Translate this page into:
Green synthesis of iron nanoparticles from Ulva lactuca and bactericidal activity against enteropathogens
⁎Corresponding author. bensybotany2000@gmail.com (Asha D.V. Bensy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Antimicrobial resistance has emerged as one of the major public-health issues in recent times. The multi-drug resistant opportunistic bacterial pathogens, Staphylococcus aureus, Escherichia coli, and Salmonella typhimurium cause urinary tract, respiratory infections and diarrhea. The prevalence in the hospital environment increases multi-drug resistant bacteria and beta-lactam antibiotics (carbapenems) are highly effective against these pathogens, however development of drug resistance against these antibiotics may lead to increased mortality. Hence, it is of utmost significance to develop alternative antibiotics against these drug-resistant bacteria. In this study, we have synthesized iron nanoparticles using the water extract of marine algae (Ulva lactuca) collected from Tamilnadu coast, India. The nanoparticles were characterized by UV-spectroscopy, transform infrared spectroscopy, and Scanning electron microscopy. The green synthesized nanoparticles have the size of 30–40 nm and have potent biological activities. The synthesized nanoparticles showed anticancer activity against HeLa and DLD-1 cell lines. The methanol extract of U. lactuca showed maximum activity against E. coli (24 ± 2 mm), followed by S. typhimurium (23 ± 1 mm), B. cereus (19 ± 1 mm), P. vulgaris (17 ± 2 mm), and S. aureus (16 ± 2 mm). Antibacterial disc diffusion analysis of nanoparticles showed improved activites than algal extract. The iron nanoparticles showed activity against S. aureus (24 ± 1 mm), E. coli (29 ± 1 mm), and S. typhimurium (31 ± 2 mm). The minimum inhibitory concentration values of nanoparticles against diarrhoea causing bacteria varied widely. U. lactuca extract was highly effective against S. typhimurium (25 µg/mL), followed by E. coli (30 µg/mL), and S. aureus (45 µg/mL). These results clearly demonstrate that iron nanoparticles could be developed as alternate therapeutics against carbapenems resistant bacteria and against types of cancers.
Keywords
Marine algae
Aqueous extract
Capping agent
Iron nanoparticles
Antibacterial
Anticancer
1 Introduction
The natural and synthetic metals, polymers and metallic alloys offer various explicit properties and make them smart for various biomedical applications (Vijaya et al., 2017). Metal nanoparticles have remarkable applications in the areas of catalysis, optoelectronics, environmental applications, diagnostic biological probes and in various devices (Wagner et al., 2004). These nanoparticles have widespread application and these metal nanoparticles have unique properties (Thomas et al., 2019). Meal nanoparticles are generally synthesized using chemical and physical processes, which allow making them with desired properties. The methods such as, oxidation, deposition precipitation, anodization, convenstional heating and hydrothermal methods are being used to synthesize the metal nanoparticles (Badineni et al., 2021). Moreover, these synthesis methods are generally labour-intensive and expensive and are hazarouds to the living organisms and to the natural environment. Green synthesis of nanoparticles has several advantages over physical and chemical method as it is eco-friendly, cost-effective and easily scalable for batch production and it does not require toxic chemicals, high temperature and energy. Green synthesis of nanoparticles offer controlled growth, crystal growth, better influence and improved stability (Valsalam et al., 2019; Anand et al., 2020; Mani et al., 2021; Ezhilarasi et al., 2016). The green synthesized nanoparticles have antibacterial, antifungal (Valsalam et al., 2019; Renuka et al., 2020), cytotoxic (Ezhilarasi et al., 2016; Kaviyarasu et al., 2017), antimicrobial and photochemical (Raja et al., 2018; Sathiyaraj et al., 2021; Jayaprakash et al., 2017) applications. Green synthesis of iron nanoparticles employing either plant extracts or biological samples has emperged as an alternative and simple to costly chemical synthesis (Kasinathan et al., 2016; Kennedy et al., 2014; Prakash et al., 2016; Panimalar et al., 2022). Plant extracts-mediated green synthesis of nanoparticles can be several advances compared with biological samples as it does not require any aseptic environment and culture methods (Dhivahar et al., 2020). Several studies were performed on the green synthesis of iron nanoparticles in recent years (Anand et al., 2020; Mani et al., 2021; Ezhilarasi et al., 2016).
