Translate this page into:
Exogenous methyl jasmonate (MeJA) altered phytochemical composition and enhanced the expression of PatAACT gene of in vitro culture-derived patchouli var. Sidikalang (Pogostemon cablin Benth.)
⁎Corresponding author at: Department of Biology, Institut Teknologi Sepuluh Nopember, Surabaya, 60111, Indonesia. nuruljadid@bio.its.ac.id (Nurul Jadid)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The demand for economically valuable terpenoid-derived compounds, especially those extracted from patchouli plants, is experiencing exponential growth. These compounds are potentially applied in a wide range of industrial sectors including food manufacturing, perfumeries, and pharmaceuticals. Nevertheless, there is a need for further development in patchouli plant cultivation to align with market demands. Therefore, employing robust in vitro-based propagation methods could offer significant advantages. Furthermore, this technique is also used for plant metabolites enhancement. This strategy applies the methyl jasmonate (MeJA) elicitor to boost the production of secondary metabolites. In Indonesia, patchouli var. Sidikalang is extensively cultivated in many areas. This study aims to evaluate the effect of naphtaleneacetic acid (NAA) and 6-benzylamino purine (BAP) to increase plant growth via in vitro culture. Furthermore, we also investigate the effect of MeJA on the metabolite profiles and on the AACT gene expression. Our findings revealed that the combination of 2.5 µM of BAP and 0.25 µM of NAA resulted in optimal growth and high rate of organogenesis across all growth parameters. Our data also successfully identified 50 dominant compounds, each with varying percentage areas. Notably, patchouli alcohol emerged as the primary compound in the tricyclic sesquiterpene group, consistently present in all treatment groups. Furthermore, the expression of the PatAACT gene significantly increased by 7.42-fold after treatment with MeJA at 100 µM and by 2,9-fold after treatment with MeJA at 300 µM, compared to the control. Altogether, our findings might offer new insight in P. cablin propagation and new strategy for patchouli-derived metabolites using exogenous MeJA.
Keywords
Elicitor
Hormone
Methyl jasmonate
Patchouli oil
Pogostemon cablin var. Sidikalang
1 Introduction
Indonesia hosts around eighty percent of the global medicinal plant species. However, many species still need to be explored, even though some of them have been scientifically verified (Jadid et al., 2018a). Patchouli (Pogostemon cablin Benth.) is a Lamiaceae aromatic tropical plant possessing.. around 140 natural compounds including terpenoid, flavonoid and saponin derivatives (Swamy & Sinniah, 2016). The main component of essential oil is sesquiterpenes, where tricyclic sesquiterpenes (patchouli alcohol) are the most common compounds. To date, around 15 identified patchouli oil constituents have been identified, with the five most prominent components include patchouli alcohol (33 %), δ-guaiena (23 %), α-guaiena (16 %), seychellena (6.9 %), and α-patchoulena (5.5 %) (Chen et al., 2019).
Patchouli essential oil (PEO) is used commercially in the manufacture of perfumes, cosmetics and aromatherapy which gives a calming effect. In addition, the biological effects of patchouli include antioxidant, pain relieving, anti-inflammatory, anticoagulant and anti-microbial activities (Swamy et al., 2010). The world's largest producer of patchouli oil is Indonesia, supplying between 75 % to 90 % of the global demand on an annual basis. In addition, Indonesia's patchouli exports fluctuate with an export increase rate of 700 tons to 2,000 tons of patchouli oil per year (Astuti et al., 2022).
Conventional patchouli oil production can be done through direct extraction from plant organs. However, the availability of the plant become the major limitation. Furthermore, the widespread production of patchouli has been constrained by the reoccurrence of issues such as pathogenic virus, nematodes, and insects. Vegetative cutting for propagation is a gradual process and proves inadequate for extensive-scale cultivation. Therefore, plant tissue culture techniques could be an alternative to mass propragate the patchouli plants (Swamy et al., 2010).
Direct regeneration of shoots is one method of in vitro plant propagation that provides several advantages, including high propagation rates, and low somaclonal variation frequency. In many cases, the use of plant growth regulators such as cytokinin (6- benzylaminopurine (BAP)) and auxin (naphthaleneacetic acid (NAA)) might accelerate proliferation of the plants (Hardjo et al., 2019; Jadid et al., 2024). Paul et al. (2010) reported that the addition of combined NAA and BAP increased the number of shoots in patchouli in vitro propagation and shoot elongation. However, several studies have shown that different plant varieties have different responses to the application of plant growth regulators (Avila-Victor, 2023).
Some studies showed that abiotic elicitors might enhance the production of plant natural products. One of the abiotic elicitors commonly used is methyl jasmonate (MeJA), which could be applied through in vitro or in vivo (Wang et al., 2023b). However, in vivo application offers an effective and economically efficient (Ahmed et al., 2020). Gas chromatography and gene expression analysis are used to determine the plant phytochemical content (Wang et al., 2023a). In patchouli plants, the biosynthesis of patchoulol, a key sesquiterpene compound, is facilitated through the mevalonate diphosphate (MVD) pathway, involving crucial enzymes encoded by the AACT (Acetyl-CoA acetyltransferase), HMGR (3-hydroxy-3-methylglutaryl-coenzyme A reductase), and MVD (Mevalonate diphosphate decarboxylase) genes. Among those enzymes, AACT initiates the pathway by catalyzing the condensation of acetyl-CoA molecules to form acetoacetyl-CoA, a precursor essential for terpenoid synthesis. However, these genes work together to orchestrate the MVD pathway, providing the necessary substrates and enzymatic reactions for the production of patchoulol in patchouli plants (Tang et al., 2019).
Previous studies revealed that MeJA induces the expression of PatAACT, PatHMGR, and PatMVD genes (Chen et al., 2020). However, the expression of these genes is generally specific to each individual plant genotypes. There is currently no reported study on the expression of genes associated with patchouli oil biosynthesis in the Sidikalang variety of patchouli. Therefore, this study was conducted to in vitro propagate, determine the effect of MeJA elicitor on the secondary metabolite profiles and the expression of PatAACT of Pogostemon cablin var. Sidikalang.
