Translate this page into:
Green synthesis and antimicrobial efficacy of titanium dioxide nanoparticles using Luffa acutangula leaf extract
⁎Corresponding author. babukmg@gmail.com (Ranganathan Babujanarthanam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present study deals with the synthesis of titanium dioxide (TiO2) nanoparticles using Luffa acutangula leaf extract and explore the antimicrobial potential of synthesized nanoparticles. The biosynthesized TiO2 nanoparticles were characterized using different spectroscopic and microscopic techniques. The absorption spectrum of synthesized TiO2 nanoparticles were primarily characterized by Ultraviolet visible (UV–vis) spectrophotometer. The functional groups associated with the TiO2 nanoparticles and the Luffa acutangula leaf extract were examined by Fourier Transform Infrared (FTIR) spectroscopy. The crystalline structure of nanoparticles were analyzed by X-ray diffraction (XRD) examination. Morphological characters were examined by Scanning Electron Microscopy (SEM) and Transmission Electron Microscope -Selective Area Electron Diffraction (TEM - SAED). Presence of elemental composition of synthesized TiO2 nanoparticles were characterized by Energy Dispersive X-ray (EDX). The antimicrobial properties of the TiO2 nanoparticles were observed to be highly toxic against bacterial strains are Bacillus subtilis (B. subtilis), Escherichia coli (E. coli), Enterococcus faecalis (E. faecalis), Klebsiella pneumonia (K. pneumoniae), Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa (P. aeruginosa) and the fungal strains are Aspergillus flavus (A. flavus), Aspergillus niger (A. niger), Rhizopus oryzae (R. oryzae) and Sclerotium rolfsii (S. Rolfsii). The zone of inhibition was estimated by disc diffusion assay and moreover, minimum inhibitory concentration was evaluated by the micro broth dilution assay. It can be concluded that titanium dioxide nanoparticles manifest a strong antimicrobial action and thus can be developed as a novel type of antimicrobial materials for the cure of microbial infections.
Keywords
TiO2 nanoparticles
Luffa acutangula
Morphological characterize
Antimicrobial action
Microscopy analysis
1 Introduction
Nanotechnology has attained vast attention over time, and it involves synthesizing and developing different nanomaterial (Kasinathan et al., 2016; Panimalar et al., 2022; Rathnakumar et al., 2019; Magdalane et al., 2018; Aina et al., 2018) and it is an accelerating field of recent research with desirable applications in medicine and electronic and has been developing very fast in recent generation, impacting on distinct areas such as environment and economy (Mani et al., 2021; Magdalane et al., 2021; Loo et al., 2018; Srinivasan et al., 2020). It is fundamentally concerned about the synthesis of nanoparticles (NPs) and their application in different fields of science, medicine, designing and imaging (Ocsoy et al., 2013; Jayakumar et al., 2022). Nanoparticles are characterized as building blocks of nanotechnology and the size ranging from 1 to 100 nm in diameter (Hoffmann et al., 1995; Fabrega et al., 2010; Fang et al., 2012). The foremost feature of nanoparticles is their surface area to volume aspect ratio, enabling them to combine with other particles easier. Moreover, metal nanoparticles have been intensively used in biology because they are biocompatible in nature and can interact with proteins, receptors, and nucleic acids because of their size range (Gelis et al., 2003; Trouiller et al., 2009; Jayaseelan et al., 2013). These nanoparticles are regarded as susceptible to bind targeting agents after appropriate functionalization for delivery of those biomolecules to the targeting sites (Ahmad et al., 2015; Zdyb and Krawczyk, 2014; Dadkhah et al., 2014). Currently, nanoscale particles have been tremendously applied to the advancement of novel antimicrobial agents for the treatment of pathogenic microorganisms. Among the prevailing antimicrobial substances, nanoscale materials are of immense attention because of their eminent effectiveness and reactivity (Gerhardt et al., 2007; Prakash et al., 2012; Chen et al., 2014). Administration of nanoparticles as antibacterial drug is a new and ingenious approach, which is a cost-effective measure against pathogenic microorganisms (Xiaoming, 2015; Pu et al., 2013; Zhao et al., 2015). Over the last few years, titanium dioxide nanoparticles (TiO2 NPs) have been broadly utilized as an eco-friendly and clean photocatalyst, due to its optical properties, apex chemical stability and less toxic nature (Ahmed et al., 2016; Ahmad et al., 2010; Manjula et al., 2018; George et al., 2022). Moreover, titanium dioxide are the most important materials for beauty care products, drugs, skin care materials specially to protect skin from UV rays, whiteness, opacity to products like paints, food colorants, papers, toothpastes, plastics, and inks (Kaviya et al., 2021; Orudzhev et al., 2020; Mani et al., 2021; Geethalakshmi and Sarada, 2010; Willner et al., 2007). It is likewise utilized in wide potential applications in acaricidal action, antibacterial, pharmaceuticals and solar cell (Ambika and Sundrarajan, 2016; Duan et al., 2015; Weir et al., 2012; French et al., 2009). The less hazardous and biocompatible nature of TiO2 NPs find its applications in biomedical sciences, for example pharmaceutical industries as well as in bone tissue engineering (Roopan et al., 2012; Velayutham et al., 2012; Kayalvizhi et al., 2022). There are a few strategies available for synthesis of titanium dioxide nanoparticles like chemical micro emulsion, wet chemical method, vapor phase process, hydrothermal process, microwave-assisted technique and solvothermal technique (Kirthi et al., 2011; Singh and Lillard, 2009; Li et al., 2014; Hu et al., 2010; Brown et al., 2012).
Now a days, some consideration has been focused on the biological route for the synthesis of nanoparticles (Nithiyavathi et al., 2021; Orudzhev et al., 2021; Rajeswari et al., 2021). The utilization of environmentally friendly plant materials such as leaves roots and fruits for the synthesis of nanoparticles serves various advantages because of their non-harmful nature as well as safety and ecological properties (Gong et al., 2007; Allahverdiyev et al., 2011; Anitha and Miruthula, 2014; Dandge et al., 2010). The biological technique utilizing plant extracts has received more consideration than physical and chemical strategies. Furthermore, synthetic drugs formulated by employing distinct strategies in the laboratory and these are the medicines which are not existing in nature. Despite the fact that, herbal medicines are less potent in comparison to synthetic drugs in some cases but still these are consider less toxic or having few side effect in contrast to synthetic drugs (Vanajothi et al., 2012; Abdelhalim et al., 2012; James and Cohen, 1980; Rajakumar et al., 2012). Overcome of these limitations, biosynthesized nanoparticles are widely used in the biomedical applications due to its drug compatibility and ecofriendly nature because of harmful synthetic compounds are not used in this technique. Using plant extracts as an emerging technology for synthesis of different metal oxide nanoparticles (Hunagund et al., 2016; Colthup, 1950) because the plant extract containing various phytochemicals for example, flavonoids, tannins, and proteins and so forth, are the main point of interest to control the shape and size of the nanoparticles (Wiley, 2001; Vizhi et al., 2016). Accordingly, the existence of different surfactants and stabilizers are beneficial to have a control over the development of the nanoparticles. TiO2 NPs are well recognized as a versatile multifunctional material because of their superior chemical stability under physiological environment (Philip, 2010; Rajakumar et al., 2011). Recent studies reported that the TiO2 NPs were synthesized from peel extract of Annona squamosa, aqueous leaf extract of Catharanthus roseus and the microorganism Bacillus subtilis for biomedical applications (Jayaseelan et al., 2013; Fu et al., 2001; Salunkhe et al., 2011; Sathishkumar et al., 2009). The high degree of microbial diseases and their multidrug resistant properties make the scientists to develop a new type of antimicrobial agents (Sundrarajan et al., 2017; Gelover et al., 2006). An advanced and emerging approach to drug development is the utilization of metal nanoparticles as new forms of antimicrobial agents. Therefore, the current interest in the researchers due to the developing microbial resistance against metal particles, antibiotics and the development of resistant strains and the TiO2 NPs have exhibited significant antibacterial action (Zhang and Chen, 2009; Vazquez-Munoz et al., 2014; Holt and Bard, 2005; Vinayagam et al., 2021). Titanium dioxide plays a more supportive role in our environmental purification because of its antifogging effect, photo induced super-hydrophilicity and less toxic nature (Singh et al., 2021; Vijayakumar et al., 2020; Geetha et al., 2018). These properties have been applied in eliminating microbes and toxic organic materials from air and water, also in self-sterilizing surfaces in medical centres.