Macroalgae or sea weeds are important natural resources and are used in various applications. Macroalgae play a critical role in the development of novel molecules and they are widely consumed traditioanally and are well known for their nutritional and antibacterial properties. Among various bioactive molecules, pigments, fatty acids, polyphenols, polysaccharides, proteins and peptides are important molecules (Andryukov et al., 2019). The chemical composition of macroalgae is affected by various factors including, seasonal variation, geographical place, environmental conditions and species and developmental stages. Seaweeds are potential source of various bioactive molecules including, pigments, polyphenols, peptides and proteins (Rengasamy et al., 2019). The development of drug resistance among bacteria is increasing in recent times and this drug resistance of pathogenic bacteria is a severe concern of public health management. Hence it is required to develop alternative medicines from natural sources like macro algae to find the alternative lead molecules. These natural sources have little or no side effects when compared with the synthetic antibiotic molecules (Al-Ansari et al., 2021). From seaweeds, a variety of molecules have been extracted and these molecules have antimicrobial and synergistic activities. Macro algae are the rich sources of polyphenolic compounds and these polyphenols contain various molecules including phlorotannins. In addition with these molecules, macroalgae are potent sources of flavonols, bromophenols, and catechins. Polyphenols from medicinal plants have antimicrobial properties (Wu et al., 2020; Al-Dhabi et al., 2020; Malar et al., 2019).
Macroalgae contain various naturally avialable pigments including, chlorophylls, phycobilins and carotenoids. Phycobiliproteins are one of the pigments detected in seaweeds and is water soluble in nature. In the case of peptides and proteins, their antibacterial properties are mainly associated with their amphiphilic nature, which effectively allows them to interact with non-polar and polar sites of the membranes of the bacteria. The binding interaction leads to the development of pores on the surface, causing severe cell wall disruption and rupture of cell membrane (Arokiyaraj et al., 2014; Ilavenil et al., 2016; Jans et al., 2020). The secondary metabolites from the algae showed antibactieral activity against bacteria such as, B. subtilis, S. typhi, K. pneumoniae, and P. aeruginosa (Valsalam et al., 2019). Iron nanoparticles are effective against bacterial and fungal pathogens because of their catalytic, magnetic, thermal and electrical characteristics (Arasu et al., 2019). Moreover, iron nanoparticles show reactivity, requiring more stabilization after or during the synthesis of nanoparticles to prevent aggregation and oxidation of nanoparticles over time. Although many stabilizing agents are proposed, the use of marine macro algae has various advantages, because of the presence of polysaccharides, and gum effectively control the size of nanoparticles. The main aim of this study is to determine anticancer activity of green synthezied nanoparticles and to study antibacterial potential against carbapenems resistant bacteria.
2 Materials and methods
2.1 Macroalga
The macroalga, Ulva lactuca (green algae) was selected based on availability throughout the year and the traditional knowledge of its biological activities. It was collected from South East coast of India at depth range between 1 and 5 m. The selected seaweed was characterized using the manual authenticated by Botanical Survey of India (BSI/SRC/5/23/2020/Tech/885).
2.2 Algal extract preparation
The collected seaweed was washed, dried and powdered using a mechanical grinder. To the five gram dried powder, the solvent such as, ethanol, ethyl acetate, acetone, chloroform and methanol were added and kept for over night. The Erlenmeyer flask was placed on a rotary shaker incubator (30 ± 2 °C) at 150 rpm and the sample was filtered using whatman’s no 1 filter paper. The filtrate was dried under reduced pressure using an evaporator and the dried powder was used for phytochemical and antibacterial screening.
2.3 Phytochemical screening
Phytochemicals such as, flavonoids, alkaloids, phenolic compounds, tannins, steroids, saponins, terpenoids, and glycosides were assayed with minor modifications (Yadav et al., 2014). Iron chloride test (III) was used for the determination of tannins. Aluminum chloride test was applied for flavonoids determination. Acetic anhydride test was used for the determination of terpenoids, and sulphuric acid test was used for the determination of steroids. Dragendorff method was used for the determination of alkaloids (Ganame et al., 2021).
