Translate this page into:
The interplay between bisphenol A and algae – A review
⁎Corresponding authors at: Marine Environmental Change Physiology, State Key Laboratory of Marine Environmental Science, Xiamen University (Xiang-An campus), Xiamen, Fujian 361102, China. azizullah@uoh.edu.pk (Azizullah Azizullah), ksgao@xmu.edu.cn (Kunshan Gao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
A complex interplay occurs between algae and bisphenol A (BPA) BPA adversely affects algal growth and other functions. Algae remediate and/or biodegrade BPA. Increase in BPA concentration decreases BPA-degradation potential of algae. There are several research gaps that need further work.
Abstract
Bisphenol A (BPA) is a synthetic organic compound used as raw material in many industrial products, particularly epoxy resins and polycarbonate plastics. Due to its extensive applications, increasing contamination in the environment and adverse effects on living organisms, BPA has been regarded as a pollutant of emerging concern. In aquatic environments, it has been detected ubiquitously in concentrations ranging from few ng L−1 to several µg L−1. BPA has been found toxic to a number of aquatic organisms from diverse taxa at all trophic levels. In terms of photosynthetic organisms, a complex interplay occurs between BPA and algae. BPA adversely affects algae by inhibiting several physiological and biochemical processes; at the same time, algae potentially biodegrade and/or remediate the environmental BPA. In this review article these complex interactions between algae and BPA are elaborated. The effects of BPA on different parameters of algae including growth, light-harvesting pigments, photosynthesis, respiration, morphology and macromolecules are discussed. Considering the reported EC50/IC50 values of BPA for algae growth and following the criteria of the EU-Directive 93/67/EEC for classifying aquatic pollutants, BPA can be classified as toxic and harmful to algae (having EC50 in the range of 1–10 and 10–100 mg L−1). The oxidative stress caused by BPA resulting in lipids peroxidation of cellular membranes is the most possible cause of BPA toxicity in algae. In addition, the abilities of different algae to remove BPA by adsorption, accumulation and biodegradation are also compared and discussed. Algae remove BPA from water mainly by biodegrading it with monohydroxy-BPA and BPA-glycosides as the most common intermediates. Algae generally remove BPA more efficiently at low doses of BPA (lower than 1 mg L−1) but increase in the concentration of BPA greatly reduces the biodegradation efficiency of algae. For an in-depth understanding, the molecular responses of algae to BPA exposure are reviewed. This manuscript provides comprehensive information on the subject matter that would be useful for both academia and policy makers working in this domain.
Keywords
Algae
Aquatic environments
Biodegradation
Bisphenol A
Interplay
Toxicity
1 Introduction
Bisphenol A (BPA) [2,2-bis(4-hydroxyphenyl) propane] is a synthetic organic compound consisted of two phenol rings connected by methyl functional groups. It has many industrial applications, particularly in the production of epoxy resins and polycarbonate plastics (Björnsdotter et al., 2017; Chiu et al., 2018). About 27% of epoxy-based resins and 71% of polycarbonate-based plastics involve BPA as a raw material (Duan et al., 2019). Consequently, BPA find its applications in a number of products like water pipes, medical equipment, tubing, coating and packing materials, thermal papers, electrical equipment, flame retardants, and numerous other plastic-based products (Björnsdotter et al., 2017; Wang et al., 2016a). Due to its wide-spread applications in different products, there has been a tremendous increase in BPA global production with time that touched 5.2 million metric tons in 2008, reached 8 million metric tons in 2016 and is estimated to reach 10.6 million metric tons by 2022 (Björnsdotter et al., 2017; Experts, 2016).
The widespread use of BPA has resulted in its ultimate growing discharges and accumulation into different compartments of the environment including air, water, soil and even biota (Michałowicz, 2014). Over the last two decades, a number of papers or reports have been published around the globe on the presence of BPA in environmental matrices like air, soil, river water, sediment, ground water, raw leachate, waste landfill leachate, raw sewage sludge, tape water, food materials and biological samples (Björnsdotter et al., 2017; Huang et al., 2012; Kleywegt et al., 2011; Lee and Peart, 2000; Michałowicz, 2014; Yamamoto et al., 2001). Because of its extensive applications, consequent contamination in the environment and toxicity to living organisms (especially endocrine disruption), BPA has been regarded as a contaminant of emerging concern (Berhane et al., 2017). Due to increasing concerns about its toxicity and frequent occurrence in the environment, some countries have banned BPA for certain specific applications as summarized by Björnsdotter et al. (2017) and Zheng et al. (2019). Although the half-life of BPA in surface water under aerobic conditions and at environmentally relevant concentrations of 0.05 to 0.5 µg L−1 is reported to be very short (3–6 days) (Kleĉka et al., 2001), it is still being found accumulated in the bodies of freshwater and marine organisms (Corrales et al., 2015). Aquatic organisms at different trophic levels like algae, crustacean and fish have widely been reported to be adversely affected upon exposure to BPA (Alexander et al., 1988; Belfroid et al., 2002; Li et al., 2017; Li et al., 2018).
The toxicity of BPA to aquatic organisms is a well-established fact now. It can be deleterious to aquatic organisms in doses as low as below 1 µg L−1 (Oehlmann et al., 2005). BPA can cause cytotoxicity, genotoxicity, neurotoxicity, reproductive toxicity and endocrine disruption in the exposed organisms (Bonefeld-Jørgensen et al., 2007; Li et al., 2016; Wang et al., 2017b; Wu et al., 2017; Yang and Hong, 2012). The estrogenic effects of BPA on aquatic organisms are of great concern. For example, Nucella lapillus (sea snail) and Marisa cornuarietis (freshwater snail) were turned in to superfemales with increased oocyte production and enlarged accessory pallial sex glands when exposed to 1 µg L−1 of BPA (Oehlmann et al., 2005). At a concentration of 1.5 mg L−1, BPA adversely affected the digestive, nervous, and reproductive systems in Japanese medaka (Li et al., 2016). The toxic concentration may vary depending upon the taxon and species, but BPA toxicity has been confirmed in diverse groups of aquatic organisms including algae, amphibians, annelids, crustaceans, fish, hydra, mollusks, insects and rotifers as summarized by Guo et al. (2017a).
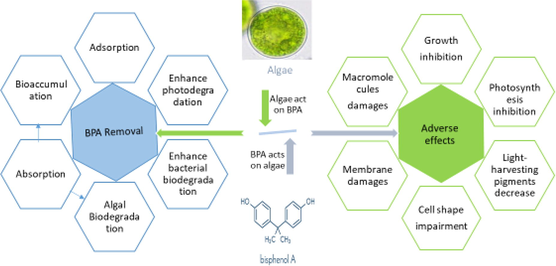
Algae and cyanobacteria are primary producers in aquatic food chains and play a very important role in the overall functioning and structure of both freshwater and marine ecosystems. They are inevitably exposed to different pollutants, including BPA, released in to the aquatic environments (Abdel-Hamid, 1996; Ben Ouada et al., 2018a; Li et al., 2009; Zhang et al., 2014a). BPA has been found toxic to algae inhibiting several physiological and biochemical processes like cell growth, photosynthesis and light-harvesting pigments in diverse groups of algae (Ben Ouada et al., 2018b; Ji et al., 2014; Zhang et al., 2014a). These primary producers also contribute to the cleaning and remediation of aquatic environments by removing certain pollutants like toxic metals, insecticides, herbicides, and phenolic compounds from water (Doshi et al., 2008; Jonsson et al., 2001; Kanekar et al., 2004; Rodríguez-Bernaldo de Quirós et al., 2010; Tahira et al., 2019; Zhang et al., 2011; El-Sheekh et al., 2012; Kanekar et al., 2004; Munoz et al., 2003; Norvill et al., 2016; Sethunathan et al., 2004). Like many other organic pollutants, algae potentially absorb, accumulate and biodegrade the environmental BPA (Ben Ouada et al., 2018a; Guo et al., 2017b; Hirooka et al., 2005; Ji et al., 2014; Li et al., 2009). It shows that an interplay between algae and BPA occurs, i.e., BPA adversely affects algae by causing toxicity while algae at the same time biodegrade BPA (Fig. 1). Several studies with diverse information have been published individually on both aspects of this interplay between algae and BPA. Collecting, summarizing and interpreting these diverse data in the form of a single comprehensive review article would be a good contribution to literature that would facilitate researchers and policy makers working in this field.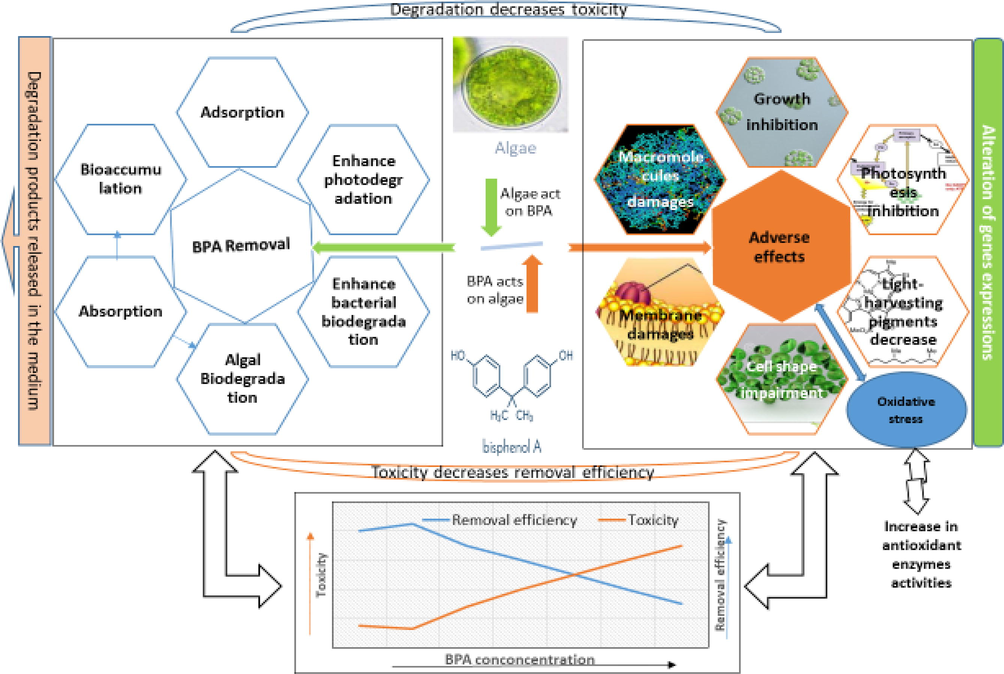
Graphical presentation of the interplay between BPA and algae. BPA adversely affects different parameters in algae while algae remove BPA from the medium in different ways. The increase in toxicity decreases degradation efficiency of algae, while degradation by algae reduces BPA concentration in the medium and hence its toxicity. The lower figure presents that increase in BPA concentrations increases BPA toxicity and decreases removal efficiency of algae. At low concentrations of BPA, slight stimulatory effects are depicted.
The main objectives of this study are (1) to briefly overview BPA contamination in freshwater and marine environments; (2) to summarize the adverse effects of BPA on various processes in different algae; and (3) to scrutinize BPA removal and biodegradation potentials of different algae. The effects of BPA on different parameters of algae like growth, light-harvesting pigments, photosynthesis, respiration, morphology and macromolecules (proteins, lipids and carbohydrates) are discussed. The oxidative stress caused by BPA in algae resulting in membranes lipids peroxidation and algae responses to this oxidative stress by activating antioxidant agents are elaborated. Furthermore, the ability of different algae to remove BPA by adsorption, accumulation and biodegradation is also presented. Since physical and chemical attributes of natural aquatic environments fluctuate spatio-temporally, an attempt is made to explain how different environmental factors influence the interactions between BPA and algae. This review provides comprehensive information on BPA and algae interactions that will be helpful in understanding the fate of BPA and its interactions with primary producers in aquatic environments. It will also facilitate decision makers in planning future strategies for controlling BPA pollution, and will be useful in selection of algal species for remediation of BPA in wastewater treatment plants. This review also identifies several research gaps that point the way to new research directions.
2 BPA contamination and fate in aquatic environments
The occurrence of BPA is almost ubiquitous in aquatic environments as it has widely been reported in various water types including freshwater, marine water, wastewater, ground water and drinking water as well as in sediments from aquatic environments across the world (Barnes et al., 2008; Boyd et al., 2004; Huang et al., 2012; Makinwa and Uadia, 2015; Yamamoto et al., 2001; Yamazaki et al., 2015). Many studies reported BPA presence in varying concentrations in both fresh and marine environments and data of some selected studies from different parts of the world is summarized here as representative concentrations (Suppl Table 1). An overview of the overall data on BPA contamination in aquatic environments reveals that in majority of cases BPA in both fresh and marine waters was found below 1 µg L−1 (Suppl Table 1). However, in some cases comparatively higher contamination was found, as for example, in river and sea waters from Turkey its concentrations were detected in the range of 4.62–29.92 µg L−1 (Ozhan and Kocaman, 2019). Similarly, water samples from sixteen different rivers in Taiwan contained BPA in the range of 0.01–44.65 μg L−1 (Lee et al., 2013). Usually, water resources near to industrial and commercial cities contain higher levels of BPA (Huang et al., 2012). BPA contamination was also reported in ground water. U.S. Geological Survey in a report of 2000 placed BPA among the top five most frequently occurred organic compounds in ground water of the United States (Barnes et al., 2008). In comparison to water, sediments were generally found with higher concentrations of BPA (Suppl Table 1).
Sources of BPA contamination in aquatic environments include both pre-consumer and post-consumer sources (Corrales et al., 2015). Major sources of its contamination in aquatic environments include manufacturing plants synthesizing BPA and using it as raw materials, landfill sites, wastewaters and effluents, waste treatment plants, and improper disposal of BPA-containing products (Corrales et al., 2015; Li et al., 2017). BPA concentrations crossing 17,000 µg L−1 have been observed in hazardous waste landfill leachates from Japan (Yamamoto et al., 2001). Untreated domestic and municipal sewage has also been related to migration of BPA-based products in water bodies (Zheng et al., 2019). Waste effluents from industries using BPA as raw materials have been a major cause of BPA pollution in water as they contains BPA even after treatment (Yamamoto et al., 2001). Usually most of the BPA in wastewater (nearly 90%) is removed in wastewater treatment plant (WWTP), but still some of it remains there after treatment (Dorn et al., 1987; Fuerhacker, 2003). A review by Corrales et al. (2015) reveals that WWTPs across the globe contained BPA concentrations ranging from non-detectable to 370 µg L−1. Effluents from sewage treatment plant have been regarded as a major source of BPA contamination in Yunliang River in Nanjing, China (Zheng et al., 2019). Similarly, urban wastes discharges from the city of Seoul have resulted in increased BPA concentration in Han River in South Korea. At upstream sites in this river BPA concentration was found to be 4.6 ng L−1 which enhanced to 241 ng L−1 downstream after receiving effluents from metropolitan area of Seoul (Yamazaki et al., 2015). BPA levels near wastewater plants and landfills are usually reported high in water bodies, but degradation and dilution result in lowering the level with increasing distance from the source. Other possible sources of BPA contamination in water are leaching from degradation of waste plastics and synthetic leather, migration from PVC hoses used for drainage and watering and leaching from epoxy-resin tanks (Yamamoto et al., 2001).
After its release into the environment several biotic and abiotic mechanisms disperse and degrade BPA. In water, it may be subjected to biodegradation, photodegradation and adsorption to suspended solids, but usually does not hydrolyze or volatilize (Li et al., 2019; NCBI, 2020). Depending on the prevailing condition like pH, turbidity and water turbulence, BPA half-life in water ranges from 66 h to 160 days (Im and Löffler, 2016). BPA, in aqueous media, may undergo light induced transformation via photocatalysis or photooxidation after absorption of radiation in the UV range or interaction with hydroxyl radicals, respectively (Howard et al., 1990; Peng et al., 2006). During photolysis, photons are absorbed by the degrading chemical species which initiate its chemical breakdown. The resulting intermediates of photolysis of BPA include phenol, 4-isopropylphenol and a semi-quinone derivative of BPA (Peng et al., 2006). Photooxidation causes BPA degradation through naturally occurring reactive oxygen species (ROS) including hydroxyl radicals (OH•), peroxide radicals (ROO•) and singlet oxygen (O) produced by light irradiation (Peng et al., 2006; Reddy et al., 2018). In the presence of certain chemical species like nitrite and nitrate, solar radiations generate hydroxyl radicals from water that cause transformation of BPA (Reddy et al., 2018). Several efficient methods for removal of BPA and other contaminants from aquatic environment are summarized in recently published review articles (Zhang et al., 2021; Li et al., 2021; Yu et al. 2021; Liang et al., 2021).
In addition to abiotic removal, BPA in natural water undergoes biotic degradation as many different species of plants, algae, fungi, and bacteria are capable of adsorbing, accumulating, transforming and biodegrading BPA (Chang et al., 2014; Gulnaz and Dincer, 2009; Husain and Qayyum, 2013; Michałowicz, 2014; Xiao et al., 2020). For example, bacteria of several different genera from various compartments of the environment were reported to metabolize BPA under aerobic conditions (Husain and Qayyum, 2013; Im and Löffler, 2016). Bacterial degradation of BPA in water may follow several different metabolic pathways with different routes, but oxidative skeletal rearrangement giving 4-hydroxybenzoate (HBA), 4-hydroxybenzaldehyde (HBAL), 4-hydroxycumyl alcohol (HCA), 4-isopropenylphenol (IPP), 4-hydroxyacetophenone (HAP), and hydroquinone (HQ) as intermediates is considered as the most probable route (Im and Löffler, 2016). Similarly, fungi were found to potentially degrade BPA by certain enzymes like lignin peroxidases, manganese peroxidases, versatile peroxidases, and laccases (Husain and Qayyum, 2013). Among these fungal enzymes, laccases from different fungi were found to have high potential for BPA degradation and transformation giving carboxylic derivatives as degradation products (Bilal et al., 2019; Daâssi et al., 2016). Algae also have an efficient role in the biodegradation of BPA in aquatic environments, which is discussed later in this article.
3 Effect of BPA on algae
In laboratory studies, the effect of a given toxicant on living organisms is generally assessed by determining the no observed effect concentration (NOEC) and EC50/IC50 (as defined below) values for specific end points (e.g. cell growth) in the test organism (Azizullah and Häder, 2018). NOEC is the highest tested concentration of a substance that does not cause any visible or observed effect on the studied end point (e.g. growth) of the bioassay organism. EC50 or IC50 is the concentration of a substance which causes 50% of the observed effect or causes 50% of the inhibition. Sometimes effective concentration (EC) is also used in determining toxicity, which is considered as the lowest tested dose of a substance that caused a significant effect. From the available literature, we extracted EC and/or EC50/IC50 values of BPA, depending upon the availability of data, for different parameters of algae and used these indices in describing the impact of BPA on algae. Different biochemical, morphological and physiological aspects like cell growth, cell shape, photosynthesis, respiration, light-harvesting pigments and other related parameters of algae were reported to be affected by exposure to high concentration of BPA (Tables 2–5). *under dark and ** light conditions. *At both concentrations inhibition was observed in initial days and was later recovered. ** In dark adapted cells, stimulation was caused *** in light adapted cell, inhibition was caused. *decrease was observed.
Algae
Group
Habitat
BPA conc. tested (mg L−1)
Exposure duration (days)
EC (mg L−1)
IC50/EC50 (mg L−1)
Reference
Alexandrium pacificum
Dinoflagellate
Marine
0.002 & 0.02
(2 & 20 µg L−1)7
0.002
–
(M'Rabet et al., 2018)
Chlamydomonas mexicana
Green algae
Freshwater
1–50
5
25
44.8
(Ji et al., 2014)
Chlorella fusca VAR. VACUOLATA
Green algae
Freshwater
2.283–36.53
(10–160 µM)7
9.13
–
(Hirooka et al., 2005)
Chlorella pyrenoidosa
Green algae
Freshwater
0.1–10
6
1
–
(Duan et al., 2019)
Chlorella pyrenoidosa
Green algae
Freshwater
1.6–50
1–7
44.9 (96 h)
(Li et al., 2017)
Chlorella pyrenoidosa
Green algae
Freshwater
1–50
1–7
1
46.04–89.39
(Zhang et al., 2014a)
Chlorella pyrenoidosa
Green algae
Freshwater
1–50
5–30
No effect
–
(Zhang et al., 2014a)
Chlorella sorokiniana
Green algae
Freshwater
10–50
7
20
–
(Eio et al., 2015)
Chlorella vulgaris
Green algae
Freshwater
1–50
5
25
39.8
(Ji et al., 2014)
Chlorella vulgaris
Green algae
Freshwater
2–50
10
20* 10**
–
(Wang et al., 2017a)
Chlorella vulgaris
Green algae
Freshwater
5–80
7
20
–
(Gulnaz and Dincer, 2009)
Cochlodinium polykrikoides
Dinoflagellate
Marine
0.1–500
3
25
68.15
(Ebenezer and Ki, 2012)
Cyclotella caspia
Diatom
Marine
4–12
4
4
7.96
(Li et al., 2008)
Desmodesmus subspicatus
Green algae
Freshwater
7–42
3
–
19.6
(Tišler et al., 2016)
Ditylum brightwellii
Diatom
Marine
0.05–20
3
–
0.039
(Ebenezer and Ki, 2016)
Graesiella sp.
Green algae
Hot water spring
1–75
1–5
1
19.5–24
(Ben Ouada et al., 2018b)
Monoraphidium braunii
Green algae
Freshwater
4–10
4
–
10
(Gattullo et al., 2012)
Navicula incerta
Diatom
Marine
1–5
4
–
3.37
(Liu et al., 2010)
Picocystis sp.