Luffa acutangula belongs to the family Cucurbitaceae, is usually known as ridge gourd and it is utilized as vegetable in Asian nations. The whole plant of Luffa acutangula L. (L. acutangula) is therapeutically significant and is utilized widely in Indian traditional system of medicines (Parasuraman et al., 2019; Anju et al., 2019). For the purpose of killing the parasite, ridged gourd’s leaf extract is applied on sores that have occurred due to guinea worms. Also, leaf sap is useful in curing conjunctivitis as an eyewash. For the preparation of herbal products, the fruits and seeds are extensively used for treating venereal disease, specifically gonorrhoea. It is also noted in Mauritius that these seed are consumed to remove intestinal worms. Besides, the application of leaf juice on skin to cure eczema. The seed as well as the plant is insecticidal, indicating the potential antioxidant activity (Ramesh et al., 2021; Ramesh et al., 2022; Ramesh et al., 2021; Radhakrishnan et al., 2020). The juice of leaves is likewise utilized for jaundice, antitumor, antidiabetic, eczema, and dysentery conditions (Paramanantham et al., 2018; Akhtar et al., 2016; Habimana et al., 2011). Seed oil used for dermatitis, a fruit extract of L. acutangula was noted to possess stronger antibacterial and antifungal activity. The scope of this work was to scrutinize the antimicrobial activity of synthesized TiO2 NPs using leaf extract of L. acutangula against pathogens. The current work aims to utilize the biological route for the synthesis of TiO2 NPs and characterized using various spectroscopic and microscopic methods for the analysis of structure, morphology, and optical properties (Rajkumari et al., 2019; Parasuraman et al., 2019; Renuka et al., 2020; Nalini et al., 2019). The TiO2 NPs was synthesised by biological reduction which anticipate appropriate capping agent for the stability of the synthesized nanoparticles and exhibits excellent antibacterial and antifungal activities (Buszewski et al., 2018; Lonnen et al., 2005; Mani et al., 2021; Akiba et al., 2005).
2 Experimental section
2.1 Preparation of leaves extraction
The leaves of Luffa acutangula (Family: Cucurbitaceae) were gathered from Nattamangalam Village, Vellore, India, and the gathered plant leaves were approved (PARC/2017/3522) by Professor. P. Jayaraman, Director, Institute of Herbal Botany, Plant Anatomy and Research centre, Chennai, Tamil Nadu, India. The newly gathered leaves sample was surface cleaned with running faucet water for eliminate soil followed by refined water and shade dried for 7–8 days at room temperature. The dried sample was powdered in a blender and sieved to get uniform size range and put away at 4 °C for additional investigations. From that, 5 g of leaves sample was risen in 100 ml of refined water taken in 500 ml Erlenmeyer flask at 60 °C for 15 min. At that point, the concentrate was separated utilizing Whatman No.1 filter paper. The filtrate was utilized for the synthesis of titanium dioxide nanoparticles.
2.2 Biogenic synthesis of titanium dioxide nanoparticles
In the present studies, the synthesis of nanoparticles of titanium dioxide was done using an organic strategy. For biogenic synthesis of titanium dioxide (TiO2) nanoparticles, 100 ml of 1 mM titanium sulfate (bought from Hi Media Laboratories Ltd., India) solution was mixed for 2 hrs. From that, 80 ml of titanium sulfate solution was added to 20 ml of aqueous leaves extract at room temperature under blended condition for 6 hrs. After the response of plant extracts with titanium sulfate, the incorporated nanoparticles turned dark green to light green in shading. Moreover, the concoction was exposed to centrifugation at 10,000 rpm for 10 min. Consequently, made pellets were gathered and decontaminated by repeated centrifugation to eliminate unbound phytochemicals and the subsequent pellet was air dried for assessment of TiO2 NPs.
2.3 Characterization of synthesized titanium dioxide nanoparticles
After synthesis, the titanium dioxide nanoparticles were exposed to characterization by different insightful estimations. UV–Visible (UV–Vis) spectroscopy is a methodology employed for assessing the light that is acclimatized and dispersed by a model. The integrated TiO2 nanoparticles solution was put between a light source and a photograph finder and the intensity of absorbed light is estimated by Shimadzu 3600 spectrometer and worked at a resolution of 1 nm ranging from 200 and 800 nm ranges. The absorbance can be used to measure the concentration of solution by using Beer Lambert's Law. It is the most widely used method to confirm the formation of nanoparticles and to characterize the electronic structure and the optical features of nanoparticles, as the absorption bands are associated to the diameter and aspect ratio of metal nanoparticles. The presence of functional groups and the binding property of the titanium oxide nanoparticles were determined by Fourier Transform Infrared (FTIR) spectroscopy. Infrared spectroscopy was done in JASCO-4600 spectrometer with straightforward wafers containing the titanium dioxide nanoparticles to be dissected and KBr as cover (9:1 dilution). Spectra were recorded at a goal of 4 cm−1 more than 4000–500 cm−1. The structure of the crystalline pertaining to the integrated TiO2 NPs were gotten by utilizing X-ray diffraction (XRD) (Bruker D8, Advance) spectroscopy which utilized Cu Kα (λ = 1.5406 Å) radiation for the Bragg's Law ranging from 10 to 80° on the 2θ scale and working at 40 kV and at a current of 30 mA. The phase of the nanocrystalline particles were likewise confirmed by utilizing XRD. The morphological characters of the synthesized TiO2 NPs were analyzed by utilizing scanning electron microscopy (ZEISS (EBO 180) Scanning Electron Microscopy (SEM) equipped with EDX) and elemental components of the synthesized nanoparticles were recognized by Energy Dispersive X-ray (EDX) investigation with a same Model. The TiO2 NPs were dried and to acquire a powdered structure. At that point, 10 mg of the TiO2 NPs were redispersed in ethanol and were placed in thin films on carbon coated copper grids. SEM micrographs were obtained with a finder of helper electrons at magnification of 50,000×, where the accelerating voltage was 5 kV and the working distance was adjusted to around 3 mM. The shape, size and distribution of the synthesized TiO2 NPs were explored by transmission electron microscopy (TEM) (TEM, FEI Tecnai 20 G2 20 S-TWIN High Resolution Transmission Electron Microscopy). A drop of diluted aqueous solution consisting of synthesized TiO2 NPs were set on the Holey carbon TEM grid followed by drying prior to placing them into the TEM test chamber. TEM microscopic pictures were taken by analyzing the prepared grids and were recorded at an acceleration voltage of 200 kV. Chosen zone of electron diffraction was a crystallographic experimental technique accomplished inside a TEM. Selective Area Electron Diffraction (SAED) was utilized to distinguish crystal structures and examine crystal defects of nanosized particles.