2.4 Green synthesis of iron nanoparticles
The green seaweed sample was washed with tap water, followed by double distilled water (Fig. 1A), and it was ground mechanically (Fig. 1B). The finely ground algal powder was stored at −20 °C. About 5 g freeze dried sample was boiled with 500 mL double distilled water in an Erlenmeyer flask and stirred continuously for 30 min. The algal extract was cooled to room temperature, filtered using a vaccum filtration unit and stored at −20 °C. Ferric sulphate (FeSO4) was used for the preparation of iron nanoparticles. Iron nanoparticles were prepared by mixing 25 mL algal extract and 25 mL 0.1 M FeCl3 and the mixture was stand for 2 h at ambient temperature (30 ± 2 °C). The final suspensions were further centrifuged and washed with ethanol (three times) and then dried at 40 °C and nanoparticles were obtained. The aqeous algal extract acts as reducing, stabilizing and capping agent in iron nanoparticles synthesis.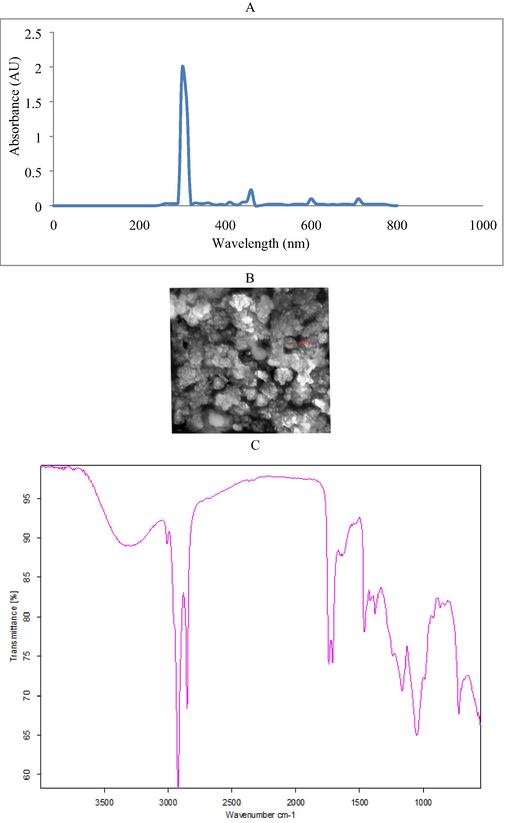
UV–Visible spectrophotometry analysis of green synthesized iron NPs (A), SEM image of iron NPs (B) and FT-IR spectrum of green synthesized iron NPs (C).
2.5 Characterization of NPs
The green synthesized nanoparticles were subjected to FT-IR analysis. FT-IR analysis of sample was prepared as KBr Disc was carried out over 4000–400 cm−1 resolution on a Perkin Elmer Spectrum 65 FT-IR Spectrometer. The morphology of the nanoparticles was characterized using a Scanning Electron Microscopy (JEOL-MODEL 6390) after coating on copper grid for 5 min on mercury lamp.
2.6 Anticancer activity of nanoparticles
The drug resistant pattern of the bacterial strains was determined using various commercial antibiotics. These bacteria were cultured in nutrient broth medium and incubated at 37 °C for 18 h. After 18 h, it was cultured on Mueller Hinton Agar plates and the selected antibiotics were placed on it. These include ampicillin, cotrimoxazole, aztreonam, ciprofloxacin, amoxicillin, oxacillin, and penicillin. The plates were incubated for 24 h and the antibiotic sensity was analyzed. The selected strains were resistant against more than three antibiotics and considered as drug resistance (Wang et al., 2020; Kalaiyarasi et al., 2020; Baazeem et al., 2021). HeLa and DLD-1 cell lines were procured from National Centre for Cell Sciences (NCCS), Pune, India and maintained in Dulbecco’s modified Eagles medium (DMEM) (Sigma-Aldrich, USA). It was cultured in tissue culture flask with DMEM medium containing antibiotics (Amphoteracin B – 2.5 µg/mL; Streptomycin – 100 µg/mL; Penicillin – 100 U/mL). These were maintained in a humidified 5% CO2 incubator at 37 °C. The nanoparticle was suspended in 1 mL DMEM and vortexed using a cyclomixer. It was filter sterilized using 0.22 µm syringe filter. Then the NPs were serially diluted in 500 µL of DMEM (100 µg, 50 µg, 25 µg, 12.5 µg, 6.25 µg) and loaded in the corresponding wells. After 24 h treatment, inverted phase contrast microscope was used to detect the morphology of the cells, including granulation, shrinking of cells and vacuolization in the cytoplasm of the cells. MTT assay method was used for the determination of anticancer property of iron NPs. The percentage of growth inhibition was calculated using the formula:
2.7 Anti-diarrheal activity of algal extract and nanoparticles
