Translate this page into:
Encapsulation of MERS antigen into α-GalCer-bearing-liposomes elicits stronger effector and memory immune responses in immunocompetent and leukopenic mice
⁎Corresponding author at: Department of Basic Health Sciences, College of Applied Medical Sciences, Qassim University, Buraydah 51452, Saudi Arabia. a_khan@qu.edu.sa (Masood Alam Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Here, we prepared a liposome-based vaccine formulation containing Middle East Respiratory Syndrome Coronavirus papain-like protease (MERS-CoV-PLpro).
Methods
A persistent leukopenic condition was induced in mice by injecting cyclophosphamide (CYP) three days before each dose of immunization. Mice were immunized on days 0, 14 and 21 with α-GalCer-bearing MERS-CoV PLpro-encapsulated DPPC-liposomes (α-GalCer-MERS-PLpro-liposomes or MERS-CoV PLpo-encapsulated DPPC-liposomes (MERS-PLpro-liposomes), whereas the antigen emulsified in Alum (MERS-PLpro-Alum) was taken as a control. On day 26, the blood was taken from the immunized mice to analyze IgG titer, whereas the splenocytes were used to analyze the lymphocyte proliferation and the level of cytokines. In order to assess the memory immune response, mice were given a booster dose after 150 days of the last immunization.
Results
The higher levels of MERS-CoV-PLpro-specific antibody titer, IgG2a and lymphocyte proliferation were noticed in mice immunized with α-GalCer-MERS-PLpro-liposomes. Besides, the splenocytes from mice immunized with α-GalCer-MERS-PLpro-liposomes produced larger amounts of IFN-γ as compared to the splenocytes from MERS-PLpro-liposomes or MERS- PLpro-Alum immunized mice. Importantly, an efficient antigen-specific memory immune response was observed in α-GalCer-MERS-PLpro-liposomes immunized mice.
Conclusions
These findings suggest that α-GalCer-MERS-PLpro-liposomes may substantiate to be a successful vaccine formulation against MERS-CoV infection, particularly in immunocompromised individuals.
Keywords
Immunization
Liposome
MERS-CoV
NKT cell
- Liposome, Vaccine, MERS-PLpro, Infectious diseases
-
Immune response
Abbreviations
1 Introduction
Immunocompromised individuals have shown greater susceptibility to viral infections, including influenza virus, cytomegalovirus, Herpes simplex virus, Severe Acute Respiratory Syndrome Corona virus 2 (SARS-CoV2) and Middle Eastern Respiratory Syndrome corona virus (MERS-CoV) (Bosaeed and Kumar, 2018, Vora and Englund, 2015, Lai et al., 2020). MERS-CoV was first isolated in Saudi Arabia from a patient suffering from severe respiratory problems (Faridi, 2018). The healthcare workers are considered the major victims of MERS-CoV infection (Alshammari et al., 2018).
Upon activation with alpha-Galactosylceramide (α-GalCer) NKT cells produce Th1 and Th2 cytokines as well (Brutkiewicz, 2006). The activation of NKT cells leads to the maturation of dendritic cells (DCs) and B cells (Fig. 1). NKT cells have shown critical role during viral infections, including Hepatitis B virus (HBV), Influenza A virus (IAV), Respiratory Syncytial virus (RSV) and Herpes Simplex virus (HSV) (Khan and Khan, 2021; Grubor-Bauk et al., 2003).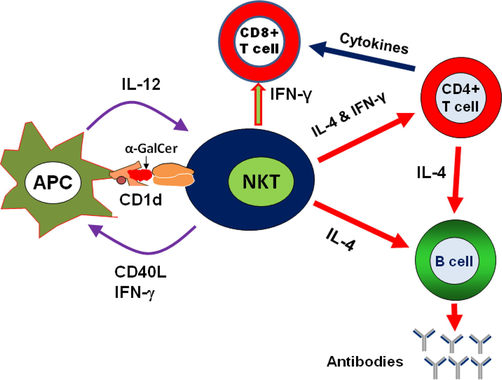
The stimulation of NKT cells by α-GalCer induces the secretion of Th1 and Th2 cytokines. IFN-γ (Th1 cytokine) stimulates T cells, whereas IL-4 (Th2 cytokine) activates B cells that contributes to humoral immunity.