Green algae
Saline lakes
1–75
1–4
25
>75
(Ben Ouada et al., 2018b)
Prorocentrum minimum
Dinoflagellate
Marine
0.01–10
3
–
1.506
(Ebenezer and Ki, 2016)
Prorocentrum minimum
Dinoflagellate
Marine
0.01–10
3
–
1.51
(Guo et al., 2012)
Scenedesmus obliquus
Green algae
Freshwater
1.6–50
1–7
–
33.9 (4 day)
(Li et al., 2017)
Scenedesmus obliquus
Green algae
Freshwater
1–50
1–7
1
15.59–29.16
(Zhang et al., 2014a)
Scenedesmus obliquus
Green algae
Freshwater
1–50
5–30
No effect
–
(Zhang et al., 2014a)
Scenedesmus quadricauda
Green algae
Freshwater
1–20
4
2
13.233
(Xiang et al., 2018a)
Selenastrum capricornutum
Green algae
Freshwater
0.78–10
4
2.16
2.73
(Alexander et al., 1988)
Skeletonema costatum
Diatom
Marine
0.72–15
4
0.72
1
(Alexander et al., 1988)
Stephanodiscus hantzschii
Diatom
Marine
0.01–9
4
5
8.65
(Li et al., 2009)
Tetraselmis suecica
Green algae
Marine
0.5–100
3
–
15.55
(Ebenezer and Ki, 2016)
Ulva pertusa
Green macroalga
Marine
6.25–100
4
12.5
23.84
(Yang and Hong, 2012)
Algae name
Algae group
Habitat
BPA conc. tested (mg L−1)
Exposure duration (days)
EC (mg L−1)
EC50 (mg L−1)
Reference
Alexandrium pacificum
Dianoflagellate
Marine
0.002 & 0.02
(2 & 20 µg L−1)7
0.002
(2 µg L-1)–
(M'Rabet et al., 2018)
Chlamydomonas mexicana
Green algae
Freshwater
1–50
5
25
–
(Ji et al., 2014)
Chlorella pyrenoidosa
Green algae
Freshwater
0.1–10
6
1
–
(Duan et al., 2019)
Chlorella pyrenoidosa
Green algae
Freshwater
1–50
1–7
1–25
–
(Zhang et al., 2014a)
Chlorella pyrenoidosa
Green algae
Freshwater
1–50
5–30
No effect
–
(Zhang et al., 2014a)
Chlorella sorokiniana
Green algae
Freshwater
10–50
7
20
–
(Eio et al., 2015)
Chlorella vulgaris
Green algae
Freshwater
1–50
5
25
–
(Ji et al., 2014)
Cyclotella caspia
Diatom
Marine
4–12
4–20
4–8
–
(Li et al., 2008)
Desmodesmus sp.WR1
Green algae
Freshwater
1–13.5
10
No effect
–
(Wang et al., 2017c)
Ditylum brightwellii
Diatom
marine
0.05–20
3
–
0.037
(Ebenezer and Ki, 2016)
Monoraphidium braunii
Green algae
Freshwater
4–10
2–4
2–10
–
(Gattullo et al., 2012)
Navicula incerta
Diatom
Marine
1–5
4
No effect
–
(Liu et al., 2010)
Scenedesmus obliquus
Green algae
Freshwater
1–50
1–7
1–10
–
(Zhang et al., 2014a)
Scenedesmus obliquus
Green algae
Freshwater
1–50
5–30
10–50
–
(Zhang et al., 2014a)
Scenedesmus quadricauda
Green algae
Freshwater
1–20
4
1
–
(Xiang et al., 2018a)
Skeletonemu costarum
Diatom
Marine
0.72–15
4
0.72
1.8
(Alexander et al., 1988)
Stephanodiscus hantzschii
Diatom
Marine
0.01–9
4
3
–
(Li et al., 2009)
Algae species
Group
Habitat
BPA conc. tested (mg L−1)
Exposure duration (days)
EC (mg L−1)
Reference
Fv/Fm
Alexandrium pacificum
Dinoflagellate
Marine
0.002 & 0.02
(2 & 20 µg L−1)1–7
0.002 and 0.020*
(M'Rabet et al., 2018)
Chlorella pyrenoidosa
Green algae
Freshwater
0.1–10
6
1
(Duan et al., 2019)
Desmodesmus sp.WR1
Green algae
Freshwater
1–13.5
1–10
3
(Wang et al., 2017c)
Graesiella sp.
Green algae
Hot water spring
1–75
1–5
1
(Ben Ouada et al., 2018b)
Monoraphidium braunii
Diatom
marine
4–10
4
2**
10***(Gattullo et al., 2012)
Picocystis sp.
Green algae
Saline lakes
1–75
1–5
1–25
(Ben Ouada et al., 2018b)
Picocystis sp.
Green algae
Saline lakes
1–75
1–5
1–25
(Ben Ouada et al., 2018a)
Scenedesmus quadricauda
Green algae
Freshwater
1–20
4
10
(Xiang et al., 2018a)
O2 method
Alexandrium pacificum
Dinoflagellate
Marine
0.002 & 0.02
1–7
0.002 and 0.020*
(M'Rabet et al., 2018)
Graesiella sp.
Green algae
Hot water spring
1–75
1–5
1–10
(Ben Ouada et al., 2018b)
Picocystis sp.
Green algae
Saline lakes
1–75
1–5 days
1–10
(Ben Ouada et al., 2018b)
Picocystis sp.
Green algae
Saline lakes
1–75
1–5 days
1–10
(Ben Ouada et al., 2018a)
Algae
Group
Habitat
BPA conc. (mg L−1)
Exposure duration
(days)APX
SOD
POD
CAT
GST
LPO
Reference
Chlorella pyrenoidosa
Green algae
Freshwater
1–50
4
__
1
__
1
__
__
(Zhang et al., 2014a)
Chlorella vulgaris
Green algae
Freshwater
2–50
1–10
__
2
__
2
__
__
(Wang et al., 2017a)
Cyclotella caspia
Diatom
Marine
4–12
4
__
4
__
__
__
__
(Li et al., 2008)
Graesiella
Green algae
Hot water spring
1–75
1–5
1–10
__
__
1–25
1–25*
1–10
(Ben Ouada et al., 2018b)
Navicula incerta
Diatom
Marine
1–5
4
__
4
5*
__
4
__
(Liu et al., 2010)
Picocystis
Green algae
Saline lake
1–75
1–5
10–25
__
__
25–50
25
10
(Ben Ouada et al., 2018b)
Picocystis
Green algae
Saline lake
1–75
1–5
10–25
__
__
10–25
25
10
(Ben Ouada et al., 2018a)
Scenedesmus obliquus
Green algae
Freshwater
1–50
4
__
25
__
10
__
__
(Zhang et al., 2014a)
Scenedesmus quadricauda
Green algae
Freshwater
1–20
4
__
1
__
2
__
2
(Xiang et al., 2018a)
Algal species
Group
Habitat
Conc. of BPA (mg L−1)
Growth conditions (photoperiod & temp.)
Exposure duration (days)
Techniques used for BPA analysis
Abiotic removal
Total removal
Biodegradation (%)
BCF value
Reference
Picocystis sp.
Green algae
Saline lake
25, 50, 75
16:8h (L:D), 30 °C
5
HPLC
13.8–19.23%
40.4–72%
20.7–39.87%
3.9–6.86
(Ben Ouada et al., 2018a; Ben Ouada et al., 2018b)
Graesiella sp.
Green algae
Hot water spring
25, 50, 75
16:8h (L:D), 30 °C
5
HPLC
12.99–17.59%
27.77–52.63%
7.29–18.88
5.49–9.88
(Ben Ouada et al., 2018b)
Stephanodiscus hantzschii
Diatom
Marine
0.01–9
12:12 h (L:D) 20 °C
16
SPME/GC-FID
16.2–23.5%
25.6–98.8%
7–78 %
3.40–457.75
(Li et al., 2009)
Navicula incerta
Diatom
Marine
1–5
16:8h (L:D), 23 °C
4
GC–MS
__
__
3.71–37.78
͌150–261
(Liu et al., 2010)
Chlamydomonas mexicana
Green algae
Freshwater
1–50
16:8h (L:D), 27 °C
5–10
UPLC-MS
5.5–15%
8–39%
0.7–24%
2.1–23.3
(Ji et al., 2014)
Chlorella vulgaris
Green algae
Freshwater
1–50
16:8h (L:D), 27 °C
5–10
UPLC-MS
5.5–16%
6–38%
3.7–23%
1.4–29.6
(Ji et al., 2014)
Chlorella vulgaris
Green algae
Freshwater
2–50
12:12 h (L:D),
25 °C10
HPLC
__
3.425 mg (L.D)−1
__
__
(Wang et al., 2017a)
24 h dark, 25 °C
10
HPLC
__
1.53 mg (L.D)−1
__
__
Ulva prolifera
Green algae
Marine
0.1
(100 µg L−1)12:12 h (L:D)
1
UHPLC and GC–MS
0%
94.3%
__
__
(Zhang et al., 2019)
24 h light
1
UHPLC and GC–MS
__
98.50%
__
__
24 h dark
1
UHPLC and GC–MS
__
41.80%
__
__
Desmodesmus sp.WR1
Green algae
Freshwater
1–13.5
12:12 h (L:D), 22 °C
10
HPLC, LC-HRMS
Slight
18–57%
__
__
(Wang et al., 2017c)
Chlorella vulgaris
Green algae
Freshwater
20
14:10 h (L:D) 25 °C
4
GC and GC–MS
0
greater than 50%
__
__
(Gulnaz and Dincer, 2009)
Chlorella pyrenoidosa
Green algae
Freshwater
0.0108
(10.8 µg L−1) (radioactive)12:12 h (L:D) 25 °C
10
HPLC
__
__
__
106
(Guo et al., 2017a)
Chlorella fusca
Green algae
Freshwater
2.283–36.53
(10–160 µM)24 h light 27.5C
7
HPLC
0
70–95%
__
__
(Hirooka et al., 2005)
Chlorella sorokiniana
Green algae
Freshwater
10, 20, 50
12:12 h (L:D) 25 °C
7
HPLC
11.3–18.8%
__
20.7–38.5%
__
(Eio et al., 2015)
Chlorella sorokiniana + bacteria
Green algae
Freshwater
10, 20, 50
12:12 h (L:D) 25 °C
7
HPLC, GC–MS
11.3–18.8%
__
100%
__
Monoraphidium braunii
Green algae
Freshwater
2, 4, 10
12:12 h (L:D) 22 °C
4
HPLC
Negligible
35–48%
__
__
(Gattullo et al., 2012)
Monoraphidium braunii + NOM
Green algae
Freshwater
2, 4, 10
12:12 h (L:D) 22 °C
4
HPLC
Negligible
31–52%
__
__
Chlorella fusca
Green algae
Freshwater
9.13 (40 µM)
Light 25 °C
5
HPLC
0
85%
__
__
(Hirooka et al., 2003)
Dark
25 °C5
HPLC
0
22%
__
__
3.1 Effect of BPA on growth in algae
Growth of algae is one of the most important and most commonly used endpoints in ecotoxicological assessment of pollutants in aquatic environments. Growth is given the prime importance because it is comparatively easy to measure, and it reflects the ultimate and net effect of a pollutant on algae. Though sometimes the growth may show less sensitivity and may not be affected by doses of a pollutant that causes notable changes in other physiologically and ecologically important parameters of algae (Azizullah et al., 2013; Ben Ouada et al., 2018b). Inhibition of growth by BPA has been reported in many algal species of different classes and habitats, and this effect varies greatly from algae to algae as revealed from the reported EC and EC50/IC50 values (Table 1). Based on the growth inhibition test, species like Ditylum brightwellii, Skeletonema costarum, Prorocentrum minimum, Selenastrum capricornurum and Navicula incerta with EC50/IC50 values of 0.039, 1, 1.506, 2.73 and 3.37 mg L−1, respectively, can be considered as the most sensitive to BPA stress among the species reported in the reviewed literature (Table 1). A marine dinoflagellate, Alexandrium pacificum, can be considered even more sensitive as its growth was drastically and significantly inhibited by BPA at a concentration as low as 2 µg L−1 (M'Rabet et al., 2018). On the other hand, growth in algae like Chlorella pyrenoidosa (EC50: 44.9–89.39 mg L−1), Cochlodinium polykrikoides (EC50: 68.15 mg L−1), Chlamydomonas mexicana (EC50: 44.8 mg L−1) and Chlorella vulgaris (EC50: 39.8 mg L−1) was found very tolerant to BPA stress (Table 1). Picocystis also tolerated very high doses of BPA and exposure to 75 mg L−1 of BPA for five days could not inhibit its growth by more than 45% (Ben Ouada et al., 2018a; Ben Ouada et al., 2018b). Similarly, adverse effects of BPA on growth in different algal species can be seen from the effective concentrations (EC) shown in Table 1. BPA was also shown to cause reproductive toxicity in algae as confirmed by inhibition of spores release in Ulva pertusa (Yang and Hong, 2012). In a 96-h acute toxicity test with sporulation of this alga, 0, 12.35, 53.85, 99.69 and 100% inhibition of spore release was observed at 6.25, 12.5, 25, 50 and 100 mg L−1 of BPA, respectively, with an average EC50 value of 23.84 mg L−1 (Yang and Hong, 2012).
The adverse effect of BPA on growth in algae was mostly assessed for 1 to 7 days (Table 1) and only a few studies evaluated it in long-term beyond seven days (Ji et al., 2014; Li et al., 2009; Li et al., 2008; Zhang et al., 2014a). In a 10-day exposure of Chlamydomonas mexicana and Chlorella vulgaris to 1–50 mg L−1 of BPA, no significant effect on growth was observed at concentration below 10 mg L−1, while a 25 mg L−1 BPA initially caused a slight growth inhibition during initial days, but it was recovered thereafter (Ji et al., 2014). However, a 50 mg L−1 of BPA caused severe growth inhibition of 85% in C. vulgaris and little inhibition of 18% in C. mexicana after ten days (Ji et al., 2014). A 16-day experiment with growth of Stephanodiscus hantzschii under BPA exposure (0.01–9 mg L−1) revealed time and dose dependent effects (Li et al., 2009). A dose of BPA up to 1 mg L−1 had no obvious effects on algal growth irrespective of exposure time but 3 mg L−1 inhibited the algal growth during the first four days but thereafter caused growth stimulation. However, exposure to 7 and 9 mg L−1 BPA severely stopped algal growth and caused the cell to die when exposure time exceeded 8 days (Li et al., 2009). The effect of BPA on algae growth in the longest exposure time was assessed by Zhang et al. (2014a) who studied the growth of Chlorella pyrenoidosa and Scenedesmus obliquus under 0, 1, 10, and 50 mg L−1 of BPA for 30 days. BPA did not inhibit the growth of any of the two algal species, rather the growth of C. pyrenoidosa was significantly enhanced by 1 and 50 mg L−1 of BPA after 30-day exposure and the authors concluded that in long-term exposure BPA may not be harmful to algae (Zhang et al., 2014a). Utilization of chemicals generated from degradation of BPA may be a possible reason for the enhancement of algal growth. Li et al. (2008) reported time-dependent findings upon exposure of Cyclotella caspia to 4–12 mg L−l BPA for 20 days. Growth determination after 4, 8 and 16 days revealed significant inhibition of growth at all treatment of BPA as compared to the untreated control. Culture treated with the highest concentration of BPA reached the death phase by day 12, the control culture reached the death phase by day 20, but interestingly the algae at 4 and 6 mg L−1 of BPA recovered to a normal growth at day 20 and culture at 4 mg L−1 of BPA had significantly higher growth than all treatments including the control (Li et al., 2008).
The collected data (Table 1) show huge differences in growth responses of different species of algae to BPA. No doubt the comparison of data on growth inhibition in different species of algae extracted from different studies may not give reliable conclusion on comparison as the experimental conditions and procedures used were different in different studies. However, differences in sensitivity of growth to BPA in different algae are also evident from the findings of several comparative studies (Ben Ouada et al., 2018b; Ji et al., 2014; Li et al., 2017; Xiang et al., 2018a; Zhang et al., 2014a). For example, when two different species of Chlorophyta, Graesiella (microalga with cell size of 8–12 µm) and Picocystis (microalga with cell size of 2–3 µm), were exposed to 10, 25 and 75 mg L−1 of BPA for 5 days, 20, 62 and 80% growth inhibition, respectively, was observed in Graesiella but only 10, 32, and 43% decrease in the growth of Picocystis was caused at the same doses of BPA (Ben Ouada et al., 2018b), showing that the inhibitory effect of BPA on the growth of Graesiella was almost double than Picocystis. This might be due to the increase surface area exposure of Graesiella due to its larger size. Li et al. (2017) compared the sensitivity of growth in Chlorella pyrenoidosa and Scenedesmus obliquus upon exposure to 1.6–50 mg L−1 BPA for 48, 96 and 144 h. The growth of S. obliquus was inhibited at all the tested concentrations of BPA at all exposure times, but in C. pyrenoidosa stimulation of growth was observed at all doses of BPA in the initial 48 h of exposure and an inhibitory effect was shown only at higher doses of BPA and that too after 48 h (Li et al., 2017). Zhang et al. (2014a) also observed that growth in S. obliquus was more sensitivity than C. pyrenoidosa to BPA. Similarly, Chlorella vulgaris demonstrated a higher sensitivity to BPA as compared to Chlamydomonas mexicana (Ji et al., 2014). It was suggested that cell wall composition might be a possible factor in differential sensitivities of different algae to BPA stress (Xiang et al., 2018a). Periplasmic redox activities might also be partially responsible. This speculation is supported by the differential sensitivities of prokaryotic and eukaryotic systems to environmental pollutants as these organisms have different cell wall composition (Bährs et al., 2013; Gao et al., 2009; Xiang et al., 2018a). For example, the cyanobacterium Cylindrospermopsis raciborskii responded to BPA very differently than the green alga S. quadricauda (Xiang et al., 2018a). However, in depth studies are needed to determine the role of cell wall in algae sensitivity to BPA. The different capacities of different algae to absorb, accumulate and degrade BPA (as discussed later in this article) are another possible explanation for the observed differential effects of BPA on growth in different algal species (Li et al., 2017). The origin of collection and past growth conditions can also contribute to BPA sensitivity in algae. In a comparison of growth sensitivity in Picocystis and Graesiella to BPA stress, the high tolerance of Picocystis was regarded to be attained due to its wastewater origin (Ben Ouada et al., 2018b). Tolerance of some other algal species like Stephanodiscus hantzschii and Cyclotella caspia to BPA stress was also attributed to their collection from polluted sites (Li et al., 2009; Li et al., 2008). Algae growing in water bodies that continuously receives sewage or other pollutants may develop high tolerance to contaminants (Aguilera and Amils, 2005; Ben Ouada et al., 2018b; Rehman et al., 2007). Furthermore, different algae have different defense mechanisms to cope with stress (discussed here later) that can be another explanation for differential effects of BPA on growth in different algae.
BPA may inhibit algal growth directly by affecting cell division or indirectly by adversely affecting different pathways in physiological and biochemical processes and the net effect may come in the form of growth inhibition. BPA was found to interfere with nutrients uptake in algae that can result in poor cell growth due to decreased uptake of some nutrients (Ji et al., 2014). A microscopic study revealed that algal cells exposed to high doses of BPA had significantly enlarged cell size, broken cell wall and poorly organized organelles (Li et al., 2009). Similarly, BPA caused alterations in the expression levels of several genes involved in different cellular pathways like photosynthesis, fatty acid metabolism, glycolysis, tricarboxylic acid cycle and oxidative phosphorylation (Duan et al., 2019). Therefore, growth inhibition by BPA in algae could be through interfering with multiple processes related to energy metabolism and other related cellular functions. Depending upon the dose of BPA, growth in some algae can recover from BPA stress with increase in exposure time (Ji et al., 2014; M'Rabet et al., 2018; Zhang et al., 2014a). For instant, growth inhibition in Chlorella vulgaris and Chlamydomonas mexicana caused by 25 mg L−1 of BPA observed in the initial five days of exposure was later recovered to a certain limit when the incubation period extended to 10 days (Ji et al., 2014). Similarly, growth recovery in Chlorella pyrenoidosa and Scenedesmus obliquus from BPA stress was observed with prolonged exposure time (M'Rabet et al., 2018). This recovery can be explained by two possible hypotheses: an adaptation of algae to BPA stress with time or a decrease in BPA concentrations due to degradation with the passage of time (Zhang et al., 2014a). Stimulation of growth, particularly at low doses of BPA, has also been reported in some algae like C. pyrenoidosa, C. vulgaris and S. hantzschii (Duan et al., 2019; Li et al., 2017; Li et al., 2009; Wang et al., 2017a). Exposure to low concentrations of BPA upregulated several genes involved in energy releasing processes (glycolysis, tricarboxylic acid cycle, and oxidative phosphorylation), cellular transport and nucleotides transport (Duan et al., 2019). The upregulation of such genes indicates that algal cells accelerate energy production and materials transport for cell growth that can be a possible reason for increase in growth and other functions of algae at low concentrations of BPA (Duan et al., 2019). Stimulation of some physiological parameters at low levels of different stresses is a common phenomenon in algae as reported in different algae (Cedergreen et al., 2006; Danilov and Ekelund, 2001b; Saikia et al., 2011; Stebbing, 1982) and may be a defense strategy of algae to overcome the stress.
