Translate this page into:
In vitro leishmanicidal potential and silico study of flavonoids isolated from Pistacia integerrima Stew ex Brandis
⁎Corresponding author. abdurrauf@uoswabi.edu.pk (Abdur Rauf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Medicinal plants are the mainstay of various chemical constituents responsible for curing or mitigating different pathological and non-pathological health issues. In this regard, the current research work was subjected to finding the best effective, new and safe leishmanicidal molecules. Pistacia integerrima is an important medicinal plant growing in Asia with various ethnopharmacological uses such as skin, respiratory and hepatic disorders. The folklore of this plant in skin problems encouraged us to evaluate the isolated compounds for leishmanicidal activity.
Methods
In the current study, whole ethyl acetate extract was subjected to chromatographic analysis affording two flavonoid constituents, namely naringenin (1) and 3,5,4′-trihydroxy-7-methoxy-flavanone (2). The extract and isolated constituents were tested for leishmanicidal activity. Both compounds were docked into the target binding site (PDB ID: 5HJ9) using MOE 2016. Arginase was taken as the targeted enzyme which is an important enzyme for leishmania species’ survival. Arginase up-regulates amastin that impacts amastigote replication and survival. This approach aimed to explore the binding modes of the two isolated flavonoids and to predict their inhibitory activity against arginase.
Results
The two flavonoid compounds isolated from P. integerrima were evaluated for their anti-leishmanial activity. Compounds 1 and 2 were tested at a concentration of 5 µg/mL against the selected Leishmanial species. The percent inhibition was 70.43 and 74.76 regarding compounds 1 and 2, respectively with IC50 of 29.43 and 16.43 μM. The reference standard drug used, amphotericin-B, displayed the highest leishmanicidal effect (IC50 of 0.22 μM) with 84.09 % inhibition at half concentration compared to compounds 1 and 2. The molecular docking studies of these two compounds and the standard drug were performed in MOE software. According to the findings, compound 1, naringenin, docked well in the binding site, with a docking score of −9.28 followed by compound 2, 3,5,4′-trihydroxy-7-methoxy-flavanone with a docking score of −8.37.
Conclusion
Both of the tested samples demonstrated excellent leishmanicidal effects. This pharmacological inhibitory profile provides a strong scientific profile to the folklore of Pistacia integerrima in the treatment of skin disorders especially leishmanial. A further mechanistic study is recommended to find the extract target responsible for the said activity.
Keywords
Pistacia integerrima
Anacardiaceae
Flavonoids
Leishmanicidal activity
Docking studies
1 Introduction
Plants are the key figures for the life of human beings in various aspects. They are important for all needs of human beings in terms of clothing, shelter, food, flavors, fragrances, and especially medicine. Medicinal herbs have designed the foundations of traditional medicinal systems, including Chinese medicine, Ayurveda, and Unani (Gurib-Fakim, 2006; Naz, 2022). The practice of this medicinal plant-based therapy is found in all corners of the world in various shapes and they are considered safe and free of toxic effects by the world population. This concept of being free from adverse effects increased many folds the consumption of these plants for the treatment or mitigation of various diseases. Natural products are effective due to their secondary metabolites. A lot of secondary metabolites have been isolated and even in practice from these plants. These traditional systems have led to the discovery of key drugs like the well know central analgesic morphine and the best hepatoprotective drug silymarin. In the current modern era, human beings are facing a lot of health problems and still wait for the discovery of new natural products for the treatment of a lot of diseases. Search for novel drugs is a permanent challenge. Phytochemists first selected medicinal plants based on popular usage (Gurib-Fakim, 2006). About 5.2 billion peoples live in less developed countries and WHO has estimated that 80 % of these countries depend on traditional medicine for their primary healthcare (Davidson-Hunt, 2009). Herbal products are the backbone of traditional medicine and nearly 3.3 billion people regularly use herbal products (Liu et al., 2009; Ahvazi et al., 2012). Medicinal plants are the key sources of secondary metabolites and their biological potential is due to the presence of these bioactive compounds (Anand et al., 2019). The medicinal plant is a rich source of several bioactive compounds like Pistagremic acid (Ullah et al., 2014), Diospyrin (Ullah et al., 2015a), and 8-hydroxyisodiospryin which possess excellent biological potential (Ullah et al., 2015b).
