Therapeutic potential of Hyoscyamus niger-derived compounds: Targeting ovarian cancer through antioxidant activity and EGFR tyrosine kinase inhibition
⁎Corresponding author. lekmine.sabrina@univ-khenchela.dz (Sabrina Lekmine)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Ovarian cancer poses a significant challenge to women's health, necessitating innovative therapeutic approaches. This study investigates the therapeutic potential of compounds derived from Hyoscyamus niger, a medicinal plant with a history of traditional use, focusing on their antioxidant properties and their ability to inhibit epidermal growth factor-receptor (EGFR) tyrosine kinase a promising target in cancer therapy. LC-ESI-MS/MS analysis of H. niger extract revealed the presence of 21 phenolic compounds, with luteolin and p-coumaric acid notably exhibiting the highest concentrations. The extract displayed robust antioxidant activity across multiple assays, and its performance in the Reducing Power assay highlights its potential in reducing oxidative stress. Molecular docking results indicate that several phenolic compounds may serve as EGFR inhibitors, offering promising avenues for further investigation in cancer therapy. Notably, hyperoside emerged as a strong candidate due to its favorable binding energy and interactions within the EGFR active site. This study demonstrates the therapeutic promise of H. niger-derived compounds in targeting ovarian cancer through antioxidant activity and EGFR inhibition. Further research is warranted to validate their efficacy and safety, potentially opening up new avenues for ovarian cancer treatment.
Keywords
Ovarian cancer
LC-MS/MS
Hyoscyamus niger
Antioxidant activity
1 Introduction
The diagnosis of epithelial ovarian cancer (EOC) typically occurs in the later stages of the disease and is considered an aggressive form of cancer (Varier et al., 2023) EOC is a prevalent form of cancer and encompasses a range of subtypes, including serous, endometrioid, transitional cell tumors, clear cell, and mucinous(Zara et al., 2023). Among these, serous tumors are the most frequently observed subtype of ovarian cancer, constituting 2.5 % of all cancers among women (Alshaya et al., 2022).
Currently, the primary treatment method involves a combination of surgery and platinum-based chemotherapy (Zhao et al., 2022). However, first-line therapy proves ineffective in 60 % of cases resulting in relapse, while 50 % of patients develop resistance to chemotherapy (Schuler et al., 2023). Nevertheless, the emergence of acquired chemoresistance in the majority of ovarian cancer cases has presented a significant challenge to treatment strategies (Huffman et al., 2023). Therefore, discovering drugs that target the mechanisms of drug resistance is of paramount importance (Al-Zharani and Abutaha, 2023).
The Human Epidermal Receptor (HER) family member, Epidermal Growth Factor Receptor (EGFR), is commonly overexpressed in numerous malignancies, with a particular prevalence in ovarian cancer (Al-Zharani and Abutaha, 2023).As one of the tyrosine kinases, EGFR is mainly activated through binding with extracellular ligands, inducing receptor auto phosphorylation, and ultimately activating downstream pathways that contribute to processes such as angiogenesis, proliferation, and survival (Ahmad et al., 2022).
Studies conducted recently have revealed that the EGFR pathway is often activated in a wide range of cancer cells. Targeted inhibition of this pathway through the use kinase inhibitors has proven successful in treating ovarian, breast, and lung cancers that possess EGFR mutations (Ahmad et al., 2022).
Elevated levels of free radicals, often referred to as “oxidative stress,” have been associated with the promotion and advancement of cancer. Oxidative stress can lead to uncontrolled cell proliferation and resistance to programmed cell death (apoptosis) due to DNA mutations (Zubair and Bandyopadhyay, 2023) It is widely recognized that DNA damage induced by free radicals is a primary contributor to the development of cancer (Ola, 2022). Consequently, a critical therapeutic approach in cancer treatment may involve preventing the generation of free radicals and their subsequent oxidative harm, a task that can be accomplished through the use of antioxidants and/or free radical scavengers.
Plant-based products are gaining recognition as a promising alternative or complementary therapy to modern anti-cancer chemotherapies. The use of medicinal plants, including their derived fractions, isolated compounds, and extracts, has been a longstanding tradition in treating various types of cancer (Dehelean et al., 2021). Furthermore, certain compounds sourced from plants have been identified for their ability to resensitize resistant cancer cells, thereby restoring the effectiveness of existing chemotherapeutic agents (Dehelean et al., 2021). Given these considerations, natural products of herbal origin, known for their diverse pharmacological and therapeutic applications, hold significant interest.