Some studies evaluated the effect of BPA on algal growth in comparison to organisms from other trophic levels (consumers) that give interesting information on BPA toxicity at different trophic levels. Li et al. (2017) compared the effect of BPA on Oryzias latipes (Japanese medaka: secondary consumer), Daphnia magna (a cladoceran: primary consumer) and Chlorella pyrenoidosa and Scenedesmus obliquus (algae: primary producers). The order of acute toxicity of BPA was found as O. latipes (96 h LC50 9.4 mg L−1) > D. magna (96 h LC50 11.7 mg L−1) > S. obliquus (96 h EC50 33.9 mg L−1) and C. pyrenoidosa (96 h EC50 44.9 mg L−1) (Li et al., 2017). Though slightly differ, but still the EC50 values of both algal species are closer to each other and those of Daphnia and Oryzias are closer to each other reflecting that BPA is more toxic at consumer level (animals) than at primary producer level (algae). However, another study (Alexander et al., 1988) revealed something quite contradictory to the findings of Li et al. (2017). They evaluated the toxicity of BPA using test organisms at different tropic levels both in freshwater (alga Selenustrum capricornutum; crustacian Daphnia magna; fish Pimephales prornelas) and saline environment (diatom Skeletonemu costatum; crustacian Mysidopsis bahia; fish Menidia menidia). The EC50/IC50 obtained for freshwater organisms were as: S. capricornutum: 2.7 mg L−1 (96 h), Daphnia magna: 10 mg L−1 (48 h) and Pimephales prornelas: 4.7 mg L−1 (96 h) giving the order of sensitivity as algae > fish > crustacean. The 96 h EC50/IC50 values obtained with test for marine organisms were 1, 1.1 and 9.4 mg L−1 for S. costatum, M. bahia and M. menidia, respectively, giving the order of sensitivity as alga > crustacean > fish (Alexander et al., 1988). The study of Alexander et al. (1988) reflects growth in algae to be more sensitive than toxicity tests with crustaceans or fish. Bacteria, another trophic level, were found more tolerant than algae to BPA. For example, a comparative study of the alga C. vulgaris with the bacterium Aeromonas hydrophilia to BPA exposure revealed that the cell growth of the alga was inhibited by BPA in a dose above 20 mg L−1 but the bacterium could grow even at 120 mg L−1 of BPA (Gulnaz and Dincer, 2009).
According to the EU-Directive 93/67/EEC of the Commission of the European Communities (Commission, 1996), chemicals of environmental concern in aquatic environments are categorized in to three classes of toxicity based on their EC50 values: (1) very toxic to aquatic organisms (substances having EC50 values below 1 mg L−1), (2) toxic to aquatic organisms (substances having EC50 values in the range of 1 to 10 mg L−1), and (3) harmful to aquatic organisms (substances having EC50 values in the range 10 to 100 mg L−1), while chemicals with EC50 values above 100 mg L−1 should not be classified (Zhang et al., 2014a). According to this criterion, only to the diatom Ditylum brightwellii (EC50 = 0.039) BPA can be considered as very toxic. To the remaining species of algae BPA can be regarded as toxic to some species having EC50 values below 10 and harmful to some other species where EC50 values are above 10 but below 100 (Table 1). However, the reported concentrations of BPA in natural aquatic environments as discussed above are far lower than these values and, at present pollution level, BPA may not be a threat to the growth of algae. Nevertheless, growth in the dinoflagellate Alexandrium pacificum was affected by a very low dose of BPA (M'Rabet et al., 2018) but due to no determination of EC50 value by the authors, it cannot be interpreted here with the criteria of EU-Directive 93/67/EEC.
3.2 Effect of BPA on light-harvesting pigments in algae
Light-harvesting pigments are vital substances used by photoautotrophic organisms for light-energy absorption and its subsequent conversion to a form of energy (organic compounds) that is utilized not just by the photoautotrophs themselves but also by organisms in other trophic levels. In addition, the process releases molecular oxygen from water which is used in the vital cellular processes of aerobic respiration. Therefore, the presence of an optimum amount of these pigments in the plastids (chloroplasts) of phototrophic organisms is essential for proper functioning of the overall ecosystem (Azizullah et al., 2014; Fodorpataki et al., 2001). Environmental pollutions or stressors that impair the balance of light-harvesting pigments in photoautotrophic organisms can influence their photosynthetic performance and hence organism survival (Azizullah et al., 2012a, 2014). The composition and concentration of photosynthetic pigments are therefore widely used as endpoints in monitoring the toxicity of environmental pollutants in algae and plants (Ali et al., 2016; Azizullah et al., 2014; Markina, 2010; Shakir et al., 2016; Tahira et al., 2019). Common pollutants of aquatic environments like salinity, pesticides, heavy metals, metalloids, and detergents were frequently reported to adversely affect light-harvesting pigments (chlorophylls and carotenoids) in algae (Ahmed, 2010; Azizullah et al., 2014; González-Moreno et al., 1997; Küpper et al., 2002; Markina and Aizdaicher, 2007; Tahira et al., 2019).
Several studies reported the adverse effects of BPA on light-harvesting pigments in different algae (Table 2). While studying the effect of BPA on light-harvesting pigments in algae, most of the studies relied only on chlorophyll a and other accessory pigments (carotenoids and chlorophylls other than a) have rarely been considered. Therefore, the data extracted and shown in Table 2 is only for BPA effect on chlorophyll a. Like cell growth, the response of light-harvesting pigments (chlorophyll a) to BPA stress varied greatly in different algae and the effective concentrations ranged from as low as 2 µg L−1 in the marine dinoflagellate Alexandrium pacificum to as high as 25 mg L−1 in some other algae (Table 2). Based on the reported data, chlorophyll a pigment in A. pacificum and in the diatoms Skeletonema costarum and Ditylum brightwellii can be considered as the most sensitive to BPA exposure (Table 2). In the diatom S. costarum, a significant decrease in chlorophyll a was observed at 0.72 mg L−1 of BPA with a 96-h EC50 of 1.8 mg L−1 (Alexander et al., 1988). Similarly, in D. brightwellii, an EC50 value of 0.037 mg L−1 BPA for chlorophyll a was reported after 72 h exposure (Ebenezer and Ki, 2016). In the marine dinoflagellate A. pacificum, in a 7-day long experiment with chlorophyll a determination after every 24 h, a significant fall in the pigment was reported at BPA dose of 2 µg L−1 throughout the exposure time (M'Rabet et al., 2018). This might be the most sensitive response of light-harvesting pigments in algae to BPA stress ever reported as even at 2 µg L−1 of BPA more than 80% reduction in chlorophyll a was noted in comparison to the control. On the other hand, pigments in some species (e.g. C. mexicana and C. vulgaris) were very resistant to BPA and a decrease was noticed only at very high doses of BPA like 25 mg L−1 (Ji et al., 2014). Pigments in other algae responded to BPA at a dose of 1 mg L−1 or well above it (Table 2). Like growth, the responses of light-harvesting pigments in algae to BPA stress varied greatly from species to species, even in closely related species. For example, chlorophyll a in Chlorella pyrenoidosa and Scenedesmus obliquus was affected by BPA (1–50 mg L−1) differently both in acute (1–7 days) and chronic (5–30 days) tests (Zhang et al., 2014a). In initial four days (24–96 h) of acute test, significant decreases in chlorophyll a of C. pyrenoidosa were mostly observed at 25 and 50 mg L−1 of BPA, but in S. obliquus significant decreases were shown even at the lowest tested concentration of BPA (1 mg L−1) (Zhang et al., 2014a). Similarly, in chronic exposure BPA did not significantly affect chlorophyll a in C. pyrenoidosa, but in S. obliquus it was generally declined with increasing concentration of BPA and a significant reduction was noticed at 25 and 50 mg L−1 of BPA (Zhang et al., 2014a). Authors concluded that chlorophyll a in S. obliquus was more sensitive than in C. pyrenoidosa to BPA stress. Light-harvesting pigments in algae may also recover from the adverse effects of BPA with increase in incubation time, as for example, a recovery in Desmodesmus sp. from a stress of 13.5 mg L−1 BPA in a 10-day long experiment (Wang et al., 2017c). This recovery can be explained by the degradation (photo- or biodegradation) of BPA with the passage of time or by some protective strategy and/or adaptation of algae. At low doses, BPA may even cause stimulatory effects and increase chlorophyll content in some algae. For example, in a five days experiment 0.1 mg L−1 of BPA markedly increased chlorophyll a content in C. pyrenoidosa, particularly on the third day of growth and thereafter. On third day of growth, chlorophyll a concentration in the culture treated with 0.1 mg L−1 of BPA was 25% higher than the chlorophyll a content in the untreated control group (Duan et al., 2019). In Desmodesmus sp. even higher concentration of BPA as up to 5.5 mg L−1 caused an increase in the concentration of chlorophyll a (Wang et al., 2017c). This differential sensitivity of pigments in algae to BPA may be due to multiple and complex factors as discussed in the case of cell growth. One additional possible factor in the case of light-harvesting pigments can be the differences in thylakoids arrangement and light-harvesting proteins and pigments composition in thylakoids among different group of algae (Patty et al., 2019).
In some algae, the effect of BPA on chlorophyll a (measured per volume of culture) was similar to the effect on cell density or biomass of algae (Duan et al., 2019; Ji et al., 2014; Li et al., 2009). In such cases, the decrease in chlorophyll a was possibly not due to the direct effect of BPA on chlorophyll, but due to a decrease in cell density and biomass and hence lower concentration of pigments was found per volume of culture. Considering this factor and normalizing chlorophyll a to cell density (chlorophyll a per cell or per specific number of cells) can give more accurate information about the effect of BPA stress on pigments in algae. However, rare of the published studies on the subject matter considered this factor (Liu et al., 2010; Xiang et al., 2018a). BPA inhibited growth in the diatom Navicula incerta with a 96-h EC50 value of 3.73 mg L−1, but cellular contents of chlorophyll a and total chlorophyll c (when calculated per cell) were not significantly affected by the tested concentrations of BPA (1–5 mg L−1) (Liu et al., 2010). However, in another algal species, Scenedesmus quadricauda, a decrease in pigment concentration in response to BPA was shown even when normalized to cell density (Xiang et al., 2018a).
From overall survey of literature, it is evident that high concentrations of BPA cause a decrease in chlorophyll content of algae. However, it is not yet clearly known whether BPA inhibits the synthesis of new molecules of chlorophyll or destroys the already synthesized pigments. Zhang et al. (2014a) proposed that BPA may interfere with the synthesis of protochlorophylls or proteins and their subsequent conversion to chlorophyll. A 96-h exposure to BPA caused downregulation of several genes like hemN, acsF, chlL, chlN, chlP, crtB, pds related to chlorophyll and carotenoids synthesis in the cyanobacterium Cylindrospermopsis raciborskii (Xiang et al., 2018b). In a microscopic study Li et al. (2009) observed that when Stephanodiscus hantzschii cells were exposed to BPA above 5 mg L−1, cell organelles appeared somehow disorganized, and a gradual loss of the yellow-green color indicated a disintegration of the chloroplast and loss of chlorophyll molecules. Since BPA can lead to accumulation of reactive oxygen species (ROS) in algae, the oxidative stress may oxidize and damage the membranes of thylakoids and structure of chloroplast and hence reduces the chlorophyll content (Zhang et al., 2015). The oxidative damage of protein-pigments complexes may make the pigments prone to photodegradation. More in-depth studies are needed to understand the mechanism by which BPA affect chlorophyll in algae.
3.3 Effect of BPA on photosynthesis in algae
Photoautotrophic organisms including plants, algae and some bacteria trap light with their pigments to run photosynthesis. Environmental stresses can impair processes of photosynthesis. Photosynthetic efficiency has been used widely as a common endpoint in assessing the effects of environmental pollutants and stresses on photoautotrophic organisms (Fai et al., 2007; Petsas and Vagi, 2017). Any change in the photosynthesis of an organism caused by a toxicant can be reflected through changes in photochemical performances that can be detected using chlorophyll fluorescence measuring devices (Van Kooten and Snel, 1990). Photosynthetic rates determined by gaseous exchange or carbon fixation usually show close correlation with photosynthetic efficiency measured by fluorescence techniques (Genty et al., 1989; Seaton and Walker, 1990). Changes in photosynthesis of algae measured with chlorophyll a fluorescence method have been used very commonly in the assessment of ecotoxicity of pollutants in aquatic environments (Azizullah et al., 2014). Among the different fluorescence parameters, maximum quantum yield of photosystem II (Fv/Fm: in dark adapted cells), effective quantum yield of photosystem II (ΦPSII, or Φm or ΔF/Fm’, Fv’/Fm’: in light adapted cells), relative electron transport rate (rETR), photochemical quenching (qP) and non-photochemical quenching (NPQ) are usually used in ecophysiology and ecotoxicology studies (Petsas and Vagi, 2017; Van Kooten and Snel, 1990). These parameters give useful information on the status of reactions centres related to PSII (Henley, 1993; Schreiber et al., 1995; Seaton and Walker, 1990).
BPA can impair photosynthesis in algae as revealed by studies that monitored photosynthesis in BPA exposed algae by the classical gaseous exchange method or chlorophyll fluorescence method (Ben Ouada et al., 2018a; Ben Ouada et al., 2018b; Duan et al., 2019; Gattullo et al., 2012; M'Rabet et al., 2018; Wang et al., 2017c; Xiang et al., 2018a). A decrease in Fv/Fm was observed in several algal species like Alexandrium pacificum, Chlorella pyrenoidosa, Desmodesmus sp., Graesiella sp., Monoraphidium braunii, Picocystis sp., and Scenedesmus quadricauda when exposed to BPA (Table 3). Like growth and chlorophyll, high variations in the response of photosynthesis among different algal species to BPA exposure were found. The results obtained for the effect of BPA on photosynthesis in algae with fluorescence techniques corresponded well to the trend in the effect obtained with the measurement of gaseous emission (Ben Ouada et al., 2018b; M'Rabet et al., 2018). Ben Ouada et al. (2018b) studied the comparative sensitivity of photosynthesis in two algal species Picocystis and Graesiella to BPA by measuring their net photosynthetic activities (NPS) using oxygen evolution method and quantum yield of photosystem II (Fv/Fm) using chlorophyll fluorometer. A decrease in photosynthesis measured by both techniques in both the species of algae was observed with increasing concentration of BPA and increasing exposure time. The effect was more pronounced in Graesiella than in Picocystis. A five days exposure to 1–25 mg L−1 BPA inhibited NPS to a maximum of 40% in Picocystis but the same dose caused 80% decrease in NPS in Graesiella. The observed effects were even more pronounced at higher concentrations of BPA (50–75 mg L−1), where the decrease in NPS of Picocystis reached 82% but 100% inhibition of NPS was caused in Graesiella (Ben Ouada et al., 2018b). BPA-induced changes in photosynthetic efficiency of both algae measured by the florescence technique followed almost the same trend as that by O2 evolution method. A decrease in Fv/Fm in both algae was noted with increase in BPA concentration and exposure time. However, like the NPS, the observed effect was significantly less pronounced in Picocystis than in Graesiella (Ben Ouada et al., 2018b). At doses below 50 mg L−1 of BPA Fv/Fm in Picocystis was not reduced very prominently during the whole exposure duration, however, in Graesiella, a reduction of 60% in Fv/Fm was observed. At 75 mg L−1 BPA, the inhibition of Fv/Fm in Picocystis reached a maximum of 73% but to 90% in Graesiella after five days (Ben Ouada et al., 2018b). Similar findings were reported by Ben Ouada et al. (2018a) in Picocystis when exposed to BPA and the photosynthesis was measured as NPS and as Fv/Fm. M'Rabet et al. (2018) also observed a similarity in trend of the inhibitory effect of BPA on photosynthesis measured by chlorophyll fluorescence techniques (measuring Fv/Fm) and oxygen evolution method (measuring gross primary productivity GPP) in a marine dinoflagellate A. pacificum in a 7-day experiment. The observed effect of BPA on photosynthesis in this alga measured as Fv/Fm and as GPP showed a linear correlation (M'Rabet et al., 2018). The similar pattern in photosynthetic inhibition measured by gaseous method and as Fv/Fm suggest that BPA caused a proportion of PSII reaction centers to be photodamaged or inactivated and hence inhibited photosynthesis as revealed by NPS (Ben Ouada et al., 2018b).
Like Fv/Fm, relative electron transport rate (rETR) is also measured as an indicator of photosynthesis and is used to characterize the photochemical efficiency and the proportion of open oxidized reaction centers of PSII (Baumann et al., 2009). BPA was found to impair rETR and increase photoinhibition in algae (M'Rabet et al., 2018; Xiang et al., 2018a). For example, upon exposure to 20 µg L−1 of BPA for 7 days, a slight decrease in rETR (increased photoinhibition) was reported in Alexandrium pacificum with increasing light intensity (M'Rabet et al., 2018). Similarly, BPA at concentrations of 10 and 20 mg L−1 caused significant inhibition of rETR in the green microalga Scenedesmus quadricauda and at 5 and 10 mg L−1 in the cyanobacterium Cylindrospermopsis raciborskii after 96 h exposure (Xiang et al., 2018a). These findings are further confirmed by a significant decrease in photosynthetic light use efficiency by BPA in algae like A. pacificum and S. quadricauda and in the cyanobacterium C. raciborskii (M'Rabet et al., 2018; Xiang et al., 2018a).
Most of the studies in evaluating BPA toxicity used Fv/Fm as end point. However, the response of Fv/Fm and effective or actually functional quantum yield (quantum yield in light adapted cells) in algae may respond to BPA stress differently as reported by Gattullo et al. (2012). The effect of 2, 4 and 10 mg L−1 BPA on photosynthesis alone and in combination with natural organic matter (2, 5 and 20 mg L−1) in the green alga Monoraphidium braunii after two and four days exposure revealed that the maximum quantum yield (Fv/Fm) and the actual quantum yield (QPSII) of PSII were affected quite differently. After 2 days exposure, no significant effect of BPA alone nor of its combination with NOM was observed on Fv/Fm, while NOM alone at 5 and 20 mg L−1 caused an increase in Fv/Fm. After 4 days exposure an increase in Fv/Fm was shown by all doses of BPA alone and by almost all its combination with NOM (Gattullo et al., 2012). In comparison the effect on QPSII was quite different where some stimulation was observed at low doses of BPA, but a significant inhibition was caused by the highest applied dose of BPA (10 mg L−1), both after 2 and 4 days of exposure to BPA alone or in combination with NOM (Gattullo et al., 2012). However, Xiang et al. (2018a) did not notice any difference in the response of Fv/Fm and ΔF/Fm’ to BPA stress in Cylindrospermopsis raciborskii and Scenedesmus quadricauda and both the parameters were found significantly impaired at 10 and 20 mg L−1 of BPA.
In addition to Fv/Fm and rETR, photochemical quenching (qP) and non-photochemical quenching (NPQ) are used as indices of photosynthesis. These parameters provide useful information on photosystem (PSII) efficiency for utilizing the absorbed light. qP is used as an index of the conversion of the absorbed light energy into photochemical energy while NPQ shows the efficiency of dissipating access energy as heat and is related to photoprotection of the photosystem (Adams and Demmig-Adams, 2004; Müller et al., 2001; Ruban, 2016). BPA was found to affect both qP and NPQ in algae as reported in a comparative study on Cylindrospermopsis raciborskii (cyanobacteria) and Scenedesmus quadricauda upon exposure to different concentrations of BPA for 96 h (Xiang et al., 2018a). At low concentrations of 0.1 to 5 mg L−1, BPA caused an increase in the qP value in C. raciborskii but an inhibition was found when BPA concentration reached 10 mg L−1. In S. quadricauda, no stimulation of qP was shown at lower concentrations of BPA but a strong decrease was noted at BPA concentration of 10 mg L−1 or above (Xiang et al., 2018a). Calatayud and Barreno (2001) proposed that a decrease in qP can be due to the inhibition of the Calvin cycle, which reduces the reoxidizing primary electron acceptor (Q) capacity. BPA stress may cause closure and functional inactivation of some of the PSII reaction centers that result in the accumulation of QA -AM at acceptor side (Wang et al., 2010). In S. quadricauda, a tremendous increase of NPQ was observed at high concentrations of BPA indicating that excess light energy was dissipated as a heat to protect photosystem against the photodamage caused by BPA, however, in C. raciborskii a significant decrease in NPQ was caused by BPA (Xiang et al., 2018a). The additional energy in C. raciborskii might be dissipated in some other way or the dissipation capacity as heat in this species might have been impaired by BPA.
BPA may inhibit photosynthesis in algae by damaging and reducing the efficiency of different components of the photosynthetic machinery (Ben Ouada et al., 2018a; M'Rabet et al., 2018; Xiang et al., 2018a). In the photosynthetic machinery, PSII is generally considered as the major target of stresses (Ashraf and Harris, 2013), therefore, the damage or inactivation of PSII can be the main cause of photosynthesis impairment by BPA (Ben Ouada et al., 2018a; Xiang et al., 2018a). The stress caused by elevated doses of BPA can disturb biochemical and physiological processes by damaging cellular architecture (Li et al., 2009). BPA may damage light-harvesting pigments proteins complexes (LHC) in algae and reduces their ability to trap light energy (Xiang et al., 2018a). This is further supported by the adverse effects of BPA on different light-harvesting pigments in algae as discussed in this article. Ben Ouada et al. (2018a) and Qiu et al. (2013) proposed that due to the presence of phenolic hydroxyl group (–OH) in BPA, it may take electrons during its degradation in the medium and thus impair the transfer of electrons between PSII and PSI reaction centers, which can be another possible reason for photosynthesis inhibition by BPA. The impact of BPA on the expression of photosynthesis related genes confirms the adversity of BPA to photosynthesis in algae as discussed later in this article (Duan et al., 2019; Xiang et al., 2018b).