2.4 Antimicrobial activity of synthesized TiO2 nanoparticles
2.4.1 Antibacterial activity by disc diffusion assay
The antibacterial action of the synthesized TiO2 nanoparticles were evaluated against the bacterial pathogens of Bacillus subtilis (B. subtilis) (ATCC 6051), Escherichia coli (E. coli) (MTCC-1677), Enterococcus faecalis (E. faecalis) (ATCC 2912), Klebsiella pneumonia (K. pneumonia) (NCTC 9633), Staphylococcus aureus (S. aureus) (MTCC-3160) and Pseudomonas aeruginosa (P. aeruginosa) (MTCC-4030) strains. Disc diffusion technique was adopted to monitor the antibacterial activity of synthesized titanium dioxide nanoparticles. Exponential bacterial cultures were seeded into Muller Hinton agar and impregnated with sterile discs. The discs were loaded with titanium dioxide nanoparticles with various concentrations (20, 30 and 40 µg/ml) and empty sterile disc was used as a control. The impregnated discs were kept on the surface of the agar and incubation of the plates was done overnight at room temperature. The experiment was performed in triplicates and the formation of the clear zone of inhibition was computed.
2.4.2 Minimal inhibitory concentration against bacterial pathogens
The lesser concentration of each antibacterial agent that hinder the development of the microorganisms were tested by Minimum Inhibitory Concentration (MIC) using microbroth dilution method and was detected by lack of turbidity matching with a negative control. The TiO2 nanoparticles were dissolved in 10% Dimethyl sulfoxide (DMSO). The MIC of TiO2 NPs were determined as the minimal concentration of the TiO2 NPs repressing the visual development of the test cultures like S. aureus (MTCC-3160), K. Pneumoniae (NCTC 9633), P. aeruginosa (MTCC-4030), E. faecalis (ATCC 2912), B. subtilis (ATCC 6051), and E. coli (MTCC-1677). The titanium dioxide nanoparticles were dissolved in 10% DMSO. The various concentrations of 100, 80, 60, 40, 20, 10 and 5 µg/ml were serially diluted in a 96 well plate and inoculated with 5 µl of bacterial culture. After inoculation, the plates were incubated for 24 hrs at 37 °C for cultural growth. The intensity of the culture of each well was read at 600 nm and it compared with the untreated control.
2.4.3 Antifungal action by disc diffusion assay
The potato dextrose agar (PDA) was prepared, sterilized, and poured on to sterile petri plates. The cultures of Aspergillus flavus (A. flavus) (ATCC 10124), Aspergillus niger (A. niger) (ATCC 1015), Rhizopus oryzae (R. oryzae) (ATCC 24563) and Sclerotium rolfsii (S. rolfsii) (ATCC 62666) were swabbed on the PDA plates. The various concentrations of 20, 30 and 40 µg/ml of titanium dioxide nanoparticles were loaded on sterile disc separately and placed inverted on the swabbed plate. Empty sterile disc was kept as control. The plates were kept for 48 hrs incubation and the zone of inhibition was estimated.
2.5 Statistical examination
Statistical analysis was done by the SPSS (Statistical Package for Social Sciences) version 20.0, Chicago, IL, USA programming. Examinations were done in sets of three and the information was communicated as mean ± standard error (SE) by the One-Way ANOVA test and the individual correlations were obtained by Duncan's technique. A difference was deliberated as significant at p < 0.05.
3 Results and discussion
3.1 Optical properties of synthesized TiO2 NPs
UV–visible spectroscopic examination was an efficacious technique to explore if the precursors are totally diminished. The colour change was observed by visible in L. acutangula leaves extract during the incubation with titanium sulfate solution. The pure titanium sulfate without aqueous leaves concentrate of L. acutangula did not show any colour difference (Fig. 1a) and there was no confirmation for the synthesis of titanium dioxide nanoparticles. Following the reaction of L. acutangula extract with titanium sulfate, the colour was changed into light green during 6 hrs stirred conditional incubation period after which there was no noteworthy changes occurred (Fig. 1b) (Hu et al., 2010; Brown et al., 2012). This characteristic colour variation was because of the excitation of the surface plasmon resonance in the synthesis of TiO2 NPs. Now, the UV–visible absorption of TiO2 nanoparticles were recorded in an optical range from 200 to 600 nm. Fig. 2 represents the UV–visible absorption spectrum of TiO2 nanoparticles. The maximum absorption peak was observed at 322 nm, which was a preliminary sign for the synthesis of TiO2 nanoparticles. In previous research for the green synthesized TiO2 nanoparticles, noticed that the absorption peak was at 342 nm. As contrasted on previous report, the synthesized titanium dioxide nanoparticles show comparative absorption peak (Nithiyavathi et al., 2021).
Visual observation of (a) Titanium sulphate solution with L. acutangula leaves extract (b) Biosynthesized titanium dioxide nanoparticles.
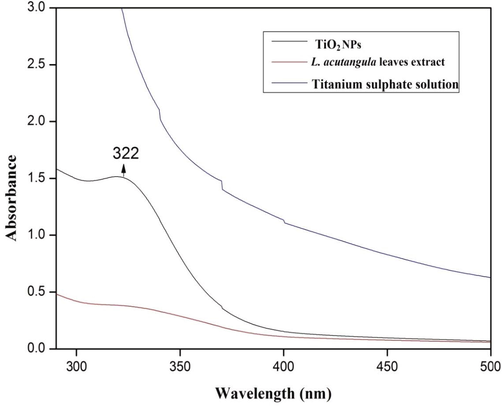
UV–Vis absorption spectra of the synthesized TiO2 NPs, L. acutangula leaves extract and Titanium sulphate solution.
3.2 Functional groups analysis
FTIR technique was performed to analyse the occurrence of functional groups that was the reasonable for the reduction of TiO2 nanoparticles. The results were recorded in the range of 4000 to 400 cm−1 at a resolution of 2 cm−1 in the KBr pelletization technique. Fig. 3 represents the FTIR spectrum of L. acutangula leaves concentrate and TiO2 NPs. The FTIR range of the synthesized TiO2 nanoparticles shows the band intensities in various regions such as 598, 1132, 1381, 1611, and 3393 cm−1 and the peaks were corresponded to the presence of alkyl halides, carboxylic acid, nitro group, amines, and phenol may have taken part during the process of nanoparticle synthesis. The spectral bands were noticeable for L. acutangula leaves extract at 606, 1077, 1399, 1630, and 3401 cm−1 and those were corresponded to the presence of alkyl halides, aliphatic amines, alkanes, amide, and amine. The presence of organic groups were because of titanium particle reduction through organic source (Kirthi et al., 2011; Nithiyavathi et al., 2021; Gong et al., 2007). Consequently, it very well might be expected that the compounds were the capping ligands of the nanoparticles (Allahverdiyev et al., 2011; James and Cohen, 1980; Rajakumar et al., 2012). In the current investigation, the association of titanium dioxide nanoparticles with the biomolecules of L. acutangula leaves extract indicated that the intense peaks at 598, 1132, 1381, 1611, and 3393 cm−1 relative shift in position and intensity distribution were confirmed by FTIR. The primary difference between the spectra was the proper conversion of varied peaks, this occurrence shows that biomolecules in L. acutangula leaves extract such as alkaloids, phenols, saponins, tannins, terpenoids and triterpenoids were responsible for the biotransformation of TiO2 NPs.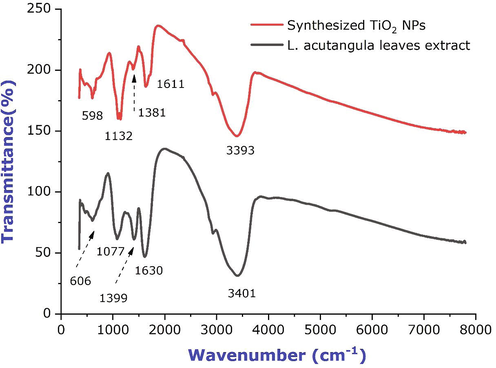
FTIR spectra of L. acutangula leaves extract and synthesized titanium dioxide nanoparticles.
