Translate this page into:
Biochanin A prevents buccal pouch carcinogenesis by enhancing carcinogen detoxification and antioxidant status in hamsters
⁎Corresponding author. drlvbiolab@gmail.com (Vennila Lakshmanan) vennilajnr@gmail.com (Vennila Lakshmanan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
This study objective was to ascertain the drug biochanin A (BCA) impacts on the management of oral squamous cell carcinoma (OSCC) caused by the chemical 7,12-dimethylbenz[a]anthracene (DMBA) in male Syrian golden hamsters.
Methods
The oral cancer was induced by the application of a DMBA (0.5 %) thrice a week for the duration of fourteen weeks in the buccal pouch. The study assessed various parameters, including volume, incidence, and burden of tumors within the hamsters' buccal pouches. Additionally, it employed colorimetric methods to measure the levels of carcinogen-detoxifying agents, lipid peroxidation, and antioxidant activity. The histopathological examination was assessed in the buccal tissues, as well.
Results
The hamsters exposed to DMBA exhibited the growth of tumors throughout the buccal pouches, accompanied by notable changes in lipid peroxidation, antioxidant levels, and carcinogen-detoxifying agents. However, oral administration of BCA (20 mg/kg bw) every second day for fourteen weeks showed significant alteration in the histopathological changes and reduced incidence, volume, and burden of tumors in DMBA-exposed hamsters. BCA established substantial anti-lipid peroxidation effects and the ability to enhance antioxidant and carcinogen-detoxifying agent levels in DMBA-exposed animals.
Conclusion
The outcomes of this investigation emphasize the chemopreventive and antioxidant capability of BCA against DMBA-induced oral carcinoma in hamsters.
Keywords
Antioxidants
DMBA
Detoxification
BCA
Oral carcinogenesis
Lipid peroxidation
1 Introduction
Oral cancer ranks as the 11th most prevalent malignancy worldwide. In 2024, the World Health Organization (WHO) projects that there will be 384,864 newly reported instances of oral cancer and 197,384 related deaths (Borse et al., 2020). Oral cancer is the formation of malignant tumors in the oral cavity, comprising of the lips, the floor of the mouth, tongue, cheekbones, firm and muscular palates, and pharynx. Risk factors include cigarette and alcohol usage, human papillomavirus (HPV) infection, poor oral hygiene, betel nut chewing, and exposure to certain chemicals and carcinogens (Panaseykin et al., 2023). A thorough oral cavity examination is required for diagnosis, frequently followed by a biopsy of suspected lesions for microscopic investigation. 7,12-dimethylbenz[a]anthracene (DMBA) utilization in the development of oral cancer in hamsters is a commonly employed method for investigating the development and origins of oral cancer (Gimenez-Conti and Slaga, 1993).
When DMBA interacts with the DNA of oral epithelial cells, DNA adducts, and genetic alterations are formed during the beginning stage. These alterations can impair normal cellular functioning and result in the formation of precancerous cells (Liu et al., 2015). The hamster model permits researchers to explore multiple aspects of oral carcinogenesis, such as tumor growth, metastasis, and therapy response. It offers the chance to investigate the molecular pathways, genetic changes, and immune responses involved in the progression of oral cancer (Desai, 2018).
DMBA-induced peroxidation of lipids yields numerous reactive lipid peroxidative products. These lipid peroxidation products have been linked to cancer growth, by influencing cellular processes such as enzyme activity, gene expression, and protein modification. Antioxidant defense systems in the cells, including the enzymes like glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD), can battle against the harmful consequences of lipid peroxidation (Vengaimaran et al., 2023). However, cancer cells frequently display changes in these antioxidant mechanisms, which increase their sensitivity to peroxidation-induced lipid damage (Shao et al., 2008). Prevention of cancer refers to using natural or manufactured substances to prevent, postpone, or reverse cancer development (Kraft et al., 2019). Phase 1 and Phase II collaborate to digest and remove carcinogens and other potentially hazardous compounds from the body (Rahman et al., 2022). Detoxification enzymes play a major task in cancer prevention by improving the detoxification mechanism in the removal of these toxic chemicals. By modulating the activity of these enzymes through dietary interventions or pharmaceutical drugs, it may be possible to increase the body's capacity to prevent or minimize cancer development (Chen et al., 2022). The main purpose of cancer therapy is to remove the primary tumor and prevent the spread of distant metastasis. To attain this without any side effects, chemotherapeutics derived from natural sources are a newly developed field of study.
Epidemiological research has shown that isoflavone intake has lowered the rate of survival of cancer cells. Isoflavones are recognized for their potential as chemoprotective agents and can be an alternative treatment option for various illnesses (Rudzińska et al., 2023). Biochanin A(BCA) is a remarkable isoflavone with many health benefits. BCA is a naturally occurring phytoestrogen, and the supplements derived from red clover are commercially available for postmenopausal women with hot flushes (Al-Shami et al., 2023). BCA possesses potential medicinal properties, including antioxidant effects, cardiovascular benefits, anti-cancer potential (particularly in breast and prostate cancers), neuroprotection, and antimicrobial and anti-inflammatory properties (Vennila et al., 2019, Danciu et al., 2018). It is vital to emphasize that the chemopreventive ability of biochanin A against DMBA-induced hamster oral carcinogenesis was not stated by previous scientific evidence. The research sought to conduct a comprehensive investigation into the influence of BCA on the levels of detoxification enzymes (phase I and II), and antioxidants, in addition to lipid peroxidation in hamsters with oral carcinogenesis induced by DMBA.