2 Material and methods
2.1 Establishment of culture medium and in vitro inoculation of plant explants
Pogostemon cablin var. Sidikalang was obtained from the Indonesian Sweetener and Fiber Crops Research Institute, and nodal segments of the plant were used as explants. The explants underwent surface sterilization and were initially inoculated in the MS medium. Subsequently, the resulting plantlets were used as secondary explant sources for this present study.
The solid MS culture medium was prepared by mixing 4.4 g/L MS media (PhytoTech Lab®, USA), 30 g/L of sucrose (Duchefa Biochemie, Netherland), and solidifying the medium with 8 g/L of gelrite powder (PhytoTech Lab®, USA). A series of combined BAP (0; 1.25; 2.5 μM) and NAA (0; 0.25; 0.5 μM) concentrations were applied to the MS medium. The combination of both BAP (S) and NAA (A) were coded as: S1A1, 0 μM of BAP and 0 μM of NAA; S1A2, 0 μM of BAP and 0.25 μM of NAA; S1A3, 0 μM of BAP and 0.5 μM of NAA; S2A1, 1.25 μM of BAP and 0 μM of NAA; S2A2, 1.25 μM of BAP and 0.25 μM of NAA, S2A3, 1.25 μM of BAP and 0.5 μM of NAA; S3A1, 2.5 μM of BAP and 0 μM of NAA; S3A2, 2.5 μM of BAP and 0.25 μM of NAA; S3A3, 2.5 μM of BAP and 0.5 μM of NAA. Finally, the axenic secondary explant derived from nodal segment were inoculated in the culture medium and cultured at room culture at ± 25⁰ C with 16 h light of photoperiod for 50 days. Each treatment consisted of three replications.
2.2 Plant growth measurement and data analysis
The growth response was determined based on shoot formation and callogenic frequency according to Jadid et al. (2024). The equations used in this study are as follows:
2.3 Methyl jasmonate treatment and patchouli biomass measurement
The application of MeJA (Sigma-Aldrich, USA) was carried out using the hydroponic system (Deschamps and Simon, 2006). Hydroponic solution was prepared using 12.5 % MS basal medium. MeJA was dissolved using ethanol (Merck, Germany) and diluted with distilled water to make 100 and 300 µM solutions. Planlets were firstly grown in greenhouse for two weeks before being transferred to hydroponic media supplemented with MeJA for up to seven days. Measurement of patchouli plant biomass is carried out before and after being treated with MeJA. Then, the measurement data was analyzed using the Paired T-test (Minitab 19).
2.4 Patchouli oil extraction using sonication
600 mg of patchouli leaf samples were submerged in liquid nitrogen for 5 min, then crushed with a mortar to obtain sample powder. The powder was mixed with 3 mL of ethyl acetate (Merck, Germany), followed by sonication for 20 min (Fisher Scientific FB 15051). The extract was filtered using WhatmanTM filter paper (Sigma Aldrich, USA) and then collected. It was subsequently centrifuged at 6000 rpm (Thermo Scientific MySPIN 6) for 5 min at ambient temperature. The supernatant was taken and then added with anhydrous sodium sulfate solid (Merck, Germany). The clear greenish extract was collected in a glass bottle for further analysis using Gas Chromatography Mass Spectrometry (GC/MS) (Shimadzu QP2010-Plus) (Yadav et al., 2017).
2.5 Qualitative analysis of the extract using gas chromatography-mass spectroscopy
Patchouli oil was analyzed using GCMS 2010 system (Shimadzu QP2010-Plus) with an RTx- 5MS column (30 m × 0.25 mm × 0.25 µm). The condition used inlcude: injector temperature at 200 °C, helium as carrier gas, injection vol. 0.2 µL with an injection split of 1:200, at 60.5 kPa, flow rate of 13.9 mL/min, flow rate of 0.99 mL/min, linear velocity of 36.5 cm/sec and a purge flow rate of 3 mL/min. The initial temperature of the column oven was at 70 °C, then augmented by 15 °C/min to 100 °C, with 2 min hold time, followed by a 2 °C/min increase to 160 °C, with 2 min hold time, and finally ramped up by 20 °C/min to 250 °C with a 6 min hold time, resulting in a total program time is 46.5 min. The MS specifications include the ion source temperature at 200 °C, interphase temperature at 250 °C, and detector voltage at 0.70 kV; with a 0.5 s duration and a mass range of 40–550 m/z. Fragment ions (m/z SIM) utilized were 95, 124, 161 and 222 (Yadav et al., 2017).
2.6 Extraction of total RNA and gene expression analysis
Patchouli leaves were collected after seven days of MeJA treatment. The total RNA extraction was performed according to Jadid et al. (2016) and was quantified using Nano Drop (Thermo Scientific™ NanoDrop 2000, USA) (Jadid et al., 2018b). RT-qPCR was carried out utilizing 2x-SensiFAST™ SYBR-No-ROX One-Step Mix (Meridian Bioscience, USA). We measured the expression of PatAACT using the following primers: PatAACT-F (5′- TGTGCCTCTGGAATGAAAGCA–3′) and PatAACT-R (5′– CAGCATTCCATCAACCAGCG – 3′). The reference gene used in this study was P. cablin Actin gene ACT-Forward (5′ − CGACTC TGGTGATGGTGTCAG – 3′) and ACT-Reverse (5′ – CGAGAGCAATGTAGGCTAGC–3′). The reaction of RT-qPCR conditions was: 10 min for reverse transcription at 45 °C, followed by 2 min of polymerase activation at 95 °C. Finally, about 39 cycles of PCR were performed at 95 °C (5 s); 61.3 °C (10 s) and 72 °C (20 s) for denaturation, annealing and elongation, respectively. All samples were in triplicate and analyzed according to 2-ΔΔCq method.
3 Results
3.1 Impact of combined BAP and NAA on organogenesis and callogenesis responses
Different combination of BAP and NAA exhibited distinct shoot and root formation responses after 50 days of incubation. Low percentage of explants (0 to 80 %) forming roots was detected in almost all treatments (Table 1). We noted that axenic nodal explants placed in S2A1 and S3A3 treatments were not able to produce root (Fig. 1C-D). The highest percentage of rooted explants (80 %) was shown by treatment S1A2 (BAP 0 μM + NAA 0.25 μM) followed by S1A1 treatment (60 %) (Fig. 1A-B). Meanwhile, all treatments were able to induce shoot formation (varied from 50 to 100 %). The lowest shoot induction respon was demonstrated in S2A2 treatment. All nodal segment explants also exhibited callus formation. The presence of BAP in the culture medium with or without NAA apparently favored callus formation (Table 1). In contrast, zero supplementation of BAP resulted in low frequency of callus formation, which ranged from 20 to 80 %. Note: PGRs: plant growth regulators; BAP: 6-benzylamino purine; NAA: naphtaleneacetic acid.