The immunization of immunocompromised individuals has been a big challenge owing to their poor immune response to antigens. Liposomes are effective as a potent immunoadjuvant due to their ability to stimulate the immune responses (Khan and Khan, 2021; Ahmad et al., 2001). Liposome-mediated delivery of MERS-CoV PLpro can stimulate antigen-specific immune responses (Khan et al., 2022). NKT cell-ligand alpha-Galactosylceramide (α-GalCer) has the ability to equally stimulate B cells and T cells (Fig. 1). Moreover, α-GalCer has ability to augment the immunogenicity of antigens (Liu and Guo, 2017, Kim et al., 2008). Keeping into consideration the immunoadjuvant role of α-GalCer, we formulated α-GalCer-MERS- PLpro-liposomes or MERS-PLpro-liposomes. Mice were injected with α-GalCer-MERS-PLpro-liposomes or MERS-PLpro-liposomes, whereas the mice immunized with MERS-CoV-Alum used as a control. Mice immunized with α-GalCer-Lip-MERS-PLpro-liposomes exhibited higher levels of effector and memory immune responses.
2 Materials and methods
2.1 Reagents
α-GalCer, DPPC, cholesterol and ELISA kits were bought from Abcam (Cambridge, United Kingdom), whereas Phycoerythrin (PE)-conjugated PBS57-loaded CD1d tetramers were kindly provided by the Tetramer Core Facility of NIH (Atlanta, GA).
2.2 Expression and purification of MERS-CoV-PLpro
Middle East Respiratory Syndrome Coronavirus papain-like protease (MERS-CoV-PL pro) was expressed and purified by following the published procedure (Khan et al., 2022).
2.3 Preparation of liposomes and the entrapment of antigen
DPPC and Chol (7:3 M ratio) with or without α-GalCer (24 µg) were used in the preparation of liposomes as described earlier (Khan et al., 2022). The characteristics of liposomes, including the size and polydispersity index (PDI) were determined by the Malvern Nano Zeta Sizer as described earlier (Khan et al., 2022).
2.4 Mice
Swiss female mice (25 ± 5 g) were used in the present study. Mice were taken from the animal house facility of the College of Applied Medical Sciences, Qassim University, Saudi Arabia. The experimental procedures were approved by the committee of research ethics, Deanship of Scientific Research, Qassim University, Buraydah, Saudi Arabia.
2.5 Determination of cyclophosphamide (CYP) - induced immune suppression
A dose of 100 mg/kg of cyclophosphamide (CYP) was injected into each mouse through the intraperitoneal route (Khan et al., 2021).
2.6 Immunization schedule
MERS-CoV PLpro (20 μg) encapsulated in liposomal formulations or emulsified with Alum was injected into each mouse through the subcutaneous route. Mice received two booster doses on days 14 and 21. Mice, which were immunized with the first dose of α-GalCer-MERS-PLpro-liposomes, received two booster doses of MERS-PLpro-liposomes on days 14 and 21. Leukopenia induced in mice by injecting CYP (100 mg/kg) three days before each immunization dose. Mice were separated into six groups: 1. PBS, 2. Sham liposomes, 3. α-GalCer-liposomes, 4. MERS-PLpro-liposomes, 5. α α-GalCer-MERS-PLpro-liposomes, 6. MERS-PLpro-Alum.
2.7 Determination of NKT cell activation in the spleen of the immunized mice
The status of NKT cell was determined by analyzing the splenocytes stained with PE-conjugated α-GalCer-loaded CD1d tetramer and FITC-TCR-β as described in the Supplementary data.
2.8 Determination of IgG titer and the IgG isotypes
On day 5 post immunization, the blood was drawn through retro-orbital puncture and centrifuged at 1500 rpm to separate the serum. The status of antigen-specific IgG, IgG1 and IgG2a was estimated by the ELISA (Syed et al., 2003).
2.9 Determination of the proliferation of splenocytes
The proliferation assay of the splenocytes was performed by using the cell titer 96 non-radioactive BrdU calorimetric ELISA kit (Abcam, Cambridge, UK) and following the instructions provided by the manufacturer (Khan et al., 2022).
2.10 Determination of Th1 and Th2 cytokine
Three mice from were sacrificed to take out the spleen in order to prepare its single cell suspension as mentioned above. The cells were stimulated with 20 µg/ml of antigen and incubated for 48 h at 37 °C. The cells stimulated with 20 µg/ml of ovalbumin were used as a control. The amounts of IFN-γ, IL-4, IL-12 and IL-13 were analyzed as described earlier (Khan et al., 2022).