The bacteria used were, E. coli, S. typhimurium, B. cereus, P. vulgaris and S. aureus for antibacterial assay. These bacterial strains were cultured individually in nutrient broth medium (Himedia, Mumbai, India). The growth was monitored continuously at 600 nm using a UV–Visible spectrophotometer. The methanol fraction of macroalga was evaluated for antibacterial activity against carbapenems resistant bacteria. The methanol extract showed maximum phytochemical components, hence it was selected. Antibacterial plates were prepared using MHA medium. About 20 µL sample was loaded and incubated for 37 ± 1 °C in an incubator and antibacteiral activity was assayed in mm zone of inhibition. Broth dilution method was selected for the determination of minimum inhibitory concentration (MIC). The extract tubes were inoculated with 0.1 mL inoculum (3 × 107 CFU/mL) and incubated at 37 °C for 24 h. The visible growth was analyzed after incubation and the MIC value was determined (Al-Dhabi et al., 2020; Al-Ansari et al., 2021).
3 Results
3.1 Phytochemical screening
The algal extract of U. lactuca L. showed the presence of various phytochemicals and the result was described in Table 1. The ethanol extract U. lactuca showed the presence of alkaloids, phenolic compounds, tannins, saponins and glycoside. In methanol extract almost all the tested phytochemicals were determined except glycosides. In acetone extract six phytochemicals were present but only two phytochemicals (flavonoids and glycosides) were absent (Table 1). + present; − absent.
Phytochemicals
Solvents
Ethanol
Ethyl acetate
Chloroform
Methanol
Acetone
Flavonoids
−
+
+
+
−
Alkaloids
+
+
+
+
+
Phenolic compounds
+
−
−
+
+
Tannins
+
−
−
+
+
Steroids
−
−
+
+
+
Saponins
+
−
+
+
+
Terpenoids
−
+
−
+
+
Glycosides
+
−
−
−
−
3.2 Characterization of nanoparticles
The aqueous extract of seaweed consists of iron precursor and it induced sudden change in the colour of the mixture and dark black suspension was observed. Instantaneous colour change was observed and the parameters such as initial aqueous extract concentration, optimum concentration of iron, reaction time were important factors. The required level of iron sulphate solution and aqueous extract was optimized. The characteristic peak between 275 and 325 nm indicated the presence of iron NPs in the reaction mixture (Fig. 1A). The diameter of the NPs synthesized using U. lactuca ranged between 20 and 40 nm in size (Fig. 1B). The analyzed NPs were uniformed size and the determined particle was spherical in shape. As seen in the Fig. 1B, the shape of the particles was almost spherical. Almost all particles were regular shape and some irregular shaped particles were also observed. These irregular shapes were analyzed because of the agglomeration process related to the Van der Waals forces and magnetic attraction among Fe-NPs. The FTIR spectrum of green synthezied nanoparticles using aqueous extracts of U. lactuca was described in Fig. 1C. The green synthesized iron NPs showed the presence of aliphatic nitro compound, alcohol, carbonyl group, alcohol group, methylene group, aromatic C–H, venyl and ether groups. The signals at 3229.25 cm−1 (OH stretching), 3039 cm−1 (CH2 stretching) were observed. The iron NPs exhibited intense and sharp peaks at about 3000 cm−1 and 1700 cm−1 due to the presence of methylene group and carbonyl group (Table 2).