Pistacia integerrima (zebrawood) is a member of the Anacardiaceae family native to Asia (Main et al., 2001; Patel et al., 2018). P. integerrima is a deciduous tree used in traditional folk medicine for curing various diseases such as phthisis, cough, vomiting, diarrhea, jaundice, asthma, chronic wounds, and dysentery, and employed as an antiseptic (Chopra et al.,1986; Khan et al., 2004). In various countries, zebrawood galls are used in herbal formulations for the treatment of diarrhea and dysentery. Aqueous extracts of that plant were found effective in the curing of hepatic injuries (Ansari et al., 1996; Ansari et al., 1993). Gall extracts of P. integerrima (Fig. 1) were also reported to have promising analgesic, hypouricemic, antioxidant, and anti-inflammatory activities (Ahmad et al., 2006; Ahmad et al., 2008). The crude extracts and isolated P. integerrima have been documented for potential anticancer, antioxidant, and xanthine oxidase inhibitory effects (Bibi et al., 2012; Ahmad et al., 2010). Various classes of bioactive secondary metabolites such as teraponids, sterols, flavonoids, phenolic compounds, and dihydromalvalic acid have been isolated from the different parts of P. integerrima (Zahoor et al., 2018; Joshi et al., 2010; Ahmad et al., 2020). These secondary metabolites have been reported to show anticancer, analgesic, antioxidant, and enzyme-inhibitory activities (Rauf et al., 2017a, 2017b, 2017c; Rauf et al., 2017a). Various class of compounds such as triterpenes and flavonoids has been isolated from various part of Pistacia integerrima which are reported as anticancer (Rauf et al., 2017b), muscle relaxant (Rauf et al., 2018; Rauf and Patel, 2017), analgesic, phosphodiesterase, glucosidase activities (Alhumaydhi et al., 2021; Bawazeer et al., 2021). The phytochemicals isolated from galls of Pistacia integerrima also exhibited excellent antimicrobial and α-glycosidase activity. Hemeg et al., 2022a; Hemeg et al.,2022b). Here, we report on the in vitro leishmanicidal potential of P. integerrima ethyl acetate extracts/compounds and the in silico study of two flavonoids 1 and 2.
Image of Pistacia integerrima Stew ex Brandis.
2 Materials and methods
2.1 Plant collection
Galls of Pistacia integerrima were collected in May 2021, from Tehsil Khall District Dir Lower, KP, Pakistan. The galls were selected at maturity. The collected specimen was brought from the Department of Botany University of Swabi, KPK, Pakistan. The identification of the specimen was by Dr. Muhammad Ilyas Department of Botany, University of Swabi, and the voucher specimen number No. UOS/Bot-102 was stored at the Botany University of Swabi, KPK, Pakistan.
2.2 Extraction and isolation
The collected galls (7.54 kg) were washed with water to remove the dust part and then cut into small pieces. These small pieces were dried under shade and converted to powder with the help of a grinder machine. The powder plant material was soaked in 50 L methanol and water (80:20) for 18 days according to the published procedure (Rauf et al., 2022a, 2022b, 2022c; Rauf et al., 2017a, 2017b, 2017c). The obtained extract was concentrated in a rotary evaporator under low temperature and pressure to obtain a dry extract (97 g). The dry extract was then dissolved in minimum amount water (200 mL) and fractionated with the help of a separating funnel to respectively non-polar (Hexane) and polar fractions (Chloroform, ethyl acetate). The ethyl acetate fraction (17.1 g) was subjected to glass column chromatography elution being ensured by a chloroform–methanol mixture (92:8, V/V)) affording 43 subfractions termed AF hereafter. Based on their TLC profiles, the sub-fractions AF-1 to AF-17 were combined and subjected to repeated column chromatograms (CC) using the same solvent mixture chloroform–methanol (92:8, V/V) yielding two compounds 1 (1.20 g; 99.93 % pure) and 2 (1.02 g; 99.91 % pure). Structures of the isolated compounds (1 & 2; Fig. 2) were elucidated by comparing their physical and spectroscopic data (FT-IR, H1 NMR, C13 NMR) with those previously reported (Rauf et al., 2015: Rauf et al., 2022a, 2022b, 2022c).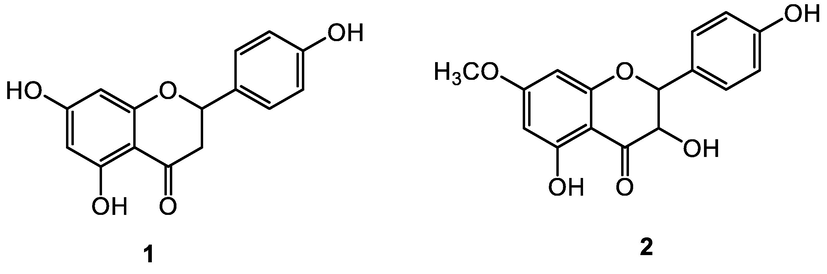
Chemical structures of the two flavonoid constituents 1 and 2 isolated from P. integerrima.