The stimulatory effects of BPA on photosynthesis in algae, reported particularly at low doses of BPA in some cases (Duan et al., 2019; Gattullo et al., 2012; Xiang et al., 2018a), can be explained by the change of membrane permeability due to BPA (Yang et al., 2014) or can be taken as a toxic excitation effect and a defense strategy of algae as discussed above. A recovery of photosynthesis from BPA stress with extending exposure time has been observed in some algae, as for example in Alexandrium pacificum where a recovery was noticed from 72 h of exposure onward (M'Rabet et al., 2018). This recovery can be due to the depletion of BPA in the culture medium by photodegradation and phycodegradation or possibly by exudate production by algae with time that can restrict BPA binding to the cell as summarized by M'Rabet et al. (2018).
3.4 Effect of BPA on respiration in algae
Respiration is one of the most vital and essential physiological activities in cell and can be impaired with environmental stresses. BPA adversely affects several physiological functions in algae as summarized in this article, an adverse effect on respiration cannot be excluded. BPA was found to impair activities of certain key enzymes involved in cellular respiration like cytochrome c oxidase, pyruvate kinase, phosphofructokinase, hexokinase and isocitrate dehydrogenase in soybean roots (Nie et al., 2015). Upregulation and inhibition of respiration related several pathways at low and high doses of BPA, respectively, were reported in the green alga C. pyrenoidosa (Duan et al., 2019). A transcriptomic study revealed downregulation of respiration related 45 genes in the cyanobacterium Cylindrospermopsis raciborskii (Xiang et al., 2018b). However, the effect of BPA on this one of the most vital cellular processes in algae was evaluated very rarely. M'Rabet et al. (2018) attempted to assess the effect of BPA alone and in combination with another plastic derived chemical (DEHP) on a marine alga Alexandrium pacificum. Both the chemicals alone as well in combinations significantly reduced the rate of respiration in the alga over a 7-day long experiment. The two chemicals in combination caused more severe effects. Their results revealed that with increasing time (1–7 days) the rate of respiration increased in the control as well as in cultures of algae treated with BPA, DEHP and their combination, however, it was very evident that the difference in the respiration rate between control and treatments increased with increase in exposure time and the most evident difference was shown after 72 h and thereafter (M'Rabet et al., 2018). Authors further investigated that the impact of both the chemicals on respiration was less than the gross primary productivity (photosynthesis), which was attributed to a possible contamination of algal culture with bacteria and the oxygen consumption was not just of the A. pacificum cells, but also of the associated microflora. Their further investigation of the specific respiration rate (Respiration/Cell density) revealed no significant difference in respiration of control and treated algae during the whole course of experiment and the changes in respiration due to BPA and DEHP were attributed to the changes in the total biomass caused by these chemicals (M'Rabet et al., 2018). To the best of our knowledge, no other study on BPA effect on algal respiration was found.
3.5 Effects of BPA on proteins, lipids and carbohydrates in algae
Abiotic stresses in the form of physicochemical changes or pollutants can cause changes in macro organic compounds like proteins, carbohydrates and fats composition in algae, which can be a result of changes in different metabolic pathways of algae in response to the stress conditions (Ji et al., 2014). Like physiological, morphological and other biochemical aspects of algae discussed here, BPA can evidently interfere with the composition of fats, carbohydrates and proteins in algae as reported in marine diatom N. incerta and freshwater microalgae Chlorella vulgaris and Chlamydomonas mexicana (Ji et al., 2014; Liu et al., 2010). In a cyanobacterium BPA downregulated several genes related to protein and fatty acid metabolism (Xiang et al., 2018b). In N. incerta, 96 h exposure to BPA (1–5 mg L−1) caused a significant reduction in cellular contents of total protein at a dose of 3 mg L−1 or above with a 43% decrease at the highest tested concentration (5 mg L−1) as compared to the control (Liu et al., 2010). Like total proteins, the content of polysaccharides was significantly decreased in N. incerta upon exposure to BPA at 2 mg L−1 or above and a 70% decrease was observed in polysaccharide at 5 mg L−1 of BPA after 96 h (Liu et al., 2010). In contrast to N. incerta, BPA exposure caused a slight increase in carbohydrates contents in freshwater algae C. vulgaris and C. mexicana (Ji et al., 2014). In these two algae, exposure to 25 and 50 mg L−1 of BPA increased the carbohydrates contents by 9.6 and 6.5%, respectively, as compared to the control. In C. vulgaris there were 40.4% of carbohydrates in culture grown in 25 mg L−1 of BPA in comparison to 33.5% carbohydrates in the control culture, while in C. mexicana in comparison to 33.6% in the control, 39.6% carbohydrates were quantified in cultures grown at 50 mg L−1 of BPA (Ji et al., 2014). Another phenolic compound, 2–4-dichlorphenol also caused a significant increase in carbohydrate content in the diatom Skeletonema costatum (Yang et al., 2002). Since carbohydrates are used both as structural components in the cell wall and as a source of energy (stored form) in the metabolic processes of plants and algae (Carrieri et al., 2010; Ji et al., 2014), an effect on carbohydrates reflects an influence on structural, functional and metabolic aspects of algae (Ji et al., 2014; Li et al., 2008). Many environmental factors like light, temperature, salinity and nutrients status affect carbohydrates contents in algae due to alteration in metabolic pathways (Carrieri et al., 2010; Ji et al., 2013; Liu et al., 2010; Salama et al., 2013). The differential responses of carbohydrates to BPA in different algae (Ji et al., 2014; Liu et al., 2010) show that different algae may respond differently to BPA stress regarding energy storage and metabolism.
BPA significantly increased lipid contents in both marine and freshwater algae. In N. incerta a significant increase in total lipids was caused by BPA at a dose above 2 mg L−1 and a tremendous increase of 134% in comparison to the control was observed at 5 mg L−1 of BPA (Liu et al., 2010). A detail study on different fatty acids in C. mexicana and C. vulgaris reveals a similar trend, i.e. an increase in lipids in both algae upon exposure to BPA (Ji et al., 2014). Measuring fatty acids methyl ester (FAME) revealed that 9.3 and 8.9% production of FAME both in C. mexicana and C. vulgaris was found in the control that were increased to 11.4 and 10.6% at 10 mg L−1 of BPA, respectively (Ji et al., 2014). Exposure to 6 mg L−1 of 2,4-dichlorophenol also led to an enhancement of lipid content in the marine diatom S. costatum (Yang et al., 2002). A correlation of the increase in lipid content with the decrease in algal growth suggests that under BPA stress algae may change the pattern of energy utilization by diverting the energy towards lipid storage than using it for growth (Ji et al., 2014; Liu et al., 2010). Increase in lipids content of algae is regarded as an indicator of toxicant stress and it enhances the bioconcentration of hydrophobic and lipophilic organic toxicants and algae used it as a protective measure by reducing the bioavailability of these compounds (Halling-Sørensen et al., 2000; Ji et al., 2014). However, the saturated and unsaturated fatty acids may respond differently to BPA stress. In C. vulgaris and C. mexicana BPA caused a decrease in saturated fatty acid but an increase in unsaturated fatty acids (Ji et al., 2014). The increase in unsaturated fatty acids can be due to the membrane damage caused by oxidative stress of BPA (as discussed here later), which can influence the functions of algal cell membranes and metabolic processes (Li et al., 2008). Further in-depth studies are needed on the effect of BPA on the content and metabolism of lipids, proteins and carbohydrates in algae. This will also help in understanding the effect of BPA on nutritional value of algae used as food by consumers in the aquatic ecosystems.
3.6 Effect of BPA on morphology of algae
Morphological features of an organism may have an important role in its ecological position and adaptation to the environment. Algae are known to change their shape in response to changes and pollution in the surrounding environment (Azizullah et al., 2012a,b; Murray, 1981; Takenaka et al., 1997). Pollutants or stresses that can interact with the cell wall, plasma membrane, cytoskeleton or homoeostasis of algal cells can change the cell morphology of algae. This change in cell shape in some aquatic organisms like Euglena is very sensitive to changes in the environment and has widely been used as endpoint in ecotoxicological assessment of aquatic pollutants (Ahmed, 2010; Azizullah et al., 2012b; Azizullah et al., 2013; Conforti, 1998; Danilov and Ekelund, 2001a; Tahira et al., 2019).
Studies are there revealing strong impact of BPA on morphological features of algae (Gattullo et al., 2012; Li et al., 2009; Li et al., 2008; Xiang et al., 2018a). In a cyanobacterium Cylindrospermopsis raciborskii and green alga Scenedesmus quadricauda dose dependent prominent changes in cell morphologies have been noticed after 96 h exposure to BPA (Xiang et al., 2018a). The morphological changes have been determined microscopically as changes in trichome length in C. raciborskii and cell morphology as four cells, three cells, two cells, single cell and empty cell in S. quadricauda. After exposure to BPA, cells of C. raciborskii were grouped as trichome with length of 0–50 µm, 50–250 µm, and above 250 µm. With the increasing concentration of BPA, the percentage of trichome with shorter length increased in C. raciborskii culture. In the control culture, around 93% of trichomes length was above 250 µm which dropped to 85% even at the lowest dose of BPA tested (0.1 mg L−1). This effect tremendously increased with increase in BPA dose and at 20 mg L−1 of BPA around 92% cells had a trichome length of 0–50 µm with almost no cell with a length above 250 µm (Xiang et al., 2018a). Similarly, visible changes in S. quadricauda morphology have been noticed in BPA exposed cultures. Many colonies splitted into single cells or groups of two or three cells as compared to four cells colonies in the control, and even completely empty cells have frequently been observed at higher concentrations of BPA. At 20 mg L−1 of BPA, 24.47% of the cells were empty cells (Xiang et al., 2018a). BPA at a concentration of 10 mg L−1 significantly reduced cell size in another alga Monoraphidium braunii after 2 and 4 days exposure (Gattullo et al., 2012). In contrast, BPA at a concentration above 6 mg L−1 significantly increased cell volume in a marine alga Cyclotella caspia as concluded in a detailed 20-day microscopic study by Li et al. (2008). At lower dose (4 mg L−1) some effect on cell morphology of this alga was observed in initial days that was later recovered. A 6 mg L−1 of BPA increased the length of the pervalvar axis in some cells of C. caspia from 9.8 µm (in control) to 19.85 µm on day 4. A dose of 8 mg L−1 of BPA after 12 and 16 days exposure caused approximately three fold increase in cell length as compared to the control (Li et al., 2008). Similarly, an increase in cell size of Stephanodiscus hantzschii was observed after treatment with BPA (5 mg L−1) (Li et al., 2009).
Like other parameters, cell morphology also showed variation in response to BPA from species to species. No significant effect on cell size was noticed in the diatom Navicula incerta upon exposure to BPA and some other endocrine disrupting chemicals for 96 h (Liu et al., 2010). BPA might impair cell morphology by changing cell surface area to volume ratio, as cell surface area to volume ratio had a positive correlation with sensitivity to environmental pollutants (Miao et al., 2005; Xiang et al., 2018a). As discussed in this article BPA can interfere with cellular membranes and other cytoarchitectures, it may disturb cytoskeleton and cell homeostasis that can be a cause of changes in cell shape. Li et al. (2009) attributed the increase in length of algal cell by BPA to a halted cell division caused by this chemical.
4 BPA-induced oxidative stress and antioxidant responses of algae
Many of the abiotic environmental stresses are known to adversely affect living system by causing oxidative stress through increasing the production of reactive oxygen species (ROS) like hydroxyl radical (OH•), superoxide anion radical (O2−), singlet oxygen (O) and hydrogen peroxide (H2O2) (Li et al., 2008). ROS are normally produced in cell during the aerobic respiration (Buzadžić et al., 1990; Murthy et al., 2005; Ray et al., 2012). In an optimum concentration, ROS may not be harmful to cell and are used in cell signaling of some important cellular processes (Galvez‐Valdivieso and Mullineaux, 2010; Ray et al., 2012). However, the stressful conditions like exposure to toxic substances enhance the production of ROS through aerobic metabolism (Choo et al., 2004; Ray et al., 2012). The disruption of the electron transfer between the PSII and PSI reaction centers in chloroplast by stress can also lead to access accumulation of ROS (Asada, 2006; Sies, 1997). ROS produced in excess amount attack essential molecules like polyunsaturated fatty acids, proteins and nucleic acids in the cell causing cell injury of cellular organelles and disturbance of metabolic and physiological processes (Ali et al., 2016; Doyle et al., 2009; Imlay and Linn, 1988; Shakir et al., 2018). The most common impairment caused by ROS excess is lipid peroxidation of cellular membranes caused as a result of attack on the unsaturated fatty acids (Wang et al., 2015). The oxidative damage caused by ROS is therefore commonly determined by measuring the lipids peroxidation as thiobarbituric acid reactive substances (TBARS: products of lipids peroxidation) or more specifically as malondialdehyde (MDA: major contributor of TBARS) content (Asakawa and Matsushita, 1979; Dinakar et al., 2012; Mateos et al., 2005; Tahira et al., 2019). The oxidative stress is also determined by the direct measurement of ROS concentrations and by determination of the increase in antioxidant enzymes’ activities in the cell (Ali et al., 2016; Ben Ouada et al., 2018b; Shakir et al., 2018).
Many of the hydrophobic organic pollutants like BPA tend to accumulate in cellular lipids inducing oxidative stress and consequent lipid peroxidation (Ji et al., 2014; Lee et al., 2014). It has widely been reported that BPA exposure can trigger the overproduction of ROS in different organisms that lead to oxidative stress (Ali et al., 2016; Gassman, 2017; Tao et al., 2016; Wang et al., 2015; Yang et al., 2009). BPA-induced oxidative stress has been reported in several algal species like Chlorella pyrenoidosa, Chlorella vulgaris, Scenedesmus quadricauda, Scenedesmus obliquus, Navicula incerta, Picocystis sp. and Graesiella sp. (Ben Ouada et al., 2018a; Ben Ouada et al., 2018b; Li et al., 2008; Liu et al., 2010; Wang et al., 2017a; Xiang et al., 2018a; Zhang et al., 2014a) as revealed by lipids peroxidation or enhancement of the activities of antioxidant enzymes (Table 4). By oxidative stress and consequent lipids peroxidation, BPA affects the fatty acid profile of algae that can influence the functions of cell membranes and metabolic processes in algae (Li et al., 2008). Exposure to BPA caused increased lipids peroxidation in Picocystis and Graesiella as revealed by MDA content and the observed effect increased with the increase in BPA concentration and exposure duration (Ben Ouada et al., 2018b). However, the peroxidation damage caused in the two algal species was distinctly different in pattern and severity. In Picocystis, the peroxidation was not very evident during the initial days and MDA content did not exceed 2-fold of the control value even at the highest tested concentration of BPA (75 mg L−1) in the first three days of exposure. It enhanced to at most five times of the control only after five days at the highest tested dose of BPA. In contrast, a significant increase in lipid peroxidation in Graesiella was noticed even at the lowest tested concentration of BPA and it exceeded seven times the control levels even after one day of BPA treatment. After five days of exposure the effect got more severe in this alga and a tremendous increase in MDA content in the culture treated with 50 and 75 mg L−1 of BPA was observed (Ben Ouada et al., 2018b). Based on the results for MDA formation demonstrated that BPA caused more severe oxidative stress and higher lipids peroxidation in Graesiella than in Picocystis. Similar differences were revealed in a comparative analysis of lipids peroxidation in a green alga S. quadricauda and a cyanobacterium Cylindrospermopsis raciborskii when exposed to BPA in a concentration range of 0.1 or 1 to 20 mg L−1 (Xiang et al., 2018a). As compared to the control, both the exposed species exhibited a significant increase in MDA content at BPA dose above 5 mg L−1 but the effect was much higher in the green alga than in the cyanobacterium. Additionally, unlike S. quadricauda where a tremendous increase in MDA content was seen, a significant decrease in MDA content was noticed in C. raciborskii at the highest tested dose of BPA (20 mg L−1) (Xiang et al., 2018a). Different levels of oxidative damages caused by BPA in different algal species may be attributed to differences in their metabolic activities and antioxidative defense systems.
To cope with the oxidative stress, living organisms have developed certain antioxidant defense mechanisms including enzymatic and non-enzymatic agents like superoxide dismutase (SOD), catalase (CAT), peroxidases (POD), glutathione S-transferase (GST), ascorbic acid, that protect cells from oxidative damage by scavenging ROS. SOD functions to remove ROS by catalyzing the dismutation of O2− to O2 and H2O2, the latter is then converted to water by CAT and POD (Morelli and Scarano, 2004). CAT exists in the mitochondria and peroxisomes and transforms H2O2 into water and oxygen (Madsen et al., 1998). POD catalyzes the oxidation of many organic compounds with H2O2 and detoxify H2O2 by converting it into water (Morelli and Scarano, 2004). GST detoxifies hydrophobic toxic substances by catalyzing their enzymatic conjugation with GSH (Higgins and Hayes, 2011). APX is applied by the cell to scavenge H2O2 produced in the chloroplast (Kwon et al., 2002). The activities of these enzymes are nowadays widely measured as indicator of cellular oxidative stress caused by environmental stresses.
BPA exposure significantly provoked the activities of antioxidant enzymes in algae as reported in several studies (Table 4). Overexpression of these enzymes in algae has been linked with a protection against BPA stress, as for example a decrease in lipid peroxidation and a consequent protection of cellular structures like photosystem (Ben Ouada et al., 2018a,b; Xiang et al., 2018a). Different algae may handle the BPA-induced oxidative stress differently by overexpressing different enzymes depending upon the dose and exposure duration of BPA stress. For instant, the two algae Chlorella pyrenoidosa and Scenedesmus obliquus responded to oxidative stress of BPA differently both during acute (96 h) and chronic exposure (5–30 days) as determined by SOD and CAT activities (Zhang et al., 2014a). In acute tests, activities of both enzymes in C. pyrenoidosa were higher than in S. obliquus. C. pyrenoidosa showed an increase in the activities of both CAT and SOD even at the lowest tested dose of BPA (1 mg L−1), but S. obliquus showed an increase in CAT and SOD activities at BPA doses of 10 and 25 mg L−1 or above, respectively (Zhang et al., 2014a). In chronic observations, SOD activity of C. pyrenoidosa was more sensitive to BPA than that of S. obliquus while CAT activity of S. obliquus was more responsive than in C. pyrenoidosa (Zhang et al., 2014a). Similarly, Picocystis and Graesiella responded to oxidative stress of BPA (1–75 mg L−1) differently in a five days long test as revealed by differential activities of APX, CAT and GST (Ben Ouada et al., 2018b). Picocystis significantly increased the activity of GST with increase in the concentration of BPA and exposure time but in Picocystis a dose and time dependent decrease in the activity of GST was observed. A similar trend was reported for APX activity, i.e. it was over-expressed in Picocystis and under-expressed in Graesiella, particularly at higher doses of BPA and longer exposure of 4 and 5 days. In initial days of exposure to BPA, CAT activity was stimulated in a similar fashion in both the species at BPA concentrations ranging from 1 to 25 mg L−1; however, at higher concentrations (50–75 mg L−1), stable over-expression of CAT was shown only in Picocystis where in Graesiella a decrease in the activity of this enzyme was started from the second day onward (Ben Ouada et al., 2018b). Similar differences in protective mechanisms against BPA-induced oxidative stress by different algae were reported in other studies (Li et al., 2008; Xiang et al., 2018a). Moreover, algae may differently handle the oxidative stress induced by different chemicals. For example, a marine diatom N. incerta depended against four different endocrine disrupting chemicals, including BPA, nonylphenols (NPs), 17 a-ethynylestradiol (EE2), and estradiol (E2) differently as revealed by the response of the three antioxidant enzymes SOD, GST and POD (Liu et al., 2010). SOD and GST were increased upon exposure to all the four chemicals but to different extents, however, POD was decreased in response to BPA, NP2 and EE2 but increased in response to E2. In this diatom, in response to a 5 mg L−1 of BPA, SOD and GST activities were increased by 52.5% and 37.1%, respectively, and POD activity was declined by 19.9% (Liu et al., 2010). In such a scenario, it is possible that the H2O2 generated by the action of SOD may not be completely reduced by POD, and the residual H2O2 will cause oxidative damages to the algal cells (Liu et al., 2010). Algae may deploy different enzymes at different exposure time against BPA-induced stress. For example, in Picocystis and Graesiella CAT activity was stimulated since the first day of BPA exposure, while APX and GST were activated later (Ben Ouada et al., 2018a,b).
At very high concentration, BPA may harm the antioxidant defense system of algae and inhibit or decreases the expression of antioxidant enzymes (Ben Ouada et al., 2018a; Li et al., 2008; Liu et al., 2010; Wang et al., 2017a). This decrease can be due to the inactivation of these enzymes by the ROS production beyond the scavenging capacity of enzymes or due to the exhaustion of these enzymes in attempting to eliminate the generated ROS (Pigeolet et al., 1990). With prolonged exposure to BPA, particularly at low doses, the gradual decline in antioxidant enzymes as compared to initial days indicates that an oxidative balance between production and scavenging of ROS in algae is re-established through the regulation of self-protection mechanisms (Li et al., 2008). The decrease in BPA concentration due to degradation with increase in exposure time, as discussed in this article, can be another possible factor for the corresponding decrease in the antioxidant response of algae with prolonged exposure time (Wang et al., 2017a).
The variation in responses of the antioxidant enzymes in algae to BPA stress is a complex phenomenon and might be dependent on a number of factors like the productions of different ROS in different concentrations by different doses of BPA in different algae, structural and functional differences in algal species, main target of BPA in an alga (organelle level) and the prevailing environmental conditions. Physicochemical conditions and environmental factors (e.g. light intensity, photoperiod, pH etc.) may also influence the antioxidant defense responses of algae to BPA stress. However, unfortunately studies can rarely be found in literature on this aspect of algae-BPA interactions except Wang et al. (2017a) who studied the expression of SOD and CAT in C. vulgaris upon exposure to BPA under light and dark conditions (photoautotrophic and heterotrophic growth). They concluded that both the enzymes expressed differently under light and dark conditions with higher activities of both enzymes under light conditions. Further in-depth studies are needed to investigate the antioxidative defense mechanisms in algae against BPA under different environmental conditions.