Treatments
PGRs Concentration (μM)
Percentage of root formation (%)
Percentage of
shoot formation (%)
Percentage of callogenesis (%)
BAP
NAA
S1A1
0
0
60
80
60
S1A2
0
0.25
80
100
20
S1A3
0
0.5
0
100
80
S2A1
1.25
0
0
100
100
S2A2
1.25
0.25
50
50
100
S2A3
1.25
0.5
40
80
100
S3A1
2.5
0
20
100
100
S3A2
2.5
0.25
20
100
100
S3A3
2.5
0.5
0
100
100

Effect of combined BAP and NAA on organogenesis and callogenesis after 50 days of incoulation. A: S1A1; B: S1A2; C: S3A3; D: S2A1; E: S3A2; F: S1A3. 1: root; 2: callus; 3: adventitious shoots. (Scale bar = 1 cm). Abbreviations: BAP, 6-benzyl amino purine; NAA, naphtaleneacetic acid; S1A1 (control), 0 μM of BAP and 0 μM of NAA; S1A2, 0 μM of BAP and 0.25 μM of NAA; S3A3, 2.5 μM of BAP and 0.5 μM of NAA; S2A1, 1.25 μM of BAP and 0 μM of NAA; S3A2, 2.5 μM of BAP and 0.25 μM of NAA; S1A3, 0 μM of BAP and 0.5 μM of NAA.
3.2 Effect of BAP and NAA combination on root, shoot and leaves proliferation
Combination of BAP and NAA siginificantly influenced roots, shoots, and leaves proliferation on P. cablin in vitro culture (p-values < 0.05) (Table 2). Highest number of root (6.3 roots per explants) were observed in S1A2 treatment, followed by treatment with 1.25 μM of BAP and 0.25 μM of NAA (S2A2) (3.7 roots per explants). These results suggest that 0.25 μM of NAA is required to induce P. cablin root proliferation in vitro. Meanwhile, high level of NAA (0.5 μM) concentration inhibited root proliferation (Table 2). We noted that three treatments resulted in zero root proliferation. They include the S1A3 treatment (0 μM BAP + 0.5 μM NAA), S2A1 (1.25 μM BAP + 0 μM NAA) and S3A3 (2.5 μM BAP + 0.5 μM NAA). *Mean values with different letters in the same column are significant at p-value ≤ 0.05 based on One Way ANOVA and Tukey Test with 95 % confidence.
Treatments
Average number of roots ± SD
Average number of shoots ± SD
Average number of leaves ± SD
S1A1
0.3 ± 0.58ab
2.0 ± 1.00c
10.3 ± 3.21e
S1A2
6.3 ± 1.51a
2.0 ± 0.00c
11.3 ± 1.53de
S1A3
0.0 ± 0.00b
3.5 ± 1.12abc
19.5 ± 6.36cde
S2A1
0.0 ± 0.00b
2.3 ± 1.53bc
21.7 ± 2.08bc
S2A2
3.7 ± 1.73ab
6.5 ± 1.12ab
32.5 ± 0.71ab
S2A3
1.0 ± 0.02ab
5.7 ± 0.58abc
22.7 ± 3.06bc
S3A1
1.0 ± 0.23ab
2.0 ± 1.00c
20.7 ± 3.06 cd
S3A2
0.7 ± 0.05ab
7.0 ± 1.83a
42.5 ± 2.12a
S3A3
0.0 ± 0.00b
3.0 ± 0.00abc
25.0 ± 5.29bc
All treatments induced shoot proliferation, which varied between 2.0 and 7.0 shoots per explants. Our results showed the highest average number of shoots (7.0 shoots per explants) in the S3A2 treatment (2.5 μM BAP + 0.25 μM NAA). Meanwhile, the lowest average of shoot number (2.0 shoots per explants) was obtained in three treatments, including S1A1 (0 μM BAP + 0 μM NAA), S1A2 (0 μM BAP + 0.25 μM NAA) and S3A1 (2.5 μM BAP + 0 μM NAA). Our results also demonstrate that shoot proliferation requires higher cytokinin (BAP) concentration than auxin (NAA).
BAP and NAA treatments also affected in vitro patchouli leaves production (p-values < 0.05). The production of patchouli leaves was consistent with shoot proliferation. The number of leaves ranged from 10.3 to 42.5 leaves per explants (Table 2). Highest number of leaves was obtained from S3A2 (BAP 2.5 μM + NAA 0.25 μM) (Fig. 2). Meanwhile, treatment S1A1 demonstrated the lowest number of leaves (10.3 leaves per explants). Furthermore, we highlighted that higher amount of BAP concentration, compared to NAA, is responsible for the formation and proliferation of patchouli leaves.
Effect of combined BAP and NAA on leaf formation after 50 days of incoulation. A: S1A1 (control); B: S1A2; C: S2A2; D: S3A2. 1: leaf. (Scale bar = 1 cm). Abbreviations: BAP, 6-benzyl amino purine; NAA, naphtaleneacetic acid; S1A1 (control), 0 μM of BAP and 0 μM of NAA; S1A2, 0 μM of BAP and 0.25 μM of NAA; S2A2, 1.25 μM of BAP and 0.25 μM of NAA, S3A2, 2.5 μM of BAP and 0.25 μM of NAA.
