Translate this page into:
Evaluation of the antimicrobial and antifungal activity of endophytic bacterial crude extracts from medicinal plant Ajuga turkestanica (Rgl.) Brig (Lamiaceae)
⁎Corresponding authors. rahulmedcure@gmail.com (Rahul Datta), d_jabborova@nuu.uz (Dilfuza Jabborova)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Ajuga turkestanica (Lamiaceae) is an endemic species of flora of Uzbekistan and is widely used in traditional medicine from its aboveground and root parts. In the course of a scientific study strains of endophytic bacteria with antimicrobial activity were isolated from the underground and upper parts of the plant. Twelve bacterial strains were selected to obtain crude extracts with methanol and ethyl acetate. The was studied pathogenic test of crude extracts of selected endophytic bacterial strains against bacterial strains and plant phytopathogenic fungi. It has been established that crude extracts isolated from strains of endophytic bacteria are effective against pathogenic test microbes. All extracts have an inhibitory effect pathogen test strains against gram-positive Staphylococcus aureus-91, Bacillus subtilis-5, gram-negative Escherichia coli − 221, Pseudomonas aeruginosa − 225 and Candida albicans-247. The crude extracts showed a minimum inhibitory concentration of 3.125 µg/mL − 50 µg/mL, inhibiting the growth of test strains of the pathogen. The range of action of all extracts at the minimum inhibitory concentration was as follows: E. coli 6.25 µg/mL − 25 µg/mL, B. subtilis 12.5 µg/mL −50 µg/mL, S. aureus −3.122 µg/mL − 50 µg/mL, P. aeruginosa 12.5 µg/mL −50 µg/mL. The inhibitory effect of all endophytic bacterial strains on plant phytopathogenic fungi was as follows: Fusarium oxysporium: 3–5 cm, 44% −70% and Fusarium proliferatum: 4.1–7 cm, 27.8% − 56%. Our findings provide new insights into the antimicrobial activities of B. mojavensis (M11) and B. amyloliquefaciens (M13) natural endophytes, suggest this species may a promising candidate as a biocontrol agent to confer resistance to F. oxysporium and F. proliferatum disease and other phytopathogens in medicinal plants.
Keywords
Endophytic bacteria
Ajuga turkestanica
Antimicrobial activity
Antifungal activity
Crude extract
1 Introduction
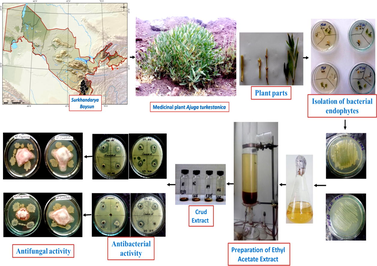
Endophytic bacteria are natural factories synthesizing pharmacologically important biologically active secondary metabolites diverse in structure and function of synthesized metabolites. In terms of biodiversity endophytic bacteria are one of the most diverse species present on earth. This is why the study of endophytic bacterial bioresources that colonize the body of medicinal plants is of great interest. Endophytic bacteria produce secondary metabolites of various natures due to their extensive colonization of the host plant body and their biodiversity. Singh et al. (2017) reported that the formation of bioactive compounds effective against pathogenic microbes in strains of endophytic bacteria isolated from plants with pharmacologically important medicinal properties has been established. Strains of endophytic bacteria are associated with all plants in the natural ecosystem, colonizing intercellular and intracellular spaces in plant tissues. Endophytic bacteria have been isolated from various plants such as common bean (Phaseolus vulgaris) (Costa et al., 2012), medicinal Glycyrrhiza species (Li et al., 2012), pea (Pisum sativum L.) (Tariq et al., 2014), Ajuga turkestanica (Mamarasulov et al., 2022) and Pennisetum glaucum (Gupta et al., 2022). (See Table 1)
Bacterial Isolates
Weight of the methanolic crude extract (g)
Bacterial isolates
Weight of the methanolic crude extract (g)
M1
0.15
M7
0.10
M2
0.11
M9
0.13
M3
0.24
M10
0.23
M4
0.11
M11
0.16
M5
0.16
M12
0.28
M6
0.81
M13
0.18
Endophytic bacteria form biologically active compounds that protect against herbivores and pathogenic microorganisms. Endophytec bacteria synthesize flavonoid, terpenoid, phenolic nature and many other biologically active secondary metabolites (Egamberdieva et al., 2020; Jabborova et al., 2020a, 2020b). Plants with medicinal properties, which are currently widely used in modern pharmaceuticals, are an important object in the search for productive strains of endophytic bacteria. The role of secondary metabolites synthesized by endophytic bacteria in the formation of biologically active substances of medicinal plants can be significant. Crude extracts obtained by extraction of endophytic bacterial strains have antibacterial and phytopathogenic fungal, diabetic and anticancer properties (Gunatilaka et al., 2006; Guo et al., 2008; Hardoim et al., 2015; Jabborova et al., 2019).
As the basis of this scientific article focusing on the antimicrobial and antifungal activity of endophytic bacteria we have attempted to investigate some endophytic bacteria that have the property of producing biologically active metabolites against plant phytopathogenic fungi and pathogenic microbial strains. It is noteworthy that the bioactive properties of medicinal plants have been identified as a result of scientific studies that depend on endophytic microorganisms that colonize its body. A. turkestanica is a popular medicinal plant endemic to the Boysun Mountains of Uzbekistan and its body has been found to contain biologically active components that have cytotoxic, antioxidant and antimicrobial effects (Mamadalieva et al., 2013). The purpose of the study was to search for strains of endophytic bacteria synthesizing bioactive metabolite compounds against pathogenic and phytopathogenic microbes.
We hypothesize that endophytic bacteria that have the ability to produce bioactive substances with activity against pathogenic and phytopathogenic microbes based on the medicinal properties of the medicinal plant A. turkestanica. Based on this, the objectives of our study include: i) investigation on the pathogenic test of crude extracts of selected endophytic bacterial strains against bacterial strains and plant phytopathogenic fungi; ii) determination on the antifungal activity of strain endophytic bacteria against phytopathogenic fungi.
2 Materials and methods
2.1 Fermentation in Liquid Medium
Endophyte bacterial strains were incubated in Nutrient broth medium (0.5% peptone, 0.3% yeast extract, 0.5% NaCl, 0.25% glucose, distilled water) at 28 ± 2 °C for 72 h on an incubator shaker (Shaker; Incubator, MaxQ 6000, Thermo Fisher, 240) with periodic shaking at 180 rpm (Santos et al., 2015).
2.2 Preparation of crude extracts of methanol and ethyl acetate from liquid fermentation medium
The fermentation liquid of bacterial strains was extracted with organic solvents, ethylacetate and methanol. In this process, an equal volume of organic solvent is added to the fermentation liquid and shaken vigorously. Then wait ten minutes until two distinct immiscible layers form. The organic phase containing secondary metabolites is separated using a separating funnel. In the next step, it was dried in a vacuum evaporator to separate crude metabolites from the organic phase. The crude extract was then dissolved in dimethyl sulfoxide and stored at 4 °C for further experiments.