2.11 Evaluation of long-term humoral and T cell memory immune response
After 150 days of the last immunization, a single dose of α-GalCer-MERS-PLpro-liposomes or MERS-PLpro-liposomes or MERS-PLpro-Alum was injected in mice in order to assess the memory immune response. Fresh mice (first time immunized) were used as a control. On days 0, 7, 14 and 21 post-immunization, the blood was taken to analyze the level of total IgG. The effect of booster dose was also measured by analyzing the proliferation of splenocytes as mentioned above.
2.12 Secretion of cytokines after a booster dose of immunization
The amounts of IFN-γ, IL-4, IL-12 and IL-13 were quantified by ELISA as described in the methods section of the supplementary data.
2.13 Statistical analysis
The results were analyzed by one-way analysis of variance (ANOVA) using GraphPad Prism software, version 6.0 (La Jolla, CA, USA). The Turkey post-hoc test was performed to compare various groups. A value of p < 0.05 was considered statistically significant.
3 Results
3.1 Characterization of liposomes
The mean size of α-GalCer-MERS-PLpro-liposome was 139 nm and PDI value was found to be 0.265, whereas those of MERS-PLpro-liposomes were found to be 140 nm and 0.331.
3.2 CYP administration results in the depletion of the leukocyte numbers in the systemic circulation and spleen
The administration of CYP induced the condition of temporary leukopenia in the systemic circulation on days 3 and 5 post-CYP injection (Fig. 2A). The leukocytes started their recovery from day 7 and completely recovered to almost normal level by day 11 (Fig. 2A). Similarly, the numbers of splenocytes were reduced in CYP-injected mice on day 3, and thereafter started their recovery from day 5 post-CYP administration (Fig. 2B).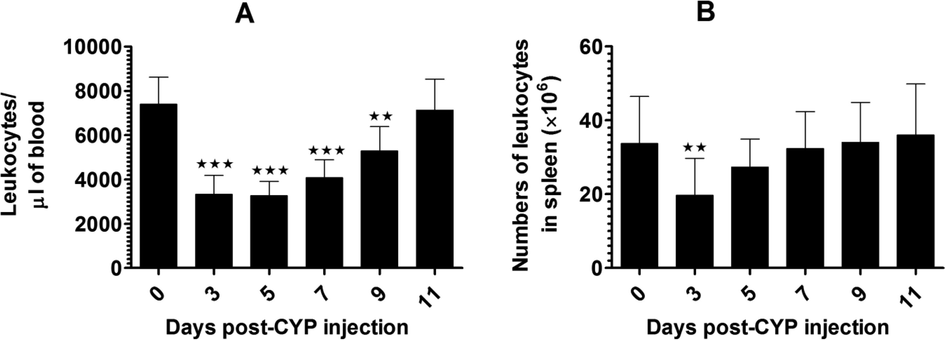
CYP administration induces leukopenia in (A) Blood, (B) Spleen. ** (p < 0.01) and *** (p < 0.001), Days post-CYP injection vs. day 0.
3.3 Administration of α-GalCer results in the proliferation of NKT cells
Mice immunized with α-GalCer-liposomes showed the activation of CD1d/α-GalCer + TCR-β positive splenocytes (Fig. 1, supplementary data).
3.4 Immunization with α-GalCer-MERS-PLpro-liposomes induced higher production of IgG and IgG2a isotype
There was the highest level of antibody titer in the sera of immunocompetent mice injected with α-GalCer-MERS-PLpro-liposomes followed by those immunized with MERS-PLpro-liposomes or MERS-PLpro-Alum (Fig. 3A). On the other hand, immunized leukopenic mice had lower antibody titer (Fig. 3A, 3B). Despite this, α-GalCer-MERS- PLpro-liposomes effectively stimulated greater antibody generation in leukopenic mice (Fig. 3B).
α-GalCer-MERS-PLpro-liposomes immunization enhanced the secretion of antibody (A, B) and IgG2a/IgG1 (C, D) in immunocompetent and leukopenic mice. The data are expressed as the mean ± SD of three independent values.