3.3 Short term exogenous MeJA treatment did not affect the growth of patchouli derived from in vitro culture
Patchouli plant obtained from in vitro culture were then moved to the greenhouse and grown in polybag for two weeks before being placed into the hydroponic container containing 12.5 % MS medium with 0 μM, 100 μM and 300 μM of MeJA. The fresh weight of the patcouli plants was measured before and after MeJA treatment. Our results demonstrated that MeJA did not statistically affect fresh weight of the patchouli plants (Fig. 3). Slight reduction of fresh weight was observed in patchouli plants treated with 0 μM and 300 μM of MeJA. In addition, plants treated with 100 μM of MeJA demonstrated more reduction (20.7 %) in fresh weight compared to other treatments (Fig. 3).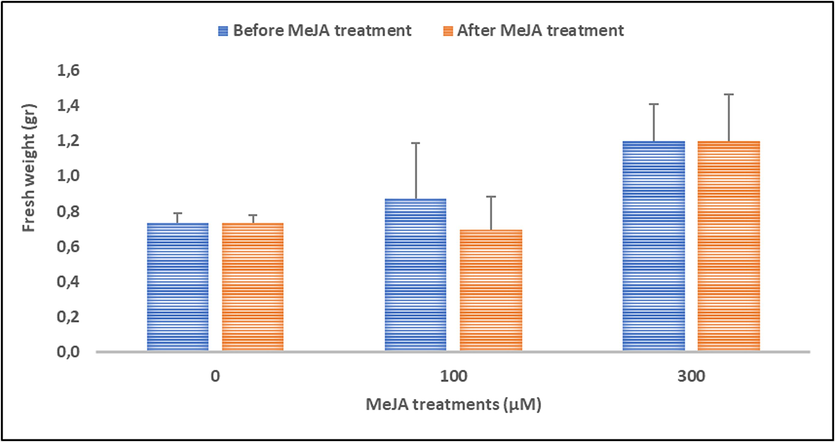
Effect of Methyl Jasmonate (MeJA) treatment on the fresh weight of patchouli plants. Blue and red histograms represent fresh weight of patchouli plants before and after MeJA treatments, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4 Effect of MeJA on phytochemical composition of p. Cablin var. Sidikalang
Following the MeJA treatment, leaves of the treated P. cablin plants were subjected to phytochemical composition analysis using GC–MS. We obtained 50 compounds detected during analysis (Suppl. 1). Nevertheless, among those compounds, 16 metabolites were similarly found in all treatments with distinct percentage of areas (Table 3). It indicated different concentration of each metabolite. We noted that 1,2,3,3-D4-Trans-1,2-Dihydroxy-Cyclopentane covered around 63.55 % of peak area in 0 μM of MeJa, followed by 100 μM and 300 μM of MeJA representing 67.73 % and 64.19 % of peak areas, respectively. Other metabolites found in high percentage of areas were 1,3,5-Cycloheptatriene and 2H-Pyran-2-one, 3-acetyl-4-hydroxy-6-methyl-. Meanwhile, lowest percentage of area covered in the chromatogram (Suppl. 2). We also identified patchouli alcohol in all MeJA treatments (0; 100 and 300 μM) (Table 3). This compound signifies the presence of patchouli oils in the leaves extract of patchouli plants treated with MeJA.
Name of the compounds
0 μM
100 μM
300 μM
Peak Area (%)
%Area
%Area
1,2,3,3-D4-Trans-1,2- Dihydroxy-Cyclopentane
63.55
67.73
64.19
1,3,5-Cycloheptatriene (CAS)
12.63
9.14
8.43
2-Pentanone, 4-Hydroxy-4- Methyl-
1.97
2.36
2.25
1-Pentene, 4,4-dimethyl-1,3- diphenyl-1-(trimethylsilyloxy)-
0.83
0.40
0.44
1-Octen-3-ol (CAS)
0.32
0.31
0.34
Neophytadiene
0.24
0.24
0.23
Benzoic acid, 2-hydroxy-, methyl ester (CAS)
0.47
0.46
0.45
α-Guaiene
1.31
0.37
0.74
Seychellene (CAS)
0.51
0.15
0.29
α-Patchoulene (CAS)
0.39
0.11
0.20
Cyclohexanol, 4-(1,1-dimethylethyl)- (CAS)
0.09
0.08
0.09
Pentadecane (CAS)
1.36
1.93
2.07
Cyclohexanone, 2,3,3-trimethyl-2-(3-methyl-1,3-butadienyl)-, (Z)- (CAS)
0.12
0.07
0.14
Patchouli alcohol
2.49
0.72
1.39
2H-Pyran-2-one, 3-acetyl-4-hydroxy-6-methyl-
2.99
2.58
5.39
1H-Purin-6-amine, [(2-fluorophenyl) methyl]- (CAS)
0.06
0.13
0.05
Our analysis also discovered 18 natural compounds found exclusively in patchouli plants with no MeJA treatment (Table 4). In the 0 μM MeJA treatment, the compounds with the largest peak areas were 1-trimethylsilyloxy-2-(3′-methoxy-4′-trimethylsilyloxyphenyl) ethane (0.41 %), 2,2,3-Trimethyloxirane (0.30 %), and N-(1-benzofuran-5-yl) acetamide (0.32 %). Conversely, tetradecane had the smallest peak area at 0.04 %. Overall, these compounds (Table 4) were detected between retention times of 3.23 and 46.04 min (Suppl. 2).
Name of the compounds
Retention Time
Peak area (%)
(min)
%Area
1-trimethylsilyloxy-2-(3′-methoxy-4′-trimethylsilyloxyphenyl) ethane
3.23
0.41
Oxirane, trimethyl- (CAS)
3.31
0.30
Acetamide, N-(5-benzofuroxanyl)-
3.41
0.32
3,9-Dioxa-6-thiaundecane, 2,10-dimethyl-
3.89
0.20
Derivatized melilotate
4.26
0.14
Cyclopentasiloxane, decamethyl- (CAS)
6.72
0.05
Tetradecane (CAS)
17.76
0.04
α-humulene (CAS)
20.51
0.08
Patchoulene (CAS)
20.96
0.13
2H-Pyran, 2-(7-heptadecynyloxy) tetrahydro- (CAS)
21.63
0.07
Kauren-18-ol, acetate, (4β)- (CAS)
22.86
0.05
1,2-Benzenedicarboxylic acid, diethyl ester (CAS)
27.61
0.08
1,1,4,7-tetramethyldecahydro-1 h-cyclopropa[e]azulen-4-ol
31.38
0.19
Hexadecane, 2,6,11,15-tetramethyl- (CAS)
32.19
0.05
Heptadec-8-ene
32.82
0.07
Tetracosamethylcyclododecasiloxane
41.54
0.05
Quercetin 7,3′,4′-trimethoxy
45.68
0.07
Eicosamethylcyclodecasiloxane
46.04
0.19
We discovered 26 compounds that were found only in patchouli plants treated with 100 µM of MeJA (Table 5). The retention time of these metabolites ranged from 2.32 to 45.18 min (Suppl. 2). The compounds with the highest peak area in the 100 µM MeJA treatment were Formic acid, butyl ester (3.42 %), Butanal, 3-hydroxy (0.54 %) and Pentanal, 3-(acetyloxy)-2,2,4-trimethyl- (0.45 %). While the compounds with the lowest peak area are Trans-Caryophyllene (0.06 %), Propanal, 3- hexylimino-2-nitro- (0.06 %) and Pentanal (0.06 %).