2.3 Fundamentals of selection of microorganisms
The bacteria and fungi selected for this study were selected because of their strong pathogenic role including their ability to cause gastrointestinal diseases such as dysentery and many other diseases.
2.4 Tested microorganisms
The test microorganisms used to determine antimicrobial activity are bacteria that are conditionally pathogenic to humans (S. aureus-91, B. subtilis − 5, gram-negative bacteria P. aeruginosa − 225 and E. coli- 221 microscopic fungi C. albicans-247). Two plant phytoratogens F. oxysporum 316 and F. proliferatum 516 fungi were also used to determine the antifungal activity of endophytic bacteria. All test cultures used were registered and stored at the Institute of Microbiology of the Academy of Sciences of Uzbekistan.
2.5 Determination of antibacterial activity of bacterial crude extracts
2.5.1 Well Diffusion Method
Preparation of pathogen test culture inoculants. Bacteria grown on Nutrient agar were obtained in flasks containing 5 mL of sterile distilled water (Marson et al., 2014). The resulting suspension was brought to a turbidity equivalent to 0.5 McFarland standard to bring it to a state of 1.5 × 108 CFU/mL. The test strains were then planted as a lawn on the entire surface of the Müller Hinton agar medium with a sterilized L-shaped glass rod and kept at 37 ± 2° C for 15 min. The test strains were then cut into 6 mm pits on the surface of the planted medium. Endophytic bacteria were then infused into the pits in an amount of 10 μL from crude extracts of ethyl acetate. Penicillin antibiotic was used for positive control and Whatman No.1 paper soaked in 6 mm dimethyl sulfoxide was used for negative control. In the next step, the test cultures were incubated for 18–24 h at 37 ± 2° C. After incubation the zone of inhibition of the extracts was measured in millimeters. The experiments were repeated three times to confirm the reliability of the results.
2.5.2 Disc diffusion method
The antimicrobial activity of the methanolic crude extract of endophytic bacteria against strains of pathogenic bacteria was determined by the disk diffusion method (Photolo et al., 2020; Makuwa et al., 2021; Kebede et al., 2021; Ababutain et al., 2021).
Selected test strains (S. aureus-91, B. subtilis-5, гpaмм мaнфий бaктepиялap E. coli- 221 вa P. aeruginosa-225, microscopic fungus C. albicans-247) were grown nutrient nroth medium at 37 ± 2° C for 24 h. The cultured test strains were diluted in 20 mL sterilized distilled water. The turbidity level of the strains used for the test was adjusted with a McFarland 0.5 standard (0.5 × 108 CFU/mL). Pathogen test bacterial strains were then inoculated on a lawn using a pre-prepared nutrient agar medium medium sterilized L-shaped glass rod. Methanol extract of endophytic bacteria was soaked in 6–8 mm sterile Whatman No.1 paper. The nutrient was then placed in a Petri dish containing pathogenic bacterial strains grown in a medium. A penicillin antibiotic was used as a positive control, and dimethyl sulfoxide solution was used as a negative control. Petri dishes were kept in a thermostat at a temperature of 37 ± 2C° (Memmert Made in Germany Schutzart DIN EN 60529 - IP20) for 18–24 h. At the end of the incubation period, the zones of inhibition around the disks impregnated with extracts were measured and compared with the values of the zone of inhibition of the penicillin antibiotic.
2.5.3 Minimum inhibitory concentration of crude methanol extracts
Minimum inhibitory concentrations of methanol crude extracts of endophytic bacteria were carried out in sterilized round-bottomed 96-well microplates (Veiga et al., 2019; Rodríguez et al., 2022). Separate microplates were used for each pathogen strain. 200 μL of nutrient broth (S. aureus, E. coli, P. aureginose, B. sibtilis) and Sabouraud broth (C. albicans) were poured into each well of the plates. Methanolic extracts of 100 μL endophytic bacteria were added to the first well of microplates (line A) (columns 1 and 10). As a positive control, the antibiotic ceftriaxone was added to the eleventh column (an agent against pathogen test strains). Methanolic extracts of 100 μL endophytic bacteria were added to the first well of microplates (row A) (columns 1 and 10). Ceftriaxone antibiotic was added to the eleventh column (an agent against pathogen test strains) for positive control. In the next step, the microplate was taken 100 μL from line A and diluted successively to line B and other wells. As a result the following dilution ratios are (A) 1:2, (B) 1:4, (C) 1:8, (D) 1:16, (E) 1:32, (F) 1:64, (G) 1:128, (H) 1:256 was formed. 20 μL of pathogen test strains inoculation (0.5 McFarland 108 colony-forming unit CFU/mL) was then added to each well. The twelfth column (Nutrient broth) used for the negative control was left unchanged. The microplate was then incubated for 24 h at a temperature of 37 ± 2°. After the storage time, 20 μL of 2,3,5-triphenyltetrazolium chloride solution was added to each well and kept for 2 h. After incubation color development was observed in one well. If a red color develops in the wells the test indicates that the strains have grown. If color does not develop we can conclude that the growths of the strains are inhibited by the extracts. Wells without color development in the microplate lines represent the minimum inhibitory concentration of the methanolic crude extracts.
2.6 Antagonistic effects endophytic bacteria on phytopathogenic fungi
The antifungal activity of endophytic bacterial strains F. Oxysporium and F. proliferatum of phytopathogenic fungi was assessed by analysis of petri dishes (Cho et al., 2007; Abdelmoteleb et al., 2017; Zhang et al., 2017; Liu et al., 2019; Xie et al., 2020; Erfandoust et al., 2020). Endophyte bacterial strains were grown in Nutrient broth at 28 ± 2 °C for 24 h. In the next step potato dextrose agar (PDA) medium was prepared by adding 40 g of PDA powder to 1L distilled water and poured into sterilized Petri dishes. The bacterial strains were then harvested in a square shape at a distance of 2 cm from the center of a Petri dish filled with PDA medium using an inoculation loop. Then phytopathogenic fungi selected for antifungal activity were from the mycelium (6 mm) and placed in the center of the Petri dish. The distance between strains of endophytic bacteria and phytopathogenic fungi was 2–3 cm. Then Petri dishes were stored at a temperature of 28 ± 2 °C for 7 days. At the end of the incubation period the growth inhibition of the phytopathogenic fungal colony was determined by measuring in millimeters in diameter. The percentage of growth inhibition of phytopathogenic fungi used in the experiment was calculated using the formula Trivedi.
[%]
Where R1 = radial growth of control test strains, R2 = radial growth of test test strains (Trivedi et al., 2008). All treatment was replicated five.