5 BPA removal and biodegradation by algae
In aquatic environments, BPA may undergo different attenuation processes like photodegradation, chemical oxidation, adsorption on non-living and living surfaces, and bioaccumulation and biodegradation by living organisms (Li et al., 2019). In aqueous medium, BPA may abiotically degrade with time as reported in several studies (Ben Ouada et al., 2018b; Eio et al., 2015; Ji et al., 2014; Li et al., 2009; Liu et al., 2010; Wang et al., 2017c). For example, in bioremediation of BPA with marine alga Stephanodiscus hantzschii, 16–23.5% of the initial BPA (0.01–9 mg L−1) was degraded abiotically after a period of 16 days (Li et al., 2009). Similarly, in a 5-day removal test of BPA with green algae Graesiella and Picocystis, Ben Ouada et al. (2018b) found that 13–19% of the initial 25–75 mg L−1 BPA was removed abiotically from the medium without algal involvement. In another phycoremediation study involving microalgae Chlorella vulgaris and Chlamydomonas mexicana, 5.5–15.5% abiotic removal of the initial 1–50 mg L−1 BPA was observed after 10 days (Ji et al., 2014). The abiotic degradation of BPA in water is mainly the result of photodegradation as concluded by several studies (Eio et al., 2015; Ji et al., 2014; Li et al., 2009; Wang et al., 2017c). When the abiotic control medium (medium with BPA but without algae) having 10, 20 and 50 mg L−1 of BPA was simultaneously placed in dark and light for 7 days, no significant change in the concentration of BPA was observed in the dark set of experiment but 11.0–18.8% decrease in the residual concentration of BPA was shown under the light conditions (Eio et al., 2015). However, Zhang et al. (2019) in a short-term study of 24 h did not detect any decrease in the residual concentrations of BPA in the medium without algae, concluding that abiotic loss due to photodegradation and volatilization was negligible at least in 24 h. Similarly, no significant abiotic degradation of BPA in a 4-days experiments using 2–10 mg L−1 initial BPA was noticed (Gattullo et al., 2012). Exceptions can be there (Gattullo et al., 2012; Zhang et al., 2019) but generally abiotic degradation of BPA in water occurs, though the rate of degradation vary.
Algae are capable of adsorbing, accumulating, transforming and biodegrading BPA and were found to efficiently remove BPA from the medium in several laboratory based studies (Ben Ouada et al., 2018b; Guo et al., 2017a; Ji et al., 2014; Wang et al., 2017a). BPA removal from the aqueous medium by algae, particularly at low initial concentrations of BPA, was always found much faster than that by abiotic processes (Table 5). Depending upon the algal species and level of BPA contamination, up to 99% of the initial BPA can be removed from water by applying algae. For example, the marine alga Stephanodiscus hantzschii removed 25.6–98.8% of the initial 0.01 to 9 mg L−1 BPA from the medium after 16 days growth (Li et al., 2009). Similarly, Ulva prolifera removed 94.3 and 98.5% of the initial 100 µg L−1 BPA when grown under a 12:12 h (dark:light) photoperiod and continuous light, respectively (Zhang et al., 2019). Data extracted from literature and summarized in Table 5, reveal that maximum efficiencies of different algae for BPA removal were in the range of 35–98.8% and minimum efficiency in the range of 6–70% at the initial concentrations of BPA tested in the range of 100 µg L−1 to 75 mg L−1. This efficiency can vary greatly from species to species of algae and is strongly influenced by the exogenous BPA in the medium as discussed later in this section. Different potentials of different algae for BPA accumulation and removal can be attributed to variation in several physiological and structural factors of algae. The accumulation of hydrophobic compounds like BPA by phytoplankton is influenced by factors like growth rate, biomass, organism structure, cell size, and cell wall structure etc. (Staniszewska et al., 2015). Algae may remove BPA from the medium in different ways like by adsorbing it to the surface layers, accumulating it inside, and biodegrading or transforming it into other compounds (Ben Ouada et al., 2018b; Guo et al., 2017a; Hirooka et al., 2005; Ji et al., 2014; Li et al., 2009; Zhang et al., 2019).
Depending upon the algal species, varying proportions of the applied BPA were observed to be removed from the medium by getting adsorbed on algae surface. After 5 days growth in the medium with 25 to 75 mg L−1 BPA, Graesiella and Picocystis removed 6–12% and 5–12% of the initial BPA, respectively, by adsorption processes (Ben Ouada et al., 2018b). However, Eio et al. (2015) found that only a very small fraction of the applied BPA was removed as surface adsorption by Chlorella sorokiniana. Since BPA is a hydrophobic compound, its adsorption to algal surface can be due to its hydrophobic binding to proteins on the algal cell wall (Endo et al., 2007) or due to its physical trapping in the extracellular polymers (Kawaguti et al., 2006) as explained by Ben Ouada et al. (2018b). This is further elaborated by Guo et al. (2017b) who exposed Chlorella pyrenoidosa to 14-C labelled BPA and monitored extractable and non-extractable BPA residue in cells for 10 days by measuring radioactivity levels. In the initial days of exposure (after 2 days), the extractable BPA residues per dry weight of cells was higher than the bound residues, but the bound fraction of residues per dry weight rapidly increased after 4 days, and after 10 days it was 2.6 times higher than the extractable residue (Guo et al., 2017b). It suggests that BPA itself or its metabolites can likely form bound residues in algae cells by strongly conjugating with structure macromolecules in cell like protein, pectin, cellulose, and hemicellulose in the cell wall (Guo et al., 2017b; Wang et al., 2016b).
Algae do not just adsorb BPA on cell surface, but also absorb and bioaccumulate it inside the cell. Several studies reported that different species of algae like Chlamydomonas mexicana, Carteria cerasiformis, Chlorella vulgaris, Chlorella pyrenoidosa, Cyanophora paradoxa, Coelastrum reticulatum, Graesiella sp., Gonium pectorale, Micractinium pusillum, Picocystis sp., Pseudokirchneriella subcapitata, Stephanodiscus hantzschii, Scenedesmus quadricauda, Scenedesmus acutus and Ulva prolifera, take in, accumulate and biodegrade BPA (Ben Ouada et al., 2018a; Ben Ouada et al., 2018b; Guo et al., 2017b; Ji et al., 2014; Li et al., 2009; Nakajima et al., 2007; Zhang et al., 2019). BPA can be considered among the highly bioaccumulated suspected endocrine disrupters in phytoplankton. For example, among the investigated three compounds including BPA, 4-tertoctylphenol and 4-nonylphenol in the Gulf of Gdansk (Southern Baltic Sea), BPA was the most bioaccumulated compound in phytoplankton (Staniszewska et al., 2015). The detected concentrations of BPA in phytoplankton were more than ten times higher than 4-tertoctylphenol and 4-nonylphenol (Staniszewska et al., 2015). Transfer and biomagnification of BPA through algae in the food chain has also been reported (Guo et al., 2017b). However, due to its biodegradation by algae and release of the resulted products back into the medium, usually smaller amount of BPA may be found inside the algal cells (Nakajima et al., 2007). Upon five days growth in medium with 25, 50 and 75 mg L−1 of initial BPA, Graesiella and Picocystis accumulated 4 and 2% of BPA, respectively in their cells (Ben Ouada et al., 2018b). The internal cellular accumulation of BPA in algae increases with increase in the exogenous application of BPA up to certain extent. C. vulgaris and C. mexicana accumulated significantly higher quantity of BPA at initial BPA concentration of 25 and 50 mg L−1 as compared to at 1, 5 and 10 mg L−1, both after 5 and 10 days of growth (Ji et al., 2014). Similarly, comparatively very high bioaccumulation of BPA in S. hantzschii was reported at initial higher doses of BPA than at initial lower doses after 4, 8 and 16 days of growth, but the difference was more prominent on day eight (Li et al., 2009). After eight days of growth at initial 5, 7 and 9 mg L−1 of BPA, maximum cellular amounts of BPA in S. hantzschii were 11.53, 35.30 and 45.44 ng BPA (mg fresh weight)−1, respectively (Li et al., 2009). This high cellular accumulation at external higher concentrations of BPA causes toxicity to algae and decreases their BPA biodegradation and removal efficiency as discussed later. Algae could accumulate BPA in higher quantity and more rapidly during the early or middle periods of growth than in the later stages of growth as observed in C. fusca, C. mexicana and S. hantzschii (Hirooka et al., 2005; Ji et al., 2014; Li et al., 2009).
The active uptake and bioaccumulation of BPA was confirmed by Guo et al. (2017b) in C. pyrenoidosa using 14-C labelled radioactive BPA. In a 10 days experiment, the radioactivity in the medium decreased while that in the Chlorella pyrenoidosa cells increased with time and at the end of experiment 15% of the initial radioactivity was detected in the algal cells. The 14-C labelled BPA study also showed that BPA accumulation (as shown by accumulated radioactivity) in cells reached to the maximum level after two days (1362 ng (g dry algal cells)−1) and a sharp decrease was observed by day 4 (Guo et al., 2017b), which further confirm higher accumulation of BPA by algae in the early growth phases. Similarly, the intake of BPA in Ulva prolifera was confirmed by using fluorescent BPA and laser confocal scanning microscopy (LCSM), a real-time tool for in situ observation of organic pollutant in the cell (Zhang et al., 2019). Further investigation of the removal of BPA by U. prolifera using living and dead (head-killed) algal biomass revealed that BPA fluorescence could be seen only in live U. prolifera and not in dead mass, which interpreted active uptake and accumulation of BPA by U. prolifera (Zhang et al., 2019). It can be concluded that algae accumulate BPA in their cells, however, different algae may accumulate BPA to different levels as revealed by the growth of eight different algal species in 2 mg L−1 of BPA for eight days (Nakajima et al., 2007). Cyanophora paradoxa, Pseudokirchneriella subcapitata subcapitata, Carteria cerasiformis, Scenedesmus quadricauda, Micractinium pusillum, Coelastrum reticulatum, Gonium pectorale, and Scenedesmus acutus accumulated 3, 2, 2, 1, 1, 1, 1 and 0.5 µg BPA (mg dry weight)−1, respectively (Nakajima et al., 2007).
Bioconcentration factor (BCF) is an index of the accumulation of chemical substances by living organisms and is calculated as a ratio of the concentration of a substance detected in the organism to its concentration in the surrounding medium. A higher BCF value indicates the higher bioaccumulation capacity of an organism and higher bioaccumulation tendency of the chemical. Based on BCF values for a specific pollutant, plants are generally categorized in to three groups: (1) excluders – plants with BCF values below 1, (2) accumulators – plants with BCF values of 1 to 1000 and (3) hyperaccumulators – plants with BCF values higher than 1000 (Islam et al., 2013; Rascio and Navari-Izzo, 2011). BCF values of BPA calculated for some algal species like Chlamydomonas mexicana, Chlorella vulgaris, Chlorella pyrenoidosa, Picocystis sp., Graesiella sp., Nacula incerta and Stephanodiscus hantzschii are shown in Table 5. Reported BCF values for algae are mostly below 100, with the exception of N. incerta and S. hantzschii where the BCF values reached to a maximum of 261 and 457, respectively (Table 5). BCF values of algae for BPA are strongly influenced by the initial concentrations of BPA. For example, 10- days BCF values of BPA obtained for C. mexicana and C. vulgaris at 1 mg L−1 initial BPA were 23.3 and 29.6 which dropped to 2.1 and 1.4 at 50 mg L−1 initial BPA, respectively (Ji et al., 2014). Very prominent changes in BCF values with changes in BPA dose and exposure time were observed for S. hantzschii when calculated after 4, 8, 12 and 16 days growth in 0.01 to 9 mg L−1 of BPA (Li et al., 2009). Significantly higher BCF values were observed at lower concentrations of BPA (0.01 and 0.10 mg L−1) than at higher concentrations. At 0.01 mg L−1 BPA, the BCF reached the maximum value of 457.75 on day eight. At the next higher BPA concentration (0.10 mg L−1), the BCF value was 322.85 on day 16, while the highest BCF at 1 mg L−1 BPA was 21.79 on day 12. At 3, 5, 7 and 9 mg L−1 BPA, the maximum BCF values were 5.59, 3.40, 6.39 and 6.25, respectively (Li et al., 2009). Since reported BCF values of BPA for all studied algae (Table 5) are above 1 but below 1000, algae can be regarded as accumulator of BPA as per classification given above. However, it is important to be mentioned that BCF value in the case of biodegradable substances like BPA may not give very accurate information on the removal potential of an organism. If the substance is degraded after intake, a lower concentration of the substance will be detected in the organism that would result in a lower BCF value. Similarly, a decrease in the concentration of the substance in the medium due to degradation by different factors will also affect BCF value in another way. Therefore, BCF value are mostly used for the purpose of interpreting bioaccumulation potential only.
The reduction in the amount of BPA in the culture medium (% removal) in the presence of algae was always found significantly and much higher than the quantity of BPA adsorbed and bioaccumulated by algal cells (Ben Ouada et al., 2018b; Eio et al., 2015; Gulnaz and Dincer, 2009; Hirooka et al., 2005; Ji et al., 2014; Li et al., 2009; Liu et al., 2010; Zhang et al., 2019). This led to a unanimous conclusion that algae not only take in BPA but actively biodegrade it, and biodegradation is the main process of BPA removal by algae. For example, Chlorella sorokiniana removed 38.5, 30.7, and 20.7% of the initial BPA concentration of 10, 20, and 50 mg L−1, but the BPA accumulated and adsorbed by algal cells was collectively less than 1% (Eio et al., 2015). Similarly, Picocystis and Graesiella accumulated 2 and 4% but removed 40–72% and 27–52%, respectively, of the initial 25 to 75 mg L−1 BPA (Ben Ouada et al., 2018b). N. incerta at initial BPA concentrations of 0.001, 0.01 and 0.1 mg L−1, accumulated only around 0.5% of BPA but biodegraded 30.62–37.78% of the initial same doses (Liu et al., 2010). The mass balance study by Zhang et al. (2019) revealed that Ulva prolifera removed 94.3% of the initial 100 µg L−1 BPA but only 37.6% of the total amount of BPA was obtained from the algae biomass. This low BPA recovery from the algal cell as compared to the high removal efficiency indicates that the absorbed BPA was subsequently metabolized and degraded in the algae (Zhang et al., 2019). Apart from some photodegradation, algal surface adsorption and bioaccumulation, the main process that can be accounted for rapid and efficient removal of BPA by algae is biodegradation (Ben Ouada et al., 2018b; Eio et al., 2015; Li et al., 2009; Liu et al., 2010; Zhang et al., 2019). Algae absorb BPA in its original form, biodegrade it by metabolic process and the biodegradation products are released back into the medium (Nakajima et al., 2007; Wang et al., 2017a). Some studies suggest that algae may also facilitate degradation of BPA by synthesis of extracellular enzymes contributing to the breakdown of BPA or by the production of some active oxygenic species which could induce the photodegradation of BPA (Otto et al., 2015; Peng et al., 2006). Degradation of BPA by algal species like C. vulgaris, C. fusca and C. sorokiniana significantly reduced its endocrine disrupting activity (Eio et al., 2015; Gulnaz and Dincer, 2009; Hirooka et al., 2005). Algae potential for BPA removal in laboratory-based studies was confirmed in natural field-based experiment in coastal water near Rushan city in China (Zhang et al., 2019). Water samples from sites in sea with green tide bloom of U. prolifera and adjacent site without green bloom were collected and analyzed for BPA concentration. The mean concentration of BPA in zone without green tide was 884.86 ng L−1 which was much higher than the BPA concentration of 62.38 ng L−1 found in the green tide blooming zone. These findings proved the role of algae like U. prolifera in removing BPA from contaminated coastal waters (Zhang et al., 2019).
BPA removal and biodegradation efficiency of algae is generally decreased with increasing BPA concentration above a certain limit as reported for several algae like Stephanodiscus hantzschii, Desmodesmus sp., Nacula incerta, Chlamydomonas mexicana, Chlorella vulgaris, Chlorella fusca, Picocystis sp., Graesiella sp. and Chlorella. sorokiniana (Ben Ouada et al., 2018b; Eio et al., 2015; Hirooka et al., 2005; Ji et al., 2014; Li et al., 2009; Liu et al., 2010; Wang et al., 2017c). A decrease in the % removal efficiency of BPA by S. hantzschii with the increase in initial BPA concentration was shown and at the end of 16-days experiment with initial BPA concentrations of 0.01, 0.1, 1, 3, 5, 7 and 9 mg L−1, removal rates were 88.3%, 98.8%, 91.9%, 61.2%, 48%, 28.1%, and 25.6%, respectively (Li et al., 2009). C. fusca in seven days growth removed more than 95% of BPA at the initial concentrations between 10 and 80 µM, but removal efficiency dropped to 70% when BPA concentration was increased to 160 µM (Hirooka et al., 2005). After ten days growth at initial BPA concentrations of 1, 3, 5.5 and 13.5 mg L−1, BPA removal efficiency of Desmodesmus was 57%, 25%, 18% and 26%, respectively, indicating a decrease in removal rate with increase in BPA concentration (Wang et al., 2017c). C. mexicana and C. vulgaris, respectively, in ten days growth biodegraded 24 and 23% of the 1 mg L−1 initial BPA but degradation capacity dropped to 6.2 and 5.2% at 10 mg L−1 and to 0.7 and 3.7% at BPA concentrations of 25 mg L−1 or above (Ji et al., 2014). However, at initial concentrations ranging below 1 mg L−1, BPA removal by algae may increase with increasing BPA concentration as reported in U. prolifera (Zhang et al., 2019). At initial BPA doses of 50, 100, 200, 500 and 1000 µg L−1, the 5-days removal efficiency of U. prolifera increased with increase in BPA concentration and reached from 94.30% at 50 µg L−1 to 99.81% at 1000 µg L−1 (Zhang et al., 2019), and a positive correlation between initial concentration of BPA and removal rate was observed. Similarly, an increase in initial BPA from 0.01 mg L−1 to 0.1 mg L−1 increased the removal efficiency of S. hantzschii from 88.3% to 98.8% but a further increase in concentration to 1 mg L−1 dropped removal efficiency to 91.9% but it was still higher than that at 0.01 mg L−1 (Li et al., 2009). The observed decrease in BPA removal efficiency of algae with increase in BPA concentrations beyond a certain limit was attributed to high accumulation of BPA inside algae and subsequent toxicity and growth inhibition (Ben Ouada et al., 2018b; Ji et al., 2014; Li et al., 2009). At high concentration of BPA, increased accumulation of BPA inside the algal cells causes cellular toxicity that impairs physiological and biochemical processes of the cell and hence decreases their biodegradation efficiency, while at low concentrations of BPA, algae can grow well and rapidly degrade the accumulated BPA (Ji et al., 2014; Li et al., 2009).
Though many studies investigated the removal and remediation of BPA by algae, comparatively few studies went in to the detail of determining intermediate products and degradation pathways (Eio et al., 2015; Gulnaz and Dincer, 2009; Hirooka et al., 2005; Nakajima et al., 2007; Wang et al., 2017c). The algal-biodegradation intermediate products of BPA are generally reported to be monohydroxy-BPA and BPA-glycosides formed as a result of hydroxylation and glycosylation as reported in early studies (Hirooka et al., 2005; Nakajima et al., 2007). However, BPA degradation mechanisms and consequent intermediate products may vary considerably among algal species (Li et al., 2009). A recent study revealed that BPA degradation and transformation by some algae may involve hydroxylation, one carbon unit transference, oxidative cleavage of carbon–carbon bond and glycosylation as revealed in Desmodesmus sp.WR1 (Wang et al., 2017c). BPA degradation by this alga gave around ten different intermediates as obtained from the analyses by HPLC and LC-HRMS (Table 6). Monohydroxy-BPA and 2-hydroxy-3-hydroxymethy-BPA were formed from BPA by hydroxylation and one carbon unit transference, respectively (Wang et al., 2017c). The BPA itself and the two metabolites (monohydroxy-BPA and 2-hydroxy-3-hydroxymethy-BPA) were further converted into BPA glycoside, monohydroxy-BPA glycoside and 2-hydroxy-3-hydroxymethy-BPA glycoside by glycosylation; and 4-isopropenyl-phenol, 4-isopropanol-phenol, 4-isopropenyl-benzene-1,2-diol, 4-isopropanol-benzene-1,2-diol, and 3-hydroxymethyl-4-(1-hydroxy1-methyl-ethyl)-benzene-1,2-diol by the oxidative cleavage of carbon–carbon bond (Wang et al., 2017c). This study, in addition to monohydroxy-BPA and BPA-glycoside intermediates of BPA degradation by algae reported in earlier studies, for the first time reported 2-hydroxy-3-hydroxy methyBPA; monohydroxy-BPA glycoside and 2-hydroxy-3-hydroxymethyBPA glycoside as well as different monophenols of BPA and its derivative as intermediates of BPA degradation by algae (Wang et al., 2017c). BPA degradation pathways may not be similar in all algae and vary from species to species. For example, Hirooka et al. (2005) in Chlorella fusca and Nakajima et al. (2007) in eight different species of freshwater microalgae did to not observe the oxidative cleavage of BPA, but this pathway probably operates in Desmodesmus sp.WR1 (Wang et al., 2017c). In a test of BPA removal by algae (C. sorokiniana) alone and by an algal-bacterial system, Eio et al. (2015) reported that the alga removed BPA from the medium, but degradation products could not be confirmed, while a total of nine degradation intermediates were reported for degradation by the algal-bacterial system (Table 6).