The Hyoscyamus genus is a member of the Solanaceae family and includes approximately twenty species found in various regions, such as Irano-Turanian, Euro-Siberian, Saharo-Arabian, and Mediterranean as reported in reference (Chen et al., 2023). Three species of this genus grow in Algeria, including H. muticus L, H. niger L. and H. albus L (Quezel, P. and Santa, 1963). Hyoscyamus niger, commonly known as black henbane, is an annual or biennial herbaceous plant found in roadsides, rocky terrains, and uncultivated areas (Giglou et al., 2023). Black henbane contains important compounds that have been used as medicine for a long time, making it an essential resource for the pharmaceutical industry (Giglou et al., 2023).
The primary objective of this study is to investigate the therapeutic potential of H. niger-derived compounds in the context of ovarian cancer. This research encompasses in vitro assessment of the extract's antioxidant activity, the identification of phenolic components using LC-MS/MS, and an in-silico evaluation of their anticancer activity targeting the epidermal growth factor kinase receptor. By comprehensively exploring these aspects, we aim to contribute valuable insights into the development of potential novel therapies for ovarian cancer, with a focus on combating drug resistance and oxidative stress within cancer cells.
2 Materiel and methods
2.1 Plant material
The aerial components of H. niger were harvested from the willaya of biskra in the southeastern of Algeria. The meticulous botanical identification of the plant was performed by Dr Azzedine Zeraib associate professor at department of agronomy in Abbes Laghrour University of Khenchela, Algeria. In adherence to rigorous scientific protocols, the Biotechnology, Water, Environment and Health Laboratory attributed a distinct voucher code (No. 454./ 40) to the plant specimen.Subsequently, the collected material underwent air-drying and was then finely ground into a powder. The extraction process closely followed the procedures outlined by (Bendjedid et al., 2021; Benslama et al., 2023; Lekmine et al., 2022, 2021, 2020). To obtain hydro-alcoholic extracts (70 % ethanol), we employed 10 g of powdered plant material. The powdered plant material was mixed with ethanol and left to dissolve for 24 h at ambient room temperature. Subsequently, the mixture underwent filtration, and the resulting filtrate was concentrated utilizing a rotary evaporator (Hahnvapor) at 40 °C. The resulting residue from the hydro-alcoholic extract was gathered and stored at 4 °C for subsequent utilization (Lekmine et al., 2023).
2.2 Equipment and Chromatographic parameters
The extracts were introduced into a Shimadzu® 8045 UPLC system (Kyoto, Japan). connected to a triple quadrupole mass analyzer from Shimadzu® Corporation. These extracts were solubilized in methanol of HPLC-grade quality and subsequently filtered through a 0.2 µm PTFE filter. Chromatographic separation was accomplished utilizing a Shimpack C18 reversed-phase column (2.7 µm, 2 x 150 mm). A gradient elution was administered at a flow rate of 0.2 mL/min, utilizing solvent A (water) and solvent B (acetonitrile). The elution initiated at 10 % B for 5 min, incrementally ascending to 30 % B until 15 min, followed by a progression to 70 % B until 22 min, then elevated to 80 % B until 30 min, and eventually restored to 10 % B by 35 min. The mass detection was conducted under the negative Electrospray Ionization (ESI) mode, wherein the interface temperature was set at 300 °C, desolvation temperature at 526 °C, cone gas flow was adjusted to 50 L/h, and the nebulizing gas flow was fine-tuned to 3 L/min. For the purpose of collision-induced dissociation (CID) MS/MS measurements, collision energy was modulated individually for each peak within the range of 20–50 eV. The mass detection spanned the range of m/z 100–1200. Data processing was undertaken using the Lab Solutions software (Lekmine et al., 2023).
2.3 Antioxydant activities
The details of all biological activity studies are provided as Supplementary Material. CUPRAC, Reducing Power, Beta Carotene, DMSO Alcalin, SNP (IC50), Phenonthroline, and Hydroxyl Radical method were used to estimate the antioxidant potential.
2.4 Statistical evaluation
The tests were conducted in triplicate on the samples. The data are expressed as means ± standard deviation. Variance analysis was carried out using SPSS version 13.0. A P value of less than 0.05 was deemed statistically significant, and pairwise comparisons were conducted using Tukey's test.
2.5 Computational analysis
2.5.1 Preparation of receptor structures for molecular docking
The 3D crystal structure of the epidermal growth factor receptor kinase in complex with its inhibitor 4-anilinoquinazoline (ID: 1 M17) was obtained from the Protein Data Bank (https://www.rcsb.org/) at a resolution of 2.60 Å. To prepare the structure for further analysis, water particles and heteroatoms were removed, and hydrogen atoms and Gasteiger charge were added using UCSF Chimera 1.15 software. After energy minimization, the protein's 3D structure was saved in mol2 format.
2.5.2 Preparation of ligands structures for molecular docking
The 2D chemical structures of the 21 phenolic molecules of H. niger were procured in SMILES files from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Each of these molecules was enhanced geometrically and prepared in the same manner described above. The molecules were then saved in mol2 format.