2.7 Statistical analyses
The experimental data were analyzed with the StatView Software using ANOVA.
3 Results
3.1 Yield of extracts
High amounts of crude extracts were obtained from endophytic bacteria B. amyloliquefaciens ssp. plantarum (M6), B. subtilis ssp. (M10) and P.chlororaphis (M12).
3.2 Agar well diffusion assay of secondary metabolites extracts
Endophytic bacteria were screened by Agar well diffusion method of ethyl acetate crude extract (Table 2; Fig. 1). The crude ethyl acetate extract of endophytic bacterial strains exhibited antimicrobial activity against opportunistic microbes. The results obtained showed that P. kilonensis (M1) ethyl acetate extract inhibited pathogen test strains (E. coli 12 ± 1.3; B. subtilis 6 ± 1.0; P. aeruginosa 11 ± 0.41; S. aureus 14 ± 1.2; C. albicans 12 ± 1.3). B. subtilis ssp. (M10). We can see that endophytic bacterium ethyl acetate crude extract inhibited the growth of the following test strains (E. coli 11 ± 1.1; B. subtilis 13 ± 1.6; P. aeruginosa 11 ± 1.0; S. aureus 16 ± 1.3; C. albicans 26 ± 1.1). (See Fig. 2.Fig. 3Table 3)
Bacterial Isolates
Endophytic bacteria
Inhibition diameter zone (mm)
E.
coli
B.
subtilis
P.
aeruginosa
S.
aureus
C. albicans
M1
P. kilonensis
12 ± 1.3
6 ± 1.0
11 ± 0.41
14 ± 1.2
12 ± 1.3
M2
B. amyloliquefaciens ssp. plantarium
11 ± 1.0
11 ± 1.6
14 ± 0.4
14.6 ± 1.0
10 ± 1.2
M3
P. putida
11.7 ± 1.4
9 ± 1.1
5.6 ± 0.1
13.8 ± 0.0
8 ± 1.0
M4
B. subtilis ssp.
7 ± 1.0
6 ± 0.2
6 ± 1.0
10 ± 1.3
9 ± 1.0
M5
P. graminis
9 ± 1.3
10 ± 1.1
9 ± 0.45
14 ± 0.10
10 ± 1.0
M6
B. amyloliquefaciens ssp. plantarium
9 ± 1.2
13 ± 1.0
10 ± 1.2
14.6 ± 1.0
14 ± 1.1
M7
S. colletis
8 ± 1.0
10 ± 1.0
8 ± 0.12
12 ± 1.0
21 ± 1.3
M9
B. amyloliquefaciens
0 ± 0
8 ± 1.2
0 ± 0
12.4 ± 0.1
24 ± 1.0
M10
B. subtilis ssp.
11 ± 1.1
13 ± 1.6
11 ± 1.0
16 ± 1.3
26 ± 1.1
M11
B. mojavensis
11 ± 1.0
14 ± 1.2
12 ± 1.0
14 ± 1.3
8 ± 1.0
M12
P. chlororaphis
8 ± 1.2
9 ± 1.2
12 ± 0.1
10 ± 1.3
10 ± 1,21
M13
B. amyloliquefaciens ssp. plantarium
12 ± 1.1
9 ± 0.1
9 ± 1.1
9.6 ± 1.2
11 ± 1.1
Positive control
Penicillin
0 ± 0
15 ± 1.2
0 ± 0
0 ± 0
14 ± 1.3
Negative control
Dimethyl sulfoxide
0 ± 0
0 ± 0
0 ± 0
0 ± 0
0 ± 0
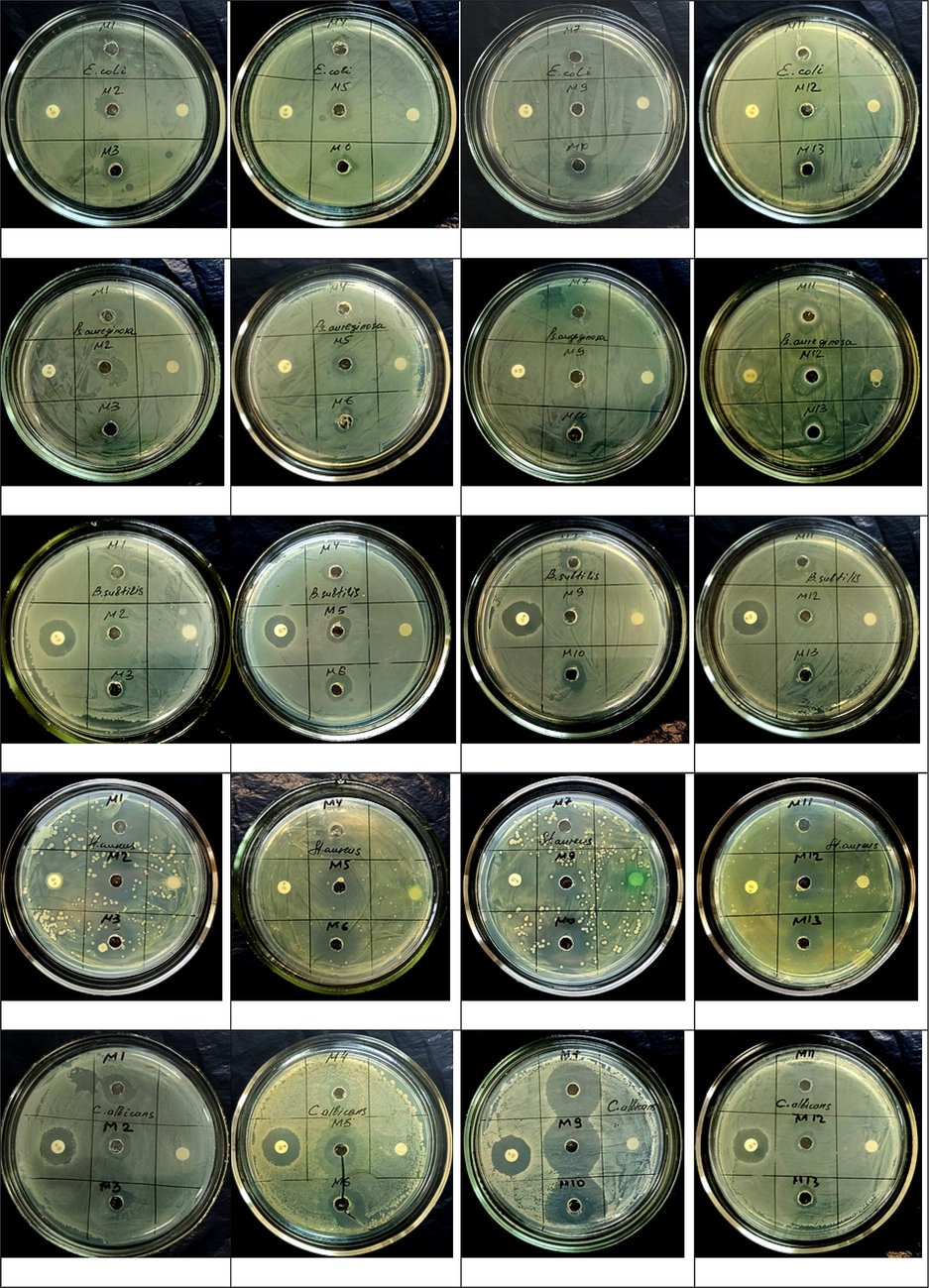
Antimicrobial activity of ethyl acetate crud extracts of well diffusion method.