The ratio of IgG2a/IgG1 was 1.25 in the immunocompetent mice immunized with α-GalCer-MERS-PLpro-liposomes as compared to 1.05 and 0.89 in mice immunized with MERS-PLpro-liposomes or MERS-PLpro-Alum, respectively (Fig. 3C). The leukopenic mice immunized with α-GalCer-MERS-PLpro-liposomes had IgG2a/IgG1 equal to 1.05 (Fig. 3D).
3.5 Lymphocytes from α-GalCer-MERS-PLpro-liposomes immunized mice had increased proliferation
Immunization with α-GalCer-MERS-PLpro-liposomes resulted in a greater proliferation of splenocytes. On the contrary, the splenocytes from the leukopenic mice responded with lower proliferation (Fig. 4A, 4B). Importantly, the leukopenic mice immunized with α-GalCer-MERS-PLpro-liposomes showed greater proliferation of splenocytes as compared those immunized with MERS-PLpro-liposomes or MERS-PLpro-Alum (Fig. 4B).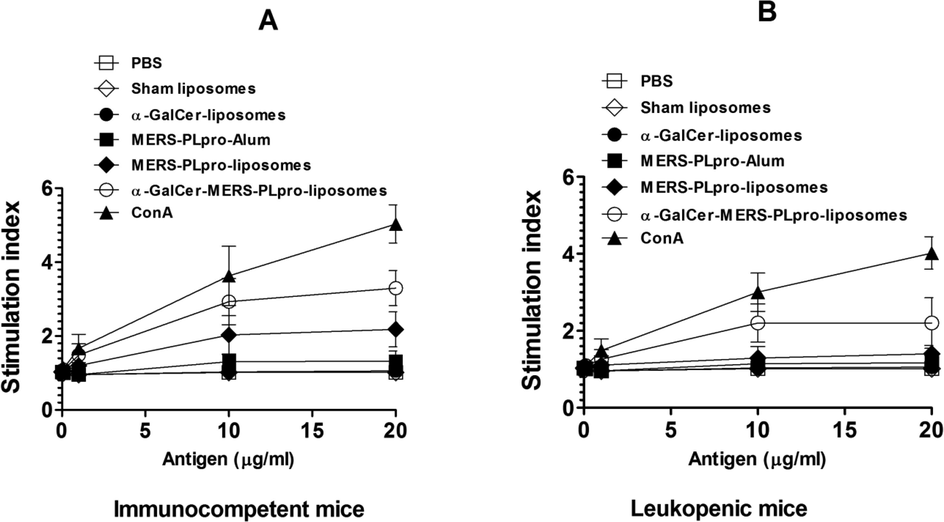
The lymphocytes from α-GalCer-MERS-PLpro-liposomes immunized mice showed greater proliferation in response to antigen stimulation in (A) Immunocompetent, (B) leukopenic mice.
3.6 Immunization with α-GalCer-MERS-PLpro-liposomes resulted in higher production of IFN-γ
The splenocytes from α-GalCer-MERS-PLpro-liposomes immunized mice produced 540 ± 88 pg/ml of IFN-γ, whereas those immunized with MERS-PLpro-liposomes secreted 215 ± 35 pg/ml (Fig. 5A). On the other hand, splenocytes from the leukopenic mice immunized with α-GalCer-MERS-PLpro-liposomes produced 302 ± 88 pg/ml of IFN-γ, whereas those immunized with MERS- PLpro-liposomes secreted 112 ± 28 pg/ml (Fig. 5B). IL-4 level was found to be 342 ± 58 pg/ml in immunocompetent mice immunized with α-GalCer-MERS-PLpro-liposomes followed by MERS-PLpro-liposomes and MERS-PLpro-Alum (Fig. 5C). However, the amount of IL-4 was found to be 418 ± 48 pg/ml in the leukopenic mice immunized with α-GalCer-MERS-PLpro-liposomes (Fig. 5D).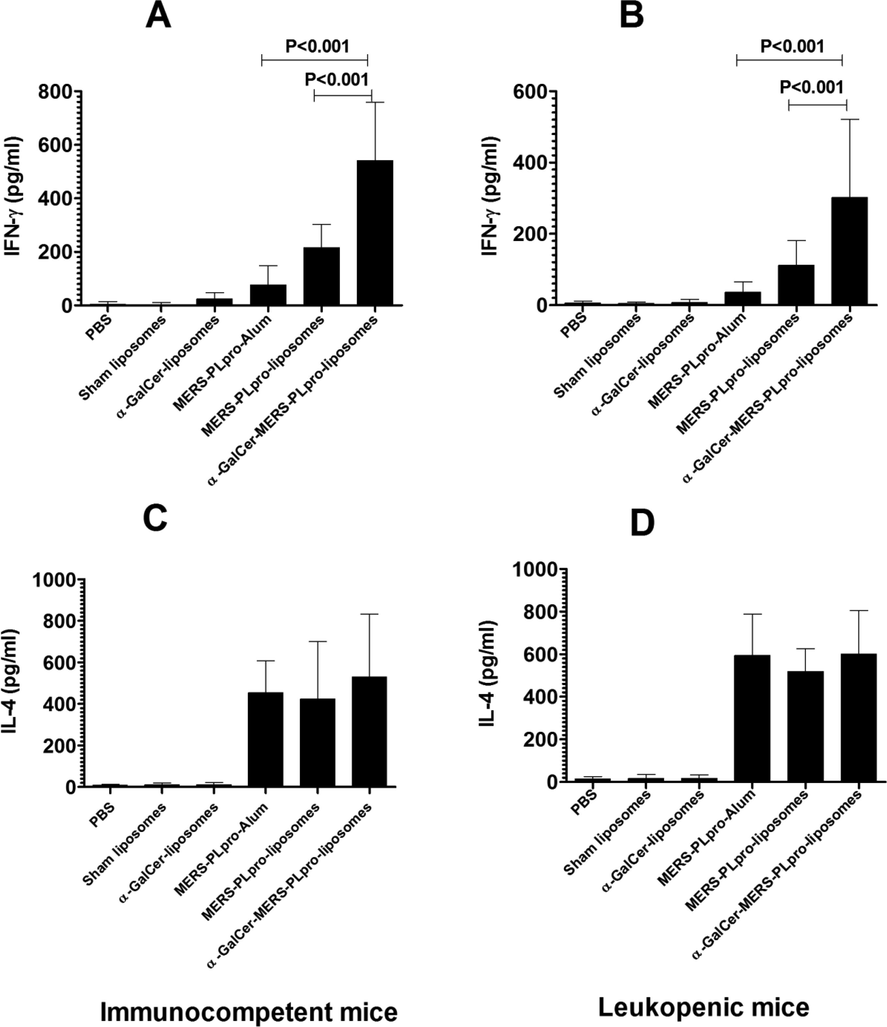