2.5.3 Molecular docking simulations
AutoDock Vina Tool (Trott and Olson, 2010) was applied to the molecular docking aim with the flexible ligand and rigid receptor methods and default parameters. The docking protocol was ascertained in order to evaluate the docking approach used in this study. To accomplish this, the prepared 1 M17 receptor and the extracted co-crystallized ligand 4-anilinoquinazoline were redocked. The RMSD value calculated between the co-crystallized and redocked ligand was determined to verify the precision of the docking configuration. A grid box was built to encircle the spatial localization of the co-crystallized ligand of the receptor. Only the ideal ligand poses were considered, and the results were reported in terms of binding affinity. The tools Discovery Studio 3.0 and PyMol were employed to depict the various interactions between the ligands and their protein target.
3 Results and discussion
3.1 LC-ESI-MS/MS analysis of the H. Niger
The LC-ESI-MS/MS analysis of the H. niger extract yielded notable findings, as illustrated in Fig. 1 and Table 1. The investigation unveiled the presence of 21 phenolic compounds, with the most substantial concentrations observed in Luteolin (22,241.0 ± 1.4 µg/g extract) and p-Coumaric acid (22,228.3 ± 3.1 µg/g extract). Notably, no significant difference was observed between these two compounds (p < 0.05). Furthermore, the analysis identified several other compounds with high concentrations, including Chlorogenic acid (16,627.3 ± 3.7 µg/g extract), Gallic acid (15,123 ± 3.1 µg/g extract), Rutin (14,548.2 ± 1.8 µg/g extract), and Apigenin (10,229.9 ± 1.7 µg/g extract). Moderately concentrated compounds, such as Rosmarinic acid, Hyperoside, Kaempferol, and Naringenin, were also detected, with concentrations ranging between 6598 ± 3.2 and 1223 ± 2.2 µg/g extract. Additionally, Tannic acid, Hesperetin, Quercetin, Quinic Acid, Fisetin, Vanillin, and Protocatechuic Acid were registered with values between 971 ± 1.3 and 125.3 ± 1.7 µg/g extract. In contrast, Salicylic Acid, tr-Caffeic acid, Malic acid, and Coumarin were detected in trace amounts, all measuring less than 100 µg/g extract.
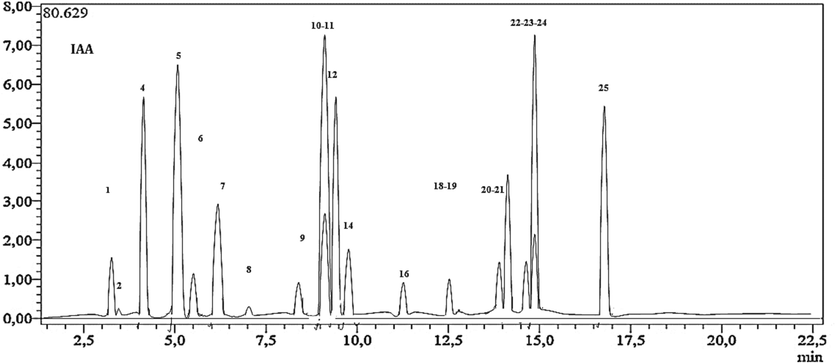
- LC-MS/MS chromatogram of H. niger ethanolic extract.