3.3 Structural analysis
The synthesis of TiO2 nanoparticles by L. acutangula leaf extract was examined by XRD estimations. The crystal phase composition of TiO2 NPs were determined by the XRD estimations was done over the diffraction angle (2θ) 10° to 80° utilizing the Cu Kα radiation. The Bragg's reflections at 2θ estimations of 25.47°, 28.30°, 29.51°, 31.65°, 40.42°, 48.98° and 54.69° could be indexed to the (1 0 1), (1 1 0), (2 2 2), (1 0 0), (1 1 1), (2 0 0) and (1 0 5) orientations. These outcomes confirmed the presence of nano-crystalline type of TiO2 nanoparticles (Fig. 4). The principle peak of 2θ = 28.30° matches the (1 1 0) crystallographic plane of rutile type of TiO2 NPs, representing that the nanoparticles structure was in rutile structure when compared with the Joint Committee on powder Diffraction Standards (JCPDS) data (File No. 89-4202) (33). This pattern reflects the shape of the wave elements of the electronic eigenstates of Ti\O\Ti\O chain on the TiO2 (1 1 0)/H2O interface (Anitha and Miruthula, 2014; Dandge et al., 2010). The diffraction patterns were consistent with the planes (1 1 1) and (1 1 0) of pure Face-Centered Cubic (FCC) titanium structure (Vanajothi et al., 2012).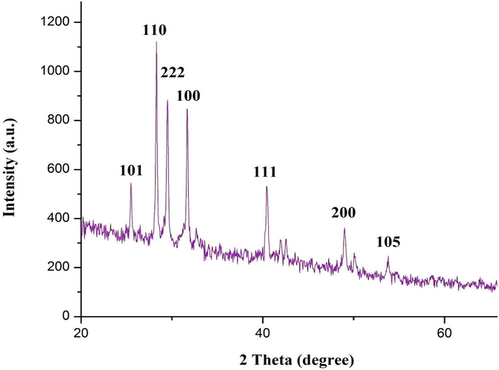
XRD pattern of the synthesized TiO2 NPs.
3.4 SEM-EDX investigation
Several researchers talked about the shape and size of nanoparticles using scanning electron microscopy (Abdelhalim et al., 2012; James and Cohen, 1980). In the current investigation, the SEM examine was carried out to understand the morphology and size distribution of titanium dioxide nanoparticles. Fig. 5a and b represents the SEM picture of TiO2 NPs, the SEM picture indicated that it was made out of NPs mostly aggregates, precise shape and size, which ascribed because of the significant function of L. acutangula leaves extract with the help of biological activity. The particles size decreases inversely correlated to increase the surface to volume ratio (Rajakumar et al., 2012; Hunagund et al., 2016). The synthesized TiO2 NPs were having a hexagonal cluster and the size range of around 10 to 49 nm, which magnified in 10,000×. EDX was utilized to investigate the constituents of elements from the synthesised TiO2 NPs. Fig. 5c represents that the EDX spectrum of TiO2 NPs, the peaks around 0.5 and 0.6 were relevant to the binding energies of titanium and oxygen resembles to the titanium dioxide NPs. The peaks recorded at 7 keV corresponded to the copper grid used for analysis. This outcome confirmed the existence of elemental compounds in the titanium dioxide NPs lacking any impurity peaks and specifies that the synthesized NPs were high purity and the weight and atomic weight percentage (%) was appeared in Fig. 5c.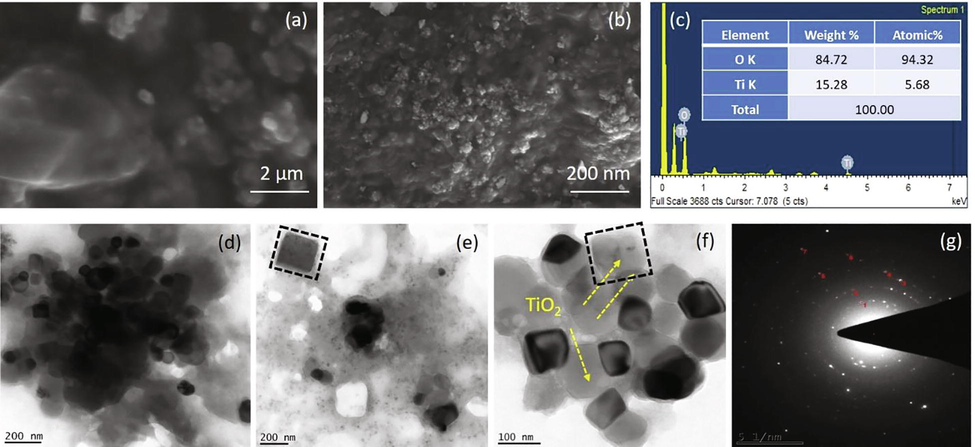
Characterization analysis (a) and (b) SEM micrograph of synthesized TiO2 NPs, (c) Energy-dispersive X-ray spectroscopy exhibiting the chemical components of the synthesized TiO2 NPs, (d)-(f) Transmission electron microscopic analysis of TiO2 NPs, and (g) SAED pattern of TiO2 NPs.
3.5 TEM-SAED examination
TEM is one of the important amazing assets which gives the structure and size information of the nanoparticles. Consequently, the definite shape and size of TiO2 NPs were done with TEM. The TEM picture of TiO2 NPs states that the nanoparticles were hexagonal in shape, with a size range of about 10 to 49 nm, in which not many nanoparticles were agglomerated (Fig. 5d-f). At highest magnification, the TEM micrograph exposed that TiO2 NPs were not in very close physical connection but rather isolated by constant distance with certain divergences. The capping of TiO2 NPs have likewise been seen under TEM micrograph. This capping might be due to the presence of phytochemicals in the extract. Fig. 5g displays the SAED pattern for the synthesised TiO2 NPs. The noticed TiO2 NPs were a constant hexagonal in structure with a diameter ranging from 5 to 7 nm. On the other hand, a diffused rings and a brilliant spot in the SAED pattern specify that the nanoparticles were well crystallized in nature.