2 Methodology
2.1 Compounds
The cancer-causing substance DMBA and the experimental medication biochanin A were supplied by Sigma-Aldrich Chemical Pvt. Ltd., located in Bangalore, India. We procured the remaining high-quality analytical chemicals required for this research from the Hi-media Laboratory in Mumbai, India.
2.2 Trial design
The Institutional Animal Ethics Committee of Annamalai University (AU-IAEC/PR/1319/6/22) approved the experimental design in Annamalai Nagar, India. The Annamalai University Ethical Committee for Animal Care adhered to Indian National Law regarding the welfare and utilization of animals in their care.
In this study, 36 male Syrian hamsters were randomly distributed into six groups, each comprising six individuals. Fig. 1 depicts the experimental method employed in the existing investigation. Later a fourteen-week treatment period, euthanasia was carried out on all animals through cervical dislocation. Several metrics were measured and quantified, including animal body weight, growth rate, tumor weight, and tumor features. Plasma was centrifuged at 1000 rpm for 15 min from whole blood for biochemical examination. Buccal tissue was removed from all the animals, homogenized using an appropriate buffer, and centrifuged. The resultant supernatant was analyzed biochemically. The buccal mucosa tissues were initially immersed in a 10 % formalin solution for fixation to facilitate histopathological examinations. Afterward, they underwent standard processing and embedding with paraffin. Using a rotary microtome, gently cut thread sections of 2–3 μm thickness. These sections were then subjected to staining with hematoxylin and eosin, which enables the visualization and differentiation of cellular structures and tissue components.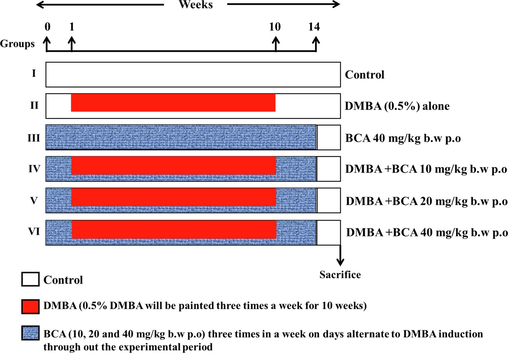
Experimental protocol.
2.3 Tumor induction
Syrian golden hamsters were given buccal pouch painting three times each week for a total of 14 weeks to cause OSCC. DMBA solution (0.5 %) prepared in liquid paraffin was used to paint the animals for the induction of cancer.
2.4 Biochemical evaluation
2.4.1 Assessment of by-products resulting from lipid peroxidation
We determined thiobarbituric acid reactive substances (TBARS) levels in both plasma and oral mucosa using established techniques. The Yagi method (1987) was employed to measure TBARS in plasma samples, while the Ohkawa et al. method (1979) was used for buccal mucosa samples (Yagi and Masumoto, 1987; Ohkawaet al., 1979). In short, both types of samples were treated with thiobarbituric acid, forming a pink color. The color intensity was then measured at 530 nm for plasma samples and 532 nm for buccal mucosa samples. The resulting absorbance directly correlated with the concentration of TBARS, indicating the extent of peroxidation of lipids.
2.4.2 Assessment of antioxidants, both enzymatic and non-enzymatic
The assessment of SOD activity was conducted following the procedure outlined in Kakkar et al.'s (1984) methodology in plasma and buccal mucosa (Kakkarand Viswanathan, 1984). This test uses a 520 nm wavelength to evaluate the color that results from a 50 % suppression of the nicotinamide adenine dinucleotide (NADH)-phenazinemethosulphate (PMS)-nitroblue tetrazolium (NBT) formation. The activity of the enzyme was determined by quantifying the enzyme quantity required to achieve a 50 % reduction in the level of nitroblue tetrazolium (NBT). In the buccal and plasma mucosa, the activity of CAT was tested according to Sinha (1972). This test measured the enzyme's H2O2 consumption, and the resultant color was evaluated at 620 nm. The enzyme activity was measured in micromoles of H2O2 absorbed per minute. The Rotruck et al. (1973) technique was utilized to determine the GPx activity in buccal mucosa and plasma. The quantity of GSH was estimated in the buccal mucosa and plasma using Beutler et al. (1963) method. The absorbance of the 412 nm yellow color produced by the interaction of plasma and buccal pouch with 5,5-dithiobis-2-nitrobenzoic acid has been directly associated with the quantity of reduced glutathione. Plasma vitamin E levels were assessed colorimetrically, following the procedure outlined by Desai in 1984 (Desai et al., 1984). When combined with a bathophenanthroline-phosphoric acid reagent, plasma vitamin E produced a pink-colored compound detected at 536 nm. The fluorometric technique established by Palan et al. (1991) was utilized to determine the vitamin E levels in the buccal mucosa (Palan et al., 1991). The amount of vitamin C in plasma was measured using the technique established by Omaye et al. (1979). When 2,4-dinitrophenylhydrazine was used to treat dehydro-ascorbic acid, which forms when copper oxidizes vitamin C, it produced a colored product with an absorbance of 520 nm.