2.3 Leishmanicidal activity
The two isolated compounds were tested for their leishmanicidal activity as per the standard procedure described in the literature (Shah et al., 2019). The two isolated flavonoids were tested against leishmanial spp I, e L. major. The selected species were cultured at 25 ± 2 °C on the RMPI-1640 medium following literature recommendations (Shah et al., 2018). Ten percent heated-inactivated FBS was added to the medium. The culture was centrifuged (2000 rpm) out at the logarithmic growth phase of the promastigote and washed with saline. The culture was diluted with fresh medium (up to 106 cells/mL). The procedure was performed in 96-well microplates. The first row was supplemented with 180 mL of the medium while the rest of the wells were filled with 100 mL. The isolated flavonoids (1 and 2) were diluted in 5 mL DMSO and added to the medium and the pathogen culture was added to all wells. The negative control well was filled with DMSO and the positive control well was filled with the reference antileishmanial drug used in this study, amphotericin-B. The above-filled microplates were properly handled and inoculated for 72 h. After this time, the leishmanicidal potential was counted. The experiment was repeated three times and the inhibition percentage was calculated as per the reported methods (Shah et al., 2018).
2.4 Docking studies
The ChemDraw Ultra 8.0 was used to draw the 2D structures of the two isolated flavonoid constituents, naringenin 1, and the 3,5,4′-trihydroxy-7-methoxy-flavanone 2. The crystal structure of leishmanial Mexicana arginase PDB ID 5HJ9 was retrieved from the PDB (Protein Data Bank) Hai et al., 2016. Energy minimization was fixed and charges were added by the MOE software. The minimized structure was used to dock the compounds in the active site of the receptor. Furthermore, the best score compounds were selected and visualized in the MOE software for protein–ligand interaction analysis (Malayeri et al., 2017).
3 Results
The two flavonoid compounds isolated from P. integerrima were evaluated for their anti-leishmanial activity, as presented in Table 1. Compounds 1 and 2 were tested at a concentration of 5 µg/mL against the selected Leishmanial species. The percent inhibition was 70.43 and 74.76 regarding compounds 1 and 2, respectively with IC50 of 29.43 and 16.43 μM. The reference standard drug used, amphotericin-B, displayed the highest leishmanicidal effect (IC50 of 0.22 μM) with 84.09 % inhibition at half concentration compared to compounds 1 and 2.
Tested sample
Concentration
Percent effect
IC50 (µM)
Ethyl acetate Extract
5 µg/mL
64.09 ± 0.80
41.98 ± 1.98
Compound 1
5 µg/mL
70.43 ± 0.70
29.43 ± 0.23
Compound 2
5 µg/mL
74.76 ± 0.43
16.43 ± 0.65
Amphotericin-B
2.5 µg/mL
84.09 ± 0.40
0.22 ± 0.09
To investigate the nature of the interactions between the two flavonoids and the targets, both compounds were docked into the target binding site (PDB ID: 5HJ9) using MOE 2016 (Mirzaei et al., 2020). The targeted enzyme was arginase, which is an important enzyme for leishmania species’ survival. Arginase up-regulates amastin that impacts amastigote replication and survival. This approach aimed to explore the binding modes of the two isolated flavonoids and to predict their inhibitory activity against arginase. Table 2 shows the overall docking scores and interactions of the tested compounds with arginase. The two flavonoids showed a high binding affinity with arginase though being lower than that of Amphotericin-B. According to the findings, compound 1 docked well in the binding site, with a docking score of −9.28 Fig. 3 (a-c). The oxygen atom of the standard compound forms 2H-bond acceptor interactions with Ser 150 and ASN 143 and one H-donor interaction with the Asp 195 residue of the receptor. The O atom of compound 2 forms two H bond donor interactions with ASP 194 and GLU197. Compound 2 forms one H-bond acceptor interactions and one pi-pi interaction. The O 18 atom of the ligand forms one interaction and 6 ring forms one donor interaction with HIS 139 of the receptor. The interaction images of the two compounds and the reference are shown in Figs. 3-8.