Algal species
BPA initial concentration (mg L−1)
Exposure duration (days)
Analytical techniques
Detected intermediates
Reference
P. subcapitata
10
10
HPLC, FAB-MS, 1H NMR
BPA-mono-O-bD-glucopyranoside
(Nakajima et al., 2007)
S. acutus
10
10
HPLC, FAB-MS, 1H NMR
BPA-mono-O-bD-glucopyranoside
(Nakajima et al., 2007)
C. reticulatum
10
10
HPLC, FAB-MS, 1H NMR
BPA-mono-O-bD-glucopyranoside
(Nakajima et al., 2007)
S. quadricauda
10
10
HPLC, FAB-MS, 1H NMR
BPA-mono-O-bD-galactopyranoside
(Nakajima et al., 2007)
C. fusca
2.283–36.53
(10–160 µM)7
HPLC, LC-MS
monohydroxybisphenol A
(Hirooka et al., 2005)
Desmodesmus sp.WR1
1–13.5
10
HPLC, LC-HRMS
Monohydroxy-BPA; 2-hydroxy-3-hydroxymethy-BPA; BPA glycoside; Monohydroxy-BPA glycoside; 2-hydroxy-3-hydroxymethy-BPA glycoside; 4-isopropenyl-phenol; 4-isopropanol-phenol; 4-isopropenyl-benzene-1,2-diol; 4-isopropanol-benzene-1,2-diol; 3-hydroxymethyl-4-(1-hydroxy1-methyl-ethyl)-benzene-1,2-diol
(Wang et al., 2017c)
C. vulgaris
20
9
GC and GC–MS
4-(1-hydroxy-2-methylprop-1-enyl) phenol
(Gulnaz and Dincer, 2009)
C. sorokiniana + bacteria
10, 20, 50
7
HPLC, GC–MS
2,2-bis(4-hydroxyphenyl)-1-propanol; 1,2-bis(4-hydroxyphenyl)-2-propanol; 2,2-bis(4-hydroxyphenyl) propanoic acid; p-hydroxybenzoylmethanol; p-hydroxybenzaldehyde; p-hydroxyacetophenone; p-hydroxybenzoic acid; p-hydroquinone; hydroxy-BPA
(Eio et al., 2015)
6 Molecular responses of algae to BPA exposure
BPA adversely affects diverse morphological, physiological and biochemical processes in algae, while algae biodegrade BPA. BPA may trigger a cascade of cellular responses in algae involving the expression of different genes and their products related to the affected processes and defense mechanisms of algae as wells as related to BPA biodegradation pathways of algae. To understand the underlying mechanism of algae-BPA interaction, studies on molecular level are important. In ecotoxicological studies, expressions of heat shock proteins (Hsps) and their genes have been the most commonly studied molecular biomarkers (Al-Whaibi, 2011; Jacob et al., 2017; Kim et al., 2014; Kobayashi et al., 2014; Neumann et al., 1994; Sathasivam and Ki, 2019). Hsps is a group of highly conserved and ubiquitously expressed molecular chaperones divided into five major families, including Hsp100, Hsp90, Hsp70, Hsp60 and small Hsp (Al-Whaibi, 2011). In addition to other functions in the cell, Hsps play important role in defense mechanisms against different environmental stresses like temperature, UV radiations, toxic metals, O2 stress, endocrine disrupting chemicals (EDCs) and many others (Jacob et al., 2017).
The responses of three families of Hsps including Hsp90, Hsp70 and Hsp20 (small Hsp) to BPA have been studied in the dinoflagellate Prorocentrum minimum and diatom Ditylum brightwellii (Guo et al., 2012; Guo and Ki, 2012; Lee et al., 2014). Hsp20 expression in D. brightwellii and Hsp90 in P. minimum studied in response to 48 exposure to a wide range of BPA (0.01–10 mg L−1) showed no significant changes in their expression levels in the respective algal species (Guo and Ki, 2012; Lee et al., 2014). The expression levels of Hsp20 and Hsp90 in the mentioned algae was also not affected by another related endocrine disrupter (PCB) (Guo and Ki, 2012; Lee et al., 2014). However, the expression of Hsp70 monitored in P. minimum in response to 0.01 to 10 mg L−1 BPA gradually increased with increase in BPA concentrations with a significant enhancement in expression level at 2.5 mg L−1 or above of BPA. At 10 mg L−1 of BPA, the expression level of Hsp70 was the highest and 1.7-fold higher than the untreated control (Guo et al., 2012). Findings of these studies (Guo et al., 2012; Guo and Ki, 2012; Lee et al., 2014) suggest that members of Hsp20 and Hsp90 may have a very non-significant or no involvement in instant responses of the studied algae to BPA, though the same families of Hsps responded well to heat and copper (Cu) stresses in the same algal species. However, members of the Hsp70 family have a role in the algal response to BPA-induced toxicity, at least in the studied species. Furthermore, the maximum expression response of Hsp70 in P. minimum to BPA was observed at 12 h and thereafter a decrease in the expression level was shown suggesting that Hsp70 expression was more sensitive to BPA at initial stages of exposure (Guo et al., 2012). The responses of different families of Hsps to environmental stresses in different organisms and in the same organism to different stresses may differ as can be concluded from the responses of different Hsp families to different stresses in different algae (Guo et al., 2012; Guo and Ki, 2012; Lee et al., 2014).
Algae exposure to BPA have generally shown some stimulatory effects at lower doses but inhibitory effects at high doses of BPA, suggesting some hermetic effects. Duan et al. (2019) attempted to elucidate the mechanism of this hermetic effect of BPA in C. pyrenoidosa upon exposure to 0.1 mg L−1 (stimulatory concentration) and 10 mg L−1 (inhibitory concentration) of BPA for 72 h using transcriptomic approaches. Exposure to low (0.1 mg L−1) BPA caused 216 genes to be upregulated, and 142 genes to be downregulated. The high dose of BPA (10 mg L−1) resulted in upregulation of 3133 and downregulation of 12,142 genes (Duan et al., 2019). Gene oncology (GO) enrichment analysis of differentially expressed genes (DEGs) revealed that no downregulated GO term was significantly enriched at low concentration of BPA, but certain upregulated genes related to cellular transport and cellular respiration were significantly enriched (Duan et al., 2019). For example, DEGs including ATPF1B, COX1, COX3, and ndhB involved in oxidative phosphorylation were upregulated by low doses of BPA (Duan et al., 2019). ATPF1B encodes ATP synthase subunit beta which regulates the rate of ATP synthesis and is essential for mitochondrial cellular oxidative phosphorylation (Zuo et al., 2017). Several other genes involved in energy releasing processes like glycolysis, tricarboxylic acid cycle, and oxidative phosphorylation were upregulated at low BPA (Duan et al., 2019). The upregulation of such genes indicates the increase in the synthesis of cellular ATP, that in turn activates different regulators of cell cycle and promotes cell proliferation and division. Furthermore, at low BPA doses the enrichment in GO term of DEGs related to nucleotide transport and cellular transport shows that cells accelerate energy production and materials transport for cell growth (Duan et al., 2019) that can be possible reason for increase in growth and other functions of algae at low concentrations of BPA as observed in many studies and can possibly be a strategy of algae to overcome BPA stress.
At high concentration of BPA, many differentially expressed genes in Chlorella pyrenoidosa related to different cellular metabolic processes were downregulated and significantly enriched in GO term. Several genes associated with the process of glycolysis and TCA cycles were downregulated (Duan et al., 2019). BPA also inhibited the expression of around 45 genes related to respiration in the cyanobacterium Cylindrospermopsis raciborskii (Xiang et al., 2018b). The gltA encoding citrate synthase and IDH1, IDH2, and IDH3 encoding isocitrate dehydrogenase, were downregulated by high BPA in C. pyrenoidosa (Duan et al., 2019). Downregulation of isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase by BPA was also observed in C. raciborskii (Xiang et al., 2018b). Citrate synthase has a key role in controlling metabolism speed and rate of turnover of the TCA cycle (Walsh and Koshland, 1985), while isocitrate dehydrogenase catalyze the oxidative decarboxylation of isocitric acid producing ketoglutarate in TCA (Al-Khallaf, 2017; Lv et al., 2018) which is further processed by α-ketoglutarate dehydrogenase (Xiang et al., 2018b). A downregulation of these genes means a disturbance or inhibition of TCA, a complex metabolic pathway of amino acids, carbohydrates and lipids (Halarnkar and Blomquist, 1989; Sweetlove et al., 2010). The downregulation of photosynthesis gene PsbA at high concentration of BPA in C. pyrenoidosa (Duan et al., 2019) can be the evident reason of photosynthesis inhibition in algae by BPA as reported in several studies (Table 3). A total of 50 genes related to photosynthesis, light-harvesting pigments and reactions center including the most common psbA, psbB, psbC, psbD, apcA, apcB, cpcA, and cpcB related to photosynthesis and hemN, acsF, chlL, chlN, chlP, crtB, pds related to chlorophyll and carotenoids synthesis, were all downregulated in the cyanobacterium C. raciborskii upon 96 h growth in BPA (Xiang et al., 2018b). The upregulation and downregulation of relative mRNA expression levels of important genes like those encoding ATP synthase, NADH dehydrogenase and D1 proteins of PSII (PsbA) after exposure to low and high dose of BPA, respectively, were confirmed by RT-qPCR in C. pyrenoidosa (Duan et al., 2019). In addition to genes involved in photosynthesis and respiration cited here, downregulations of several genes related to many other important processes in cells were caused by BPA (Duan et al., 2019; Xiang et al., 2018b) which may explain the adversity of BPA to different cellular processes and metabolic pathways and consequent cell growth.
A transcriptomic study in the green alga Desmodesmus sp.WR1 shed light on the molecular mechanism of BPA degradation by algae. Exposure to 13.5 mg L−1 of BPA for 2–5 days caused differential expression of many genes in this alga (Wang et al., 2017c). Identification of DEGs followed by GO enrichment analysis revealed that BPA caused upregulation of 2516 DEGs and downregulation of 4846 after 2 days while upregulation of 2556 DEGs and downregulation of 5662 DEGs after 5 days exposure. Further analysis of GO molecular functions (GOMF) of upregulated DEGs encoding proteins showed significant enrichment of upregulated DEGs involved in glucosyltransferase, UDP-glucosyltransferase, UDP-glycosyltransferase and oxidoreductases activities (Wang et al., 2017c). The relative mRNA expression of genes determined by RT-qPCR further confirmed that the glycosyltransferase gene (Unigene0000428) and oxidoreductase gene (Unigene0003885) were significantly upregulated in Desmodesmus sp.WR1 by BPA (Wang et al., 2017c). Since oxidoreductase enzymes are involved in the oxidative degradation of phenolic compounds (Fregapane and Salvador, 2013; Taticchi et al., 2013), overexpression of oxidoreductases encoding genes in Desmodesmus sp.WR1 validates BPA degradation via oxidative processes by this alga (Wang et al., 2017c) as discussed above. UDP-glycosyltransferase catalyzes glycosylation of phenolic compounds by transferring UDP-activated sugar moieties to small molecules like flavonoids, antibiotics and phenolics, as the sugar acceptors (Hyung Ko et al., 2006), which can be responsible for the production of BPA glycosides in alga (Wang et al., 2017c). These findings demonstrated that genes involved in cleavage, oxidative hydroxylation and glycosylation of BPA are upregulated in Desmodesmus sp.WR1 upon exposure to BPA that explain BPA degradation through different routes (Wang et al., 2017c).
7 Effect of environmental factors on algae-BPA interactions
Different environmental factors like light, temperature, nutrients and organic matter in water may influence the interactions between algae and BPA (Staniszewska et al., 2015; Wang et al., 2017a; Zhang et al., 2019). The interactions of BPA and a green alga (Chlorella vulgaris) studied under light and dark conditions, in a 10-day experiment using 2–50 mg L−1 initial concentrations of BPA, revealed clear differences between the two conditions in the toxicity of BPA to the alga as well as in the removal of BPA by the alga (Wang et al., 2017a). Under the light conditions, this alga tolerated BPA up to 20 mg L−1 and thereafter a toxicity was shown as determined by cell growth, while under the dark condition the alga could tolerated BPA up to 10 mg L−1 and thereafter sever growth inhibition was caused. Supplying light energy seems to increase the tolerance, probably, by providing energy and resources to cope with the stressors. Furthermore, under the light condition stimulatory effects while under the dark inhibitory effects of BPA were more prominent (Wang et al., 2017a). Under light conditions, algae also showed higher rate of BPA degradation than in dark (Hirooka et al., 2003; Hirooka et al., 2005; Wang et al., 2017a; Zhang et al., 2019). For example, at an initial concentration of 50 mg L−1, C. vulgaris removed 15.79 mg L−1 of BPA which was 50% higher than that removed under the dark conditions (7.30 mg L−1) (Wang et al., 2017a). The maximum BPA removal rate by C. vulgaris was 3.425 mg (L·d)−1 under light conditions which was much higher than 1.53 mg (L·d)−1 under the dark (Wang et al., 2017a). Similarly, Chlorella fusca removed 85% of the initial 40 µM BPA in 120 h when grown under light conditions but removed only 22% of the same dose of initial BPA when grown under dark conditions (Hirooka et al., 2003). In a 24 h experiment, Ulva prolifera was applied to remove BPA under different photoperiods including continuous light, 12:12 h (dark: light) photoperiod and complete darkness. The removal efficiency under complete darkness (41.8%) was found much lower than that under continuous light (98.5%) or 12:12 h photoperiod (94.3%) (Zhang et al., 2019). But the difference in the BPA removal efficiency between cultures of U. prolifera grown under continuous light and 12:12 h photoperiod was not significantly different (Zhang et al., 2019). Similarly, Hirooka et al. (2005) observed that BPA removal by C. fusca under continuous light and under 8:16 h light:dark photoperiod was not very different. It reflects that the removal of BPA by algae may not be significantly influenced by the length of the illumination period (Zhang et al., 2019). However, the complete darkness significantly reduces algal potential for BPA removal. Beside light period, irradiance intensity can also influence BPA degradation efficiency of algae as evident from BPA removal by C. fusca under different intensities of light including 2, 9, 18 and 36 W m−2 (Hirooka et al., 2005). A regular decrease in BPA removal was shown with the decrease in the light intensity so that the BPA removal was 98% at 36 W m−2 and dropped to 82% at 2 W m−2 and further decreased to 27% in the complete dark (Hirooka et al., 2005). Light deficiency could inhibit photosynthesis and hence may impair energy to other metabolic processes that may subsequently led to a decrease in BPA removal efficiency of algae. The inhibition or decrease in growth rate of algae with decreasing light can be another possible explanation for the decrease in BPA removal potential of algae (Hirooka et al., 2005; Zhang et al., 2019).
Temperature is another important environmental factor that can influence BPA and algae interactions. An increase in temperature from 10 °C to 30 °C significantly enhanced BPA removal by Ulva prolifera (Zhang et al., 2019). The increase in BPA removal was very prominent when the temperature changed from 10 to 20 °C but the increase was slight when it further increased from 20 to 30 °C. The removal constant (k = 0.088 h−1) calculated under 10 °C (lowest temperature) was almost 1.5 times smaller than that (K = 0.129 h−1) obtained at 30 °C (highest temperature) (Zhang et al., 2019). Like temperature, increase in the concentrations of nutrients such as nitrates and phosphates significantly enhanced BPA remediation capacity of U. prolifera when tested under three gradients of nitrates and phosphates as 150 µΜ NO3−/15 µΜ PO4−2 (high), 50 µΜ NO3−/5 µΜ PO4−2 (medium) and 0 µΜ NO3−/0 µΜ PO4−2 (low). The alga removed 99.9%, 97.3% and 94.3% of the initial BPA after 24 h growth in high, medium and low nutrients concentrations, respectively, and the removal rate (k value) dropped from 0.234 to 0.123 h−1 as the nutrient concentration decreased from maximum to minimum (Zhang et al., 2019). It indicates that enrichment of nitrogen and phosphate in eutrophic coastal zones may significantly facilitate BPA removal by algae like U. prolifera. In marine ecosystem, variation in salinity level can be a factor influencing the interactions of pollutants with algae. However, studies can hardly be found on the impact of fluctuations in salinity level on BPA interactions with algae. Zhang et al. (2019) attempted to assess the impact of changing salinity on BPA removal by U. prolifera at three different salinity levels (16, 24, and 32 g L−1). There was no significant change in the BPA removal rate with the increase in salinity from 16 to 32 g L−1 (Zhang et al., 2019). The no effect of salinity on BPA removal efficiency in U. prolifera was attributed to the high tolerance of this alga to salinity variations (Rybak, 2018).
In natural environment, a pollutant may affect aquatic organisms not only by its individual toxicity but may also synergize or antagonize the effect of other chemicals present in the environment. M'Rabet et al. (2018) studied the effect of BPA in combination with di-2-ethylhexyl phthalate (DEHP), another plastic derived pollutant of aquatic environment, on the marine dinoflagellate A. pacificum. Both the compounds were toxic to Alexandrium pacificum when applied individually as revealed by the adverse effects on growth rate, photosynthesis, respiration and light-harvesting pigments, but more severe toxicities were observed when BPA and DEHP were applied in combination, both at low and high concentrations (M'Rabet et al., 2018). Similarly, a synergistic effect was observed when BPA was tested in combination with isobutylparaben (IBP) using sporulation in Ulva pertusa as end point (Yang and Hong, 2012). Studies revealed that natural organic matter (NOM) and sediments particles in water may reduce BPA toxicity to algae (Chang et al., 2014; Gattullo et al., 2012). NOM, mostly consisted of humic substances, is one of the most abundant and reactive components of aquatic environments (Gattullo et al., 2012) affecting the physicochemical characteristics of aquatic ecosystems (Jackson and Hecky, 1980). NOM was observed to interfere with the fate of different xenobiotics present in the natural environments (Loffredo and Senesi, 2006; Pan et al., 2008, 2009). Gattullo et al. (2012) studied the effect of NOM on the interplay between BPA and algae using the green alga Monoraphidium braunii as a test organism and different concentrations of BPA (2, 4 and 10 mg L−1) and NOM (2, 5 and 20 mg L−1 of DOC). The cell size and density, photosynthetic efficiency (Fv/Fm and ΦPSII) and the content of chlorophyll a were used as endpoints for algal-toxicity assessment, while residual BPA in the medium was monitored to determine BPA removal efficiency of the alga. NOM increased the algal growth and chlorophyll a content and protected the alga from the adverse effect of low doses of BPA (up to 4 mg L−1), but no significant protective role was noticed against higher concentrations of BPA (Gattullo et al., 2012). NOM did not have any significant effect on the BPA removal efficiency of M. braunii (Gattullo et al., 2012). In an interesting study, Chang et al. (2014) monitored the effect of sediment particles of various sizes (2–2000 µm) on the aerobic degradation of BPA and its toxicity to the alga C. vulgaris. Upon exposure to sediment particles of various sizes alone, the growth of the alga as measured by chlorophyll a content increased by 75–84% and particles of 500–2000 µm size yielded the highest chlorophyll a content. An increase in the size of particles increased the degradation of BPA and decreased its toxicity to C. vulgaris (Chang et al., 2014).
In aquatic ecosystems, not just the presence of different chemicals but the interactions of different organisms at different or same trophic levels may also affect the ultimate toxicity of pollutants to an organism. For example, a decrease in the toxicity of BPA to algae (Chlorella sorokiniana) was observed when it was exposed to BPA as a co-culture with bacteria in an algal-bacterial system consisting of C. sorokiniana (alga) and BPA-degrading bacteria (Eio et al., 2015). The alga was exposed to 10, 20 and 50 mg L−1 of BPA as monoculture as well as in algal-bacterial system and the phycotoxicty (using algal growth rate and chlorophyll a) and removal of BPA were monitored. BPA in a dose of 20 and 50 mg L−1 significantly inhibited the growth of alga in the monoculture, but in the algal-bacterial system rather an increase in the algal growth was observed at the same tested concentrations of BPA. The specific growth rate (SGR) of the alga when exposed to BPA alone dropped from 0.8 to −1.1 day−1 with increase in BPA concentration from 0 to 50 mg L−1. But the SGR of the alga in the algal-bacterial system increased from 0.3 to 1 day−1 when BPA increased from 0 to 50 mg L−1 (Eio et al., 2015). Not only the adverse effect of BPA on chlorophyll a was eliminated but a stimulation in pigment concentration was observed. At the end of 7-day experiment, the concentrations of chlorophyll a in C. sorokiniana when grown alone reached 1.9, 1.2, and 0.24 mg L−1 at 10, 20, and 50 mg L−1 of BPA, respectively, in comparison to 2.2 mg L−1 in the control revealing a significant decrease in the pigment at 20 and 50 mg L−1 of BPA. But when the alga was exposed to same doses of BPA as a co-culture with bacteria, chlorophyll a concentration was 1.8, 1.9, and 2.2 mg L−1 at initial BPA concentration of 10, 20, and 50 mg L−1, respectively, in comparison to 1.3 in the control indicating a significant increase in the concentration of chlorophyll a (Eio et al., 2015). The authors concluded that bacterial growth in the algal-bacterial system reduced the concentration of BPA in the medium by its rapid degradation and hence a decrease in its toxicity to alga. For instant, in the algal-bacterial system BPA initial concentration of 50 mg L−1 rapidly dropped to a residual concentration of <27 mg L−1 after two days (Eio et al., 2015). The stimulation of algal growth by BPA in the algal-bacterial system was suggested to be possibly due the fact that bacteria released CO2 from BPA biodegradation that was utilized by algae; however, this hypothesis was not further investigated. Authors also did not explain the relatively low SGR of alga (0.3 day−1) in the untreated algal-bacterial culture (0 mg L−1 BPA) as compared to 0.8 day−1 in the untreated monoculture of alga. Similarly, there was a decrease in the pigment concentration of alga (control) when grown in combination with bacteria. There was 2.2 mg L−1 chlorophyll a in C. sorokiniana when grown alone but it dropped to 1.3 mg L−1 when grown in combination with bacteria in the absence of BPA (Eio et al., 2015).