Immunization of mice with α-GalCer-MERS-PLpro-liposomes produced higher amounts of IFN-γ (A, B), and almost similar levels of IL-4 (C, D) in immunocompetent and leukopenic mice. The values are representatives of the mean ± SD of three independent experiments.
3.7 α-GalCer-MERS-PLpro-liposomes induced superior antibody memory immune response
The titer of of IgG increased much faster (25 fold) in α-GalCer-MERS-PLpro-liposomes immunized mice. On the other hand, the mice immunized with MERS-PLpro-liposomes and MERS-PLpro-alum had 15-fold and 12-fold IgG titer on day 14 (Fig. 6A). Interestingly, the leukopenic mice immunized with α-GalCer-MERS-PLpro-liposomes also responded well to a booster dose and had about 20-fold IgG titer (Fig. 6B).
Immunization with α-Galcer-MERS-PL pro-liposomes induced a long-term antibody memory immune responses in (A) Immunocompetent and (B) leukopenic mice. The results are representatives of two autonomous values.
3.8 Superior lymphocyte memory immune response in mice immunized with α-GalCer-MERS- PLpro-liposomes
A booster dose with α-GalCer-MERS-PLpro-liposomes induced higher proliferation of splenocytes as compared to those from MERS-PLpro-liposomes or MERS-PLpro-Alum immunized mice (Fig. 7A, 7B). However, the cells from immunocompetent mice immunized α-GalCer-MERS-PLpro-liposomes showed superior proliferation as compared those from leukopenic mice.
Immunization with α-Galcer-MERS-PLpro-liposomes elicited long-term T cell memory immune responses in (A) Immunocompetent and (B) leukopenic mice. The values are representatives of two experiments.