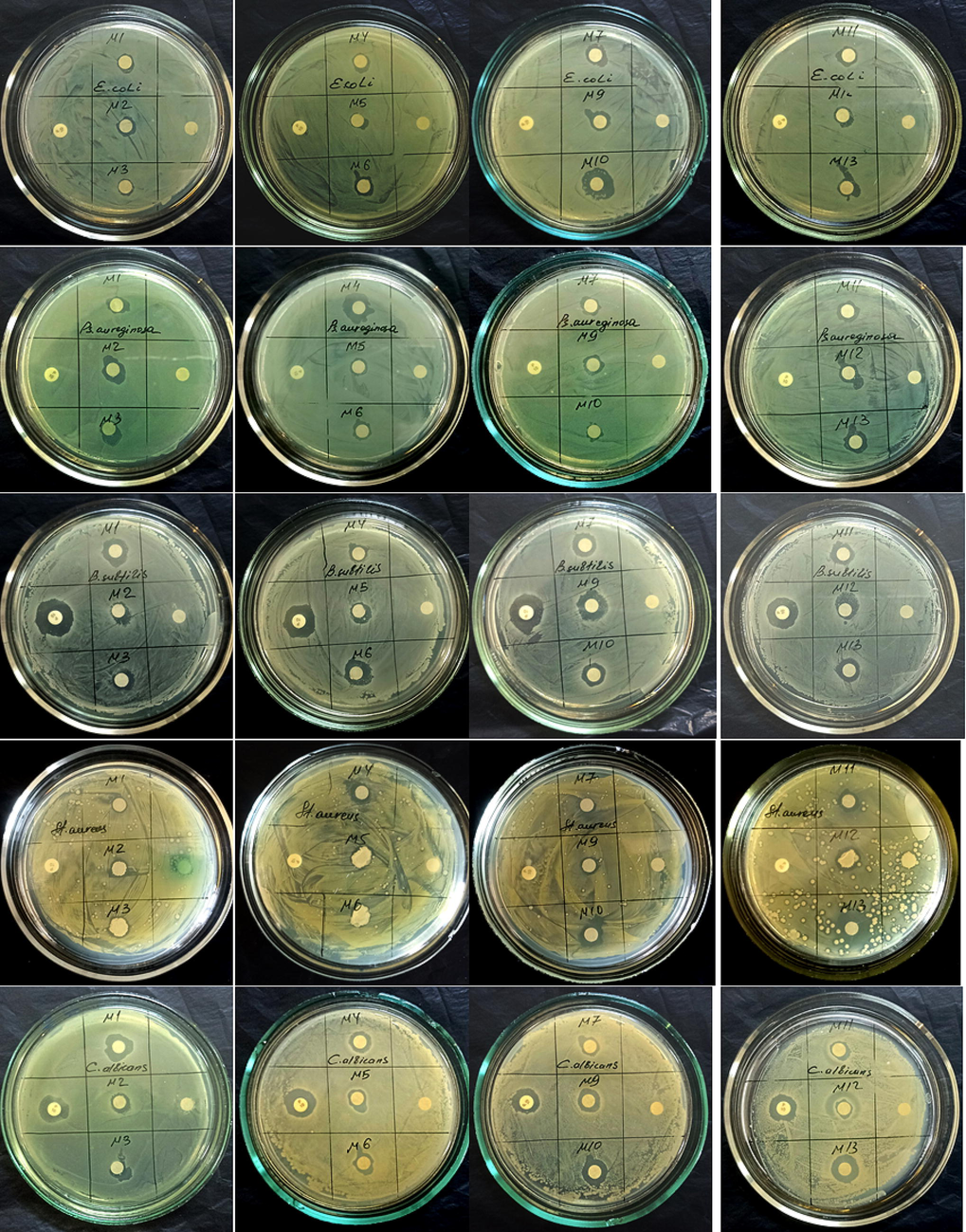
Antibacterial activity of methanol crude extracts by disc diffusion method.
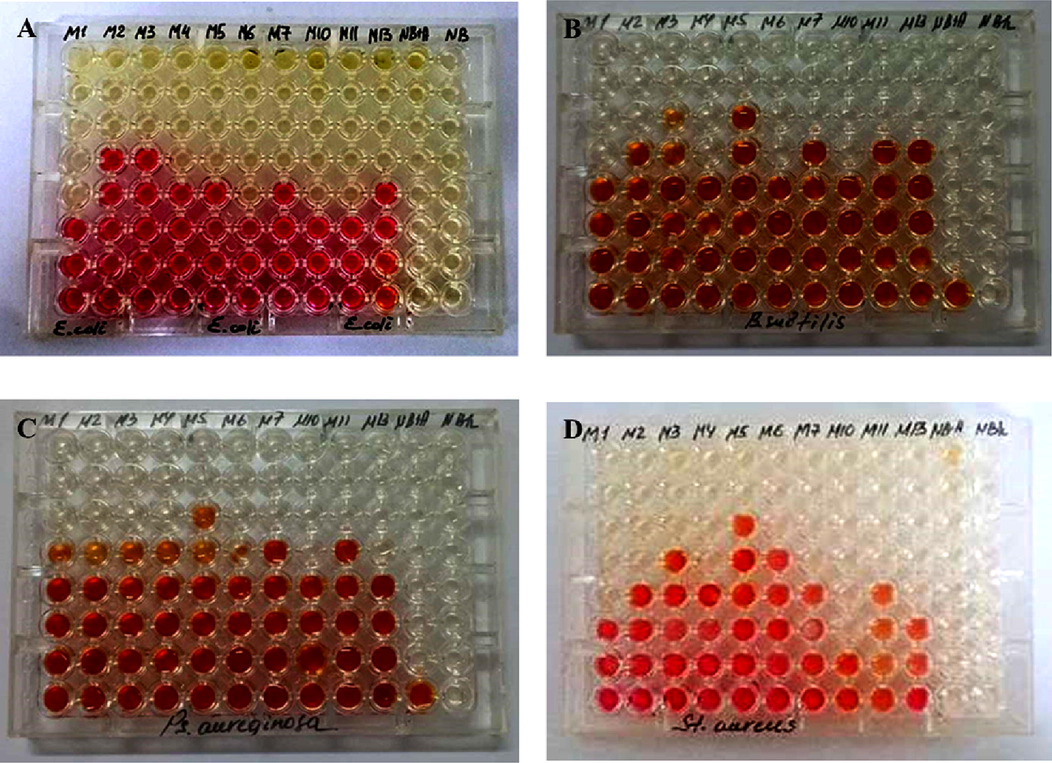
Appearance of methanol crude extracts of endophytic bacteria against pathogenic test strains on a microplate with the addition of a solution of triphenyltetrazolium chloride A) E. coli; B) B. subtilis; C) P. aeruginosa; D) S. aureus.