Name of the compounds
Retention time (min)
Peak area (%)
Formic acid, butyl ester (CAS)
2.32
3.42
Butanal, 3-hydroxy- (CAS)
3.24
0.54
O-methoxy-α, α-dimethylbenzyl alcohol
3.41
0.38
Pentanal, 3-(acetyloxy)-2,2,4-trimethyl- (CAS)
3.51
0.45
4-Aminobutyraldehyde diethyl acetal
3.91
0.28
3-octanol (cas)
3.99
0.13
Benzene, 1,3-dichloro- (CAS)
4.44
0.31
Trans-Caryophyllene
18.69
0.06
Hexadecane, 1-chloro- (CAS)
21.16
0.07
3–2-Valeryl-5-methyl-1,2,4-cyclopentane-trione
27.41
0.14
Octadecane (CAS)
28.68
0.18
1-heptadecanol (cas)
32.84
0.08
Cyclohexane, 1,5-diisopropyl-2,3-dimethyl-
33.41
0.12
Octadecane (CAS)
34.18
0.21
Propanal,3-hexylimino-2-nitro-
39.98
0.06
1r-4 t-acetamido-2,3t-epoxy-cyclohexanol
40.38
0.14
Octadecane, 5-methyl- (CAS)
40.52
0.09
Methyl 10-methoxycarbonyl-17- oxooctadecanoate
40.74
0.18
2-hydroxy-3-(tetradecanoyloxy)propyl Myristate
41.10
0.24
Acetonitrile, 2-[4,6-bis(dimethylamino)-1,3,5-triazin-2-yloxy]-
41.33
0.12
3-Methyl-1,1-cyclobutanedicarboxylic acid
41.86
0.09
2-hydroxy-3-(tetradecanoyloxy)propyl Myristate
42.29
0.12
Pentanal (CAS)
42.43
0.06
N,N-Diethyl allylthiourea
42.53
0.22
2-hydroxy-3-(tetradecanoyloxy)propyl
Myristate42.68
0.22
Hexanedioic acid, dioctyl ester (CAS)
45.18
0.15
Another 21 compounds were obtained exclusively from patchouli treated with 300 µM (Table 6). Their retention time ranged from 2.30 to 46.03 min (Suppl. 2). The compounds with the highest area in the 300 µM MeJA treatment were Acetic acid, butyl ester (3.79 %), Cyclopentene, 1-ethenyl-3-methylene (1.77 %) and Xylopyranoside, methyl 4-azido-4-deoxy-,β.-L- (0.71 %). While the compounds with the lowest area were Cyclooctene, 3- methyl- (0.05 %) and 1,2-Ethanediamine, N,N'-bis(2-aminoethyl)- (0.05 %) (Table 6). These overall results suggest that short term exogenous treatment of methyl jasmonate might alter phytochemical composition of patchouli var. Sidikalang.
Name of the compounds
Retentian time (min)
Peak area (%)
Acetic acid, butyl ester (CAS)
2.30
3.79
Cyclopentene, 1-ethenyl-3-methylene-
2.96
1.77
Xylopyranoside, methyl 4-azido-4-deoxy-, β-L- (CAS)
3.29
0.71
Acetamide, N-(6,7-dihydro-6-oxo-1H- purin-2-yl)-
3.41
0.70
Ethyl amyl carbinol
4.00
0.40
Benzene, 1,2,4-trimethyl-
4.09
0.16
Benzene, 1,4-dichloro- (CAS)
4.43
0.38
Dodecane, 4,6-dimethyl-
17.77
0.06
α-gurjunene (cas)
20.96
0.06
1-Heptadec-1-ynyl-cyclohexanol
22.30
0.06
Cyclopentaneacetic acid, 3-oxo-2-(2-pentenyl)-, methyl ester, [1α, 2α (Z)]-
30.66
0.07
Pogostol
31.38
0.12
Hexadecane, 2-methyl- (CAS)
32.17
0.06
Cyclooctene, 3-methyl-
32.36
0.05
1-pentadecanol (cas)
32.83
0.11
Inacid
35.31
0.14
1,2-Ethanediamine, N,N'-bis(2-aminoethyl)-
40.59
0.05
Trans-cyclopenten-3,4-diol
40.96
0.06
Cyclononasiloxane, octadecamethyl
42.87
0.06
Hexanedioic acid, bis(2-ethylhexyl) ester (CAS)
45.20
0.07
Silicone oil
46.03
0.14
3.5 Short term of exogenous methyl jasmonate treatment enhanced the expression of Acetyl-CoA acyltransferase (AACT) gene
The PatAACT gene expression of P. cablin treated plants was analyzed using qRT-PCR. Our results showed that 100 µM and 300 µM of MeJA treatments greatly increased the expression of the PatAACT gene (Fig. 4). 100 µM of MeJA was able to induce the expression of PatAACT 7.42 folds compared to untreated plants. While the PatAACT gene expression was also increased by 2.9 folds after 300 µM of MeJA treatment, compared to control plants.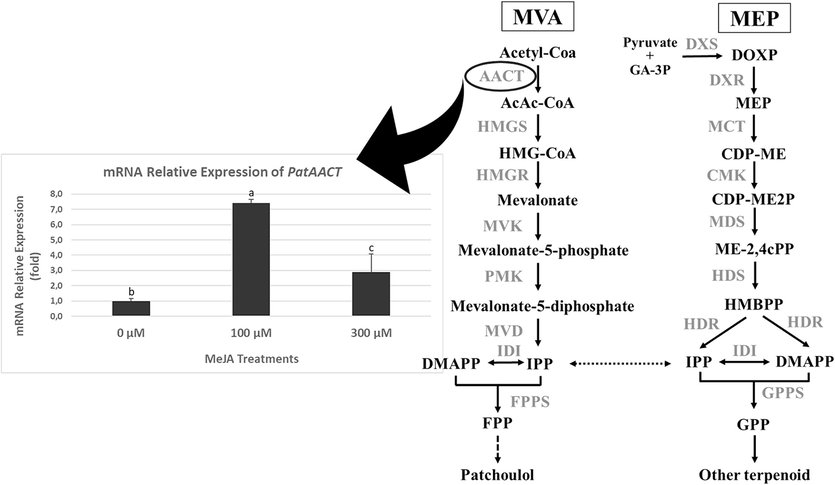
The PatAACT gene expression of Pogostemon cablin after short-term treatment using methyl jasmonate (MeJA) and Biosynthetic pathway of patchoulol (adapted from Tang et al., 2019). Abbreviations: MVA, mevalonate; MEP, methylerythritol-4-phosphate; AACT, acetoacetyl-CoA thiolase; HMGS, 3-hydroxy-3-methylglutaryl-CoA synthase; HMGR, 3-hydroxy-3-methylglutaryl-CoA reductase; MVK, mevalonate kinase; PMK, 5-phosphomevalonate kinase; MVD, 5-diphosphomevalonate decarboxylase; IDI, isopentenyl diphosphate-dimethylallyl diphosphate isomerase; FPPS, farnesyl diphosphate synthase; DXS, 1-deoxy-D-xylulose-5-phosphate synthase; DXR, 1-deoxy- D-xylulose-5-phosphate reductoisomerase; MCT, 2-C-methyl- D-erythritol-4-phosphate cytidyl transferase; CMK, 4-(cytidine 50 −diphospho)-2-C-methyl-D −erythritol kinase; MDS, 2-C-methyl-D −erythritol-2,4-cyclodiphosphate synthase; HDS, −hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate synthase; HDR, −hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate reductase; GPPS, geranyl diphosphate synthase.
4 Discussion