2.5 Identification of detoxification agents
Omura and Sato (1964) developed an approach to measure the cytochrome P450 and cytochrome b5 levels in the buccal mucosa and liver. To determine the amount of cytochrome P450, a pigment was produced when the reduced cytochrome P450 reacted with carbon monoxide, and at 450 nm the absorbance of this pigment was measured. On the other hand, cytochrome b5 levels were measured utilising the variation in cytochrome oxidised and reduced spectra.
The technique established by Habig et al. (1974) method was used to obtain the level of glutathione S-transferase (GST) activity in the buccal mucosa as well as in the liver. This enzymatic assay involves forming a conjugate between 1-chloro-2,4-dinitrobenzene and GSH, and the resultant reaction was detected at 540 nm. The glutathione reductase (GR) activity in the buccal mucosa and liver was also examined using the Carlberg and Mannervik (1985) method. At 340 nm, the level of this enzymatic activity was quantified.
2.6 Statistical evaluation
The data is presented as mean values with their corresponding standard deviations (S.D.). Statistical differences in the biochemical parameters were assessed using a one-way analysis of variance (ANOVA) charted by Duncan's Multiple Range Test (DMRT). Statistical implication was determined by examining the P values, when P < 0.05 was achieved, findings were declared statistically significant.
3 Results
Table 1 displays the occurrence, dimensions, and overall tumor load in the test subjects and the untreated group of animals. In the group of animals that exclusively received DMBA (group 2), a 100 % tumor development rate was observed, along with a tumor burden of 470.46 mm3 and an average tumor volume of 78.41 mm3. However, the tumor’s occurrence, volume, and burden were significantly reduced (p 0.05) in hamsters with BCA treatment after DMBA exposure (groups 4, 5, and 6). Notably, untreated control (Group 1) and drug-alone treated animals did not develop tumors (Group 3). The 20 and 40 mg/kg bw dosages of BCA showed notable effects in DMBA-treated hamsters among the given three dosages (10, 20, and 40 mg/kg bw). According to our research, BCA at 20 mg/kg BW is more efficient than BCA at 40 mg/kg BW. The tumor volume was determined by means of the formulation V = 4/3π(D1/2)(D2/2)(D3/2), with D1, D2, and D3 representative the three tumor diameters in millimeters, and (in mm) being the total number of rats with tumors. Data for the six animals in each group is presented as the mean ± SD. In columns (a-d), values that do not share a collective superscript significantly vary from one another at p < 0.05 (determined by DMRT).
Groups
Mean body weight
Cumulative tumor count
Tumor incidence
Total volume (mm3)/hamsters
Tumor burden (mm3)/hamsters
Control
108 ± 5.36a
0/6
–
–
–
DMBA
87 ± 6.20b
6/6
100 %
78.41 ± 7.4
470.46 ± 52.04
BCA alone (40 mg/kg b.w)
126 ± 13.35a
0/6
–
–
–
DMBA + BCA (10 mg/kg b.w)
98 ± 5.21c
2/6
33 %
22.82 ± 8.2
45.64 ± 12.29
DMBA + BCA (20 mg/kg b.w)
109 ± 9.14d
0/6
–
–
–
DMBA + BCA (40 mg/kg b.w)
112 ± 7.21d
0/6
–
–
–
Fig. 2 illustrates the macroscopic presentation of oral mucosa in both normal and trial animals. Fig. 3 represents the histological variations seen in the epithelium of the buccal pouch of each group’s experimental and untreated control animals. We observed a 100 % incidence of tumor development in control animals that received only DMBA (group II), which was characterized by hyperplasia, dysplasia, severe hyperkeratosis, and a highly specialized squamous cell carcinoma (SCC). However, the distinct SCC was not perceived in the buccal pouch epithelium of animals that received both DMBA as well as BCA. The epithelium of these animals exposed mild hyperplasia and displayed significant keratosis. Conversely, in the control animals (group I), intact and well-specified layers of epithelium were observed.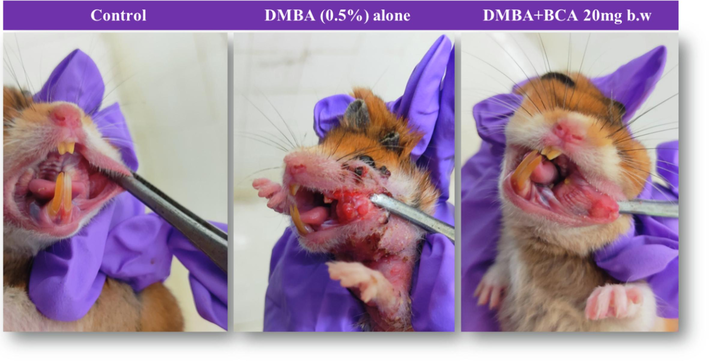
The visual representation of the oral mucosa in the untreated and dmba exposed animals is depicted. the normal buccal pouch serves as the control. well-differentiated squamous cell carcinoma is shown for animals exposed to dmba alone, while precancerous changes in the oral epithelial layers are observed in animals treated with dmba + BCA (20 mg BW).