Wave number (cm−1)
Components (peak)
Functional groups
3467
O–H
Alcohol group
3039
CH2
Methylene group
2908.7
C–H
Alkenes
2798.1
O–H
Aliphatic
2312
Si-H
Silica
1718.7
C=O
Carbonyl group
1636.5
C=O
Carbonyl group
1563.1
N–H
Amine
1531.2
N–H
Amine
1070
CH2
Methylene group
1375.9
OH
Phenyl group
1250.5
C-O
Ether
1109
C-O
Ether
937.5
C–H
Venyl
968.7
C–H
Venyl
875
C–H
Venyl
850
C-Cl
Aliphatic chlorine
3.3 Anticancer activites of iron NPs
Anticancer activity was performed for the determination of viability and proliferation of the cells and used to evaluate the cytotoxic potential of nanoparticles. The viability of cells reduced at increasing nanoparticles concentration. Higher extract concentrations exhibited stronger anticancer potential. The LC50 value (samples needed for the 50% of cell death) for iron nanoparticles was 157.366 µg/mL. Results indicated that nanoparticles decreased cells viability at increasing concentrations than lower doses (Fig. 2). The anticancer activity of iron nanoparticles against HeLa cells and DLD-1 were performed. The cancer cell lines treated with nanoparticles showed noticeable cytotoxicity at higher concentrations such as 50 µg/mL and 100 µg/mL. Biosynthesized iron nanoparticles using algal extract exhibited potent anticancer activity against HeLa cells (Fig. 3) and DLD-1 (Fig. 4).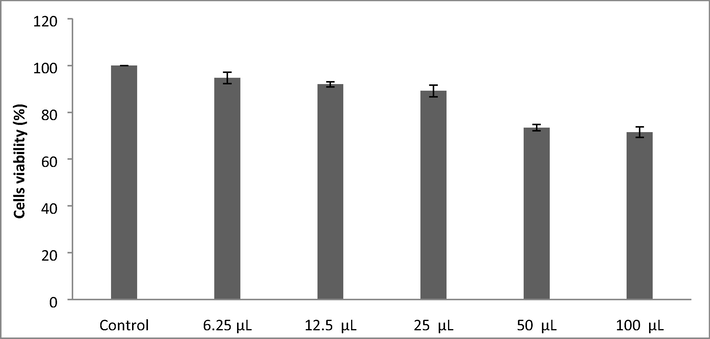
MTT assay for the determination of LC50 value of iron NPs.
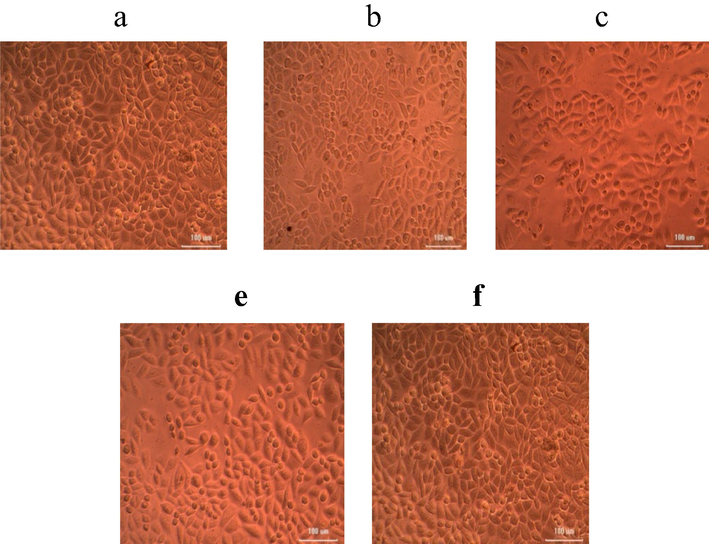
Anticancer activity of iron NPs synthesized using U. lactnca extract against HeLa cells at various concentrations of nanoparticles (a) 6.25 µg; (b) 12.5 µg; (c) 25 µg; (d) 50 µg; (e) 100 µg.