3.6 Antibacterial activity of titanium dioxide nanoparticles
The antibacterial activity of TiO2 NPs was evaluated against pathogens by disc diffusion assay such as B. subtilis, E. coli, E. faecalis, K. pneumoniae, S. aureus and P. aeruginosa. The mean of triplicates of obvious zone of inhibition (in millimetres) around each disc was estimated for each of the pathogens (Fig. 6). The capability of the TiO2 nanoparticles to inhibit bacterial growth has been listed in Table 1. Highest zone of inhibition was observed by the highest concentration of titanium dioxide nanoparticles and the maximum zone of inhibition was observed against E. coli (45 ± 0.21), followed by P. aeruginosa (43 ± 0.45), S. aureus (42 ± 0.13), K. pneumoniae (27 ± 0.54), E. faecalis (21 ± 0.41) and B. subtilis (18 ± 0.56) at 40 µg/ml concentration. Colthup et al., reported that TiO2 NPs are the mostly examined for their photocatalytic antimicrobial action among different nanoparticles (Colthup, 1950). Wiley et al., have suggested that the potential mechanisms involving the interactions between nanoparticles and biological molecules (Wiley, 2001). The microbes have a negative charge while metal oxide nanoparticles have a positive charge which creates an electromagnetic reaction between the microbes and treated material surface, once the reaction was created, the microbe was oxidized and finally leads to cell death. The interaction of nanoparticles with phosphorus or sulphur containing compounds like DNA and thiol groups of proteins can causes damage of microbe by inhibition of DNA replication and protein inactivation (Vizhi et al., 2016). They are the reason for the pits in bacterial cell walls, which leads to increased cell permeability and cell death (Philip, 2010). The recent reports were suggested that the metal nanoparticles have a durable electrostatic interaction with the cell wall of the bacteria and causes cell death (Rajakumar et al., 2011; Jayaseelan et al., 2013; Fu et al., 2001). Thus, the antibacterial action of TiO2 NPs possesses excellent ability to be utilized as antibacterial agents against microbes. Results are expressed as mean ± SE.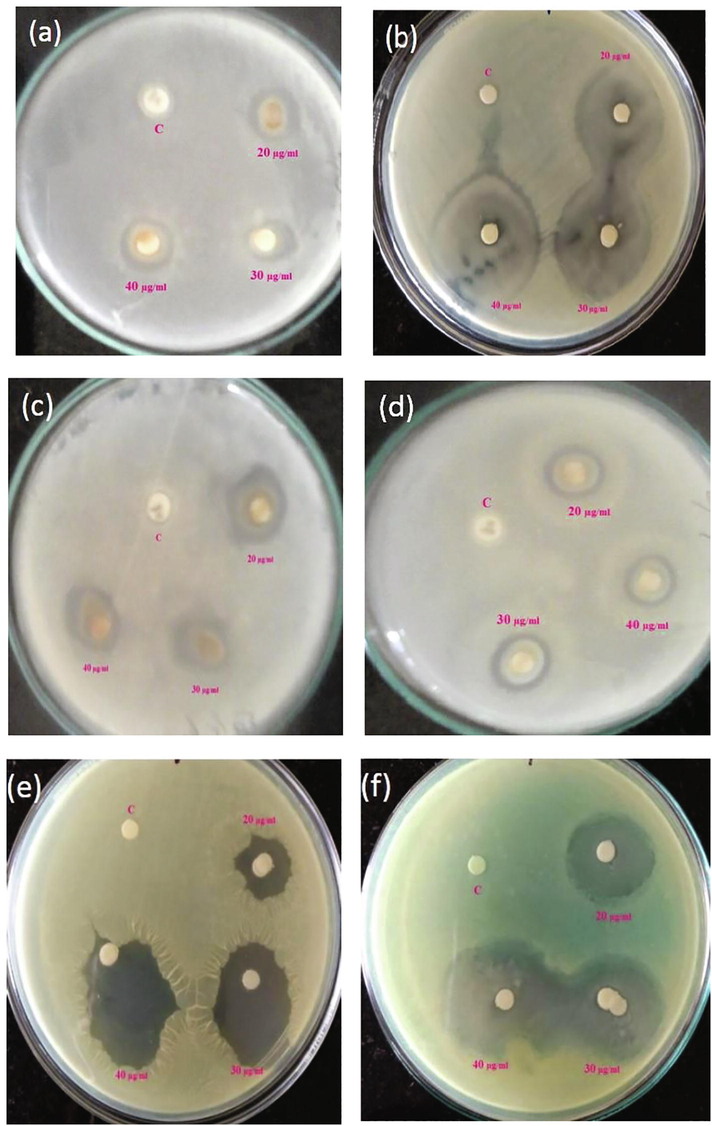
Zone of inhibition observed against (a) B. subtilis (b) E. coli (c) E. faecalis (d) K. pneumonia (e) S. aureus and (f) P. aeruginosa.
Pathogens
Zone of inhibition (mm)
TiO2 NPs (µg/ml)
20
30
40
E. faecalis
13 ± 0.35
18 ± 0.32
21 ± 0.41
B. subtilis
15 ± 0.46
16 ± 0.38
18 ± 0.56
P. aeruginosa
33 ± 0.33
36 ± 0.35
42 ± 0.45
E. coli
35 ± 0.44
42 ± 0.41
45 ± 0.21
S. aureus
21 ± 0.53
33 ± 0.48
42 ± 0.13
K. pneumonia
19 ± 0.23
21 ± 0.54
27 ± 0.54
3.7 Determination of minimal inhibitory concentration
Minimal inhibitory concentration was evaluated by microbroth dilution method using synthesized titanium dioxide nanoparticles suspension against pathogens such as S. aureus, K. pneumoniae, P. aeruginosa, E. faecalis, B. subtilis and E. coli with different concentrations of 100 to 5 µg/ml were tested and the results are given in Table 2. Turbidity within the sample would indicate development of the microorganisms. The lack of turbidity would indicate that the microorganism growth has been inhibited. The outcomes shows that the tested microorganisms were entirely inhibited at 100 to 5 μg/ml concentrations of TiO2 NPs. The MIC of TiO2 NPs against E. coli was the best affect, followed by P. aeruginosa at 5 μg/ml concentration. The moderated effect had been showed by K. pneumoniae and S. aureus and the lower effect was recorded by E. faecalis and B. subtilis at 5 μg/ml concentration. The concentration at 5 μg/ml of TiO2 NPs showed inhibition kinetics against all the tested microorganisms but no significant antibacterial action was observed at concentration less than 5 μg/ml of TiO2 NPs. The bactericidal effect of titanium dioxide has been attributed to the breakdown of bacterial outer cell membranes by the involvement of reactive oxygen species, hydroxyl radicals, which leads to cell death (Dandge et al., 2010). MIC results obtained in our study resembled those reported by Akhtar and colleagues (Salunkhe et al., 2011). The reported results of MIC are higher than those obtained by us in the present investigation, which suggests that the antimicrobial action of TiO2 NPs may be influenced by preparation technique as well as by particle size. The difference in MIC results against the test microorganisms might be due to the strains used. Consequently, we can conclude from the results of this investigation that the TiO2 NPs inhibited the growth and multiplication of all the tested microorganisms. Result are expressed as mean ± SE (N = 3). p < 0.05 statistically significant difference in groups and different letters shows significant different groups revealed by Duncan’s method.