| No Analyte | Parention(m/z) | MS2 (Collision energy) | H. niger | |
|---|---|---|---|---|
| 1 | Quinicacid | 191.0 | 85 (22), 93 (22) | 463.12 ± 22.3 L |
| 2 | Malic acid | 133.1 | 115 (14), 71 (17) | 12.6 ± 2.2q |
| 3 | tr-Aconiticacid | 172.9 | 85 (12). 129 (9) | N.D. |
| 4 | Gallic acid | 169.1 | 125 (14), 79 (25) | 15,123 ± 3.1c |
| 5 | Chlorogenic acid | 353.0 | 191 (17) | 16,627.3 ± 3.7b |
| 6 | Protocatechuicacid | 153.0 | 109 (16), 108 (26) | 125.3 ± 1.7n |
| 7 | Tannic acid | 183.0 | 124 (22), 78 (34) | 971 ± 1.3i |
| 8 | tr-Caffeic acid | 179.0 | 135 (15), 134 (24), 89 (31) | 35.1 ± 2.3p |
| 9 | Vanillin | 151.1 | 136 (17), 92 (21) | 146.5 ± 1.3n |
| 10 | p-Coumaric acid | 163.0 | 119 (15), 93 (31) | 22,228.3 ± 3.1a |
| 11 | Rosmarinic acid | 358.9 | 161 (17), 133 (42) | 6598 ± 3.2f |
| 12 | Rutin | 609.1 | 300 (37), 271 (51), 301 (38) | 14,548.2 ± 1.8d |
| 13 | Hesperidin | 611.1 | 303,465 | N.D. |
| 14 | Hyperoside | 463.1 | 300,301 | 3987. ± 1.9 g |
| 15 | 4-OHBenzoicacid | 137.0 | 93,65 | N.D. |
| 16 | Salicylicacid | 137.0 | 93,65,75 | 62.11 ± 3.6o |
| 17 | Myricetin | 317.0 | 179,151,137 | N.D. |
| 18 | Fisetin | 285.0 | 135,121 | 224.6 ± 1.1 m |
| 19 | Coumarin | 147.0 | 103,91,77 | 11.8 ± 2.3 r |
| 20 | Quercetin | 300.9 | 179,151,121 | 529. ± 2.2 k |
| 21 | Naringenin | 271.0 | 151,119,107 | 1223. ± 2.2 h |
| 22 | Hesperetin | 301.0 | 164,136,108 | 683. ± 1.2j |
| 23 | Luteolin | 285.0 | 175,151,133 | 22,241.0 ± 1.4a |
| 24 | Kaempferol | 285.0 | 217,133,151 | 1272. ± 21.6 h |
| 25 | Apigenin | 269.0 | 151,117 | 10,229.9 ± 1.7e |
| 26 | Rhamnetin | 315.0 | 165,121,300 | N.D. |
| 27 | Chrysin | 253.0 | 143,119,107 | N.D. |
|
Parent ion (m/z): molecular ions of the standard compounds (mass to charge ratio). MS2(CE): MRM fragments for the related molecular ions (CE refers to related collision energies of the fragment ions). Values in g/g (w/w) of plant methanol extract. N.D.: not detected. Values are expressed as mean ± standard deviation of five independent experiments. Values with different superscripts (a, b, c) in the same columns indicate statistically significant differences (p < 0.05). |
||||
According to the literature, these findings align with the study conducted by Amir Reza Jassbi, (Jassbi et al., 2014) who also identified similar compounds, albeit with variations in their concentrations. Notably, among these compounds, chlorogenic acid, rutin, and Quercetin-3O-Glucoside-Rhamnoside-Rhamnoside (QGRR) were detected using HPLC-Diode Array Detector-Electrospray Ionization Mass Spectroscopy (LC-DAD ESIMS) in both studies.
The phenolic compounds found in H. niger (commonly known as black henbane) can vary depending on various factors such as the plant's growth conditions and location.
Rutin had been previously detected in the seeds of H. niger (Sengupta et al., 2011). However, in our study, the sample we analyzed consisted only of the aerial parts of the plant, excluding the seeds. This variation could potentially be attributed to differences in the source of the plant material or variations in the extraction procedure (Ma et al., 2002).
3.2 Antioxidant potential
The antioxidant activity of plant extracts plays a pivotal role in numerous processes, encompassing their ability to diminish and neutralize free radicals, along with their capacity to absorb oxygen radicals. As a result, we assessed the antioxidant potential of the ethanolic extract derived from H. niger through seven antioxidant tests (as detailed in Table 2).
| Products | CUPRAC (A0.5) | Reducing Power (A0.5) |
Beta Carotene (IC50) |
DMSO Alcalin (IC50) |
SNP (IC50) | Phenonthroline (A0.5) |
Hydroxyl Radical (IC50)) |
|---|---|---|---|---|---|---|---|
| H. niger | 15.57 ± 1.2 | 4.5 ± 1.3 | 7.17 ± 2.8 | 22 ± 2.2 | 10.5 ± 2.5 | 13 ± 2.8 | 19.95 ± 2.1 |
| BHT * | 7.85 ± 1.5 | / | 9.31 ± 1.2 | / | / | 2.43 ± 1.6 | / |
| BHA * | 6.26 ± 1.4 | / | 9.63 ± 2.3 | / | / | 2.64 ± 2.8 | / |
| Α-Tocopherol* | / | 23.53 ± 2.18 | 10.43 ± 1.6 | 4.3 ± 0.85 | / | / | / |
| Ascorbic Acid * | 7.38 ± 1.2 | 6.37 ± 1.5 | / | / | 7.64 ± 0.05 | 3.48 ± 1.3 | 13.34 ± 1.14 |
| Tannic Acid * | / | 5.57 ± 1.4 | / | 3.6 ± 0.71 | / | / | / |
| Trolox * | 9.69 ± 2.1 | 5.33 ± 1.8 | / | / | 34.26 ± 2.1 | 5.51 ± 1.9 | / |
| *reference compounds. /: not tested. | |||||||
To the best of our knowledge, there is no existing research in the literature regarding the use of antioxidant techniques on H. niger. As illustrated in Table 2, the terms “IC50″ and ”A0.50 levels“ pertain to the concentration at which absorbance reaches 0.50 and the percentage of inhibitory activity at 50 %, respectively. These values were determined through a linear regression approach and are presented as mean ± standard deviation (n = 3).