Furthermore, a combination of algae and bacteria degraded BPA more efficiently than the bacteria or algae alone (Eio et al., 2015; Zhang et al., 2019). In a seven days growth, the alga alone biodegraded 38.5, 30.7, and 20.7 % of the 10, 20 and 50 mg L−1 initial BPA, respectively, but BPA was almost completely removed (below the detection limit) by the combination of algae and bacteria irrespective of the BPA initial dose (Eio et al., 2015). The BPA removal rate in the algal-bacterial system was significantly higher than the removal rate by the bacterial system alone (Eio et al., 2015; Eio et al., 2014). An enhancement in the BPA-removal efficiency by the presence of bacteria was also observed in Ulva prolifera (Zhang et al., 2019). At initial dose of 100 µg L−1, U. prolifera removed 94.3% of the BPA from the medium in 24 h when grown alone as axenic culture in sterilized medium. But 100% removal of the applied BPA was shown when U. prolifera was grown in unsterilized medium, indicating that bacteria could enhance BPA removal efficiency of U. prolifera (Zhang et al., 2019). In a subsequent experiment, BPA removal efficiency of bacteria alone was only 22.59%, which confirmed that the BPA was mainly removed by U. prolifera but it was enhanced by the presence of bacteria (Zhang et al., 2019). It was suggested that alga in addition to degradation of BPA by itself, provided O2 to BPA-degrading bacteria by photosynthesis that enhanced their degradation efficiency (Eio et al., 2015). This conclusion is supported by studies with biodegradation of other organic contaminants by algal-bacterial system reporting that O2 released by photosynthesis of algae enhanced the degradation of organic pollutants by bacteria (Borde et al., 2003; Muñoz et al., 2003). However, it is worth mentioning that the role of algae in the algal-bacterial system was not only limited to the oxygen supply but also had a significant active role in removing and degrading BPA (Eio et al., 2015).
8 Conclusions
-
Upon exposure of algae to BPA, an interplay between the two occurs (Fig. 1). BPA causes a broad range of adverse effects in algae by altering cell growth, cell morphology, chlorophyll contents, photosynthesis and cellular membranes structures and impairs the expression of several genes, particularly at high concentrations.
-
Sensitivity to BPA among different algae vary greatly as is evident from the reported EC50/IC50 and EC values for various end points in different algae. Based on the EC and EC50/IC50 values reported for growth inhibition, dinoflagellates and diatoms are more sensitive than green algae to BPA stress. This conclusion drawn from the diversely collected data (Table 1) is further supported by individual studies on the comparative sensitivities of green algae, dinoflagellates and diatoms (Ebenezer and Ki, 2016) and green algae and diatoms (Alexander et al., 1988). Ebenezer and Ki (2016) reported EC50 values of 15.55, 1.506 and 0.039 mg L−1 for growth inhibition by BPA in green algae (Tetraselmis suecica), dinoflagellate (Prorocentrum minimum) and diatom (Ditylum brightwellii), respectively. Similarly, in the study of Alexander et al. (1988) the EC50 value of 2.73 mg L−1 obtained for the green alga Selenastrum capricornutum was higher than the EC50 of 1 mg L−1 for the diatom Skeletonema costatum. The most sensitive response to BPA in term of cell growth, chlorophyll a contents and photosynthesis among algae is reported for a dinoflagellate (Alexandrium pacificum) where all these parameters were significantly inhibited by a very low dose (2 µg L−1) of BPA (M'Rabet et al., 2018).
-
Differences in cell wall composition among different groups of algae is suggested as a possible reason for distinctive sensitivities of different algae to BPA, but other structural and functional dissimilarities may also add to this differential sensitivity. However, in-depth studies on the underlying mechanisms of interspecies differences in sensitivity to BPA are lacking in literature and need further research.
-
Different parameters even in the same algae showed different sensitivity to BPA stress. For example, in Picocystis a 75 mg L−1 of BPA inhibited photosynthesis by more than 80% but growth was inhibited by less than 50% at the same concentration (Ben Ouada et al., 2018b). On the other hand, photosynthesis in Alexandrium pacificum was less sensitive than cell growth to BPA stress (M'Rabet et al., 2018).
-
Considering the reported EC50/IC50 values of BPA for algae growth and following the criteria of the EU-Directive 93/67/EEC (Commission, 1996) for classifying aquatic pollutants, BPA can be classified as toxic and harmful to algae (having EC50 in the range of 1–10 and 10–100 mg L−1). However, the reported EC and EC50/IC50 values for all parameters of all algae (except Alexandrium pacificum and Ditylum brightwellii) were found much higher than the BPA concentrations reported in natural waters and hence the possibility of BPA to cause a general toxicity to algae in natural aquatic environments is extremely low. However, the data for A. pacificum and D. brightwellii suggest that at least some species of algae can be at risk of BPA toxicity even at the presently reported concentrations of BPA in aquatic environments.
-
The adverse effects of BPA in algae can be through multiple pathways. High concentrations of BPA repressed genes associated with different physiological and metabolic processes like photosynthesis, TCA cycle, glycolysis/gluconeogenesis, fatty acid metabolism, and mitochondrial electron transport (Duan et al., 2019; Xiang et al., 2018b), which shows that its adverse effects can be through impairing different processes in algae and the net outcome may come in the form of growth inhibition.
-
One of the most possible causes of BPA toxicity in algae is the oxidative stress as revealed by the outcomes of oxidative stress markers like lipids peroxidation and expression of antioxidant enzymes in different algae.
-
At low concentrations, BPA caused some stimulatory effects in algae. Low concentrations of BPA upregulated several genes involved in energy releasing processes (glycolysis, tricarboxylic acid cycle, and oxidative phosphorylation), cellular transport and nucleotides transport in algae (Duan et al., 2019). The upregulation of such genes indicates that algal cells accelerate energy production and materials transport for cell growth that can be a possible reason for increase in growth and other functions of algae upon exposure to low concentrations of BPA, and it might be a defense strategy of algae.
-
Algae recovery from BPA toxicity with extending exposure time has been reported, at least in some cases. This recovery can be due to the depletion of BPA in the culture medium by photodegradation or phycodegradation with time or probably algae may get adapted to BPA stress with time or may produce some exudates to restrict BPA interactions with the cell.
-
Algae remove BPA from water by adsorption, bioaccumulation and biodegradation, but the main route of removal is biodegradation. Monohydroxy-BPA and BPA-glycosides have been reported as the most common intermediates in BPA degradation by algae.
-
Algae generally remove BPA more efficiently at low doses of BPA (lower than 1 mg L−1) and at environmental relevant concentrations BPA may almost completely be removed by algae. But increase in the concentration of BPA greatly reduces the biodegradation efficiency of algae. This reduction in removal efficiency is attributed to high accumulation of BPA in algae and consequent toxicity that impair algal growth and energy metabolism and hence their BPA removal efficiency.
-
Reported environmental concentrations of BPA in water and wastewater are far lower than the tolerance limit of almost all studied algae and hence algae can be applied in bioremediation processes in natural water bodies and wastewater treatment plants. However, algae have the potential to accumulate some BPA that can be passed to higher trophic levels through organisms feeding on algae.
-
BPA removal efficiencies of algae were higher under light conditions than in the dark. Other environmental factors like temperature and nutrient supply also showed a positive correlation with BPA removal efficiency of algae. Furthermore, BPA removal by algae was more efficient during the early stages of algal growth. A combination of algae and bacteria may remove BPA more efficiently and reduce its toxicity to algae.
9 Future perspectives
In addition to several research gaps identified at different points in this article, following gaps may need attention of scientists working in this field to get a further insight in the interplay between algae and BPA (Fig. 2).
-
BPA toxicity to algae has been studied fairly well, but information on the underlying mechanisms and pathways are almost lacking. Therefore, studies with a mechanistic approach are needed in this regard.
-
Many studies exist on the BPA removal efficiencies of algae, but very few studies investigated the resultant intermediates and degradation pathways, which need further studies to understand the mechanism of BPA removal by algae.
-
Biodegradation of BPA by algae have mostly been investigated in freshwater green algae, with almost negligible data on algae from marine habitat and group other than green algae.
-
A very few studies are there on the interactions of BPA with other aquatic pollutants toward toxicity in algae. In natural environments, pollutants may not interact with biota individually, but synergistic, additive or antagonistic interactions can be expected. So studies evaluating toxicity of BPA in combination with other common pollutants of aquatic environments are suggested.
-
Very limited studies are available on the impact of different environmental factors on algae-BPA interactions. Since natural aquatic environments have daily and seasonal fluctuations in factors like temperature, light, pH, nutrients and organic matter etc., more studies on the influence of these environmental factors on the toxicity of BPA to algae and on the removal of BPA by algae would not only help in understanding the interactions of algae and BPA in natural environments but would also be beneficial for application of algae in wastewater treatments plants.
-
Studies on the effect of BPA on light-harvesting pigments in algae have mostly been confined to chlorophyll a. However, different pigments may get affected by environmental stresses differently due to their different chemical structures and bonding strength in protein-pigments complexes. Therefore, studies on the effect of BPA on light-harvesting pigments other than chlorophyll a are also needed.
-
Since BPA in natural aquatic environments is usually found in low concentrations, the stimulatory effects in algae in response to low concentration of BPA and its role in algal bloom may need in-depth mechanistic studies. Studies on assessing any possible use of BPA or its degradation products as a carbon source by algae would give interesting findings.
-
Work is needed to ascertain the role of algae in the fate of BPA in aquatic food chain considering bioaccumulation and biotransformation products of BPA produced by algae and by the consumers feeding on these algae.
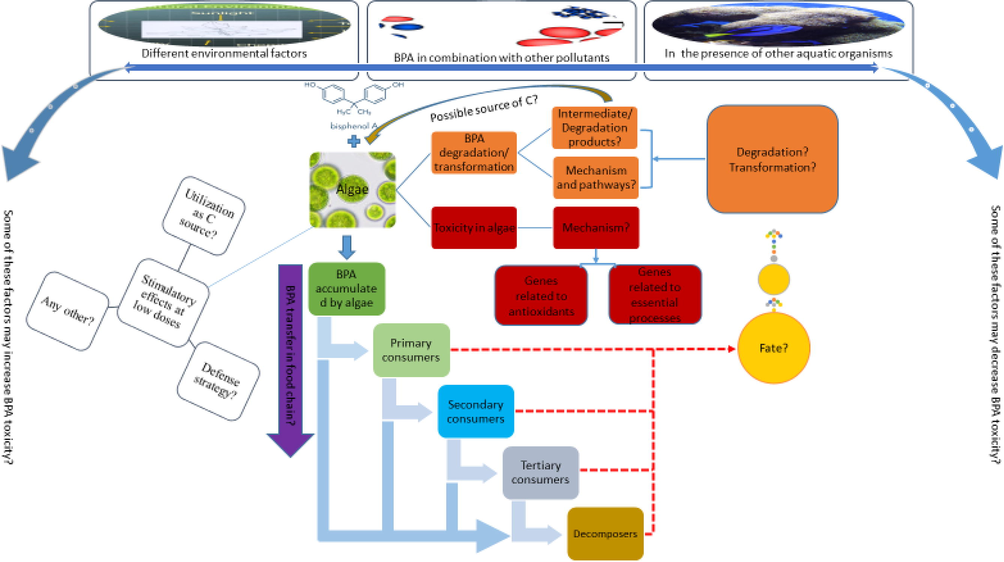
- A sketch of various research gaps identified that need further research work as explained in the section future perspectives. The punctuation mark (?) indicates the areas where no or little research has been conducted and need further studies.
Acknowledgement
This study was supported by the national key R&D program (2016YFA0601400), National Natural Science Foundation (41720104005, 41721005) China.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Development and application of a simple procedure for toxicity testing using immobilized algae. Water Sci. Technol.. 1996;33:129-138.
- [Google Scholar]
- Chlorophyll fluorescence as a tool to monitor plant response to the environment. In: Chlorophyll a Fluorescence. Springer; 2004. p. :583-604.
- [Google Scholar]
- Tolerance to cadmium in Chlamydomonas sp. (Chlorophyta) strains isolated from an extreme acidic environment, the Tinto River (SW, Spain) Aquat. Toxicol.. 2005;75:316-329.
- [Google Scholar]
- Biomonitoring of aquatic ecosystems. Erlangen, Germany: Friedrich-Alexander University; 2010. PhD thesis
- Isocitrate dehydrogenases in physiology and cancer: biochemical and molecular insight. Cell Biosci.. 2017;7:37.
- [Google Scholar]
- Plant heat-shock proteins: a mini review. J. King Saud Univ.-Sci.. 2011;23:139-150.
- [Google Scholar]
- Toxicological effects of bisphenol A on growth and antioxidant defense system in Oryza sativa as revealed by ultrastructure analysis. Ecotoxicol. Environ. Saf.. 2016;124:277-284.
- [Google Scholar]
- Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol.. 2006;141:391-396.
- [Google Scholar]
- Photosynthesis under stressful environments: an overview. Photosynthetica. 2013;51:163-190.
- [Google Scholar]
- Azizullah, A., and Häder, D.-P. (2018). A comparison of commonly used and commercially available bioassays for aquatic ecosystems. In “Bioassays”, pp. 347-368. Elsevier.
- Responses of morphological, physiological, and biochemical parameters in Euglena gracilis to 7-days exposure to two commonly used fertilizers DAP and urea. J. Appl. Phycol.. 2012;24:21-33.
- [Google Scholar]
- Sensitivity of various parameters in Euglena gracilis to short-term exposure to industrial wastewaters. J. Appl. Phycol.. 2012;24:187-200.
- [Google Scholar]
- Photosynthesis and photosynthetic pigments in the flagellate Euglena gracilis–As sensitive endpoints for toxicity evaluation of liquid detergents. J. Photochem. Photobiol., B. 2014;133:18-26.
- [Google Scholar]
- Ecotoxicity evaluation of a liquid detergent using the automatic biotest ECOTOX. Ecotoxicology. 2013;22:1043-1052.
- [Google Scholar]
- Toxicity of hydroquinone to different freshwater phototrophs is influenced by time of exposure and pH. Environ. Sci. Pollut. Res.. 2013;20:146-154.
- [Google Scholar]
- A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States — I) Groundwater. Sci. Total Environ.. 2008;402:192-200.
- [Google Scholar]
- Metal accumulation and toxicity measured by PAM—chlorophyll fluorescence in seven species of marine macroalgae. Ecotoxicol. Environ. Saf.. 2009;72:1063-1075.
- [Google Scholar]
- Occurrence of bisphenol A in surface water and uptake in fish: evaluation of field measurements. Chemosphere. 2002;49:97-103.
- [Google Scholar]
- Effect of Bisphenol A on the extremophilic microalgal strain Picocystis sp. (Chlorophyta) and its high BPA removal ability. Ecotoxicol. Environ. Saf.. 2018;158:1-8.
- [Google Scholar]
- Effect and removal of bisphenol A by two extremophilic microalgal strains (Chlorophyta) J. Appl. Phycol.. 2018;30:1765-1776.
- [Google Scholar]
- Kinetic sorption of contaminants of emerging concern by a palygorskite-montmorillonite filter medium. Chemosphere. 2017;176:231-242.
- [Google Scholar]
- Immobilization of fungal laccase on glutaraldehyde cross-linked chitosan beads and its bio-catalytic potential to degrade bisphenol A. Biocatal. Agric. Biotechnol.. 2019;19:101174
- [Google Scholar]
- Bisphenol A and replacements in thermal paper: A review. Chemosphere. 2017;182:691-706.
- [Google Scholar]
- Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ. Health Perspect.. 2007;115:69-76.
- [Google Scholar]
- Synergistic relationships in algal–bacterial microcosms for the treatment of aromatic pollutants. Bioresour. Technol.. 2003;86:293-300.
- [Google Scholar]
- Pharmaceuticals and personal care products (PPCPs) and endocrine disrupting chemicals (EDCs) in stormwater canals and Bayou St. John in New Orleans, Louisiana, USA. Sci. Total Environ.. 2004;333:137-148.
- [Google Scholar]
- Antioxidant defenses in the ground squirrel Citellus citellus 1. A comparison with the rat. Free Radical Biol. Med.. 1990;9:401-406.
- [Google Scholar]
- Chlorophyll a fluorescence, antioxidant enzymes and lipid peroxidation in tomato in response to ozone and benomyl. Environ. Pollut.. 2001;115:283-289.
- [Google Scholar]
- Boosting autofermentation rates and product yields with sodium stress cycling: Application to production of renewable fuels by cyanobacteria. Appl. Environ. Microbiol.. 2010;76:6455-6462.
- [Google Scholar]
- The occurrence of hormesis in plants and algae. Dose-response: A Publication of International Hormesis Society. 2006;5:150-162.
- [Google Scholar]
- Aerobic degradation of bisphenol-A and its derivatives in river sediment. Environ. Technol.. 2014;35:416-424.
- [Google Scholar]
- Contamination and risk implications of endocrine disrupting chemicals along the coastline of China: A systematic study using mussels and semipermeable membrane devices. Sci. Total Environ.. 2018;624:1298-1307.
- [Google Scholar]
- Oxidative stress tolerance in the filamentous green algae Cladophora glomerata and Enteromorpha ahlneriana. J. Exp. Mar. Biol. Ecol.. 2004;298:111-123.
- [Google Scholar]
- Commission, E. (1996). Technical guidance document in support of commission directive 93/67/EEC on risk assessment for new notified substances and commission regulation (EC) No. 1488/94 on risk assessment for existing substances. Office for Official Publications of the European Communities, Luxembourg, 328-334.
- Morphological changes of Euglenophyta in response to organic enrichment. In: Phytoplankton and Trophic Gradients. Springer; 1998. p. :277-285.
- [Google Scholar]
- Global assessment of bisphenol A in the environment: Review and analysis of its occurrence and bioaccumulation. Dose-Response. 2015;13
- [Google Scholar]
- Degradation of bisphenol A by different fungal laccases and identification of its degradation products. Int. Biodeterior. Biodegrad.. 2016;110:181-188.
- [Google Scholar]
- Applicability of growth rate, cell shape, and motility of Euglena gracilis as physiological parameters for bioassessment at lower concentrations of toxic substances: an experimental approach. Environ. Toxicol.. 2001;16:78-83.
- [Google Scholar]
- Effects of Cu2+, Ni2+, Pb2+, Zn2+ and pentachlorophenol on photosynthesis and motility in Chlamydomonas reinhardtii in short-term exposure experiments. BMC Ecol.. 2001;1
- [Google Scholar]
- Photosynthesis in desiccation tolerant plants: Energy metabolism and antioxidative stress defense. Plant Sci.. 2012;182:29-41.
- [Google Scholar]
- Chloroplast and reactive oxygen species involvement in apoptotic-like programmed cell death in Arabidopsis suspension cultures. J. Exp. Bot.. 2009;61:473-482.
- [Google Scholar]
- Transcriptional analysis of Chlorella pyrenoidosa exposed to bisphenol A. Int. J. Environ. Res. Public Health. 2019;16:1374.
- [Google Scholar]
- Evaluation of the sub-lethal toxicity of Cu, Pb, bisphenol A and polychlorinated biphenyl to the marine dinoflagellate Cochlodinium polykrikoides. Algae. 2012;27:63-70.
- [Google Scholar]
- Toxic effects of Aroclor 1016 and bisphenol A on marine green algae Tetraselmis suecica, diatom Ditylum brightwellii and dinoflagellate Prorocentrum minimum. Korean J. Microbiol. (미생물학회지). 2016;52:306-312.
- [Google Scholar]
- Biodegradation of bisphenol A by an algal-bacterial system. Environ. Sci. Pollut. Res.. 2015;22:15145-15153.
- [Google Scholar]
- Biodegradation of bisphenol A by bacterial consortia. Int. Biodeterior. Biodegrad.. 2014;96:166-173.
- [Google Scholar]
- Biodegradation of phenolic and polycyclic aromatic compounds by some algae and cyanobacteria. J. Bioremediation Biodegradation. 2012;3
- [Google Scholar]
- Adsorption of bisphenol A by lactic acid bacteria, Lactococcus, strains. Appl. Microbiol. Biotechnol.. 2007;74:202-207.
- [Google Scholar]
- Experts, I. (2016). Bisphenol A—a global market overview.
- Chlorophyll a fluorescence as a biomarker for rapid toxicity assessment. Environ. Toxicol. Chem.. 2007;26:1520-1531.
- [Google Scholar]
- Stress-physiological investigation of algal cell cultures in polluted media. Contrib Bot. 2001;36:101-108.
- [Google Scholar]
- Production of superior quality extra virgin olive oil modulating the content and profile of its minor components. Food Res. Int.. 2013;54:1907-1914.
- [Google Scholar]
- Bisphenol A emission factors from industrial sources and elimination rates in a sewage treatment plant. Water Sci. Technol.. 2003;47:117-122.
- [Google Scholar]
- The role of reactive oxygen species in signalling from chloroplasts to the nucleus. Physiol. Plant.. 2010;138:430-439.
- [Google Scholar]
- Effect of UV-C on algal evolution and differences in growth rate, pigmentation and photosynthesis between prokaryotic and eukaryotic algae. Photochem. Photobiol.. 2009;85:774-782.
- [Google Scholar]
- Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen.. 2017;58:60-71.
- [Google Scholar]
- Removal of bisphenol A by the freshwater green alga Monoraphidium braunii and the role of natural organic matter. Sci. Total Environ.. 2012;416:501-506.