Compound
Interacting residues
Interaction type
Docking score
E (kcal/mol)
Flavonoid 1
ASP 194 HIS 154
H-donor
pi-pi−7.32
−2.7
−0.0
Flavonoid 2
SER 150 HIS 139
H-donor
pi-pi−6.96
−2.8
−0.0
Amphotericin-B
ASP 195 SER 150 ASN 143
H-donor
H-acceptor
H-acceptor−9.70
−1.4
−1.2
−2.0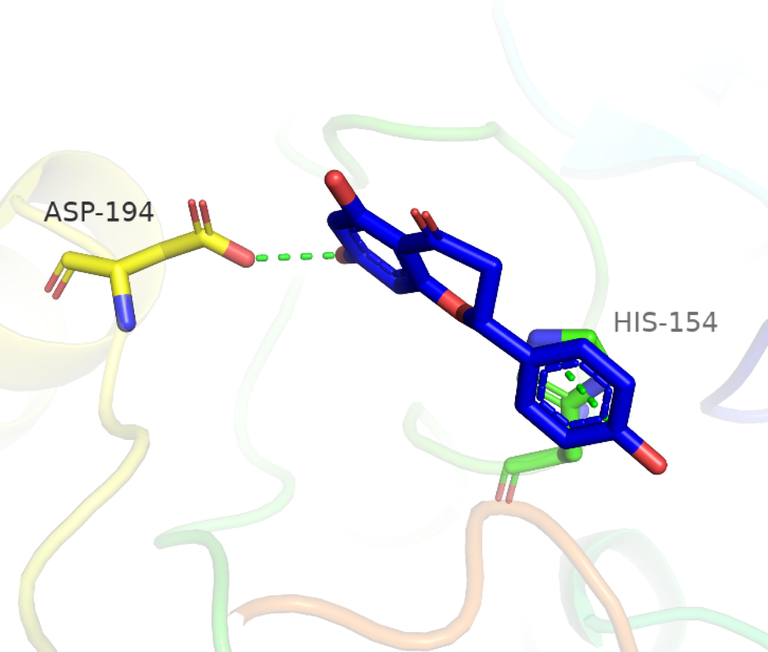
3D interaction of flavonoid 1 with active site residues of arginase.
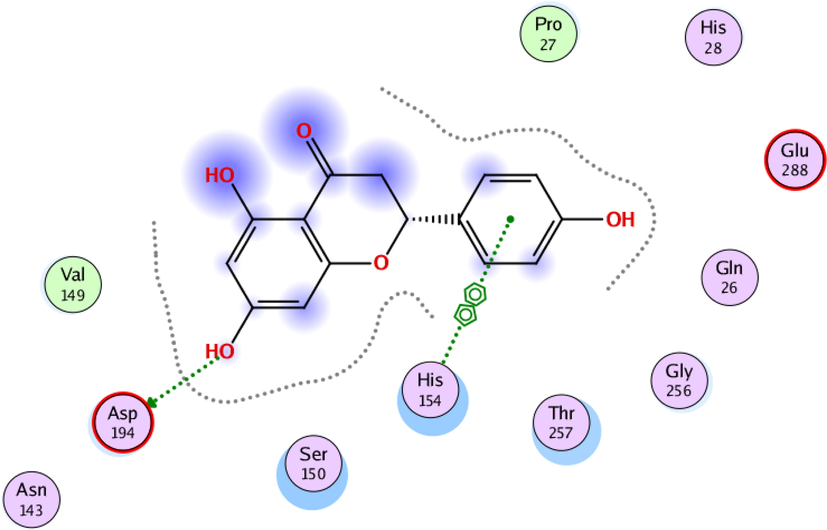
2D interaction of flavonoid 1 with active site residues of arginase.