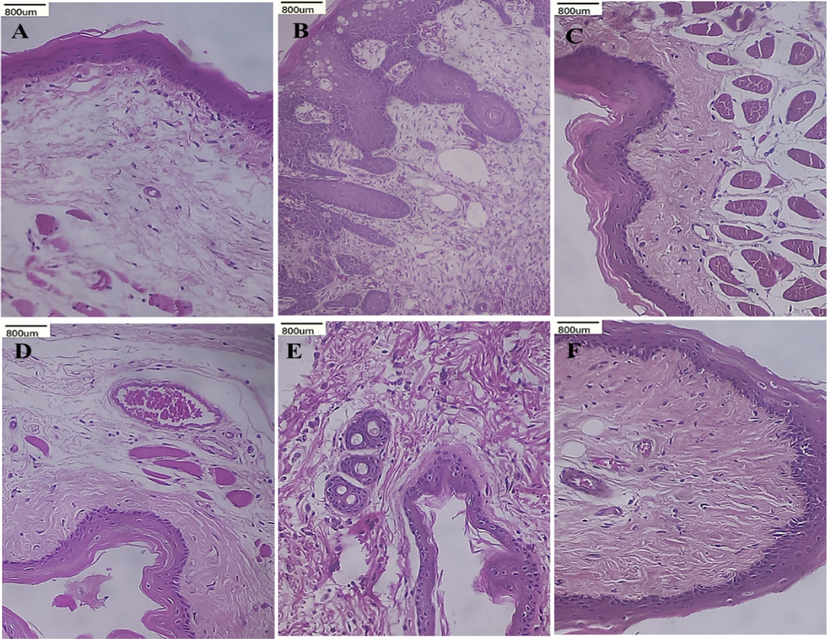
(H&E staining, x 40) shows the histopathological variations in the buccal tissue of untreated and experimental hamster.
Fig. 4 depicts the enzymatic (SOD, GPx, and CAT,) and non-enzymatic (Vitamins C, E, and reduced glutathione (GSH)) antioxidants levels and TBARS concentration in the experimental animals. TBARS were observed to be elevated in the animals with tumors (Group II), suggesting more lipid peroxidation. Furthermore, compared to the animals in the control group, the effect of both enzymatic and non-enzymatic antioxidants was considerably diminished. However, enzymatic and non-enzymatic antioxidant activity was restored to levels closer to the normal range in animals painted with DMBA (Groups V and VI) after oral treatment of BCA three times a week over a span of 14 weeks. Contrarily, compared to the control animals (Group I), hamsters fed with BCA alone (Group III) did not exhibit significant changes in antioxidant levels.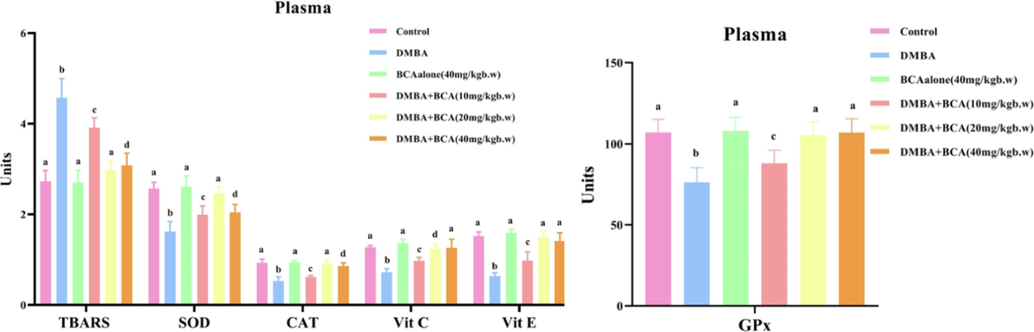
The impact of bca on tbars and antioxidant markers (sod, cat, vitamin c, vitamin e, gpx) in plasma is evident. the data is shown as the mean ± SD for six hamsters for each set. In columns (a-d), values without a shared superscript expressively differ at p < 0.05 (determined by DMRT). Unit Specifications: TBARS - nanomoles per milliliter (n mol/mL); SOD - the amount of enzyme needed to prevent 50 % NBT reduction per milliliter; CAT - micromoles of hydrogen peroxide exploited per second per milliliter (µmol H2O2/s/mL); Vit C - milligrams (mg) per deciliter (dL); Vit E - milligrams (mg) per deciliter (dL); GPx - micromoles of glutathione disbursed per minute per milliliter (µmol GSH/min/mL).