3.9 Immunization with α-GalCer-MERS-PLpro-liposomes elicited higher secretion of cytokines
A booster dose with α-GalCer-MERS-PLpro-liposomes effectively stimulated the splenocytes that secreted greater amounts of IFN-γ, IL-12 and IL-13 (Supplementary Fig. 2).
4 Discussion
Immunocompromised persons need special attention in order to immunize them against viral infections. Cyclophosphamide administration induces marked immune suppression and affects a broad spectrum of immune cells, including T cells, dendritic cells, monocytes and neutrophils (Bao et al., 2020). Earlier studies demonstrated a lower number of leukocytes in CYP-injected humans (Giang et al., 1996). Thus, it is critical to develop a vaccine formulation that can effectively induce antigen-specific immune responses in immunocompromised subjects.
Due to the versatility of lipids, various types of liposomes can be formulated in order to incorporate a variety of antigenic molecules. Liposomized antigens can induce a greater immune response since they have ability to release the antigen for a longer duration (Bernasconi et al., 2016). Moreover, liposomes can deliver antigens to APCs that cross present antigens to the cytotoxic T cells. It results in superior antigen-specific cell-mediated immune response (Dhakal and Renukaradhya, 2019; Tanaka et al., 2010). The incorporation of NKT ligands has been shown to increase the immunoadjuvant potential of liposomes resulting in the stimulation of antigen-specific CD8+ T cell responses (Bai et al., 2013; Grabowska et al., 2021).
In the current study, we measured the immunogenicity of MERS-CoV PLpro emulsified with Alum or loaded in α-GalCer-incorporated liposomes or α-Galcer-free liposomes in immunocompetent or leukopenic mice. Immunization with α-GalCer-MERS-PLpro-liposomes induced the proliferation of the NKT cells in the spleen of the mice owing to the presence of α-GalCer, whereas the mice immunized with MERS-PLpro-liposomes or MERS-PLpro-Alum did not. Vaccination with α-GalCer-MERS-PLpro-liposomes elicited higher production of antibodies as compared to those immunized with MERS-PLpro-liposomes or MERS-PLpro-Alum. α-GalCer has been reported to induce the proliferation of B cells, resulting in elevated production of antibodies (Chen et al., 2011; Rossignol et al., 2007). Notably, α-GalCer-MERS-PLpro-liposomes also augmented the production of antigen-specific antibodies in leukopenic mice. The status of a specific IgG isotype also contributes to an antiviral immunity. IgG1 contributes to Th2-type immunity, whereas IgG2a has a role in Th1-type of immunity that protects against viruses (Huber et al., 2006). Moreover, IgG2a monoclonal antibodies, not IgG1 monoclonal antibodies, can eliminate multiple viral infections (Huber et al., 2006). It suggests that IgG2a can also incite the effector immune responses. Whereas, IgG1 was reported to be a major isotype in serious COVID-19 patients. However, IgG2 isotype could not be detected in these patients (Luo et al., 2021). IgG2a isotype specific to influenza virus membrane protein M2 induced higher protection against viral infection as compared to IgG1 isotype (Van den Hoecke et al., 2017). The findings of this study illustrated that α-GalCer-MERS-PLpro-liposomes can switch IgG isotype to IgG2a in comparison to the immunization with MERS-PLpro-liposomes or MERS-PLpro-Alum. Furthermore, IgG2a has been reported to increase the proliferation of T cells, which are critical players in antiviral cell-mediated immunity (Getahun et al., 2004).
IFN-γ is an important antiviral cytokine that augments the killing activity of CD8+ T lymphocytes (Levy and García-Sastre, 2001). The splenocytes from α-GalCer-MERS-PLpro-liposomes immunized mice produced higher IFN-γ amounts that indicates the vaccine-induced antiviral immune response in the immunized mice. Furthermore, IFN-γ activates the NK cells and macrophages through the JAK-STAT signaling pathway (Mahallawi et al., 2018). Besides, IFN-γ has been shown to promote MHC class I-mediated antigen presentation (Zhou, 2009). Whereas, MERS-CoV infection antagonizes the expression of antigen presenting molecules (Menachery et al., 2018).