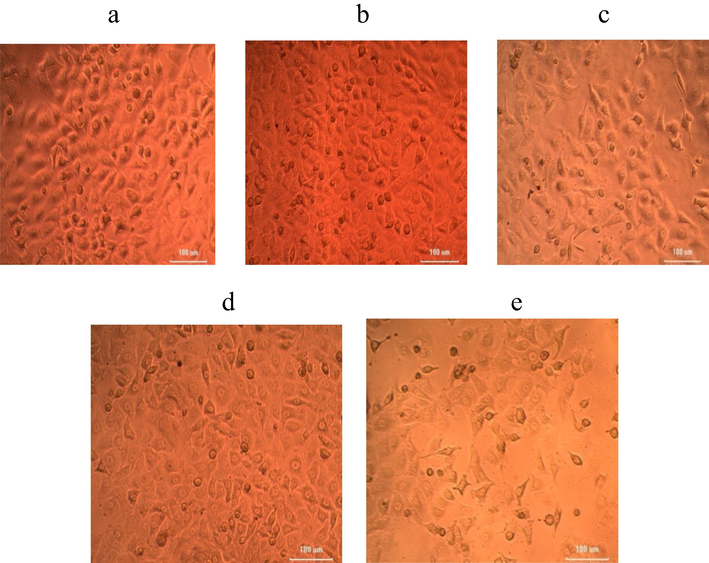
Anticancer activity of iron NPs synthesized using U. lactnca extract against DLD-1 cell lines at various concentrations of nanoparticles (a) 6.25 µg; (b) 12.5 µg; (c) 25 µg; (d) 50 µg; (e) 100 µg.
3.4 Anti-diarrheal activity of macroalgae extract and iron nanoparticles
U. lactuca extract showed maximum activity against E. coli (24 ± 2 mm), followed by S. typhimurium (23 ± 1 mm), B. cereus (19 ± 1 mm), P. vulgaris (17 ± 2 mm), and S. aureus (16 ± 2 mm) (Fig. 5A). U. lactuca mediated nanoparticles were effective aginst E. coli (29 ± 1 mm), whereas, the zone of inhibition was 17 ± 2 mm against S. aureus. Iron nanoparticles also showed potential activity against S. typhimurium (26 ± 1 mm) and P. vulgaris (22 ± 2 mm). U. lactuca extract was highly effective against E. coli and the MIC value was 50 µg/mL (Fig. 5B). The MIC values of macroalgal extract mediated iron nanoparticles showed decreased MIC values than methanol extract.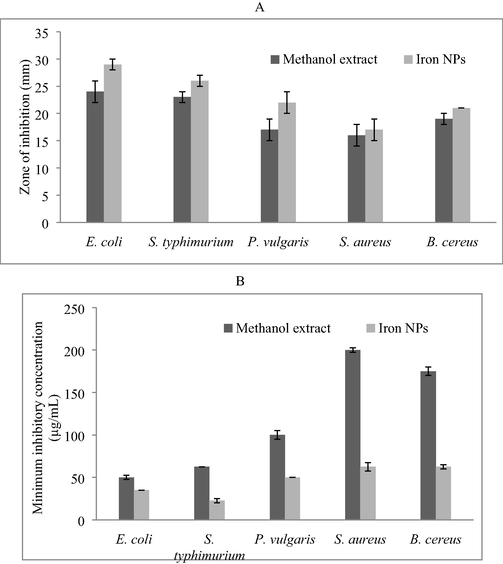
Antibacterial activities of methanol extract and iron NPs against diarrhoea cusing bacteria. Well diffusion method was used and the result was described as zone of inhibition (mm). Antibacterial activity of methanol extract and nanoparticles (A) and MIC of methanol extract and iron nanoparticles against diarrhoea cusing bacteria (µg/mL) (B).
4 Discussion
The ability of seaweed to produce various secondary metabolites of great interest has been previously described (Seca and Pinto, 2018). U. lactuca was collected from South East cost of India and solvent extracts were used for the determination of phytochemicals. The antimicrobial properties of U. lactuca have been stated earlier and the anti-bacterial activity varies on algal species, extraction methods, types of solvent and drug resistance pattern of the selected bacteria (Tan et al., 2012). Results obtained in this study revealed that, methanol was the suitable solvent for the extraction of the secondary metabolites, followed by acetone. Ethanol extraction has been indicated as the suitable method for phytochemicals, moreover, chloroform has also been reported as the suitable solvent for the extraction of phytochemicals than petroleum ether and ethanol. From the present study, it is very clear that, organic solvents have the suitable cholice for the extraction of more antimicrobial compounds. The antibacterial activity of the tested alga could be attributed to the amount and the type of free fatty acids which have a potent role in the overall defense against Gram-negative and Gram-positive bacteria (Bhuyar et al., 2020). In the case of Ulva species, palmitic acid has been considered as the one of the important fatty acids. The fatty acids and fats from marine algae play significant role in the development of various bioactive secondary metabolites and some fattacids have been showed antibacterial activity (Bhuyar et al., 2020). The phytochemicals such as, flavonoids, alkaloids, phenolic compounds, tannins, steroids, saponins, and terpenoids have been significant antibacterial activities (Mahadevi et al., 2021). In this study, the phytochemical coumpounds such as flavonoids, alkaloids, phenolic compounds, tannins, steroids, saponins, and terpenoids were determined. These results are in highly agreement with earlier studies (Mahadevi et al., 2021).