The results indicate that the H. niger extract exhibited potent activity in the Reducing Power assay, with a notably high half-maximal inhibitory concentration of 4.5 ± 1.3 µg/mL. This concentration surpassed that of α-Tocopherol, Ascorbic Acid, Tannic Acid, and Trolox, which served as standards for the Reducing Power method, with values of 23.53 ± 2.18, 6.37 ± 1.5, 5.57 ± 1.4, and 5.33 ± 1.8 µg/mL, respectively.
Additionally, the H. niger extract demonstrated a strong presence in the Beta Carotene assay, with a concentration of 7.17 ± 2.8 µg/mL. It also displayed notable activity in other assays, including SNP, Phenonthroline, CUPRAC, Hydroxyl Radical, and DMSO Alkaline assays, with values of 10.5 ± 2.5 µg/mL, 13 ± 2.8 µg/mL, 15.57 ± 1.2 µg/mL, 19.95 ± 2.1 µg/mL, and 22 ± 2.2 µg/mL, respectively. As shown in Table of LC-MS-MS, p-Coumaric acid and Luteolin are both present in very high concentrations in the H. niger extract.
These compounds have been widely demonstrated by numerous studies as effective bioactive antioxidants, According to Ismail Kiliç and Yeşim Yeşiloğlu (Kiliç and Yeşiloğlu, 2013), at a concentration of 45 μg/mL, p-Coumaric acid exhibited a remarkable inhibitory effect, reducing lipid peroxidation by 71.2 % in a linoleic acid emulsion.
Furthermore, p-Coumaric acid demonstrated significant scavenging activity against DPPH radicals, ABTS radicals, superoxide anions, hydrogen peroxide, and exhibited strong reducing power for ferric ions (Fe3+). It also displayed chelating activity for ferrous ions (Fe2+).
Collectively, these findings indicate that p-Coumaric acid possesses substantial antioxidant properties, making it a promising candidate for applications in the pharmaceutical and food industries.
On the other hand, Luteolin is a flavonoid, a type of polyphenolic compound, that is naturally found in various plants, including fruits, vegetables, and herbs. It is known for its antioxidant properties, which contribute to its potential health benefits.(Wang et al., 2011). Luteolin has the ability to neutralize and scavenge free radicals in the body. Free radicals are highly reactive molecules that can cause oxidative stress and damage to cells, proteins, and DNA. By scavenging these radicals, luteolin helps protect cells from oxidative damage.(Xiong et al., 2017).
Luteolin has anti-inflammatory properties, which can be linked to its antioxidant effects. Chronic inflammation is often associated with oxidative stress, and luteolin's ability to reduce inflammation contributes to its overall antioxidant activity (Azeem et al., 2023). Therefore, it may be suggested that the observed activity is caused by p-Coumaric acid and Luteolin and their synergic effects with other phenolic compounds.
Natural compounds found in medicinal plants, especially those with antioxidant activities, can play a significant role in cancer prevention and treatment. Antioxidant compounds like flavonoids, polyphenols, and carotenoids found in many medicinal plants help neutralize free radicals. Free radicals can damage cells and DNA, potentially contributing to cancer development. By eliminating these radicals, antioxidants can reduce the risk of cancer.(Sadiq, 2023).
3.3 Molecular docking study
Ovarian cancer stands as the most prevalent and lethal gynecological malignancy. Globally, roughly 230,000 women receive an ovarian cancer diagnosis annually, leading to the unfortunate loss of 150,000 lives (Funingana et al., 2023).
Given the detrimental side effects and the resistance observed with existing chemotherapeutic agents, the quest for the creation of more effective and less harmful anticancer drugs has become a crucial focus in drug development (Zhao et al., 2022). The results of this research constitute a crucial component of the preliminary evaluation of our compounds, which could potentially be advanced for further development as anticancer agents targeting ovarian tumors.