Plant micropropagation is essential for cultivating and preserving medicinal plants. Several studies have explored the use of plant growth regulators, predominantly auxin and cytokinin combinations, to expedite plant multiplication (Abd El-Motaleb et al., 2023). Additionally, other research has focused on leveraging callus induction and nano-elicitors to augment the plant natural products (Rahmawati et al., 2022). The synergistic combination of auxin and cytokinin has been highlighted as vital for promoting both callus and shoot formation across various tissue cultures (Fehér, 2019; Chacón et al., 2023). Our findings showed that all BAP and NAA combinations resulted in callus formation (Table 1). It is also worth noting that the P. cablin callus belongs to the compact type rather than the friable type (Fig. 1E). We also demonstrated that the presence of BAP is required for callogenesis. Previous study demonstrated the importance of BAP in inducing callus formation. Even though, it is genotype-dependent (Us-Camas et al., 2014; Lestari et al., 2019). The application of cytokinins is thought to affect callus formation by reducing the lignification of plant cell walls (Hoque et al., 2006).
Supplementation of BAP and NAA also induced shoot and root formation (Table 1). Our data are similar to those observed in Vanilla tissue culture. A greater concentration of BAP compared to NAA led to high quantity of shoots and adventitious roots (Warner et al., 2023). A similar proportion of BAP and NAA has also been utilized in Aloe elegans tissue culture (Welehaweria et al., 2023). Additionally, we observed that treatment with 0.25 μM NAA alone and in combination with 2.5 μM BAP also resulted in root and shoot multiplication, respectively (Table 2). This observation also in accordance with Kumaraswamy & Anuradha (2010) and Nguyen et al. (2013), showing that lower concentration of NAA might induce root and shoot multiplication in P. cablin in vitro culture. Higher NAA concentration than BAP also positively results in root multiplication (Seyyedyousefi et al., 2013). This is because auxin induces cell elongation and division in the cambium tissue. In contrast, a higher amount of cytokinin might inhibit root formation (Liu et al., 2022).
A higher concentration of BAP compared to NAA results in the highest shoot multiplication (Table 2). Cytokinin increases plant cell division, thereby accelerating shoot proliferation and leaf formation (Liu et al., 2022). We noticed that the increase of leaf proliferation is in line with the increase in shoot multiplication. These results are consistent with previous patchouli study (Swamy et al., 2010). A high number of patchouli leaves is important factor, since PEO found in considerable amount in leaves compared to other plant organs (Ermaya et al., 2019).
MeJA is a volatile methylester of the jasmonic acid class that acts as an elicitor in signal transduction pathways, such as increasing the phytochemical content of plants and stimulating plant growth (Yi et al., 2019; Anjalani et al., 2024). The signal transduction pathway elicited by MeJA induces certain enzymes to drive metabolic processes, resulting in the formation of defense-related metabolites (Yu et al., 2002). In this study, we have identified 50 predominant compounds across all MeJA treatments via GC–MS. Among those identified phytochemicals, 16 compounds were considered as PEO. The primary PEO identified as 1,2,3,3-D4-Trans-1,2-Dihydroxy-Cyclopentane, 1,3,5-Cycloheptatriene and 2H-Pyran-2-one, 3-acetyl-4-hydroxy-6-methyl- (which exhibited an increase in percentage area, indicating its heightened presence in the study samples) and 1,2,3,3-D4-Trans-1,2-Dihydroxy-a cyclopentane belongs to the cyclopentanol group and is commonly employed in the cosmetic industries (You et al., 2015). Conversely, the compound with the least percentage area was 1H-Purin-6-amine, [(2-fluorophenyl) methyl]-, registering at 0.06 % in the control group, 0.13 % at 100 μM, and 0.05 % at 300 μM.
The compound 2H-Pyran-2-one, 3-acetyl4-hydroxy-6-methyl-, (known as pogostone) is recognized as crucial medicinal components of PEO, following the main sesquiterpene alcohol patchoulol (Chen et al., 2019 Furthermore, pogostone displayed antifungal properties against periodontopathic bacteria (Yi et al., 2013). Other observed phytochemicals include mono-, tri-, and sesquiterpenoids; some phytosterols, flavonoid related compounds, lignin, glycosides, and some aldehydes (Swamy & Sinniah, 2015). Other study revealed 16 compounds within patchouli oil, with 5 main components: guaiene (9.95 %), seychellene (7.14 %), patchoulene (7.08 %), bulnesene (13.16 %) and patchouli alcohol (32.54 %) (Yadav et al., 2017).