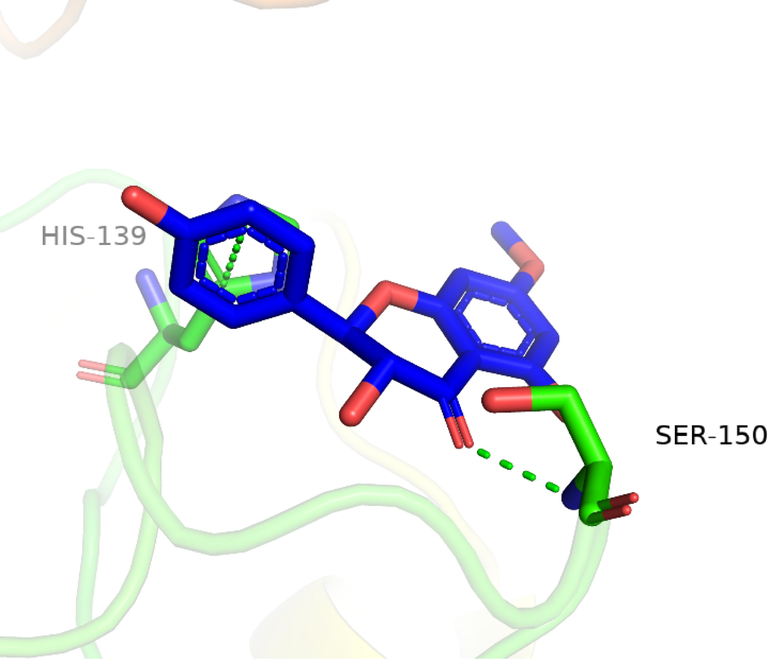
3D interaction of flavonoid 2 with active site residues of arginase.
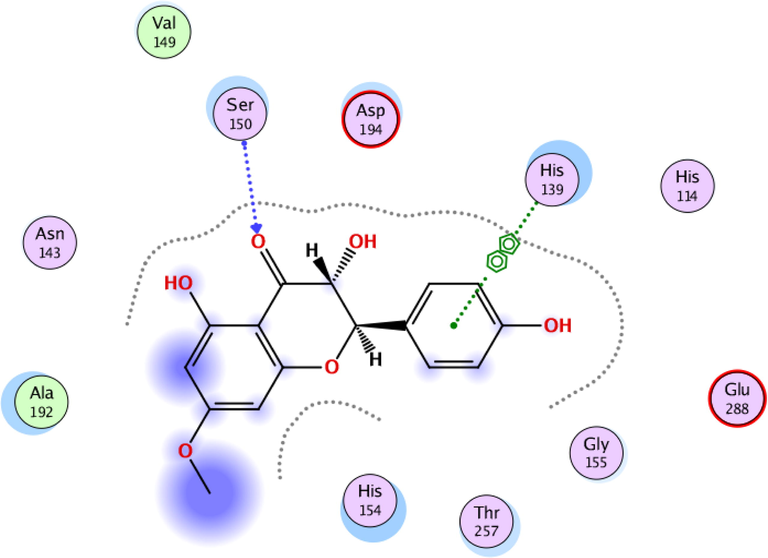
3D interaction of flavonoid 2 with active site residues of arginase.
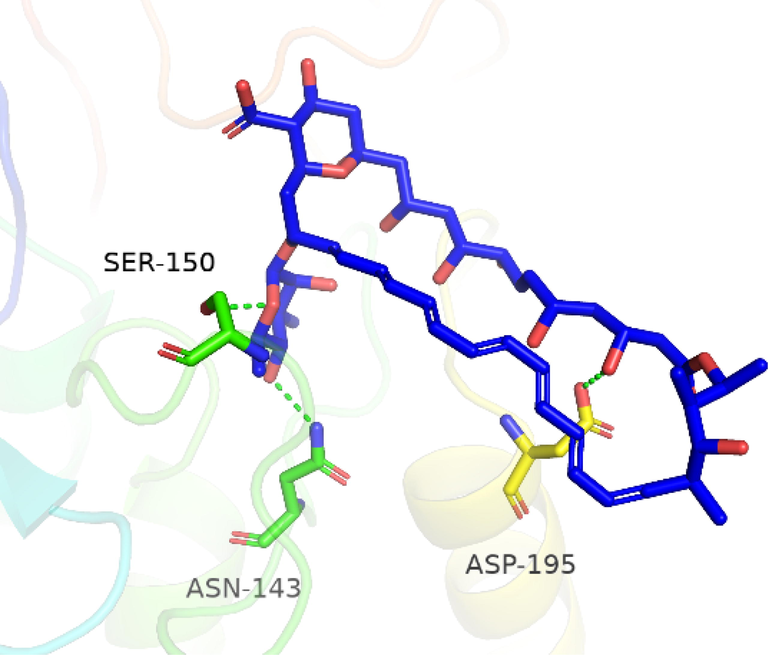
3D interaction of Amphotericin-B with active site residues of arginase.