In the present study, we provide structural insights into the protein interactions of H. niger phenolic compounds exhibiting binding energies lower than that of the co-crystallized inhibitor, 4-anilinoquinazoline. Consequently, they are considered potentially active against EGFR. The docking results for this dataset of phycompounds are presented in Table 3.The docking of these phytocompounds was conducted within the receptor's auto-phosphorylation site, a significant binding site for many kinase potent inhibitors. This analysis unveiled a protein–ligand interaction map, highlighting all potential hydrophobic and hydrophilic interaction.
| Binding energy (Kcal/mol) | Hydrogen interactions (Distance Å) | Hydrophobic interactions | Electrostatic interactions | |
|---|---|---|---|---|
| 4-anilinoquinazoline | −7.2 | Met769 (2.70), Gln767 (3.15), Thr830 (4.07) | Leu820, Ala719, Leu694, Lys721, Leu764 | – |
| Hyperoside | −8.6 | Arg817 (2.40), H16-Asp831 (2.14), H18-Asp831 (4.38), H20-Thr766 (2.63) H19-Thr766 (3.05), Cys751 (2.35), Thr830 (2.72), Pro770 (2.83) |
Leu694, Ala719, Val702, Leu820, Lys721 | Cys751 |
| Luteolin | −8.5 | Glu738 (2.57), Thr830 (3.05), Arg817 (2.51) Cys773 (3.20), Cys725 (3.17), H7-Thr766 (2.81), Thr766 (3.58) |
Leu820, Val702, Ala719 | Met742 |
| Apigenine | −8.5 | Asp813 (3.36), Thr830 (2.91), Cys773 (3.20), Lys721 (3.19), Ala719 (2.59), Thr766 (7 6 6), Glu738 (2.47) | Val702, Leu820, Lys721, Ala719 | – |
| Quercetin | −8.5 |
Thr830 (2.87), Met769 (2.80), Thr766 (4.16) Glu738 (2.53) |
Leu694, Leu820, Val702, Ala719, Lys721 | – |
| Fisetin | −8.4 | Met769(2.79), O4-Thr766 (2.90), Thr766 (3.95), Thr830 (2.92), Glu738 (2.69) | Leu694, Val702, Leu820, Ala719 | Lys721 |
| Hesperetin | −8.4 | Ala719 (2.11), Thr766 (3.53), Met742 (3.00), Thr830 (2.99), Cys773 (3.19) | Lys721, Val702, Leu820 | – |
| Chlorogenic acid | −8.2 | Leu764 (2.64), O2-Thr766 (3.19), O3-Thr766 (2.08), Thr830 (2.03), Glu738 (7 3 8), O7-Met769 (2.41), O6-Met769 (2.46) | Leu694, Leu820 | – |
| Naringenin | −8.2 | Glu738 (2.34), Cys773 (2.34), Ala719 (7 1 9), Thr766 (3.50) | Leu820, Val702, Lys721, Ala719 | |
| Kaemferol | −8.1 | Asp831 (2.26), Thr830 (2.98), Ala719 (2.33), Lys721 (3.36), Thr766 (2.49) | Leu820, Val702, Thr766, Lys721, Ala719 | Met742 |
| Rutin | −8.0 |
Thr830 (2.94), Thr766 (3.02), Phe771 (2.59), O2-Cys773 (3.78), O3-Cys773 (3.24) |
Leu694, Leu820, Val702, Lys721, Ala719 | – |
| Rosmarinic acid | −7.4 | Asp831 (2.41), H7-Met769 (2.52), O4-Met769 (3.04), Glu738 (2.44), Lys721 (3.12) | Val702, Lys721 |
In our molecular docking analysis, several phenolic compounds derived from H. niger were evaluated for their binding affinity with the EGFR, a pivotal target in cancer therapy. Among the tested compounds, hyperoside exhibited the highest binding energy, surpassing the reference compound 4-anilinoquinazoline. It formed multiple hydrogen interactions with key residues in the EGFR binding site, demonstrating strong potential as an EGFR inhibitor. Luteolin, apigenin, quercetin, and fisetin also displayed notable binding affinities and engaged in diverse hydrogen and hydrophobic interactions within the receptor's active site. Furthermore, hesperetin, chlorogenic acid, naringenin, kaempferol, rutin, and rosmarinic acid exhibited considerable binding energies, indicating their potential as EGFR inhibitors (Table 3).
In addition, the outcomes of our docking analysis indicate the significance of residues Thr830 and Met769 in forming crucial hydrogen and hydrophobic bonds with EGFR. This observation aligns with prior docking studies on various compound (Nasab et al., 2018) affirming the consistency of our findings. Interestingly, when compared to reference inhibitor 4-anilinoquinazoline, certain compounds demonstrated a favorable fit within the ATP binding gorge of the EGFR (Fig. 2). These results suggest that phenolic compounds from H. niger may hold promise as candidates for further investigation as anticancer agents targeting EGFR.
