cytokines.
5 Conclusion
In conclusion, PEMPs exposure induced kidney impairments in rats by increasing the Nrf2, kidney injury markers, inflammatory and pro-apoptotic mediators as well as OS. Additionally, PEMPs reduced the renal anti-apoptotic biomarker’s level and antioxidative genes Nevertheless, PON supplementation restored all the impairments that were induced by PEMPS intoxication.
6 Limitation of study
It is indispensable to conduct this study on human to evaluate the protective potential of PON against PEMPs induced renal toxicity in human being.
CRediT authorship contribution statement
Muhammad Faisal Hayat: Writing – review & editing, Validation, Methodology, Investigation. Maryam Javed: Writing – original draft, Methodology, Investigation, Conceptualization. Rahat Andleeb: Validation, Formal analysis, Data curation. Asma Ashraf: Writing – original draft, Methodology, Investigation, Conceptualization. Huma Naz: Visualization, Validation, Formal analysis, Data curation. Mohammad Z. Ahmed: Writing – review & editing, Resources, Funding acquisition. Ayesha Ishtiaq: Visualization, Validation, Formal analysis, Data curation.
Acknowledgments
The authors are thankful to the Researchers Supporting Project number (RSPD2024R728), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Protective effects of N acetylcysteine and vitamin E against acrylamide-induced neurotoxicity in rats. Pak. Vet. J.. 2023;43(2):262-268.
- [Google Scholar]
- Anti-hyperalgesic properties of a flavanone derivative Poncirin in acute and chronic inflammatory pain models in mice. BMC Pharmacol. Toxicol.. 2019;20:1-16.
- [Google Scholar]
- Ameliorative Effects of Rhamnetin against Polystyrene Microplastics-induced Nephrotoxicity in Rats. Pak. Vet. J.. 2023;43:623-627.
- [Google Scholar]
- Micro-and nanoplastics in the environment: Occurrence, detection, characterization and toxicity – a critical review. J. Clean. Prod.. 2021;313:127863
- [Google Scholar]
- Effects of poncirin, a citrus flavonoid and its aglycone, isosakuranetin, on the gut microbial diversity and metabolomics in mice. Molecules. 2022;27:36-41.
- [Google Scholar]
- Polyethylene microplastics trigger cell apoptosis and inflammation via inducing oxidative stress and activation of the NLRP3 inflammasome in carp gills. Fish Shellfish Immunol.. 2023;132:108470
- [Google Scholar]
- Micro-and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ. Res.. 2020;187:109677.
- [Google Scholar]
- Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ.. 2018;25(3):486-541.
- [Google Scholar]
- Study of the dermal anti-inflammatory antioxidant and analgesic activity of Pinostrobin. Heliyon.. 2022;8:10413.
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol. Environ. Mutagen.. 2007;631:55-61.
- [Google Scholar]
- Ameliorative potential of eriocitrin against cadmium instigated hepatotoxicity in rats via regulating Nrf2/keap1 pathway. J. Trace Elem. Med Biol.. 2024;84:127445
- [Google Scholar]
- Activation of caspases occurs downstream from radical oxygen species production, Bcl-xL down-regulation, and early cytochrome C release in apoptosis induced by transforming growth factor β in rat fetal hepatocytes. Hepatology. 2001;34:548-556.
- [Google Scholar]
- Urea and creatinine concentration, the urea: creatinine ratio. Acute Care 2016:1-8.
- [Google Scholar]
- Alpinetin inhibits lipopolysaccharide-induced acute kidney injury in mice. Int. Immunopharmacol.. 2015;28:1003-1008.
- [Google Scholar]
- Kidney injury molecule–1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Invest.. 2008;118:1657-1668.
- [Google Scholar]
- Sciadopitysin attenuates paraquat induced renal toxicity by modulating Nrf-2/Keap-1 pathway in male albino rats. Asian J. Agric. Biol. 2023;10:2023110.
- [Google Scholar]
- Evaluation of possible attenuative role of chrysoeriol against polyethylene microplastics instigated testicular damage: a biochemical, spermatogenic and histological study. Food Chem. Toxicol.. 2023;180:114043
- [Google Scholar]
- Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater.. 2021;401:123430
- [Google Scholar]
- Therapeutic efficacy of zingerone against vancomycin-induced oxidative stress, inflammation, apoptosis and aquaporin 1 permeability in rat kidney. Biomed. Pharmacother.. 2018;105:981-991.
- [Google Scholar]
- Poncirin and its metabolite ponciretin attenuate colitis in mice by inhibiting LPS binding on TLR4 of macrophages and correcting Th17/Treg imbalance. J. Ethnopharmacol.. 2016;189:175-185.
- [Google Scholar]
- Recovery from glycerol-induced acute kidney injury is accelerated by suramin. J. Pharmacol. Exp. Ther.. 2012;341:126-136.
- [Google Scholar]
- Toxicity study and quantitative evaluation of polyethylene microplastics in ICR mice. Polymers. 2022;14:402.
- [Google Scholar]
- Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pestic. Biochem. Physiol.. 2010;96:14-23.
- [Google Scholar]
- Microplastics in ballast water as an emerging source and vector for harmful chemicals, antibiotics, metals, bacterial pathogens and HAB species: a potential risk to the marine environment and human health. Mar. Pollut. Bull.. 2019;149:110525
- [Google Scholar]
- Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem.. 1966;16:359-364.
- [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Sci. 1973;179:588-590.
- [Google Scholar]
- Naringenin mitigates antituberculosis drugs induced hepatic and renal injury in rats. J. Tradit. Complement. Med.. 2020;10:26-35.
- [Google Scholar]
- Fungal enzymes for the degradation of polyethylene: Molecular docking simulation and biodegradation pathway proposal. J. Hazard. Mater.. 2021;411:125118
- [Google Scholar]
- Superoxide dismutase as multipotent therapeutic antioxidant enzyme: Role in human diseases. Biotechnol. Lett 2022:1-22.
- [Google Scholar]
- Estimation of the mass of microplastics ingested–A pivotal first step towards human health risk assessment. J. Hazard. Mater.. 2021;404:124004
- [Google Scholar]
- Understanding kidney injury molecule 1: a novel immune factor in kidney pathophysiology. Am. J. Transl. Res.. 2019;11:1219.
- [Google Scholar]
- A simple method for clinical assay of superoxide dismutase. Clin. Chem.. 1988;34:497-500.
- [Google Scholar]
- T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature. 2000;408:600-605.
- [Google Scholar]
- Toxic effects of exposure to microplastics with environmentally relevant shapes and concentrations: accumulation, energy metabolism and tissue damage in oyster Crassostrea gigas Environ. Pollut.. 2021;269:116169
- [Google Scholar]
- Deltamethrin-induced oxidative damage and biochemical alterations in rat and its attenuation by Vitamin E. Toxicology. 2006;227:240-247.
- [Google Scholar]
- Kidney injury molecule-1 expression in transplant biopsies is a sensitive measure of cell injury. Kidney Int.. 2008;73:608-614.
- [Google Scholar]