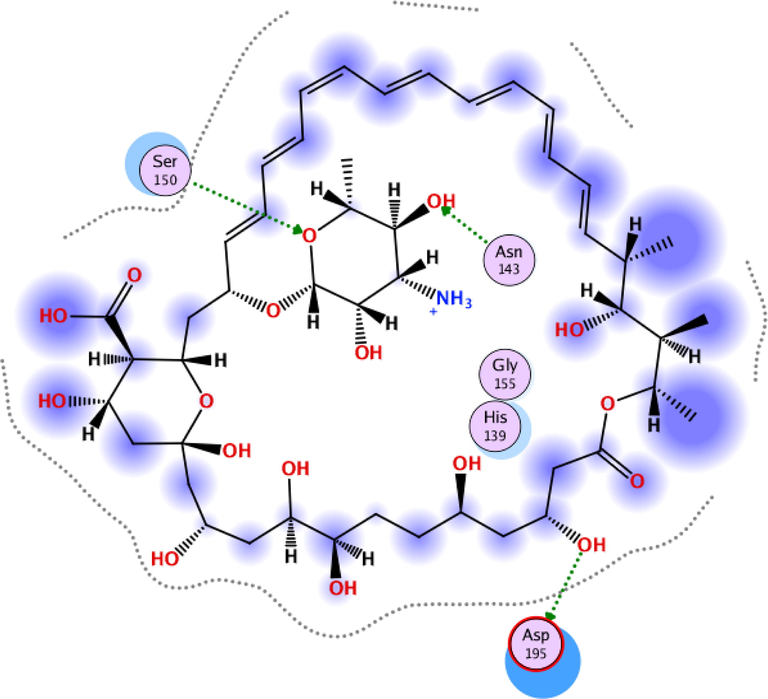
3D interaction of Amphotericin-B with active site residues of arginase.
4 Discussion
Leishmaniasis is one of the parasitic diseases which have affected a large population in various countries around the globe (Tuon et al.,2008). This dermatological disorder is considered as a neglected topical disorder (Hotez et al., 2007). Leishmania infection is attributed to leishmanial parasites and is transmitted by the bite of a sandfly (female phlebotomine) (Killick-Kendrick., 1999). The leishmanial infection is also known as black fever which is typically characterized by weight loss, weakness, fever, splenomegaly, and malaise, etc (Nazzaro et al., 2014). The most severe form of leishmaniosis is visceral, commonly called Kala- azar, while the other forms of leishmaniosis are mucocutaneous and cutaneous, the latter being the most common form of leishmaniosis (Sundar, 2002). There are thirty species of leishmania and according to some reports, human leishmaniasis is caused by 21 species out of 30. The multiple species of any pathogens are mostly responsible for drug resistance and failure of treatment. In addition to the resistance problem, poor patient compliance is also one of the major issues with this infection eradication or curing. These problems encouraged researchers to find safe, effective, and economical medicines for the treatment of this infection. Because it is a general perception that the plants based medicine are free of adverse side effects. In addition to free from toxicity, they are also economical. The current study aimed to investigate a natural remedy for the treatment of leishmaniosis as well as to minimize resistance and increase patient compliance. The two flavonoids isolated from P. integerrima extract demonstrated significant inhibition of the growth of one leishmanial specie. The percent inhibitory potential of these two compounds may be increased by performing a structure–activity relationship (SAR). The SAR might pave the way for new, effective, and economical remedies such as antileishmanial. These two flavonoids with antileishmanial activities isolated from P. integerrima can provide a strong basis for future studies. To investigate the nature of interactions, this study was strengthened by conducting comparative docking studies of the arginase receptor with the two isolated flavonoids and the standard drug. Docking studies demonstrated good qualitative agreement with experimental IC50 values. Both studies concluded that flavonoid 1 interacted more strongly with arginase than flavonoid 2.