The status of enzymatic, and non-enzymatic antioxidants and TBARS in the oral mucosa of experimental as well as the control animals is laid out in Fig. 5. The oral mucosa of experimental and control animals is shown in Fig. 5 showing the levels of enzymatic and non-enzymatic antioxidants and TBARS. Decreased TBARS levels and changes in antioxidant concentration were observed in animals with tumors (Group II), in which GSH, GPx, vitamin C and E levels were increased, while there was a decline in the activity of CAT and SOD. However, oral BCA treatment to DMBA-treated animals resulted in the decline of TBARS concentration, and restored antioxidant levels in the oral mucosa of the animals in the groups IV, V, and VI. On the other hand, Group III of hamsters that were given BCA alone did not show any notable variations in TBARS concentration and antioxidant position in comparison to the animals in the control group (Group I).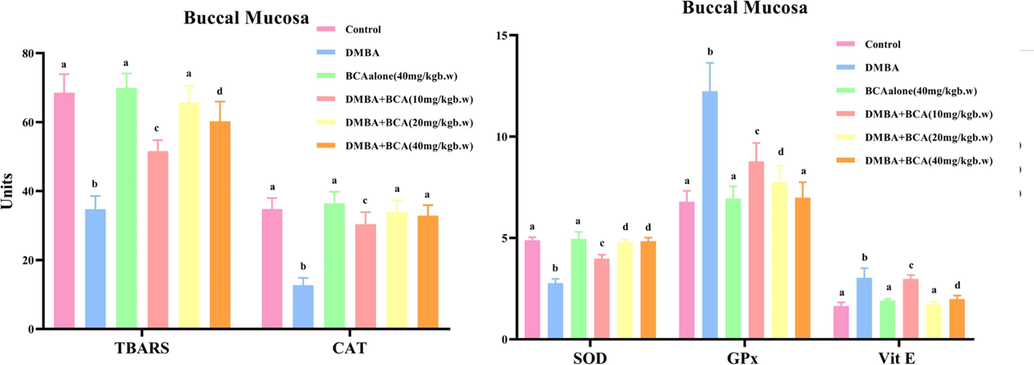
Illustrates the influence of bca on lipid peroxidation (tbars) and the antioxidant parameters in buccal mucosa (sod, cat, vitamin e, gpx). the data is presented as the mean ± SD for each set of six hamsters. Within columns (a-d), values that lack a shared superscript exhibit significant variances at p < 0.05, as determined by DMRT. Unit Specifications: TBARS – nanomoles in a milligram of protein (nmol/mg protein); SOD - the quantity of enzyme needed to prevent 50 % NBT decrease/mg of protein); CAT - µmol H2O2/s/d/mg protein; Vitamin E - milligrams per 100 g of wet tissue (mg/100 g wet tissue); GPx - µmol GSH/m/mg protein.
Fig. 6 indicates an overview of the condition of phase I and II detoxification substances in the oral mucosa of experimental and control animals. Phase I detoxification enzymes (cytochromeb5 and P450) and phase II detoxification enzymes (GST and GR) were predominantly elevated in animals with tumors (Group II). However, oral treatment of BCA to the animals subjected to DMBA treatment (Groups V and VI) led to the significant restoration of GST and GR activity, placing them within a range similar to that of the control animals. Between the control trial (Group I) and BCA-treated animals (Group III), there was no discernible difference in the levels of GST and GR.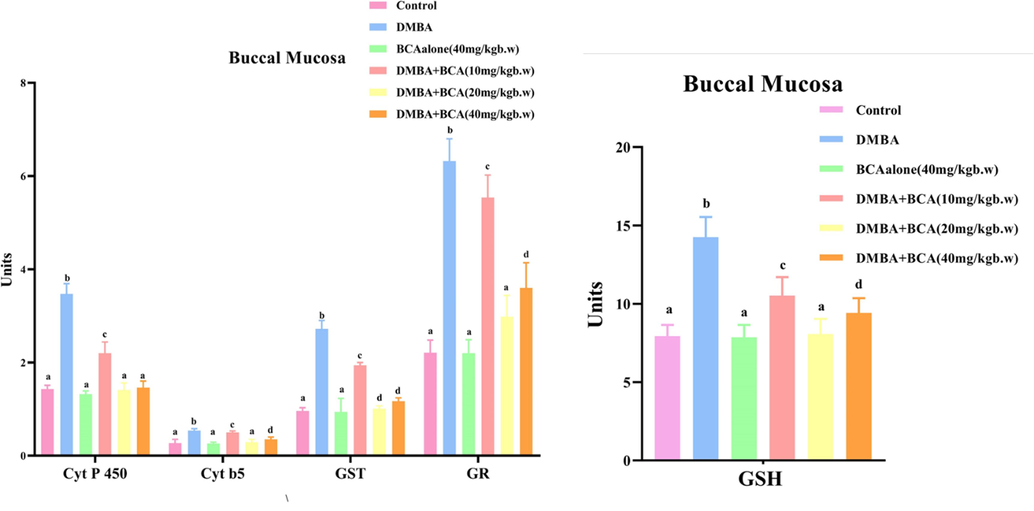
The influence of bca on the quantity of gsh and the activity of detoxification enzymes in the oral mucosa is depicted. the data is revealed as the mean ± SD for each group of six hamsters. Within columns (a-d), values that exhibit significant differences at p < 0.05 (determined by DMRT) do not share a similar superscript. Unit Specifications: Cyt P 450 - micromoles of cytochrome P450 per milligram of protein (µmol Cyt P450/mg protein); Cyt b5 - micromoles of cytochrome b5 per milligram of protein (µmol Cyt b5/mg protein); GSH - micrograms per milligram of protein (µg/mg protein); GST – micromoles of 1-chloro-2,4-dinitrobenzene associated with GSH/min/mg of protein (µmol CDNB-GSH conjugates/min/mg protein); GR –n mol CDNB conjugates/min/mg protein.