- [Google Scholar]
- The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA)-General Subjects. 1989;990:87-92.
- [Google Scholar]
- Multiple effects of salinity on photosynthesis of the protist Euglena gracilis. Physiol. Plant.. 1997;101:777-786.
- [Google Scholar]
- Biodegradation of bisphenol A by Chlorella vulgaris and Aeromonas Hydrophilia. JABS. 2009;3:79-84.
- [Google Scholar]
- Bioaccumulation and elimination of bisphenol a (BPA) in the alga Chlorella pyrenoidosa and the potential for trophic transfer to the rotifer Brachionus calyciflorus. Environ. Pollut.. 2017;227:460-467.
- [Google Scholar]
- Transcriptional responses of heat shock protein 70 (Hsp70) to thermal, bisphenol A, and copper stresses in the dinoflagellate Prorocentrum minimum. Chemosphere. 2012;89:512-520.
- [Google Scholar]
- Differential transcription of heat shock protein 90 (HSP90) in the dinoflagellate Prorocentrum minimum by copper and endocrine-disrupting chemicals. Ecotoxicology. 2012;21:1448-1457.
- [Google Scholar]
- Comparative aspects of propionate metabolism. Comp. Biochem. Physiol. B, Comp. Biochem.. 1989;92:227-231.
- [Google Scholar]
- Influence of nitrogen status on the bioconcentration of hydrophobic organic compounds to Selenastrum capricornutum. Ecotoxicol. Environ. Saf.. 2000;45:33-42.
- [Google Scholar]
- Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J. Phycol.. 1993;29:729-739.
- [Google Scholar]
- Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab. Rev.. 2011;43:92-137.
- [Google Scholar]
- Removal of hazardous phenols by microalgae under photoautotrophic conditions. J. Biosci. Bioeng.. 2003;95:200-203.
- [Google Scholar]
- Biodegradation of bisphenol A and disappearance of its estrogenic activity by the green alga Chlorella fusca var. vacuolata. Environ. Toxicol. Chem.. 2005;24:1896-1901.
- [Google Scholar]
- Howard, P. H., Sage, G., Jarvis, W., and Gray, D. (1990). Handbook of environmental fate and exposure data for organic chemicals. Volume II: solvents.
- Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts. Environ. Int.. 2012;42:91-99.
- [Google Scholar]
- Biological and enzymatic treatment of bisphenol A and other endocrine disrupting compounds: a review. Crit. Rev. Biotechnol.. 2013;33:260-292.
- [Google Scholar]
- Glycosylation of flavonoids with a glycosyltransferase from Bacillus cereus. FEMS Microbiol. Lett.. 2006;258:263-268.
- [Google Scholar]
- Fate of bisphenol A in terrestrial and aquatic environments. Environ. Sci. Technol.. 2016;50:8403-8416.
- [Google Scholar]
- Phytofiltration of arsenic and cadmium from the water environment using Micranthemum umbrosum (JF Gmel) SF Blake as a hyperaccumulator. Int. J. Phytorem.. 2013;15:1010-1021.
- [Google Scholar]
- Depression of primary productivity by humic matter in lake and reservoir waters of the boreal forest zone. Can. J. Fish. Aquat. Sci.. 1980;37:2300-2317.
- [Google Scholar]
- The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J.. 2017;15:405-414.
- [Google Scholar]
- Cultivation of microalgae species in tertiary municipal wastewater supplemented with CO2 for nutrient removal and biomass production. Ecol. Eng.. 2013;58:142-148.
- [Google Scholar]
- Biodegradation of bisphenol A by the freshwater microalgae Chlamydomonas mexicana and Chlorella vulgaris. Ecol. Eng.. 2014;73:260-269.
- [Google Scholar]
- Bioconcentration of the insecticide pyridaphenthion by the green algae Chlorella saccharophila. Chemosphere. 2001;43:321-325.
- [Google Scholar]
- Biodegradation of organophosphorus pesticides. Proceed.-Indian Natl. Sci. Acad. Part B. 2004;70:57-70.
- [Google Scholar]
- Production of isomaltulose using Erwinia sp. D12 cells: culture medium optimization and cell immobilization in alginate. Biochem. Eng. J.. 2006;29:270-277.
- [Google Scholar]
- Heavy metals induce oxidative stress and trigger oxidative stress-mediated heat shock protein (hsp) modulation in the intertidal copepod Tigriopus japonicus. Comp. Biochem. Physiol. C: Toxicol. Pharmacol.. 2014;166:65-74.
- [Google Scholar]
- Biodegradation of bisphenol a in aquatic environments: River die-away. Environ. Toxicol. Chem.. 2001;20:2725-2735.
- [Google Scholar]
- Pharmaceuticals, hormones and bisphenol A in untreated source and finished drinking water in Ontario, Canada — Occurrence and treatment efficiency. Sci. Total Environ.. 2011;409:1481-1488.
- [Google Scholar]
- Algae sense exact temperatures: small heat shock proteins are expressed at the survival threshold temperature in Cyanidioschyzon merolae and Chlamydomonas reinhardtii. Genome Biol. Evol.. 2014;6:2731-2740.
- [Google Scholar]
- Heavy metal-induced inhibition of photosynthesis: targets of in vivo heavy metal chlorophyll formation. J. Phycol.. 2002;38:429-441.
- [Google Scholar]
- Enhanced tolerances of transgenic tobacco plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against methyl viologen-mediated oxidative stress. Plant, Cell Environ.. 2002;25:873-882.
- [Google Scholar]
- The potential role of water quality parameters on occurrence of nonylphenol and bisphenol A and identification of their discharge sources in the river ecosystems. Chemosphere. 2013;91:904-911.
- [Google Scholar]
- Bisphenol A contamination in Canadian municipal and industrial wastewater and sludge samples. Water Qual. Res. J.. 2000;35:283-298.
- [Google Scholar]
- Different transcriptional responses of heat shock protein 20 in the marine diatom Ditylum brightwellii exposed to metals and endocrine-disrupting chemicals. Environ. Toxicol.. 2014;29:1379-1389.
- [Google Scholar]
- Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar. 2021;3(3):255-281.
- [Google Scholar]
- The acute toxicity of bisphenol A and lignin-derived bisphenol in algae, daphnids, and Japanese medaka. Environ. Sci. Pollut. Res.. 2017;24:23872-23879.
- [Google Scholar]
- Individual and binary mixture effects of bisphenol A and lignin-derived bisphenol in Daphnia magna under chronic exposure. Chemosphere. 2018;191:779-786.
- [Google Scholar]
- The chronic effects of lignin-derived bisphenol and bisphenol A in Japanese medaka Oryzias latipes. Aquat. Toxicol.. 2016;170:199-207.
- [Google Scholar]
- Removal of organic compounds by nanoscale zero-valent iron and its composites. Sci. Total Environ.. 2021;792:148546
- [Google Scholar]
- Toxicity of bisphenol A and its bioaccumulation and removal by a marine microalga Stephanodiscus hantzschii. Ecotoxicol. Environ. Saf.. 2009;72:321-328.
- [Google Scholar]
- Physiological responses of the alga Cyclotella caspia to bisphenol A exposure. In “Botanica Marina”. 2008;51:360.
- [Google Scholar]
- Bisphenol A attenuation in natural microcosm: Contribution of ecological components and identification of transformation pathways through stable isotope tracing. J. Hazard. Mater.. 2019;121584
- [Google Scholar]
- Cellular responses, biodegradation and bioaccumulation of endocrine disrupting chemicals in marine diatom Navicula incerta. Chemosphere. 2010;80:592-599.
- [Google Scholar]
- The role of humic substances in the fate of anthropogenic organic pollutants in soil with emphasis on endocrine disruptor compounds. Springer; 2006. p. :69-92.
- Enzymatic characterization and functional implication of two structurally different isocitrate dehydrogenases from Xylella fastidiosa. Biotechnol. Appl. Biochem.. 2018;65:230-237.
- [Google Scholar]
- Impact of two plastic-derived chemicals, the Bisphenol A and the di-2-ethylhexyl phthalate, exposure on the marine toxic dinoflagellate Alexandrium pacificum. Mar. Pollut. Bull.. 2018;126:241-249.
- [Google Scholar]
- Docosahexaenoic and eicosapentaenoic acids are differently metabolized in rat liver during mitochondria and peroxisome proliferation. J. Lipid Res.. 1998;39:583-593.
- [Google Scholar]
- A survey of the level of bisphenol A (BPA) in effluents, soil leachates, food samples, drinking water and consumer products in south-western Nigeria. World Environ. 2015;5:135-139.
- [Google Scholar]
- Effects of sodium dodecyl sulfate on the growth dynamics and physiological state of the microalga Dunaliella salina (Chlorophyta) Russ. J. Mar. Biol.. 2010;36:191-194.
- [Google Scholar]
- Influence of laundry detergents on the abundance dynamics and physiological state of the benthic microalga Attheya ussurensis (Bacillariophyta) in laboratory culture. Russ. J. Mar. Biol.. 2007;33:391-398.
- [Google Scholar]
- Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress: Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J. Chromatogr. B. 2005;827:76-82.
- [Google Scholar]
- Comparison of Cd, Cu, and Zn toxic effects on four marine phytoplankton by pulse-amplitude-modulated fluorometry. Environ. Toxicol. Chem.. 2005;24:2603-2611.
- [Google Scholar]
- Bisphenol A – Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol.. 2014;37:738-758.
- [Google Scholar]
- Copper-induced changes of non-protein thiols and antioxidant enzymes in the marine microalga Phaeodactylum tricornutum. Plant Sci.. 2004;167:289-296.
- [Google Scholar]
- Non-photochemical quenching. A response to excess light energy. Plant Physiol.. 2001;125:1558-1566.
- [Google Scholar]
- Phenanthrene biodegradation by an algal-bacterial consortium in two-phase partitioning bioreactors. Appl. Microbiol. Biotechnol.. 2003;61:261-267.
- [Google Scholar]
- Salicylate biodegradation by various algal-bacterial consortia under photosynthetic oxygenation. Biotechnol. Lett.. 2003;25:1905-1911.
- [Google Scholar]
- Control of cell shape by calcium in the Euglenophyceae. J. Cell Sci.. 1981;49:99-117.
- [Google Scholar]
- In vivo antioxidant activity of carotenoids from Dunaliella salina—a green microalga. Life Sci.. 2005;76:1381-1390.
- [Google Scholar]
- Glycosylation of bisphenol A by freshwater microalgae. Chemosphere. 2007;69:934-941.
- [Google Scholar]
- NCBI (2020). National Center for Biotechnology Information. PubChem Database. Bisphenol A, CID=6623, https://pubchem.ncbi.nlm.nih.gov/compound/Bisphenol-A (accessed on Jan. 1, 2020). Vol. 2020. National Center for Biotechnology Information.
- Heat-shock proteins induce heavy-metal tolerance in higher plants. Planta. 1994;194:360-367.
- [Google Scholar]
- Effects of bisphenol A on key enzymes in cellular respiration of soybean seedling roots. Environ. Toxicol. Chem.. 2015;34:2363-2369.
- [Google Scholar]
- Emerging contaminant degradation and removal in algal wastewater treatment ponds: Identifying the research gaps. J. Hazard. Mater.. 2016;313:291-309.
- [Google Scholar]
- Bisphenol A induces superfeminization in the ramshorn snail (Gastropoda: Prosobranchia) at environmentally relevant concentrations. Environ. Health Perspect.. 2005;114:127-133.
- [Google Scholar]
- Otto, B., Beuchel, C., Liers, C., Reisser, W., Harms, H., and Schlosser, D. (2015). Laccase-like enzyme activities from chlorophycean green algae with potential for bioconversion of phenolic pollutants. FEMS microbiology letters 362.
- Temporal and Spatial Distributions of Bisphenol A in Marine and Freshwaters in Turkey. Arch. Environ. Contam. Toxicol.. 2019;76:246-254.
- [Google Scholar]
- Humic substances. Part IV—sorption of hydrophobic organic contaminants. Environ. Sci. Pollut. Res.. 2008;15:554-564.
- [Google Scholar]
- Part V—sorption of pharmaceuticals and personal care products. Environ. Sci. Pollut. Res.. 2009;16:106-116.
- [Google Scholar]
- Circular spectropolarimetric sensing of higher plant and algal chloroplast structural variations. Photosynth. Res.. 2019;140:129-139.
- [Google Scholar]
- Photodegradation of bisphenol A in simulated lake water containing algae, humic acid and ferric ions. Environ. Pollut.. 2006;144:840-846.
- [Google Scholar]
- Effects on the photosynthetic activity of algae after exposure to various organic and inorganic pollutants. Chlorophyll. 2017;37
- [Google Scholar]
- Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech. Ageing Dev.. 1990;51:283-297.
- [Google Scholar]
- Effects of bisphenol A on growth, photosynthesis and chlorophyll fluorescence in above-ground organs of soybean seedlings. Chemosphere. 2013;90:1274-1280.
- [Google Scholar]
- Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci.. 2011;180:169-181.
- [Google Scholar]
- Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal.. 2012;24:981-990.
- [Google Scholar]
- Photocatalytic degradation of bisphenol A in aqueous media: A review. J. Environ. Manage.. 2018;213:189-205.
- [Google Scholar]
- Heavy metal resistant Distigma proteus (Euglenophyta) isolated from industrial effluents and its possible role in bioremediation of contaminated wastewaters. World J. Microbiol. Biotechnol.. 2007;23:753-758.
- [Google Scholar]
- Determination of phenolic compounds in macroalgae for human consumption. Food Chem.. 2010;121:634-638.
- [Google Scholar]
- Nonphotochemical chlorophyll fluorescence quenching: mechanism and effectiveness in protecting plants from photodamage. Plant Physiol.. 2016;170:1903-1916.
- [Google Scholar]
- Species of Ulva (Ulvophyceae, Chlorophyta) as indicators of salinity. Ecol. Ind.. 2018;85:253-261.
- [Google Scholar]
- An experimental investigation on growth stimulation (+) and inhibition (-) of algae (Oscillatoria, Chlorina and Scenedesmus quadricauda) treated with pulp and paper mill effluents. Int. J. Appl. Biol. Pharmaceut. Technol., IJABPT. 2011;2:87.
- [Google Scholar]
- Biomass, lipid content, and fatty acid composition of freshwater Chlamydomonas mexicana and Scenedesmus obliquus grown under salt stress. Bioprocess Biosyst. Eng.. 2013;36:827-833.
- [Google Scholar]
- Heat shock protein genes in the green alga Tetraselmis suecica and their role against redox and non-redox active metals. Eur. J. Protistol.. 2019;69:37-51.
- [Google Scholar]
- Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: In “Ecophysiology of Photosynthesis”. Springer; 1995. p. :49-70.
- [Google Scholar]
- Chlorophyll fluorescence as a measure of photosynthetic carbon assimilation. Proc. R. Soc. Lond. B Biol. Sci.. 1990;242:29-35.
- [Google Scholar]
- Algal degradation of a known endocrine disrupting insecticide, α-endosulfan, and its metabolite, endosulfan sulfate, in liquid medium and soil. J. Agric. Food. Chem.. 2004;52:3030-3035.
- [Google Scholar]
- Pesticide-induced oxidative stress and antioxidant responses in tomato (Solanum lycopersicum) seedlings. Ecotoxicology. 2018;27:919-935.
- [Google Scholar]
- Effect of some commonly used pesticides on seed germination, biomass production and photosynthetic pigments in tomato (Lycopersicon esculentum) Ecotoxicology. 2016;25:329-341.
- [Google Scholar]
- Oxidative stress: oxidants and antioxidants. Exp. Physiol.: Transl. Integr.. 1997;82:291-295.
- [Google Scholar]
- The role of phytoplankton composition, biomass and cell volume in accumulation and transfer of endocrine disrupting compounds in the Southern Baltic Sea (The Gulf of Gdansk) Environ. Pollut.. 2015;207:319-328.
- [Google Scholar]
- Hormesis–the stimulation of growth by low levels of inhibitors. Sci. Total Environ.. 1982;22:213-234.
- [Google Scholar]
- Not just a circle: flux modes in the plant TCA cycle. Trends Plant Sci.. 2010;15:462-470.
- [Google Scholar]
- Bio-assessment and remediation of arsenic (arsenite As-III) in water by Euglena gracilis. J. Appl. Phycol.. 2019;31:423-433.
- [Google Scholar]
- Takenaka, S., kondo, T., Nazeri, S., Tamura, Y., TOkunaga, M., Tsuyama, S., Miyatake, K., and Nakano, Y. (1997). Accumulation of trehalose as a compatible solute under osmotic stress in Euglena gracilis Z. J. Eukaryot. Microbiol. 44, 609-613.
- Oxidative stress and immunotoxic effects of bisphenol A on the larvae of rare minnow Gobiocypris rarus. Ecotoxicol. Environ. Saf.. 2016;124:377-385.
- [Google Scholar]
- The influence of the malaxation temperature on the activity of polyphenoloxidase and peroxidase and on the phenolic composition of virgin olive oil. Food Chem.. 2013;136:975-983.
- [Google Scholar]
- Hazard identification and risk characterization of bisphenols A, F and AF to aquatic organisms. Environ. Pollut.. 2016;212:472-479.
- [Google Scholar]
- The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res.. 1990;25:147-150.
- [Google Scholar]
- Characterization of rate-controlling steps in vivo by use of an adjustable expression vector. Proc. Natl. Acad. Sci.. 1985;82:3577-3581.
- [Google Scholar]
- Bisphenol A exposure may increase the risk of development of atopic disorders in children. Int. J. Hyg. Environ. Health. 2016;219:311-316.
- [Google Scholar]
- The growth behavior of Chlorella vulgaris in bisphenol a under different cultural conditions. J Environ. Anal. Toxicol.. 2017;7
- [Google Scholar]
- Bioaccumulation and biomagnification of emerging bisphenol analogues in aquatic organisms from Taihu Lake, China. Sci. Total Environ.. 2017;598:814-820.
- [Google Scholar]
- Effects of bisphenol A on antioxidant system in soybean seedling roots. Environ. Toxicol. Chem.. 2015;34:1127-1133.
- [Google Scholar]
- Identification of novel pathways for biodegradation of bisphenol A by the green alga Desmodesmus sp.WR1, combined with mechanistic analysis at the transcriptome level. Chem. Eng. J.. 2017;321:424-431.
- [Google Scholar]
- Fate and metabolism of the brominated flame retardant tetrabromobisphenol A (TBBPA) in rice cell suspension culture. Environ. Pollut.. 2016;214:299-306.
- [Google Scholar]
- Mechanism of photosynthetic response in Microcystis aeruginosa PCC7806 to low inorganic phosphorus. Harmful Algae. 2010;9:613-619.
- [Google Scholar]
- Proteomics analysis of zebrafish brain following chronically exposed to bisphenol A. Toxicol. Environ. Chem.. 2017;99:469-481.
- [Google Scholar]
- The effect of bisphenol A on growth, morphology, lipid peroxidation, antioxidant enzyme activity, and PS II in Cylindrospermopsis raciborskii and Scenedesmus quadricauda. Arch. Environ. Contam. Toxicol.. 2018;74:515-526.
- [Google Scholar]
- Chlorophyll a fluorescence and transcriptome reveal the toxicological effects of bisphenol A on an invasive cyanobacterium, Cylindrospermopsis raciborskii. Aquat. Toxicol.. 2018;200:188-196.
- [Google Scholar]
- Hazards of bisphenol A (BPA) exposure: A systematic review of plant toxicology studies. J. Hazard. Mater.. 2020;384:121488
- [Google Scholar]
- Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf.. 2015;122:565-572.
- [Google Scholar]
- Effects of bisphenol A on the growth and physiology of Microcystis aeruginosa. Saf. Environ. Eng.. 2014;21:21-32.
- [Google Scholar]
- Biodegradation and enzymatic responses in the marine diatom Skeletonema costatum upon exposure to 2,4-dichlorophenol. Aquat. Toxicol.. 2002;59:191-200.
- [Google Scholar]
- Combination effects of bisphenol A and isobutylparaben on the green macroalga Ulva pertusa. Toxicol. Environ. Health Sci.. 2012;4:37-41.
- [Google Scholar]
- Bisphenol A exposure is associated with oxidative stress and inflammation in postmenopausal women. Environ. Res.. 2009;109:797-801.
- [Google Scholar]
- Recent advances in metal-organic framework membranes for water treatment: A review. Sci. Total Environ.. 2021;800:149662
- [Google Scholar]
- Phycoremediation of coastal waters contaminated with bisphenol A by green tidal algae Ulva prolifera. Sci. Total Environ.. 2019;661:55-62.
- [Google Scholar]
- Effects of bisphenol A on chlorophyll fluorescence in five plants. Environ. Sci. Pollut. Res.. 2015;22:17724-17732.
- [Google Scholar]
- Bioaccumulation and degradation of pesticide fluroxypyr are associated with toxic tolerance in green alga Chlamydomonas reinhardtii. Ecotoxicology. 2011;20:337-347.
- [Google Scholar]
- Acute and chronic toxic effects of bisphenol A on Chlorella pyrenoidosa and Scenedesmus obliquus. Environ. Toxicol.. 2014;29:714-722.
- [Google Scholar]
- Applications of water-stable metal-organic frameworks in the removal of water pollutants: A review. Environ. Pollut.. 2021;291:118076
- [Google Scholar]
- Occurrence, distribution and ecological risk of bisphenol analogues in the surface water from a water diversion project in Nanjing, China. Int. J. Environ. Res. Public Health. 2019;16:3296.
- [Google Scholar]
- Overexpression of ATP5b promotes cell proliferation in asthma. Mol. Med. Rep.. 2017;16:6946-6952.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102050.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