5 Conclusion
The plant Pistacia integerrima Stew ex Brandis displays a multi-medicinal potential. The current flavonoids isolated in the present study from that plant, have demonstrated a significant leishmanicidal effect. Further, docking studies of these two compounds and the standard drug used to treat leishmaniosis were performed with the arginase receptor. Docking studies confirmed that the two isolated compounds docked well into the active site of arginase and form strong hydrogen bond interactions with the receptor. So the isolated compounds may possess the potential to inhibit arginase and can be the starting point for potent drugs to treat leishmaniasis.
Data availability
The data connected to this project is given in the text of this paper. The spectroscopic data of naringenin (1) and 3, 5,4’-trihydroxy-7-methoxy-flavanone (2) is available with contact authors upon request.
Acknowledgment
The authors are thankful to the Researchers Supporting Project number (RSP2023R491), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ethnobotany, ethnopharmacology, phytochemistry, biological activities and toxicity of Pistacia chinensis subsp. integerrima: a comprehensive review. Phytother. Res. 2020;34:2793-2819.
- [Google Scholar]
- Activity of polyphenolic plant extracts as scavengers of free radicals and inhibitors of xanthine oxidase. J. Basic. Appl. Sci.. 2006;58:342-344.
- [Google Scholar]
- Pharmacological basis for use of Pistacia integerrima leaves in hyperuricemia and gout. J. Ethnopharmacol.. 2008;117:478-4782.
- [Google Scholar]
- Analgesic and anti-inflammatory effects of Pistacia integerrima extracts in mice. J. Ethnopharmacol. 2010;129:250-253.
- [Google Scholar]
- Introduction of medicinal plants species with the most traditional usage in Alamut region. Iran. J. Pharm. Res.. 2012;11:185-194.
- [Google Scholar]
- In Vivo and In Silico Studies of Flavonoids Isolated from Pistacia integerrima as Potential Antidiarrheal Agents. ACS omega.. 2021;6:15617-15624.
- [Google Scholar]
- A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metabolites.. 2019;9:258.
- [Google Scholar]
- Essential oils of Pistacia integerrima galls and their effect on the central nervous system. Int. J. Pharmacogn. 1993;31:89-95.
- [Google Scholar]
- Analgesic and antiinflammatory activity of tetracyclic triterpenoids isolated from Pistacia integerrima galls. Fitoterapia.. 1996;67:103-105.
- [Google Scholar]
- Isolation of Bioactive Compounds from Pistacia integerrima with Promising Effects on Reverse Cancer Multidrug Resistance. Russ. J. Bioorg. Chem.. 2021;47:997-1003.
- [Google Scholar]
- The study of anticancer and antifungal activities of Pistacia integerrima extract in vitro. Indian. J. Pharm. Sci.. 2012;74:375-379.
- [Google Scholar]
- Glossary of Indian Medicinal Plants (Including the Supplement). New Delhi: Council of Scientific and Indust Res; 1986. p. :79-81.
- Medicinal plants: Traditions of yesterday and drugs of tomorrow. Ecological Ethnobotany: Stumbling Toward New Practices and Paradigms. Mol. Aspects. Med.. 2009;16:1-13.
- [Google Scholar]
- Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol. Asp. Med.. 2006;27:1-93.
- [Google Scholar]
- Crystal structures of Leishmania mexicana arginase complexed with α, α-disubstituted boronic amino-acid inhibitors. Acta Crystallogr. F: Struct. Biols. 2016;72:300-306.
- [Google Scholar]
- In-vitro Leishmanicidal activity and molecular docking simulations of a flavonoid isolated from Pistacia integerrima Stew ex Brandis. J. Food Qual. Article ID. 2022;6003869
- [CrossRef] [Google Scholar]
- In vitro α-glycosidase inhibition and in silico studies of Flavonoids isolated from Pistacia integerrima Stew ex Brandis. BioMed Res. Int. Article ID.. 2022;9636436
- [CrossRef] [Google Scholar]
- In vitro antioxidant and hepatoprotective activity of isolated compounds from Pistacia integerrima. Aust. J. Herb. Med.. 2010;22:94-99.
- [Google Scholar]
- Hepatocurative potentials of Pistacia integerrima in CCl4-treated rats. Sarhad. J. Agri. (Pak). 2004;20:279-285.