Bacterial Isolates
Endophytic bacteria
Inhibition diameter zone (mm)
E.
coli
B.
subtilis
P.
aeruginosa
S.
aureus
C albicans
M1
P. kilonensis
6.1 ± 1.1
11 ± 1.2
6.4 ± 1.0
5.1 ± 1.1
12 ± 1.1
M2
B. amyloliquefaciens ssp. plantarium
8.4 ± 1.0
6 ± 1.0
10 ± 1.1
6.3 ± 1.0
7.4 ± 1.2
M3
P. putida
5.7 ± 1.1
6.4 ± 1.2
9.6 ± 1.1
4.2 ± 1.0
5.4 ± 1.0
M4
B. subtilis ssp.
7.6 ± 1.1
8.4 ± 1.2
12 ± 1.0
6.6 ± 1.0
8 ± 1.1
M5
P. graminis
6.6 ± 1.2
8.6 ± 1.4
7 ± 1.1
4.2 ± 1.0
8 ± 1.0
M6
B. amyloliquefaciens ssp. plantarium
8.2 ± 1.0
10 ± 1.2
6.5 ± 1.1
4.0 ± 0.14
7.4 ± 1.0
M7
S. colletis
8.6 ± 0.13
10 ± 1.2
8 ± 1.0
0 ± 0
6.2 ± 1.0
M9
B. amyloliquefaciens
9.7 ± 0.16
12 ± 1.0
8.6 ± 1.1
6 ± 0.10
10 ± 1.4
M10
B. subtilis ssp.
12 ± 1.4
9 ± 1.6
6.2 ± 1.0
10 ± 1.1
13 ± 1.0
M11
B. mojavensis
8 ± 1.0
11 ± 1.0
10 ± 1.0
10 ± 1.0
10 ± 1.1
M12
P. chlororaphis
7.8 ± 1.0
12 ± 1.3
12 ± 1.0
10 ± 0.2
7.4 ± 1.2
M13
B. amyloliquefaciens ssp. plantarium
6.2 ± 0.3
14 ± 1.2
13 ± 1.1
12 ± 1.0
11 ± 1.2
Positive
controlPenicillin
0 ± 0
16 ± 1.0
0 ± 0
0 ± 0
14 ± 1.3
Negative control
controlDimethyl sulfoxide
0 ± 0
0 ± 0
0 ± 0
0 ± 0
0 ± 0
B. amyloliquefaciens ssp. plantarium (M6) endophytic bacteria inhibited the growth of test strains used for testing ethyl acetate crude extract (E. coli 9 ± 1.2; B. subtilis 13 ± 1.6; P. aeruginosa 10 ± 1.2; S. aureus 14.6 ± 1.0; C. albicans 14 ± 1.1). Crude extracts of ethyl acetate from endophytic bacterial strains showed high antimicrobial activity against test strains of the pathogen. The positive penicillin antibiotic showed antimicrobial activity against test strains of B. subtilis and C. albicans. A negative control test with dimethyl sulfoxide showed no antimicrobial activity against the strains. The obtained results indicate that strains of endophytic bacteria contain biologically active substances against microbes in crude extracts of ethyl acetate.
3.3 Disc diffusion assay of the methanolic crude extracts
Endophytic bacteria extracted crude extracts in methanol to determine their antimicrobial activity by disc diffusion. Methanol extracts of all endophytic bacteria showed positive antimicrobial activity to pathogen test strains. Methanolic extracts of the following endophytic bacteria showed high results in the disc diffusion method of antimicrobial activity. Methanol extract of the bacterium B. subtilis ssp. (M10) highly inhibited the growth of pathogen test strains (E. coli 12 ± 1.4; B. subtilis 9 ± 1.6; P. aeruginosa 6.2 ± 1.0; S. aureus 10 ± 1.1; C. albicans 13 ± 1.0;). Methanol extract of the bacterium B. mojavensis inhibited the growth of pathogenic test strains (E. coli 8 ± 1.0; B. subtilis 11 ± 1.0; P. aeruginosa 10 ± 1.0; S. aureus 10 ± 1.0; C. albicans 10 ± 1.1). P. chlororaphis endophytic bacterial methanolic crude extract E. coli (7.8 ± 1.0) B. subtilis (12 ± 1.3) P. aeruginosa (12 ± 1.0) S. aureus (10 ± 0.2); C. albicans (7.4 ± 1.2) exhibited high antimicrobial properties against pathogenic test strains. B. amyloliquefaciens ssp. plantarium bacteria methanol extract E. coli (6.2 ± 0.3) B. subtilis (14 ± 1.2) P. aeruginosa (13 ± 1.1) S. aureus (12 ± 1.0); C. albicans (11 ± 1.2) showed broad-spectrum antimicrobial activity against pathogen test cultures. These parameters reflect the high inhibition zone of methanol extracts of endophytic bacteria relative to pathogen test strains. Methanol extracts of endophytic bacteria showed strong activity against all test strains. These results indicate that methanol crude extracts of endophytic bacterial strains contain biologically active secondary metabolites that inhibit the growth of pathogenic bacteria. The positive control penicillin antibiotic showed antimicrobial activity against B. subtilis and C. albicans test strains. The negative control test with dimethyl sulfoxide did not inhibit the growth of the strains.
3.4 Minimal inhibitor concentration of the methanol crude extracts
Minimum inhibitory concentrations of methanol extracts of endophytic bacterial strains were performed with four pathogen test strains (E. coli, B. subtilis, P. aeruginosa, S. aureus). The following results were obtained when methanol extracts were found to have a minimal inhibitory concentration relative to the E. coli strain. The best antibacterial effect against E. coli strain is P. kilonensis (M1), B. amyloliquefaciens ssp. plantarium (M6), B. subtilis ssp. (M10) and B. mojavensis (M11) were detected in isolates (6.25 µg/mL). In the next places, the minimum inhibitory concentration of methanol crude extracts of P. graminis (M5) and B. amyloliquefaciens (M13) was 12.5 µg/mL. The best antimicrobial effect of endophytic bacterial strains methanol crude extracts against B. subtilis test strains was P. kilonensis (M1) and B. subtilis ssp. (M10) at a concentration of 12.5 µg/mL. B. subtilis ssp. (M10) methanol crude extract inhibited of S. aureus conditional pathogen strain at a concentration of 3.125 µg/mL. Strains of P. kilonensis (M1) and B. amyloliquefaciens (M13) also inhibited the of S. aureus strain at a concentration of 12.5 µg/mL. B. mojavensis (M11) and B. amyloliquefaciens ssp. (M13) methanol crude extracts of the bacterium inhibited the growth of P. aureginosa strain at a concentration of 12.5 µg/mL. The minimum inhibitory concentrations of pathogen test strains of methanol crude extracts of endophytic bacteria are given in Table 4.