Pathogens
% of cell inhibition
TiO2 NPs (µg/ml)
100
80
60
40
20
10
5
S. aureus
86.75 ± 0.36a
85.30 ± 0.27a
84.33 ± 0.24a
41.66 ± 0.36b
20.56 ± 0.22c
13.37 ± 0.46d
8.54 ± 0.35e
K. pneumoniae
86.70 ± 0.48a
76.09 ± 0.38a
63.91 ± 0.30b
45.00 ± 0.37c
30.13 ± 0.41d
15.56 ± 0.33e
7.23 ± 0.45f
E. coli
83.73 ± 0.49a
73.90 ± 0.57b
64.62 ± 0.18c
53.03 ± 0.25d
33.89 ± 0.37e
18.24 ± 0.36f
3.89 ± 0.26g
B. subtilis
93.06 ± 0.34a
83.57 ± 0.27b
79.82 ± 0.35c
59.74 ± 0.40d
37.39 ± 0.45e
23.34 ± 0.36f
15.56 ± 0.32g
E. faecalis
90.40 ± 0.19a
87.51 ± 0.38a
74.16 ± 0.25b
57.40 ± 0.40c
28.96 ± 0.30d
15.86 ± 0.46e
9.34 ± 0.36f
P. aeruginosa
84.61 ± 0.52a
72.09 ± 0.35a
66.81 ± 0.24b
53.52 ± 0.38c
29.82 ± 0.36d
17.84 ± 0.24e
6.56 ± 0.24f
3.8 Antifungal activity of titanium dioxide nanoparticles
The antifungal action of TiO2 NPs was estimated by measuring the diameter of zone of inhibitions like A. flavus, A. niger, R. oryzae and S. rolfsii. Fig. 7 shows that the obvious zone of inhibition (in millimetres) around each disc and it was estimated for each of the strains. Each concentrations showed zone of inhibition against all the fungal pathogens. The maximum zone of inhibition was shown against S. rolfsii (42 ± 0.25), followed by A. flavus (36 ± 0.56), R. oryzae (34 ± 0.71) and A. niger (27 ± 0.36) at 40 µg/ml concentration (Table 3). Highest zone of inhibition was observed by the highest concentration of TiO2 nanoparticles. Nowadays antimicrobial action of nanoparticles has been attracted interests to develop a new type of method for controlling infections by preventing at preliminary stage and inhibition of spreading of diseases (Sathishkumar et al., 2009). Because of their differential physical, chemical and biological functions of NPs were utilized in drug products, clinical diagnostic imaging and clinical treatments. The presence of inhibition of zone on culture medium shown the antifungal action of titanium dioxide NPs. To increase the inhibition of fungi growth depends upon the concentration of nanoparticles and furthermore concentration of fungal spores (Sundrarajan et al., 2017). As indicated by the investigations TiO2 NPs have antimicrobial efficiency which could be known as a self-cleaning material (Gelover et al., 2006). The antifungal action of tissue conditioner coating material with titanium dioxide photocatalyst reduce the viable fungal cells under UV light exposure (Zhang and Chen, 2009). Results are expressed as mean ± SE.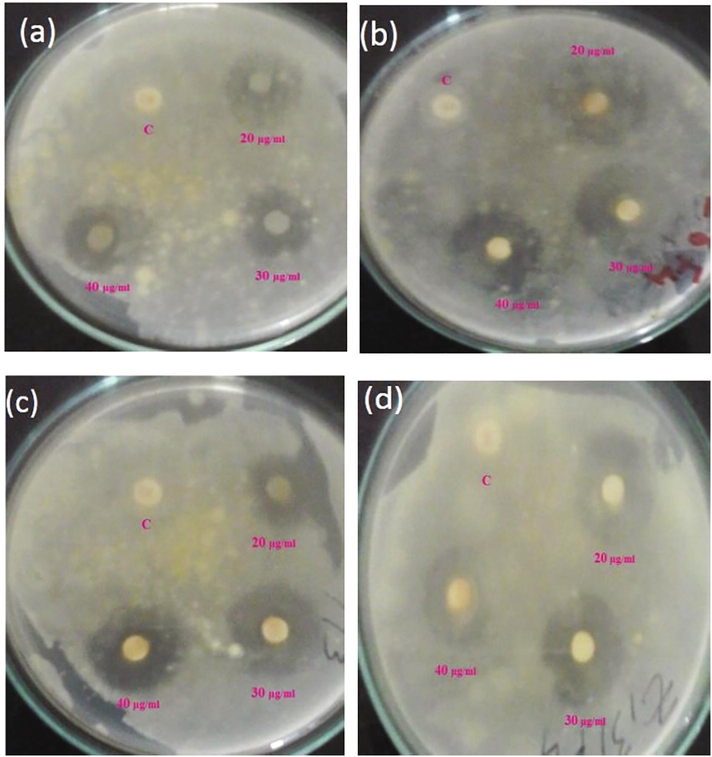
Zone of inhibition observed against different fungal pathogens (a) A. niger, (b) A. flavus, (c) R. oryzae, and (d) S. rolfsii.
Pathogens
Zone of inhibition (mm)
TiO2 NPs (µg/ml)
20
30
40
A. niger
21 ± 0.46
22 ± 0.49
27 ± 0.36
A. flavus
24 ± 0.33
30 ± 0.42
36 ± 0.46
R. oryzae
18 ± 0.35
27 ± 0.33
34 ± 0.31
S. rolfsii
24 ± 0.34
34 ± 0.31
42 ± 0.25
4 Conclusions
The investigation was initiated by the synthesis of TiO2 nanoparticles from L. acutangula leaves extract by biological route. The biological route was an attractive method for obtaining titanium dioxide nanoparticles with high surface region, porosity and monodispersity. The L. acutangula leaves extract was utilized as valuable, reducing agent for the synthesis of TiO2 NPs. This biological reduction of TiO2 NPs would be a boon for the improvement of clean, harmless and environment friendly TiO2 NPs. The synthesised TiO2 NPs were exposed to different characterizations such as UV–visible spectroscopy, FTIR investigation, XRD examination, SEM-EDX investigation and TEM-SAED investigation for their optical properties, functional groups, structure, surface morphology and elemental examinations. These outcomes suggest that they were having crystalline structure and hexagonal shape with the size of around 10–59 nm. Antimicrobial action of the synthesized titanium dioxide nanoparticles were determined against test pathogens. Disc diffusion assay and MIC were commonly used to assess the antimicrobial impacts. The disc diffusion test had been used to evaluate the effectiveness of TiO2 NPs against bacterial strains like E. coli, S. aureus, P. aeruginosa, E. faecalis K. pneumoniae and B. subtilis and the diameter of the bacterial inhibition zone depends upon the solubility and infusibility of the titanium dioxide nanoparticles. Micro broth dilution assay was used to determine the MIC of TiO2 nanoparticles against test bacterial strains. The lack of turbidity in cultural broth indicates the lowest concentration of TiO2 nanoparticles. In antifungal action, utilizing disc diffusion assay against A. niger, A. flavus, R. oryzae and S. rolfsii, the titanium dioxide nanoparticles were effectively inhibited all the test microbes as compared to control. Besides, the ability of the bacterial cell membrane plays a significant role in cell communication, which has closely related to apoptosis. The biological synthesis of TiO2 nanoparticles could be a promising process for production of other metal oxide nanoparticles which could have natural, significant, drug and clinical applications.
Acknowledgements
The authors acknowledge King Saud University, Riyadh, Saudi Arabia for funding this research through Researchers Supporting Project No. RSP 2022/11. The authors thank Sudan University of Science and Technology, Sudan for the support and encouragement.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Physical properties of different gold nanoparticles: ultraviolet-visible and fluorescence measurements. J. Nanomed. Nanotechol.. 2012;3(3):178-194.
- [Google Scholar]
- Alpha amylase assisted synthesis of TiO2 nanoparticles: structural characterization and application as antibacterial agents. J. Hazard. Mater.. 2015;283:171-177.
- [Google Scholar]
- Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surf., B. 2010;81(1):81-86.
- [Google Scholar]
- A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res.. 2016;7(1):17-28.
- [Google Scholar]
- Biosynthesis of silver nanoparticles using almond plant leaf extract and their antibacterial activity. Int. J. Eng. Sci. 2018:19227.
- [Google Scholar]
- Synthesis, characterization and antibacterial activity of titanium dioxide (TiO2) nanoparticles. FUUAST J. Biol.. 2016;6(2):141-147.
- [Google Scholar]
- Antifungal effects of a tissue conditioner coating agent with TiO2 photocatalyst. Journal of medical and dental sciences. 2005;52(4):223-227.
- [Google Scholar]
- Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol.. 2011;6(8):933-940.
- [Google Scholar]
- [EMIM] BF4 ionic liquid-mediated synthesis of TiO2 nanoparticles using Vitex negundo Linn extract and its antibacterial activity. J. Mol. Liq.. 2016;221:986-992.