- [Google Scholar]
- The biology and control of phlebotomine sand flies. Clin. Dermatol.. 1999;17:279-289.
- [Google Scholar]
- Medicinal plants used by the Tibetan in Shangri-la, Yannan. China. Ethnobiol.. 2009;5:15.
- [Google Scholar]
- Traditional use of herbs, shurbs and trees of Shogran valley, Mansehra. Pakistan. Pak. J. Biol. Sci.. 2001;4:1101-1107.
- [Google Scholar]
- Design, synthesis and biological evaluation of 7-(aryl)-2, 3-dihydro-[1, 4] dioxino [2, 3-g] quinoline derivatives as potential Hsp90 inhibitors and anticancer agents. Bioorg. Med. Chem.. 2017;25(1294–1302):2017.
- [Google Scholar]
- Design, synthesis and biological evaluation of novel 5,6,7-trimethoxy- N -aryl-2-styrylquinolin-4-amines as potential anticancer agents and tubulin polymerization inhibitors. Bioorg. Chem.. 2020;98:103711
- [Google Scholar]
- Traditional usage of Pistacia integerrima galls. Phytopharmacol. Res.. 2022;J.1:17-18.
- [Google Scholar]
- The relevance of folkloric usage of plant galls as medicines: Finding the scientific rationale. Biomed. Pharmacother.. 2018;97:240-247.
- [Google Scholar]
- Phosphodiesterase-1 inhibitory activity of two flavonoids isolated from Pistacia integerrima JL stewart galls. Evid. Based. Complement. Alternat. Med.. 2015;506564
- [CrossRef] [Google Scholar]
- Gastrointestinal Motility and Acute Toxicity of Pistagremic acid Isolated from the Galls of Pistacia integerrima. Med. Chem.. 2017;13:292-294.
- [Google Scholar]
- Fatty acid composition and biological activities of oily fractions from Pistacia integerrima roots. Chem. Nat. Comp. 2017;53:830-833.
- [Google Scholar]
- Phytochemical, ethnomedicinal uses and pharmacological profile of genus Pistacia. Biomed. Pharmacother.. 2017;86:393-404.
- [Google Scholar]
- Pistagremic acid as a broad spectrum natural inhibitor from Pistacia integerrima Stewart. Nat. Prod. Res.. 2017;31:367-368.
- [Google Scholar]
- Muscle relaxant activities of pistagremic acid isolated from Pistacia integerrima. Z. Naturforsch. C. 2018;73:413-416.
- [Google Scholar]
- Potent in vitro Phosphodiesterase 1 Inhibition of Flavone Isolated from Pistacia integerrima Galls. BioMed Res. Int. 2022:2022.
- [Google Scholar]
- Potent in vitro Phosphodiesterase 1 Inhibition of Flavone Isolated from Pistacia integerrima Galls. Biomed. Res. Int. 2022
- [CrossRef] [Google Scholar]
- Enzyme Inhibitory Activities of Extracts and Carpachromene from the Stem of Ficus benghalensis. Biomed Res. Int. Article ID. 2022;7053655
- [CrossRef] [Google Scholar]
- Potent leishmanicidal and antibacterial metabolites from Olea ferruginea. J. Asian Nat. Prod. Res.. 2019;21:679-687.
- [Google Scholar]
- Laboratory diagnosis of visceral leishmaniasis. Clin. Vaccine Immunol.. 2002;9:951-958.
- [Google Scholar]
- Treatment of New World cutaneous leishmaniasis–a systematic review with a meta-analysis. Int. J. Dermatol.. 2008;47:109-124.
- [Google Scholar]
- Density functional theory and phytochemical study of Pistagremic acid. Spectrochim. Acta A. Mol. Biomol. Spectrosc.. 2014;118:210-214.
- [Google Scholar]
- Phytochemical, spectroscopic and density functional theory study of Diospyrin, and non-bonding interactions of Diospyrin with atmospheric gases. Spectrochim. Acta A. Mol. Biomol. Spectrosc.. 2015;141:71-79.
- [Google Scholar]
- Density functional theory and phytochemical study of 8-hydroxyisodiospyrin. J. Mol. Struct. 2015;1095:69-78.
- [Google Scholar]
- Isolation and identification of phenolic antioxidants from Pistacia integerrima gall and their anticholine esterase activities. Heliyon. 2018;4:e01007.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102572.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