Bacterial Isolates
Endophytic bacterial strains and antibacterial agent
Pathogen test strains
E. coli
B. subtilis
P. aureginosa
S. aureus
concentration (µg/mL)
M1
P. kilonensis
6.25
12.5
25
6.25
M2
B. amyloliquefaciens ssp. plantarium
25
25
25
12.5
M3
P. putida
25
50
25
25
M4
B. subtilis ssp.
12.5
12.5
25
12.5
M5
P. graminis
12.5
50
50
50
M6
B. amyloliquefaciens ssp. plantarium
6.25
12.5
25
25
M7
S. colletis
12.5
25
25
12.5
M10
B. subtilis ssp.
6.25
12.5
12.5
3.125
M11
B. mojavensis
6.25
25
25
12.5
M13
B. amyloliquefaciens ssp. plantarium
12.5
25
12.5
6.25
3.5 Antifungal activity of strain endophytic bacteria against phytopathogenic fungi
The antifungal activity of endophytic bacterial strains was studied in PDA medium. Endophytic bacterial strains inhibit the growth of phytopathogenic fungi F. oxysporium and F. proliferatum. (Table 5 & Fig. 4). The strain B. subtilis ssp. inhibited the growth of the fungus F. oxysporium to 70% (3 ± 0.12) and the fungus F. proliferatum to 38.1% (6 ± 0.17). The strain of P.chlororaphis inhibited of F. oxysporium by 70% (3 ± 0.28) and the growth of F. proliferatum phytopathogen by 53% (4.5 ± 0.16). The strain of B. amyloliquefaciens ssp. also had high levels of angifungal properties (F. oxysporium 60% (4 ± 0.17), F. proliferatum 50% 4.8 ± 10). Each strain of endophytic bacteria used for the test synthesizes biologically active secondary metabolites that inhibit the growth of phytoptogenic fungi.
Bacterial Isolates
Endophytic bacteria
F. oxysporium
F. proliferatum
Zone of inhibition (sm)
Zone of inhibition (%)
Zone of inhibition (sm)
Zone of inhibition (%)
Control
10
100
9.7
97
M1
P. kilonensis
5 ± 0.14
50
4.4 ± 0.17
54.6
M2
B. amyloliquefaciens ssp. plantarium
5.6 ± 0.24
44
7 ± 0.23
27.8
M3
P. putida
5 ± 0.16
50
4.6 ± 0.15
51
M4
B. subtilis ssp.
3 ± 0.12
70
6 ± 0.17
38.1
M5
P.graminis
5.3 ± 0.36
47
6.5 ± 0.12
33
M6
B. amyloliquefaciens ssp. plantarium
3.5 ± 0.41
65
5.3 ± 0.21
45
M7
S. colletis
4.6 ± 0.17
54
6.1 ± 0.17
33
M9
B. amyloliquefaciens
4.6 ± 0.42
54
6.8 ± 0.18
30
M10
B. subtilis ssp.
3.8 ± 0.22
57
4.1 ± 0.11
57
M11
B. mojavensis
3.6 ± 0.14
56
4.2 ± 0.13
56
M12
P. chlororaphis
3 ± 0.28
70
4.5 ± 0.16
53
M13
B. amyloliquefaciens ssp. plantarium
4 ± 0.17
60
4.8 ± 10
50
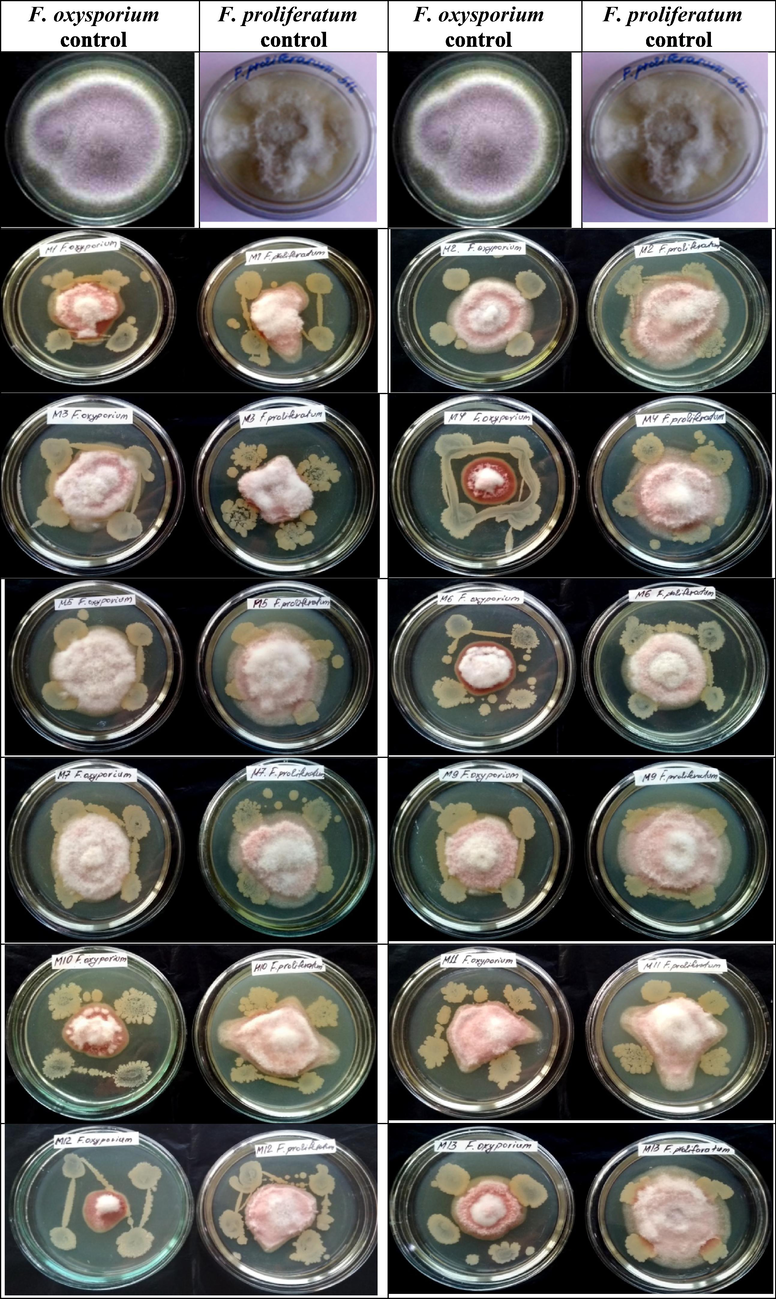
Antifungal properties of endophytic bacterial strains against phytopathogens F. oxysporium and F. proliferatum.