Our observation showed a low percentage area of patchoulol in all MeJA treatments (Table 3, 5 and 6). These results contrast with those observed in previous study, where patchoulol significantly increased after MeJA treatment. This could be attributed to the varying durations of MeJA exposure (Chen et al., 2019). For instance, exposure to MeJA for 72 h resulted in an increase of plant secondary metabolites found in Gymnema sylvestre (Chodisetti et al., 2015). Additionally, supplementation with MeJA for 24 h induced a significant amount of andrographolide in Andrographis paniculata cell culture (Sharma et al., 2015).
Patchouli alcohol is a natural compound classified as a sesquiterpenoid, a terpenoid-derived substances. A previous study revealed that sesquiterpenoid-related genes were induced after MeJA treatment in P. cablin. This includes PatAACT, which encodes acetyl coenzyme A (CoA) acetyltransferase enzyme (Tang et al., 2019). This enzyme functions in the first step of terpenoid precursor synthesis (Niu et al., 2021). The application of MeJA elevate the expression of AACT gene and consequently enhance sesquiterpene compound content in patchouli plant (Chen et al., (2019). Our data on AACT gene expression were aligned with the profile of GC–MS, noting the significant presence of compound peaks. This includes 1,2,3,3-D4-Trans-1,2-Dihydroxy- Cyclopentane and 2-Pentanone, 4-Hydroxy-4-Methyl (Table 5). The former is a compound of the cyclopentanol group used in various chemical reactions and is important in pharmaceutical intermediates, polyester resins, catechols, crystalline liquids and perfume industry (You et al., 2015). Whereas 2- Pentanone, 4-Hydroxy-4-Methyl (a diacetone alcohol) is a bioactive compound of the ketone group that functions as an antibacterial agent, possessing also sedative activity (Astuti et al., 2022).
5 Conclusions
In summary, patchouli in vitro culture has been successfully performed using dual combinations of cytokinin (BAP) and auxin (NAA). In this study, we found that the most favorable combination for both growth and organogenesis across all parameters was achieved by employing 2.5 µM of BAP and 0.25 µM of NAA. Meanwhile, it is also worth to note that short term MeJA exposure of in vitro-derived planlets exhibited no significant change in their fresh weight. Interestingly, we have recorded around 50 compounds after different MeJA concentration treatments. The variation of phtochemical profiles found in this study might relied on the over expression of PatAACT gene in both 100 and 300 µM MeJA treatments. This suggests that augmentation of terpenoid-related gene expressions might be responsible for the variation of phytochemicals found in patchouli MeJA-treated plants. The overall results provide a new insight into the use of BAP and NAA combination to boosting the pathouli propagation as well as further application of methyl jasmonate as phytochemical inducers for augmenting the metabolite content of patchouli plants.
CRediT authorship contribution statement
Nurul Jadid: Writing – review & editing, Writing – original draft, Supervision, Investigation, Funding acquisition, Conceptualization. Iro Datus Soleha: Writing – original draft, Methodology, Investigation, Formal analysis. Anisa Esti Rahayu: Visualization, Methodology, Investigation. Ira Puspaningtyas: Visualization, Methodology. Septi Anita Sari: Methodology, Investigation. Maulidia Rahmawati: Methodology, Investigation. Aunurohim: Supervision, Investigation. Dewi Hidayati: Supervision, Investigation, Conceptualization.
Acknowledgment
The authors acknowledge the support of Institut Teknologi Sepuluh Nopember, Surabaya-Indonesia through a research grant number 1702/PKS/ITS/2023.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Establishment of callogenesis and plant regeneration protocols for endemic Origanum syriacum ssp. sinaicum. J. Crop Sci. Biotechnol. 2023:1-12.
- [CrossRef] [Google Scholar]
- Salicylic acid increases flavonolignans accumulation in the fruits of hydroponically cultured Silybum marianum. Saudi Pharm. J.. 2020;28:593-598.
- [CrossRef] [Google Scholar]
- Methyl jasmonate stimulates growth and upregulates the expression of phenylalanine amonia-lyase (PAL) gene in Gynura pseudochina in vitro culture. Biodivers. J. Biol. Divers.. 2024;24:5.
- [CrossRef] [Google Scholar]
- Antidepressant-like Activity of Patchouli Oil var. Tapak Tuan (Pogostemon cablin Benth) via Elevated Dopamine Level: A Study Using Rat Model. Pharmaceuticals (basel). 2022;15(5):608.
- [CrossRef] [Google Scholar]
- Avila-Victor, C. M., Ordaz-Chaparro, V. M., Arjona-Suárez, E. D. J., Iracheta-Donjuan, L., Gómez-Merino, F. C., Robledo-Paz, A. 2023. In Vitro Mass Propagation of Coffee Plants (Coffea arabica L. var. Colombia) through Indirect Somatic Embryogenesis. Plants, 12, 6, 1237. doi: 10.3390/plants12061237.
- Influence of plant growth regulators on in vitro biomass production and biosynthesis of cytotoxic Amaryllidaceae alkaloids in Caliphuria tenera Baker. Biocatal. Agric. Biotechnol.. 2023;50:102670
- [CrossRef] [Google Scholar]
- Full- length transcriptome sequencing and methyl jasmonate-induced expression profile analysis of genes related to patchoulol biosynthesis and regulation in Pogostemon cablin. BMC Plant Biol.. 2019;19:266.
- [CrossRef] [Google Scholar]
- PatSWC4, a methyl jasmonate-responsive MYB (v-myb avian myeloblastosis viral oncogene homolog)-related transcription factor, positively regulates patchoulol biosynthesis in Pogostemon cablin. Ind. Crop. Prod.. 2020;154:112672
- [CrossRef] [Google Scholar]
- Gymnemic acid enhancement in the suspension cultures of Gymnema sylvestre by using the signaling molecules—methyl jasmonate and salicylic acid. In Vitro Cellular Developm. Biol. Plant. 2015;51:88-92.
- [CrossRef] [Google Scholar]
- Terpenoid essential oil metabolism in basil (Ocimum basilicum L.) following elicitation. J. Essent. Oil Res.. 2006;18:618-621.