The levels of detoxification enzymes (phase II) and phase I detoxification in the livers of trial and control animals are shown in Fig. 7. The GST and GR levels were remarkably curtailed in animals with tumors (Group II), suggesting a disturbance in phase II detoxification mechanisms. In contrast, significant amounts of cytochrome b5 and P450 were shown in the livers of DMBA-treated animals, which are involved in phase I detoxification, compared to the untreated group. However, phase I and II detoxifying agents were restored and brought closer to the standard range in DMBA-treated animals (Groups IV, V, and VI) after oral BCA treatment, three instances weekly over a span of 14 weeks. The status of these detoxifying agents was not significantly changed in the group I and III animals.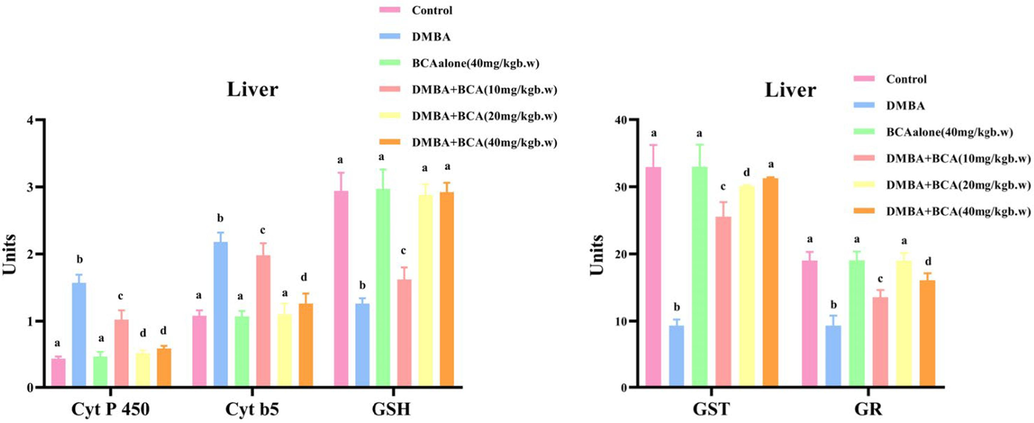
Effects of BCA on GSH levels and phase I &II detoxification enzymes in the livers of control and experimental trial animals are shown in Fig. 7. Values are obtainable as the mean ± SD for each set of six hamsters. (a-d)Values in the same column that do not have a conjoint superscript differ substantially at p < 0.05 (DMRT). Units: Cyt P 450 - Micromoles of cytochrome P450/mg protein; Cyt b5 - Micromoles of cytochrome b5/mg protein; GSH - µg/mg protein; GST -µmol of 1–chloro-2,4-dinitro-benzene conjugated with reduced glutathione per min/mg protein; GR - n mol CDNB conjugates/min/mg protein.
4 Discussion
Every year, approximately 300,000 individuals across the globe receive a diagnosis of cancer in the oral cavity. Oral carcinoma is the major frequent cancer in India. Over recent decades, numerous research efforts have been undertaken to explore cancer chemoprevention as a means of decreasing cancer-related death rates (Borse et al., 2020). This investigation will examine BCA's potential as a chemopreventive agent in buccal pouch carcinogenesis induced by DMBA in hamsters. After this experimental period, all the hamsters given DMBA alone acquired tumors. During the experimental duration, continuous observation was carried out to track the percentage of animals developing tumors and the size of tumors in animals receiving DMBA exclusively and those receiving a combination of DMBA and BCA. The chemopreventive effect of BCA in DMBA-induced buccal carcinogenesis was analyzed by evaluating antioxidants, lipid peroxidation, and detoxifying agent status as biochemical indicators.
Histopathological examination of the oral mucosa of the hamsters brushed with 0.5 % DMBA for 14 weeks, developed well-differentiated SCC that had undergone a complete differentiation process. Balakrishnan et al. (2022) reported that the cancer cells have epithelial differentiation and hyperchromatic, pleomorphic nuclei. In our study, hamsters exposed to DMBA exhibited a significant increase in hyperplasia, hyperkeratosis, and dysplasia. Oral treatment with 20 mg/kg bw BCA effectively suppressed the formation of tumors in the oral mucosa of DMBA-treated animals with mild hyperplasia and dysplasia. It is plausible due to the mechanical irritation caused by the brush after a period of ten weeks of use. The results of our research imply that BCA may act as an anti-proliferator by arresting the cell cycle during DMBA-induced oral carcinogenesis.
In our research, hamsters that were subjected to DMBA treatment alone displayed an elevation in plasma TBARS and a reduction in antioxidant levels. These changes demonstrated that the DMBA-treated hamsters had oxidative stress. The overproduction of lipid peroxidation byproducts during DMBA metabolism and leaking from injured host tissues into the plasma may be the reason for oxidative damage. The low levels of antioxidants in the plasma may be related to the tumor tissues consumption of these compounds or their role in reducing the negative effects of lipid peroxidation by-products respectively (Meulmeester et al., 2022). Comparing normal buccal mucosa tissues with tumor tissues from DMBA-treated hamsters, it was found that lipid peroxidation was raised and antioxidant levels were disrupted. Decreased polyunsaturated fatty acids (PUFA) and abnormal cell proliferation may be a cause for the decrease in lipid peroxidation by-products in cancer tissues (Li et al., 2022). There was a rise in GPx and vitamin E activity in cancer tissues, consistent with previous findings and most likely due to their cell proliferation regulatory effects. Additionally, the decreased SOD and CAT activity in the tumor tissues is consistent with data made in several malignant diseases, including oral cancer (Liao et al., 2022).