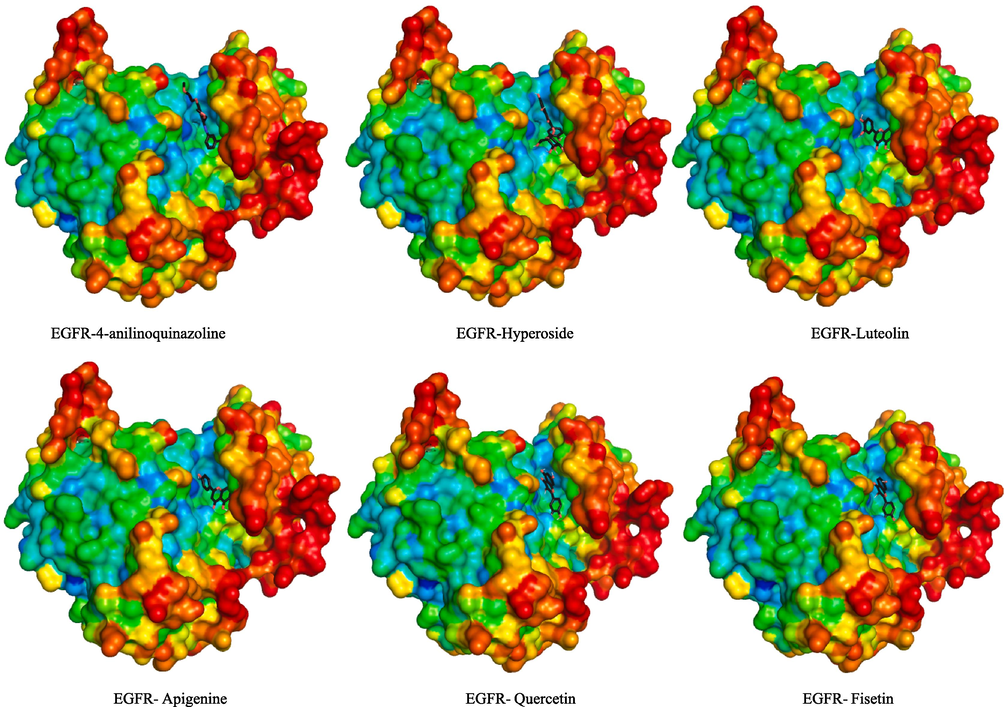
- 3D interaction model of phenolic compounds from H. niger binding to EGFR active sit.
4 Conclusion
Our study on H. niger-derived compounds reveals promising prospects for combating ovarian cancer. The extract, rich in 21 phenolic compounds, particularly luteolin and p-coumaric acid, exhibits robust antioxidant activity, positioning it as a significant candidate for addressing oxidative stress in ovarian cancer. Notably, it surpasses established standards in the Reducing Power assay. Molecular docking simulations highlight hyperoside as a compelling lead for inhibiting EGFR, suggesting a multifaceted approach to addressing ovarian cancer through antioxidant support and intervention in EGFR signaling pathways. While these findings are promising, further research, including in vivo and clinical studies, is crucial to validate efficacy and safety. H. niger-derived compounds offer a complementary avenue to existing therapies, enhancing patient well-being and mitigating treatment-related side effects, emphasizing the need for continued exploration of natural products for oncology applications.
Funding
DGRSD, PRFU project, RSPD2024R710, Biotechnology, Water, Environment and Health Laboratory, Faculty of Nature and Life Sciences, Abbes Laghrour University of Khenchela, Khenchela 40000, Algeria. Acknowledgments: The authors wish to thank the support of the Algerian Ministry of Higher Education and Scientific Research (MESRS, DGRSDT) and the National Centre for Biotechnology Research (C.R.B.T.). The authors would also like to thank Researchers Supporting Project Number (RSPD2024R710) from King Saud University, Riyadh, Saudi Arabia, for the funding support.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Characterization of fenugreek and its natural compounds targeting AKT-1 protein in cancer: Pharmacophore, virtual screening, and MD simulation techniques. J. King Saud Univ. - Sci.. 2022;34:102186
- [Google Scholar]
- Carbon nanotube-coated recombinant human surfactant protein D reduces cell viability in an ovarian cancer cell line, SKOV3, and modulates mTOR pathway and pro-inflammatory cytokine response. J. King Saud Univ. - Sci.. 2022;34:101851
- [Google Scholar]
- Phytochemical screening and GC-MS chemical profiling of an innovative anti-cancer herbal formula (PHF6) J. King Saud Univ. - Sci.. 2023;35:102525
- [Google Scholar]
- An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull.. 2023;80:241-262.
- [Google Scholar]
- Analysis of phytochemical constituents, antibacterial, antioxidant, photoprotective activities and cytotoxic effect of leaves extracts and fractions of Aloe vera. Biocatal. Agric. Biotechnol.. 2021;33
- [CrossRef] [Google Scholar]
- Phytochemical constituents of Astragalus monspessulanus and integrative analysis for its antioxidant, photoprotective, and antityrosinase activities: Experimental and computational investigation. Eur. J. Integr. Med.. 2023;60:102247
- [CrossRef] [Google Scholar]
- Efficient synthesis of biodiesel from Hyoscyamus niger L. seed oil by base catalysis. Fuel Process. Technol.. 2023;241:107630
- [Google Scholar]
- Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules. 2021;26
- [CrossRef] [Google Scholar]
- Multiparameter single-cell proteomic technologies give new insights into the biology of ovarian tumors. Seminars in Immunopathology. Springer 2023:43-59.