- [CrossRef] [Google Scholar]
- Identification of patchouli oil chemical components as the results on distillation using GC-MS. IOP Conference Series: Earth and Environmental Science. 2019;365(1):012039
- [Google Scholar]
- Callus, dedifferentiation, totipotency, somatic embryogenesis: what these terms mean in the era of molecular plant biology? Front. Plant Sci.. 2019;10:536.
- [CrossRef] [Google Scholar]
- Shoot multiplication of Pogostemon cablin var. Sidikalang and patchouli oil profile. Nusantara. Bioscience. 2019;11(2):123-127.
- [CrossRef] [Google Scholar]
- Micropropagation of water chestnut (Trapa sp.) through local varieties of Rajshahi division. Asian J. Plant Sci.. 2006;5(3):409-413.
- [CrossRef] [Google Scholar]
- Proximate composition, nutritional values and phytochemical screening of Piper retrofractum Vahl. fruits. Asian Pac. J. Trop. Biomed.. 2018;8(1):37-43.
- [CrossRef] [Google Scholar]
- Jadid, N., Mardika, R. K., Nurhidayati, T., Irawan, M. I. 2016. Reverse transcription-PCR analysis of geranylgeranyl diphosphate synthase (JcGGPPS) in Jatropha curcas L. and in silico analysis of Casbene Synthase (JcCS) among Euphorbiaceae. AIP Conference Proceedings, 1744, 020042. doi: 10.1063/1.4953516.
- Jadid, N., Anggraeni, S., Ramadani, M.R.N., Arieny, M., Mas’ud, F. 2024. In vitro propagation of Indonesian stevia (Stevia rebaudiana) genotype using axenic nodal segments. BMC Research Notes, 17, 45 (2024). doi: 10.1186/s13104-024-06703-0.
- Transcription profile data of phorbol esters biosynthetic genes during developmental stages in Jatropha curcas. Data Brief. 2018;18:700-705.
- [CrossRef] [Google Scholar]
- Micropropagation of Pogostemon cablin Benth. through Direct Regeneration for Production of True to Type Plants. Plant Tissue Cult. Biotechnol.. 2010;20(1):81-89.
- [CrossRef] [Google Scholar]
- Callus and shoot induction of leaf culture Lilium longiflorum with NAA and BAP. Nusantara Biosci.. 2019;11(2):162-165.
- [CrossRef] [Google Scholar]
- Cytokinin promotes growth cessation in the Arabidopsis root. Curr. Biol.. 2022;32(9):1974-1985.
- [CrossRef] [Google Scholar]
- The effect of roots and media constituents on trichomes and artemisinin production in Artemisia annua L. Plant Cell Rep.. 2013;32:207-218.
- [CrossRef] [Google Scholar]
- Cloning, characterization, and functional analysis of acetyl-CoA C-acetyltransferase and 3-hydroxy-3-methylglutaryl-CoA synthase genes in Santalum album. Sci. Rep.. 2021;11:1082.
- [CrossRef] [Google Scholar]
- Rapid plant regeneration, analysis of genetic fidelity and essential aromatic oil content of micropropagated plants of Patchouli, Pogostemon cablin (Blanco) Benth. – An industrially important aromatic plant. Ind. Crop. Prod.. 2010;32:366-374.
- [CrossRef] [Google Scholar]
- Nanotechnology in plant metabolite improvement and in animal welfare. Appl. Sci.. 2022;12(2):838.
- [CrossRef] [Google Scholar]
- The effect of different concentrations of NAA and BAP on micropropagation of Alstroemeria. Europ. J. Experim. Biol.. 2013;3(5):133-136.
- [Google Scholar]
- Jasmonate–induced biosynthesis of andrographolide in Andrographis paniculata. Physiologia Plantarum. 2015;2015(153):221-229.
- [CrossRef] [Google Scholar]
- In vitro multiplication of Pogostemon cablin Benth. through direct regeneration. Afr. J. Biotechnol.. 2010;9, 14:2069-2075.
- [Google Scholar]
- A comprehensive review on the phytochemical constituents and pharmacological activities of Pogostemon cablin Benth.: an aromatic medicinal plant of industrial importance. Molecules. 2016;20, 5:8521-8547.
- [CrossRef] [Google Scholar]
- Molecular identification and expression of sesquiterpene pathway genes responsible for patchoulol biosynthesis and regulation in Pogostemon cablin. Bot. Stud.. 2019;60:11.
- [CrossRef] [Google Scholar]
- In vitro culture: an epigenetic challenge for plants. Plant CellTissue and Organ Culture. 2014;118:187-201.
- [CrossRef] [Google Scholar]
- Exogenous methyl jasmonate (MeJA) enhances the tolerance to cadmium (Cd) stress of okra (Abelmoschus esculentus L.) plants. Plant Cell Tiss. Org. Cult. 2023:1-16.
- [CrossRef] [Google Scholar]
- GC-MS and UHPLC-QTOFMS-assisted identification of the differential metabolites and metabolic pathways in key tissues of Pogostemon cablin. Front. Plant Sci.. 2023;14:1098280.
- [CrossRef] [Google Scholar]
- In vitro micropropagation of Aloe elegans Tod. using offshoot cuttings. BMC. Res. Notes. 2023;16, 1:215.
- [CrossRef] [Google Scholar]
- GC-MS analysis of Essential oil of Pogostemon cablin Benth. (Patchouli oil) of Nepal. J. Plant Res.. 2017;15(1):61-65.
- [Google Scholar]
- Synthesis and antimicrobial evaluation of pogostone and its analogues. Fitoterapia. 2013;84:135-139.
- [CrossRef] [Google Scholar]
- Enhancement of phenolic compounds and antioxidative activities by the combination of culture medium and methyl jasmonate elicitation in hairy root cultures of Lactuca indica L. Nat. Prod. Commun.. 2019;1–9
- [CrossRef] [Google Scholar]
- Thermodynamic analysis of synthesis of cyclopentanol from cyclopentene and comparison with experimental data. Appl. Petrochem. Res.. 2015;5:135-142.
- [CrossRef] [Google Scholar]
- Jasmonic acid improving ginsenoside accumulation in adventitious root culture of Panax ginseng C.A. Meyer. Biochem. Eng. J.. 2002;11:211-215.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103301.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