4 Discussion
Endophyte serves as an effective method in reducing plant diseases in agriculture through biological control of various phytopathogenic bacteria and fungi using microorganisms (Jabborova et al., 2020a, 2020b). Endophytic bacteria living in communities together with medicinal plants are a rich source of biologically active secondary metabolites against phytopathogens. Endophytic bacteria live in plant tissue without causing symptoms (Bacon and White, 2000; Yu et al., 2018). Antimicrobial agents inhibit the proliferation of pathogenic bacteria and fungi by disrupting the cell wall, inhibiting the metabolism and DNA biosynthesis that take place in the bacterial cell. Antimicrobial secondary metabolites synthesized by endophytic bacteria are compounds with active action against microorganisms resistant to various drugs (Egamberdieva et al., 2021). Antimicrobial metabolites are organic compounds synthesized by these endophytic bacterial strains that limit the growth and development of harmful microbes and have a strong effect even at low concentrations (Gos et al., 2017; Islam et al., 2018; Elfita et al., 2019). B. subtilis strains have been an important source of antimicrobial compounds. Bacillus species such as B. endophyticus, B. subtilis, B. and B. insolitus improve plant growth and physiological properties in different plants (Ashraf et al., 2004; Jabborova et al., 2021; Jabborova et al., 2022). Endophyte bacterial strains have been found to have high antimicrobial activity in crude extracts of ethyl acetate and methanol. For example, we can see that the crude extract of ethyl acetate of the endophytic bacteria B. subtilis ssp. (M10) inhibited of the following test strains (E. coli 11 ± 1.1; B. subtilis 13 ± 1.6; P. aeruginosa 11 ± 1.0; S. aureus 16 ± 1.3; C. albicans 26 ± 1.1). However, methanol extract of this strain (B. subtilis ssp. M10) was highly inhibited by the growth of test strains (E. coli 12 ± 1.4; B. subtilis 9 ± 1.6; P. aeruginosa 6.2 ± 1.0; S. aureus 10 ± 1.1; C. albicans 13 ± 1.0). It is this strain of B. subtilis ssp. (M10) that methanol crude extract also inhibited the growth of test strains (E. coli 12 ± 1.4; B. subtilis 9 ± 1.6; P. aeruginosa 6.2 ± 1.0; S. aureus 10 ± 1.1; C. albicans 13 ± 1.0) to a high degree. B. subtilis ssp. (M10) endophytic bacterium methanol crude extract inhibited of pathogenic test strains S. aureus and B. subtilis at minimal (12.5 µg/mL вa 3.125 µg/mL) concentration. Mamadalieva et al. (2013) reported that the antimicrobial activity of the total extracts of the plant A. turkestanica obtained in various organic solvents against pathogenic test strains. The following results were obtained when studying the antimicrobial activity of plant aqueous (H2O), butanol (BuOH), methanol (MeOH) and chloroform (CHCl3) extracts. The plant extract (S. aureus MRSA ATCC 1000/93 6.05 ± 0.05; S. pyogenes ATCC 12344 8.8 ± 0.1) inhibited bacterial growth. Methanol extract of the medicinal plant A. turkestanica inhibited the growth of pathogenic microbes (E.coli ATCC 25922 3.15 ± 0.15; P. aeruginosa ATCC 27853 4.4 ± 0.2; C.albicans ATCC 90028 3.8 ± 0.2) in a broad spectrum. Chloroform extract of the plant reduced the growth of pathogenic test strains used in the experiment (E. coli ATCC 25922 3.3 ± 0.1; P. aeruginosa ATCC 27853 4.9 ± 0.1; C. albicans ATCC 90028 5.75 ± 0.25) (Mamadalieva et al., 2013).
There are theories according to which strains of endophytic bacteria can synthesize biologically active secondary metabolites that are structurally and physiologically similar to the host plant. A comparative comparison of the obtained results shows that endophytic bacteria also showed strong antimicrobial activity against their host plants. Endophytic bacteria confirm the presence of bioactive secondary metabolites with strong antimicrobial properties in crude extracts of methanol and ethyl acetate. Endophytic bacteria strains belonging to the Bacillus species are one of the most widely used bacterial species against phytopathogens in the development of sustainable agriculture when the activity of methanol and ethyl acetate extracts of endophytic bacteria against pathogenic microbes is studied. It also includes extracellular production of antibiotics and antimicrobial biologically active secondary metabolites, mucolytic enzymes such as chitinase, protease and 1,3-glucanase. Strains of endophytic bacteria isolated from the plant A. turkestanica showed strong antimicrobial and antifungal properties against pathogenic bacteria and plant pathogens.
5 Conclusion
The antimicrobial and antifungal activity of ethyl acetate and methanol extracts of endophytic bacterial strains isolated from the medicinal plant A. turkestanica distributed as an endemic species in the flora of Uzbekistan was studied. Experimental results showed that crude extracts of endophytic bacterial strains of B. subtilis ssp. (M10), B. mojavensis (M11) and B. amyloliquefaciens (M13) showed high antimicrobial activity against pathogenic test strains. When studying the activity of strains of endophytic bacteria against phytopathogenic plant fungi, all isolated strains inhibited the fungi F. oxysporium and F. proliferatum. Further GC–MS studies are needed to characterize biologically active secondary metabolites in crude extracts of endophytic bacterial strains.
Acknowledgments
The author is grateful to the Institute of Microbiology of the Academy of Sciences of Uzbekistan for the constant funding support during the research. This project was supported by Researchers Supporting Project Number (RSP2023R5) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Identification and antibacterial characterization of endophytic fungi from Artemisia sieberi. Int. J. Microbiol.. 2021;2021
- [CrossRef] [Google Scholar]
- Antifungical activity of autochthonous Bacillus subtilis isolated from Prosopis juliflora against phytopathogenic fungi. Mycobiology. 2017;45(4):385-391.
- [CrossRef] [Google Scholar]
- Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils.. 2004;40:157-162.
- [CrossRef] [Google Scholar]
- Bacon C.W., White J., eds. Microbial endophytes. CRC Press; 2000.
- Endophytic bacterial communities in ginseng and their antifungal activity against pathogens. Microb. Ecol.. 2007;54(2):341-351.
- [CrossRef] [Google Scholar]
- Isolation and characterization of endophytic bacteria isolated from the leaves of the common bean (Phaseolus vulgaris) Braz. J. Microbiol.. 2012;43:1562-1575.