Interestingly, a booster dose with α-GalCer-MERS-PLpro-liposomes educed strong memory immune responses. α-GalCer-MERS-PLpro-liposomes elicited a remarkably greater antibody amounts and switching of IgG isotype to IgG2a. Memory T cell responses were assessed by analyzing T cell proliferation and the levels of Th1 and Th2 cytokines. The lymphocytes from the mice immunized with α-GalCer-MERS-PLpro-liposomes exhibited larger proliferation followed by those from MERS-PLpro-liposomes or MERS-PLpro-Alum. IL-12 has been reported to have a role in IFN-γ secretion, and they together contribute to CTLs proliferation (Starbeck-Miller and Harty, 2015). IL-12 was also elevated in α-GalCer-MERS-PLpro-liposomes immunized mice. The administration of NKT cell ligands can induce the NKT cell anergy that limits the use of iNKT-cell agonists (Savage, 2014). This limitation can be overcome by incorporating α-GalCer into liposomes (Thapa et al., 2009). Therefore, α-GalCer-bearing liposomes can be used as effective immunoadjuvants to prepare an antiviral vaccine. Earlier studies evaluated the combination glycolipids and vaccine antigens against infectious diseases (Schmieg et al., 2003; Padte et al., 2011; Gonzalez-Aseguinolaza et al., 2002; Youn et al., 2007). The co-administration of 7DW8–5, an analog of α-GalCer, elevated malaria-antigen specific CTL response in primates (Padte et al., 2013).
These findings revealed that α-GalCer-MERS-PLpro-liposomes significantly enhanced MERS-CoV-PLpro-specific immune responses in immunocompetent and leukopenic mice. In addition, the splenocytes α-GalCer-MERS-PLpro-liposomes immunized mice produced larger amounts of IFN-γ that stimulates APCs and MHC class I expression. A booster dose with α-GalCer-MERS- PLpro-liposomes successfully rejuvenated the memory immune response. Thus, α-GalCer-MERS-PLpro-liposomes may ascertain to be a hopeful prophylactic preparation to protect immunocompromised persons against MERS-CoV infection. The results of iNKT cell-based vaccine formulations in mouse model can be extended and translated into human beings because of their certain common functional features in humans and mice. Future studies need to be conducted in order to develop a successful iNKT cell-based antiviral vaccine.
Acknowledgements
The authors gratefully acknowledge the Deanship of Scientific Research, Qassim University, for the financial support of this research study under the Interdisciplinary Grant No. 5575-cams1-2019-2-2-I during the 2019 academic year.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Liposome mediated antigen delivery leads to induction of CD8+ T lymphocyte and antibody responses against the V3 loop region of HIV gp120. Cell. Immunol.. 2001;210(1):49-55.
- [Google Scholar]
- Comparison of perceived and observed hand hygiene compliance in healthcare workers in MERS-CoV endemic regions. Healthcare (Basel). 2018;6(4):122.
- [Google Scholar]
- Natural killer T (NKT)-B-cell interactions promote prolonged antibody responses and long-term memory to pneumococcal capsular polysaccharides. Proc. Natl. Acad. Sci. U. S. A.. 2013;110(40):16097-16102.
- [Google Scholar]
- High-dose cyclophosphamide administration orchestrates phenotypic and functional alterations of immature dendritic cells and regulates Th cell polarization. Front. Pharmacol.. 2020;11:775.
- [Google Scholar]
- Mucosal vaccine development based on liposome technology. J. Immunol. Res.. 2016;2016:1-16.
- [Google Scholar]
- Seasonal influenza vaccine in immunocompromised persons. Hum. Vaccin. Immunother.. 2018;14(6):1311-1322.
- [Google Scholar]
- Retinoic acid and α-galactosylceramide differentially regulate B cell activation in vitro and augment antibody production in vivo. Clin. Vaccine Immunol.. 2011;18(6):1015-1020.
- [Google Scholar]
- Nanoparticle-based vaccine development and evaluation against viral infections in pigs. Vet. Res.. 2019;50(1):90.
- [Google Scholar]
- Middle East respiratory syndrome coronavirus (MERS-CoV): Impact on Saudi Arabia, 2015. Saudi J. Biol. Sci.. 2018;25(7):1402-1405.
- [Google Scholar]
- IgG2a-mediated enhancement of antibody and T cell responses and its relation to inhibitory and activating Fc gamma receptors. J. Immunol.. 2004;172(9):5269-5276.
- [Google Scholar]
- Conditioning of cyclophosphamide-induced leukopenia in humans. J. Neuropsychiatry Clin. Neurosci.. 1996;8(2):194-201.