- [Google Scholar]
- Traditional medicinal uses, phytochemical profile and pharmacological activities of Luffa acutangula Linn. Int. J. Pharmacog.. 2014;1(3):174-183.
- [Google Scholar]
- Antimicrobial photodynamic activity of toluidine blue-carbon nanotube conjugate against Pseudomonas aeruginosa and Staphylococcus aureus-understanding the mechanism of action. Photodiagn. Photodyn. Ther.. 2019;27:305-316.
- [Google Scholar]
- Nanoparticles functionalized with ampicillin destroy multiple-antibiotic-resistant isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and methicillin-resistant Staphylococcus aureus. Appl. Environ. Microbiol.. 2012;78(8):2768-2774.
- [Google Scholar]
- Antimicrobial activity of biosilver nanoparticles produced by a novel Streptacidiphilus durhamensis strain. J. Microbiol. Immunol. Infect.. 2018;51(1):45-54.
- [Google Scholar]
- Vapor-phase hydrothermal synthesis of rutile TiO2 nanostructured film with exposed pyramid-shaped (1 1 1) surface and superiorly photoelectrocatalytic performance. J. Colloid Interface Sci.. 2014;429:53-61.
- [Google Scholar]
- Synthesis and characterization of TiO2 nanoparticles by using new shape controllers and its application in dye sensitized solar cells. J. Ind. Eng. Chem.. 2014;20(6):4039-4044.
- [Google Scholar]
- Antimicrobial activity and pharmacognostic study of Luffa acutangula Roxb var amara on some deuteromycetes fungi. Int. J. Sci. Innovat. Discov.. 2010;2(1):191-196.
- [Google Scholar]
- Green chemistry for nanoparticle synthesis. Chem. Soc. Rev.. 2015;44(16):5778-5792.
- [Google Scholar]
- Location and catalytic role of iron species in TiO2: Fe photocatalysts: an EPR study. J. Photochem. Photobiol., A. 2010;211(2–3):170-175.
- [Google Scholar]
- Morphology and characterization of TiO2 nanoparticles synthesized by arc discharge. Chem. Phys. Lett.. 2012;521:86-90.
- [Google Scholar]
- Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ. Sci. Technol.. 2009;43(5):1354-1359.
- [Google Scholar]
- Surface second order optical nonlinearity of titanium dioxide sized in nanometer range. Chem. Lett.. 2001;30(4):328-329.
- [Google Scholar]
- High performance photo-catalyst based on nanosized ZnO–TiO2 nanoplatelets for removal of RhB under visible light irradiation. J. Adv. Microsc. Res.. 2018;13(1):12-19.
- [Google Scholar]
- Synthesis of plant-mediated silver nanoparticles using Trianthema decandra extract and evaluation of their antimicrobial activities. Int. J. Eng. Sci. Technol.. 2010;2(5):970-975.
- [Google Scholar]
- Assessment of the skin photoprotective capacities of an organo-mineral broad-spectrum sunblock on two ex vivo skin models. Photodermatol. Photoimmunol. Photomed.. 2003;19(5):242-253.
- [Google Scholar]
- A practical demonstration of water disinfection using TiO2 films and sunlight. Water Res.. 2006;40(17):3274-3280.
- [Google Scholar]
- Photocatalytic effect of CuO nanoparticles flower-like 3D nanostructures under visible light irradiation with the degradation of methylene blue (MB) dye for environmental application. Environ. Res.. 2022;203
- [Google Scholar]
- Titanium dioxide (TiO2) nanoparticles filled poly (D, L lactid acid)(PDLLA) matrix composites for bone tissue engineering. J. Mater. Sci. - Mater. Med.. 2007;18(7):1287-1298.
- [Google Scholar]
- Preparation and antibacterial activity of Fe3O4@ Ag nanoparticles. Nanotechnology. 2007;18(28):285604
- [Google Scholar]
- Diffusion of nanoparticles in biofilms is altered by bacterial cell wall hydrophobicity. Appl. Environ. Microbiol.. 2011;77(1):367-368.
- [Google Scholar]
- Environmental applications of semiconductor photocatalysis. Chem. Rev.. 1995;95(1):69-96.
- [Google Scholar]
- Interaction of silver (I) ions with the respiratory chain of Escherichia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemistry. 2005;44(39):13214-13223.
- [Google Scholar]
- Nanoparticle-assisted combination therapies for effective cancer treatment. Therapeutic Delivery. 2010;1(2):323-334.
- [Google Scholar]
- Biogenic and chemogenic synthesis of TiO2 NPs via hydrothermal route and their antibacterial activities. RSC Adv.. 2016;6(99):97438-97444.
- [Google Scholar]
- The measurement of residual stresses by X-ray diffraction techniques. In: Treatise on Materials Science & Technology. 1980. p. :1-62.
- [Google Scholar]
- Electrical and magnetic properties of Ni doped CeO2 nanostructured for optoelectronic applications. J. Phys. Chem. Solids. 2022;160:110369
- [Google Scholar]
- Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind. Crops Prod.. 2013;45:423-429.
- [Google Scholar]
- Biological approach to synthesize TiO2 nanoparticles using Aeromonas hydrophila and its antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2013;107:82-89.
- [Google Scholar]
- Photodegradation of organic pollutants RhB dye using UV simulated sunlight on ceria based TiO2 nanomaterials for antibacterial applications. Sci. Rep.. 2016;6(1):1-12.
- [Google Scholar]
- Synthesis and characterization of nano-hydroxyapatite/graphene oxide composite materials for medical implant coating applications. Mater. Today Proc.. 2021;36:204-207.
- [Google Scholar]
- Adsorption of copper and nickel by using sawdust chitosan nanocomposite beads–A kinetic and thermodynamic study. Environ. Res.. 2022;203:111814
- [Google Scholar]
- Biosynthesis of titanium dioxide nanoparticles using bacterium Bacillus subtilis. Mater. Lett.. 2011;65(17–18):2745-2747.
- [Google Scholar]
- Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano. 2014;8(10):10682-10686.
- [Google Scholar]
- Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Water Res.. 2005;39(5):877-883.
- [Google Scholar]
- In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front. Microbiol.. 2018;9:1555.
- [Google Scholar]
- Photocatalytic decomposition effect of erbium doped cerium oxide nanostructures driven by visible light irradiation: investigation of cytotoxicity, antibacterial growth inhibition using catalyst. J. Photochem. Photobiol., B. 2018;185:275-282.
- [Google Scholar]
- Synthesis and characterization of TiO2 doped cobalt ferrite nanoparticles via microwave method: investigation of photocatalytic performance of congo red degradation dye. Surf. Interfaces. 2021;25:101296
- [Google Scholar]
- Studies on the spectrometric analysis of metallic silver nanoparticles (Ag NPs) using Basella alba leaf for the antibacterial activities. Environ. Res.. 2021;199:111274
- [Google Scholar]
- Systematic green synthesis of silver oxide nanoparticles for antimicrobial activity. Environ. Res.. 2021;202:111627
- [Google Scholar]
- A novel biogenic Allium cepa leaf mediated silver nanoparticles for antimicrobial, antioxidant, and anticancer effects on MCF-7 cell line. Environ. Res.. 2021;198:111199
- [Google Scholar]
- Feasibility studies on avocado as reducing agent in TiO2 doped with Ag2O and Cu2O nanoparticles for biological applications. J. Bionanosci.. 2018;12(5):652-659.
- [Google Scholar]
- Development and characterization of alginate/chitosan nanoparticulate system for hydrophobic drug encapsulation. J. Drug Delivery Sci. Technol.. 2019;52:65-72.