- [CrossRef] [Google Scholar]
- Plant microbiome: source for biologically active compounds. Biodiversity and Biomedicine.. 2020;1–9
- [CrossRef] [Google Scholar]
- Antimicrobial activities of herbal plants from Uzbekistan against human pathogenic microbes. Environmental Sustainability.. 2021;4(1):87-94.
- [CrossRef] [Google Scholar]
- Antibacterial activity of Cordyline fruticosa leaf extracts and its endophytic fungi extracts. Biodiversitas J. Biol. Diversity. 2019;20(12)
- [CrossRef] [Google Scholar]
- Antifungal activity of endophytic fungi from Cupressaceae against human pathogenic Aspergillus fumigatus and Aspergillus niger. Journal de Mycologie Médicale.. 2020;30(3):100987
- [CrossRef] [Google Scholar]
- Antibacterial activity of endophytic actinomycetes isolated from the medicinal plant Vochysia divergens (Pantanal, Brazil) Front. Microbiol.. 2017;8:1642.
- [CrossRef] [Google Scholar]
- Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod.. 2006;69(3):509-526.
- [CrossRef] [Google Scholar]
- Bioactive natural products from endophytes: A review. Appl. Biochem. Microbiol.. 2008;44(2):136-142.
- [CrossRef] [Google Scholar]
- Exploring Functional Diversity and Community Structure of Diazotrophic Endophytic Bacteria Associated with Pennisetum glaucum Growing under Field in a Semi-Arid Region. Land.. 2022;11(7):991.
- [Google Scholar]
- The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev.. 2015;79(3):293-320.
- [CrossRef] [Google Scholar]
- Antibacterial activities of endophytic bacteria isolated from Taxus brevifolia against foodborne pathogenic bacteria. Foodborne Pathog. Dis.. 2018;15(5):269-276.
- [CrossRef] [Google Scholar]
- Antibacterial, antifungal, and antiviral properties of medical plants. Medically Important Plant Biomes: Source of Secondary Metabolites.. 2019;51–65
- [CrossRef] [Google Scholar]
- Isolation and characterization of endophytic bacteria from ginger (Zingiber officinale Rosc.) Ann. Phytomed.. 2020;9:116-121.
- [CrossRef] [Google Scholar]
- Plant growth promoting bacteria Bacillus subtilis promote growth and physiological parameters of Zingiber officinale Roscoe. Plant Science Today.. 2021 Jan 1;8(1):66-71.
- [CrossRef] [Google Scholar]
- Dual Inoculation of Plant Growth-Promoting Bacillus endophyticus and Funneliformis mosseae Improves Plant Growth and Soil Properties in Ginger. ACS Omega. 2022;7(39):34779-34788.
- [CrossRef] [Google Scholar]
- Effect of Bacillus subtilis 1 strain on the growth and development of wheat (Triticum aestivum L.) under saline condition. Bulgarian J. Agr. Sci.. 2020;26(4):744-777.
- [Google Scholar]
- Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: a possible alternative in the treatment of multidrug-resistant microbes. PLoS One. 2021;16:(3).
- [CrossRef] [Google Scholar]
- Biogeography of symbiotic and other endophytic bacteria isolated from medicinal Glycyrrhiza species in China. FEMS Microbiol. Ecol.. 2012 Jan 1;79(1):46-68.
- [CrossRef] [Google Scholar]
- Community structures and antifungal activity of root-associated endophytic actinobacteria of healthy and diseased soybean. Microorganisms.. 2019;7(8):243.
- [CrossRef] [Google Scholar]
- The antibacterial activity of crude extracts of secondary metabolites from bacterial endophytes associated with Dicoma anomala. Int. J. Microbiol.. 2021;2021
- [CrossRef] [Google Scholar]
- Antiproliferative, antimicrobial and antioxidant activities of the chemical constituents of Ajuga turkestanica. Phytopharmacology.. 2013;4(1):1-18.
- [Google Scholar]
- Characterization, enzymatic and biochemical properties of endophytic bacterial strains of the medicinal plant Ajuga turkestanica (Rgl.) Brig (Lamiaceae) J. King Saud Univ.-Sci.. 2022;34(6):102183.
- [CrossRef] [Google Scholar]
- Chemical assessment and antimicrobial and antioxidant activities of endophytic fungi extracts isolated from Costus spiralis (Jacq.) Roscoe (Costaceae) Evidence-Based Complementary and Alternative Medicine 2014
- [CrossRef] [Google Scholar]
- Antimicrobial and antioxidant properties of a bacterial endophyte, methylobacterium radiotolerans MAMP 4754, isolated from combretum erythrophyllum seeds. Int. J. Microbiol.. 2020;2020
- [CrossRef] [Google Scholar]
- Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Twelve Antimicrobials (Biocides and Antibiotics) in Eight Strains of Listeria monocytogenes. Biology. 2022;11(1):46.
- [CrossRef] [Google Scholar]
- Antibacterial activity of endophytic fungi from leaves of Indigofera suffruticosa Miller (Fabaceae) Front. Microbiol.. 2015;6:350.
- [CrossRef] [Google Scholar]
- Endophytic bacteria: a new source of bioactive compounds. 3. Biotech. 2017;7(5):1-14.
- [CrossRef] [Google Scholar]
- Molecular characterization and identification of plant growth promoting endophytic bacteria isolated from the root nodules of pea (Pisum sativum L.) World J. Microbiol. Biotechnol.. 2014 Feb;30(2):719-725.
- [CrossRef] [Google Scholar]
- In vitro evaluation of antagonistic properties of Pseudomonas corrugata. Microbiol. Res.. 2008;163(3):329-336.
- [CrossRef] [Google Scholar]
- Colorimetric microdilution assay: Validation of a standard method for determination of MIC, IC50%, and IC90% of antimicrobial compounds. J. Microbiol. Methods. 2019;162:50-61.
- [CrossRef] [Google Scholar]
- Antifungal activity of volatile compounds produced by endophytic Bacillus subtilis DZSY21 against Curvularia lunata. Ann. Microbiol.. 2020;70(1):1-10.
- [CrossRef] [Google Scholar]
- Diversity and antifungal activity of endophytic fungi associated with Camellia oleifera. Mycobiology. 2018;46(2):85-91.
- [CrossRef] [Google Scholar]
- Identification of antifungal substances secreted by Bacillus subtilis Z-14 that suppress Gaeumannomyces graminis var. tritici. Biocontrol Sci. Tech.. 2017;27(2):237-251.
- [CrossRef] [Google Scholar]
Further reading
- Phytochemical, pharmacological and biological properties of Ajuga turkestanica (Rgl.) Brig (Lamiaceae) Ann. Phytomed.. 2020;9:44-57.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102644.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