- [Google Scholar]
- Light-emitting diode irradiation and glycine differentially affect photosynthetic performance of black henbane (Hyoscyamus niger L.) South African J. Bot.. 2023;155:230-240.
- [Google Scholar]
- Mechanistic Insights on Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. Cancers (basel).. 2023;15:1402.
- [Google Scholar]
- HPLC-DAD-ESIMS Analyses of Hyoscyamus niger and H. reticulatus for their Antioxidant Constituents. Austin Chromatogr.. 2014;1:1-5.
- [Google Scholar]
- Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2013;115:719-724.
- [Google Scholar]
- A comparative study on chemical profile and biological activities of aerial parts (stems, flowers, leaves, pods and seeds) of Astragalus gombiformis. Biocatal. Agric. Biotechnol.. 2020;27:101668
- [Google Scholar]
- Investigation of Photoprotective, Anti-Inflammatory, Antioxidant Capacities and LC–ESI–MS Phenolic Profile of Astragalus gombiformis Pomel. Foods. 2021;10
- [CrossRef] [Google Scholar]
- Ultrasound-Assisted Extraction, LC–MS/MS Analysis, Anticholinesterase, and Antioxidant Activities of Valuable Natural Metabolites from Astragalus armatus Willd. In: In Silico Molecular Docking and in Vitro Enzymatic Studies. Antioxidants 11. 2022.
- [CrossRef] [Google Scholar]
- LC/MS-MS Analysis of Phenolic Compounds in Hyoscyamus albus L. Extract. In Vitro Antidiabetic Activity, In Silico Molecular Docking, and In Vivo Investigation against STZ-Induced Diabetic Mice. Pharmaceuticals. 2023;16
- [CrossRef] [Google Scholar]
- Ma, C.-Y., Liu, W.K., Che, C.-T., 2002. file:///D:/ben slama/cancer/ref/ITB480.pdf and Nonalkaloidal Components of Hyoscyamus n iger Seeds. J. nMa, C.-Y., Liu, W.K., Che, C.-T., 2002. Lignanamides Nonalkaloidal Components Hyoscyamus n iger Seeds. J. Nat. Prod. 65, 206–209.atural Prod. 65, 206–209.
- Exploring the interaction between epidermal growth factor receptor tyrosine kinase and some of the synthesized inhibitors using combination of in-silico and in-vitro cytotoxicity methods. Res. Pharm. Sci.. 2018;13:509.
- [Google Scholar]
- Attenuation of reactive oxygen species (ROS) generation in the cultured retinal cells under high glucose conditions. J. King Saud Univ. - Sci.. 2022;34:102227
- [Google Scholar]
- Centre (Nat. ed.). Paris: France; 1963.
- Free radicals and oxidative stress: Signaling mechanisms, redox basis for human diseases, and cell cycle regulation. Curr. Mol. Med.. 2023;23:13-35.
- [Google Scholar]
- file:///C:/Users/LENOVO BIO/Downloads/scholar (42).ris effects of aqueous methanolic extract of Hyoscyamus niger seeds result from its monoamine oxidase inhibitory and hydroxyl radical scavenging potency. Neurochem. Res.. 2011;36:177-186.
- [Google Scholar]
- AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem.. 2010;31:455-461.
- [Google Scholar]
- An Overview of Ovarian Cancer: The Role of Cancer Stem Cells in Chemoresistance and a Precision Medicine Approach Targeting the Wnt Pathway with the Antagonist sFRP4. Cancers (basel).. 2023;15:1275.
- [Google Scholar]
- Wang, G.G., Lu, X.H., Li, W., Zhao, X., Zhang, C., 2011. Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evidence-based Complement. Altern. Med. 2011.
- Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int. J. Mol. Med.. 2017;39:113-125.
- [Google Scholar]
- Dysregulation of gene expression of PTEN and AKT signaling pathway in patients of ovarian cancer: A pilot study. J. King Saud Univ. - Sci.. 2023;35:102378
- [Google Scholar]
- Escin induces apoptosis in ovarian cancer cell line by triggering S-phase cell cycle arrest and p38 MAPK/ERK pathway inhibition. J. King Saud Univ.. 2022;34:101644
- [Google Scholar]
- Small Molecule EGFR Inhibitors as Anti-Cancer Agents: Discovery, Mechanisms of Action, and Opportunities. Int. J. Mol. Sci.. 2023;24
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103103.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