DMBA’s metabolic process in the induction of tumors is by the production of free radicals and oxygenated metabolites, which induce oxidative stress by initiating lipid peroxidation. Lipid peroxidation might serve as an early diagnostic indicator for cancer (Meulmeester et al., 2022). In our study lipid peroxidation was observed in group II animals. Nevertheless, upon orally administering BCA to DMBA-treated hamsters at dosages of 10, 20, and 40 mg per kilogram of body weight, we observed progressive changes in lipid peroxidation and antioxidant levels in both plasma and buccal mucosa in a dose-dependent mode.
The improvement of lipid peroxidation inhibition and antioxidant activity was achieved, possibly due to BCA's estrogenic nature. Estrogen has a beneficial antioxidant activity as it can upregulate the antioxidant enzyme expression via the intracellular signaling pathway and potentially enhance the body's ability to combat free radicals (Magni et al., 2022). BCA also has a chromone ring system, which is the major reason for its antioxidant, antibacterial, anti-inflammatory, antiviral, and anticancer action (Huang et al., 2022). Our study indicates that BCA administration may help mitigate oxidative stress by regulating the lipid peroxidation process and levels of antioxidants during oral carcinogenesis induced by DMBA in hamsters, possibly due to its specific chemical structure containing phenolic hydroxyl groups. Among 3 doses 20 mg/kg bw of BCA is more efficient in preventing lipid peroxidation and increasing antioxidant activity.
Chemopreventive agents are required to hinder, curtail, reverse, or impede the development of tumors by preventing carcinogens' interaction with cellular macromolecules, accelerating their detoxification, and preventing their metabolic activation (Swetha et al., 2022). A large number of chemopreventive agents work by increasing GST activity. By either inhibiting the conjugation process or deactivating the reactive sites of the carcinogenic metabolites, this enzyme significantly enhances their removal. GR assumes a pivotal role in the system by actively participating in restoring and preserving the normal functions of the cell (Fotis et al., 2022). Previous research has revealed that tumor-bearing animals had increased the activities of detoxification enzymes of phase I and diminished phase II detoxification enzyme activities in the liver (Wang et al., 2022). The alterations in the detoxification systems in the liver suggest the chemical accumulation during oral carcinogenesis induced by DMBA. Following the oral treatment of BCA, the concentrations of phase I and phase II detoxification agents in the liver tissues of hamsters exposed to DMBA reverted to almost normal, demonstrating that BCA enhanced the conjugation and resulting in the removal of carcinogenic metabolites, denoting it as a potent anticancer drug.
Phase I enzymes play a crucial role in the biochemical transformation of procarcinogens to decisive carcinogens. Buccal mucosa exhibits irregular activity in phase I as well as phase II detoxification enzymes, indicating a hostile impact of harmful DMBA metabolites, particularly dihydrodiol epoxides. Notable rises of phase I and II detoxification enzyme activities were documented in the oral mucosa throughout DMBA-induced hamster oral pouch carcinogenesis (Chu et al., 2022). Our study corroborates these results. The oral administration of BCA effectively reinstated the detoxification activities to nearly normal levels in the buccal pouch of hamsters exposed to DMBA, it might be because of its specific chemical structure containing phenolic hydroxyl groups.
5 Conclusion
The current investigation elucidated the cancer preventive efficacy of BCA in hamster buccal pouch carcinogenesis induced by DMBA. This action may be due to its potential to enhance the antioxidants and counteract lipid peroxidation to control the detoxification cascade. BCA at 20 mg/kg BW is more efficient in controlling oral cancer than the other two doses in DMBA-induced carcinogenesis. The limitation of the study is to analyze the inflammatory and apoptotic mechanisms of BCA in oral carcinogenesis, which will be evaluated in future studies.
Acknowledgment
The authors sincerely thank RUSA (RUSA 2.0- 100- E- 002) for providing financial support from the RUSA 2.0 project grant. The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2023R466), King Saud University, Riyadh, Saudi Arabia. The authors thank, Department of Biochemistry and Biotechnology, Annamalai University for the laboratory facilities.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Molecular mechanisms underlying the potential neuroprotective effects of Trifolium pratense and its phytoestrogen-isoflavones in neurodegenerative disorders. Phytother. Res.. 2023;37(6):2693-2737.
- [Google Scholar]
- Anticancer and antioxidant profiling effects of Nerolidol against DMBA induced oral experimental carcinogenesis. J. Biochem. Mol. Toxicol.. 2022;36(6):e23029.
- [Google Scholar]
- Improved method for the determination of blood glutathione. J. Lab. Clin. Med.. 1963;61:882-888.
- [Google Scholar]
- Oral cancer diagnosis and perspectives in India. Sensors International. 2020;1:100046
- [Google Scholar]
- Glutathione reductase. In: Methods in Enzymology. Vol 13. Academic press; 1985. p. :484-490.