- [Google Scholar]
- Gum mediated synthesis and characterization of CuO nanoparticles towards infectious disease-causing antimicrobial resistance microbial pathogens. J. Infect. Public Health. 2021;14(12):1893-1902.
- [Google Scholar]
- Nanotechnology in plant disease management: DNA-directed silver nanoparticles on graphene oxide as an antibacterial against Xanthomonas perforans. ACS Nano. 2013;7(10):8972-8980.
- [Google Scholar]
- Atomic layer deposition of mixed-layered Aurivillius phase on TiO2 nanotubes: synthesis, characterization and photoelectrocatalytic properties. Nanomaterials. 2020;10(11):2183.
- [Google Scholar]
- Self-organization of layered perovskites on TiO2 nanotubes surface by atomic layer deposition. Mater. Today Proc.. 2021;36:364-367.
- [Google Scholar]
- Effect of Ag doped MnO2 nanostructures suitable for wastewater treatment and other environmental pollutant applications. Environ. Res.. 2022;205:112560
- [Google Scholar]
- Antimicrobial photodynamic inactivation of fungal biofilm using amino functionalized mesoporus silica-rose bengal nanoconjugate against Candida albicans. Sci. African. 2018;1:e00007
- [Google Scholar]
- Antimicrobial photodynamic activity of toluidine blue encapsulated in mesoporous silica nanoparticles against Pseudomonas aeruginosa and Staphylococcus aureus. Biofouling. 2019;35(1):89-103.
- [Google Scholar]
- P. Parasuraman, V.T. Anju, S.B. Sruthil Lal, Alok Sharan, Siddhardha Busi, K. Kaviyarasu, Mohammed Arshad, Turki M.S. Dawoud, Asad Syed, Synthesis and antimicrobial photodynamic effect of methylene blue conjugated carbon nanotubes on E. coli and S. aureus, Photochem. Photobiol. Sci. 18 (2), 563-576, 2019.
- Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2010;77(4):807-810.
- [Google Scholar]
- Synthesis of TiO2 nanoparticles with mesoporous spherical morphology by a wet chemical method. Mater. Lett.. 2012;82:208-210.
- [Google Scholar]
- One-pot microwave-assisted combustion synthesis of graphene oxide–TiO2 hybrids for photodegradation of methyl orange. J. Alloy. Compd.. 2013;551:382-388.
- [Google Scholar]
- Bio-synthesis of iron oxide nanoparticles using neem leaf cake extract and its influence in the agronomical traits of vigna mungo plant. Asian J. Nanosci. Mater.. 2020;3(1):38-46.
- [Google Scholar]
- Eclipta prostrata leaf aqueous extract mediated for the synthesis of titanium dioxide nanoparticles and its larvicidal activity against malaria vector. In: 2011 IEEE Nanotechnology Materials and Devices Conference. IEEE; 2011. p. :563-566.
- [Google Scholar]
- Eclipta prostrata leaf aqueous extract mediated synthesis of titanium dioxide nanoparticles. Mater. Lett.. 2012;68:115-117.
- [Google Scholar]
- Effect of doping concentration for the properties of Fe doped TiO2 thin films applications. Mater. Today Proc.. 2021;36:468-474.
- [Google Scholar]
- Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill and evaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. J. Photochem. Photobiol., B. 2019;201:111667
- [Google Scholar]
- Investigation of structural and optical properties of NiO nanoparticles mediated by Plectranthus amboinicus leaf extract. Mater. Today Proc.. 2021;36:268-272.
- [Google Scholar]
- Synthesis of Mn3O4 nano complex using aqueous extract of Helianthus annuus seed cake and its effect on biological growth of Vigna radiata. Mater. Today Proc.. 2021;36:184-191.
- [Google Scholar]
- Shockwave treated seed germination and physiological growth of Vigna mungo (L) in red soil environment. Physiol. Mol. Plant Pathol.. 2022;117:101747
- [Google Scholar]
- Stalling behaviour of chloride ions: a non-enzymatic electrochemical detection of α-Endosulfan using CuO interface. Sens. Actuators, B. 2019;293:100-106.
- [Google Scholar]
- Biosynthesis of silver nanoparticles using Phyllanthus emblica fruit extract for antimicrobial application, Biocatalysis and Agricultural. Biotechnology. 2020;24:101567
- [Google Scholar]
- Efficient phyto-synthesis and structural characterization of rutile TiO2 nanoparticles using Annona squamosa peel extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2012;98:86-90.
- [Google Scholar]
- Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera; Culicidae) Parasitol. Res.. 2011;109(3):823-831.
- [Google Scholar]
- Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf., B. 2009;73(2):332-338.
- [Google Scholar]
- Antioxidant and antibacterial profiling of pomegranate-pericarp extract functionalized-zinc oxide nanocomposite. Biotechnol. Bioprocess Eng.. 2021;26(5):728-737.
- [Google Scholar]
- Biogenic metal nanoparticles and their antimicrobial properties. In: Nanotechnological Approaches in Food Microbiology. CRC Press; 2020. p. :403-413.
- [Google Scholar]
- Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. J. Photochem. Photobiol., B. 2017;171:117-124.
- [Google Scholar]
- Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res.. 2009;69(22):8784-8789.
- [Google Scholar]
- Luffa acutangula and Lippia nodiflora leaf extract induces growth inhibitory effect through induction of apoptosis on human lung cancer cell line. Biomed. Prevent. Nutr.. 2012;2(4):287-293.
- [Google Scholar]
- Ultrastructural analysis of Candida albicans when exposed to silver nanoparticles. PLoS ONE. 2014;9(10):e108876
- [Google Scholar]
- Evaluation of Catharanthus roseus leaf extract-mediated biosynthesis of titanium dioxide nanoparticles against Hippobosca maculata and Bovicola ovis. Parasitol. Res.. 2012;111(6):2329-2337.
- [Google Scholar]
- Green synthesis of gold nanoparticle using Eclipta alba and its antidiabetic activities through regulation of Bcl-2 expression in pancreatic cell line. J. Drug Delivery Sci. Technol.. 2020;58:101786
- [Google Scholar]
- Bioengineered gold nanoparticles using Cynodon dactylon extract and its cytotoxicity and antibacterial activities. Bioprocess Biosyst. Eng.. 2021;44(6):1253-1262.
- [Google Scholar]
- Evaluation of antibacterial activity and cytotoxic effects of green AgNPs against Breast Cancer Cells (MCF 7) Adv. Nano Res.. 2016;4(2):129.
- [Google Scholar]
- Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol.. 2012;46(4):2242-2250.
- [Google Scholar]
- Wiley, J., 2001. Sons 1(11). New York; NY: Inc.
- Nanoparticle–enzyme hybrid systems for nanobiotechnology. FEBS J.. 2007;274(2):302-309.
- [Google Scholar]
- Synthesis and optical absorpition properies of anatase tio2 nanoparticles via a hydrothermal hydrolysis method. Rare Metal Mater. Eng.. 2015;44(5):1067-1070.
- [Google Scholar]
- Adsorption and electronic states of morin on TiO2 nanoparticles. Chem. Phys.. 2014;443:61-66.
- [Google Scholar]
- Potent antibacterial activities of Ag/TiO2 nanocomposite powders synthesized by a one-pot sol− gel method. Environ. Sci. Technol.. 2009;43(8):2905-2910.
- [Google Scholar]
- Control over the morphology of TiO2 hierarchically structured microspheres in solvothermal synthesis. Mater. Lett.. 2015;158:174-177.
- [Google Scholar]