- [Google Scholar]
- Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J. Exp. Med.. 2002;195(5):617-624.
- [Google Scholar]
- Liposomal nanovaccine containing α-galactosylceramide and ganglioside GM3 stimulates robust CD8+ T cell responses via CD169+ macrophages and cDC1. Vaccines (Basel). 2021;9(1):56.
- [Google Scholar]
- Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J alpha 281 TCR. J. Immunol.. 2003;170(3):1430-1434.
- [Google Scholar]
- Liposomal ellagic acid alleviates cyclophosphamide-induced toxicity and eliminates the systemic Cryptococcus neoformans infection in leukopenic mice. Pharmaceutics. 2021;13(6):882.
- [Google Scholar]
- Role of NKT cells during viral infection and the development of NKT cell-based nanovaccines. Vaccines. 2021;9(9):949.
- [Google Scholar]
- Liposome-mediated delivery of MERS antigen induces potent humoral and cell-mediated immune response in mice. Molecules. 2022;27(2):403.
- [Google Scholar]
- Glycolipid ligands of invariant natural killer T cells as vaccine adjuvants. Expert. Rev. Vaccines. 2008;7(10):1519-1532.
- [Google Scholar]
- SARS-CoV2 and immunosuppression: A double-edged sword. Transpl. Infect. Dis.. 2020;22(6):e13404.
- [Google Scholar]
- The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev.. 2001;12(2–3):143-156.
- [Google Scholar]
- NKT-cell glycolipid agonist as adjuvant in synthetic vaccine. Carbohydr. Res.. 2017;452:78-90.
- [Google Scholar]
- MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8-13.
- [Google Scholar]
- MERS-CoV and H5N1 influenza virus antagonize antigen presentation by altering the epigenetic landscape. Proc. Natl. Acad. Sci. U. S. A.. 2018;115(5):E1012-E1021.
- [Google Scholar]
- Clinical development of a novel CD1d-binding NKT cell ligand as a vaccine adjuvant. Clin Immunol.. 2011;140(2):142-151.
- [Google Scholar]
- A glycolipid adjuvant, 7DW8-5, enhances CD8+ T cell responses induced by an adenovirus-vectored malaria vaccine in non-human primates. PLoS ONE. 2013;8(10):e78407.
- [Google Scholar]
- Freshly isolated Valpha24+ CD4+ invariant natural killer T cells activated by alpha-galactosylceramide-pulsed B cells promote both IgG and IgE production. Clin. Exp. Immunol.. 2007;148(3):555-563.
- [Google Scholar]
- Vaccine development: NKT-cell adjuvants in conjugate. Nat. Chem. Biol.. 2014;10(11):882-883.
- [Google Scholar]
- Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J. Exp. Med.. 2003;198(11):1631-1641.
- [Google Scholar]
- The role of IL-12 and type I interferon in governing the magnitude of CD8 T cell responses. Adv. Exp. Med. Biol.. 2015;850:31-41.
- [Google Scholar]
- Antigen entrapped in the escheriosomes leads to the generation of CD4 (+) helper and CD8 (+) cytotoxic T cell response. Vaccine. 2003;21(19–20):2383-2393.
- [Google Scholar]
- Liposome-coupled antigens are internalized antigen-presenting cells via pinocytosis and cross-presented to CD8 T cells. PLoS ONE. 2010;5(12):e15225.
- [Google Scholar]
- Nanoparticle formulated alpha-galactosylceramide activates NKT cells without inducing anergy. Vaccine. 2009;27(25–26):3484-3488.
- [Google Scholar]
- Hierarchical and redundant roles of activating FcγRs in protection against influenza disease by M2e-specific IgG1 and IgG2a antibodies. J. Virol.. 2017;91(7):e02500-e3016.
- [Google Scholar]
- Cytomegalovirus in immunocompromised children. Curr. Opin. Infect. Dis.. 2015;28(4):323-329.
- [Google Scholar]
- A single intranasal immunization with inactivated influenza virus and alpha-galactosylceramide induces long-term protective immunity without redirecting antigen to the central nervous system. Vaccine. 2007;25(28):5189-5198.
- [Google Scholar]
- Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int. Rev. Immunol.. 2009;28(3–4):239-260.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102124.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