- [Google Scholar]
- Therapeutic approaches to colorectal cancer via strategies based on modulation of gut microbiota. Front. Microbiol.. 2022;13:945533
- [Google Scholar]
- Overview of human 20 alpha-hydroxysteroid dehydrogenase (AKR1C1): Functions, regulation, and structural insights of inhibitors. Chem. Biol. Interact.. 2022;351:109746
- [Google Scholar]
- Main isoflavones found in dietary sources as natural anti-inflammatory agents. Curr. Drug Targets. 2018;19(7):841-853.
- [Google Scholar]
- Polymeric drug delivery systems for intraoral site-specific chemoprevention of oral cancer. J. Biomed. Mater. Res. B Appl. Biomater.. 2018;106(3):1383-1413.
- [Google Scholar]
- Thin-layer element for interfaces and joints. Int. J. Numer. Anal. Meth. Geomech.. 1984;8(1):19-43.
- [Google Scholar]
- Risks in the European transmission system and a novel restoration strategy for a power system after a major blackout. Appl. Sci.. 2022;13(1):83.
- [Google Scholar]
- The hamster cheek pouch carcinogenesis model. J. Cell. Biochem.. 1993;53(S17F):83-90.
- [Google Scholar]
- Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249(22):7130-7139.
- [Google Scholar]
- Sesquiterpenoid-chromone heterohybrids from agarwood of Aquilaria sinensis as potent specific Smad3 phosphorylation inhibitors. J. Org. Chem.. 2022;87(12):7643-7648.
- [Google Scholar]
- Kakkar, P., Das, B. and Viswanathan, P.N., 1984. A modified spectrophotometric assay of superoxide dismutase,21(2),130-2.
- GTP cyclohydrolase 1/tetrahydrobiopterin counteracts ferroptosis through lipid remodeling. ACS Cent. Sci.. 2019;6(1):41-53.
- [Google Scholar]
- Oxidative Stress and 4-hydroxy-2-nonenal (4-HNE): Implications in the Pathogenesis and Treatment of Aging-related Diseases. J. Immunol. Res. 2022:2022-2233906.
- [Google Scholar]
- Vitamin E and metabolic health: Relevance of interactions with other micronutrients. Antioxidants. 2022;11(9):1785.
- [Google Scholar]
- Molecular mechanisms of ethanol-associated oro-esophageal squamous cell carcinoma. Cancer Lett.. 2015;361(2):164-173.
- [Google Scholar]
- Flavonoids bridging the gut and the brain: Intestinal metabolic fate, and direct or indirect effects of natural supporters against neuroinflammation and neurodegeneration. Biochem. Pharmacol.. 2022;115257
- [Google Scholar]
- Antioxidant supplementation in oxidative stress-related diseases: What have we learned from studies on alpha-tocopherol? Antioxidants. 2022;11(12):2322.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [Google Scholar]
- The carbon monoxide-binding pigment of liver microsomes. I. Evidence for Its Hemoprotein Nature. J. biolChem. 1964;239(7):2370-2378.
- [Google Scholar]
- Plasma levels of antioxidant β-carotene and α-tocopherol in uterine cervix dysplasias and cancer. Nutr. Cancer. 1991;15(1):13-20.
- [Google Scholar]
- Photodynamic therapy treatment of oral cavity cancer in patients with comorbidities. Biomed. Photon.. 2023;11(4):19-24.
- [Google Scholar]
- Rahman, M.M., Islam, M.R., Shohag, S., Ahasan, M.T., Sarkar, N., Khan, H., Hasan, A.M., Cavalu, S. and Rauf, A., 2022. Microbiome in cancer: Role in carcinogenesis and impact in therapeutic strategies. Biomedicine & Pharmacotherapy, 149, p.112898.
- Rotruck, J.T.ꎬ., Pope, A.L., Ganther, H.E., Swanson, A.B., Hafeman, D.G. and Hoekstra, W., 1973. Selenium: biochemical role as a component of glutathione peroxidase. Science, 179(4073), 588-590.
- Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients. 2023;15(8):1896.
- [Google Scholar]
- Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int. J. Biol. Sci.. 2008;4(1):8.
- [Google Scholar]
- Cancer chemoprevention: A strategic approach using phytochemicals. Front. Pharmacol.. 2022;12:809308
- [Google Scholar]
- Nano Diosgenin Abates DMBA Induced Renal and Hepatic Toxicities: Biochemical and Histopathological Evaluation on the Breast Cancer Model. Curr. Bioact. Compd.. 2023;19(4):47-67.
- [Google Scholar]
- Evaluation of In-Vitro Antioxidant Activity of Biochanin A. J. Drug Delivery Therapeutics. 2019;9(4-A):594-600.
- [Google Scholar]
- Alleviation of oral exposure to aflatoxin b1-induced renal dysfunction, oxidative stress, and cell apoptosis in mice kidney by curcumin. Antioxidants. 2022;11(6):1082.
- [Google Scholar]
- Instrumental charged-particle activation analysis of several selected elements in biological materials using the internal standard method. J. Radioanal. Nucl. Chem.. 1987;111(2):359-369.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.103067.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