In this study green synthesis of iron nanoparticles was performed using U. lactuca aqueous extract. In marine macroalgae, hydroxyl groups and sulphate present in the sulphated polysaccharides may reduce the ferric ions and obtained highly stabilized iron NPs (Tziveleka et al., 2021). The molecular mechanism of the development of ferric iron NPs has been reported previously (Bouafia and Laouini, 2021). In our study, the reduction process was monitored based on colour changes in the reaction mixture and UV–visible spectrum analysis. FT-IR analysis revealed distinct functional groups in the iron nanoparticles and the wave number at 1250.5 cm−1 revealed the presence of sulphated polysaccharide group. This sulphate group involved in FeCl3 reduction and stabilization and has been reported previously by Sahayaraj et al. (2020). Van der Waals forces of interaction have been previously proposed between nitrogen and carbon atom of the bioactive compounds (Azizi et al., 2013). Hence, the IR results revealed that iron nanoparticles were capped by metabolites present in the aqueous extract of the marine alga. The UV–Visible spectrum of green synthesized iron NPs sample gave a major absorption peak revealing the formation of iron-NPs. SEM analysis was used to determine the surface morphology and to validate the nano-structure. The SEM images of the sample showed its size as 30–40 nm. The green synthesized iron nanoparticles were determined in nanospheres form and have been previously reported by AL-Kalifawi (2015). Iron nanoparticles contact with each other due to the magnetic properties (Mondal et al., 2020).
U. lactuca extract has the potential to treat various bacterial infections and showed activity aganist E. coli (24 ± 2 mm), S. typhimurium (23 ± 1 mm), B. cereus (19 ± 1 mm), and P. vulgaris (17 ± 2 mm), and S. aureus (16 ± 2 mm). Moreover, the green syntheiszed nanoparticles showed improved antimicrobial potential than algal extract. The alga mediated iron nanoparticles have been showed bactericidal activities against various bacterial pathogens (Mashjoor et al., 2018). The iron nanoparticles was effective against most of the Gram-negative bacterial strains than Gram-positive strains. This might be due to the differences between Gram-negative and positive bacterial cell wall. The potential of iron nanoparticles mainly based on the reaction between the ions released from the nanomaterials and thiol groups of proteins. The small size particles have maximum antimicrobial potential than large size nanoparticles due to large surface to volume ratio. The iron NPs syntheiszed by Dictyota dicotoma (brown algae) has been active against various pathogenic bacteria (Chandran et al., 2016). Iron nanoparticles involved in electromagnetic attraction between the negative charges of bacterial cell wall and the positive charges of nanoparticles, which oxidize and kill bacteria (Mondal et al., 2020). The anticancer activity of iron NPs against HeLa and DLD-1 revealed dose dependent activity. Green syntheisized nanoparticles have potential anticancer activity against various cancer cell lines. Recently, the anticancer actiivty of nickel oxide, cadmium selenide nanoparticles and iron nanoparticles have been tested against MCF-7, and A549 cell lines (Mani et al., 2021; Anand et al., 2020; Kaviyarasu et al., 2017).
5 Conclusions
In this study, green synthesis of iron nanoparticles was performed using crude water extract of U. lactuca. SEM analysis of the green synthesized NPs showed spherical shape. The IR results revealed relative peaks to the iron NPs. The iron NPs showed activity against HeLa cells and DLD-1 cells and was dose dependent. Furthermore, the methanol extract and iron NPs showed potential against multi-drug resistant bacterial pathogens. Consequently, the present investigation revealed that the aqueous extract of U. lactuca is a low-cost capping and stabilizing against to improve the stability and maintain biological properties of iron NPs for antimicrobial and anticancer treatments.
Acknowledgements
The authors acknowledge King Saud University, Riyadh, Saudi Arabia for funding this research through Researchers Supporting Project No: RSP 2022/11.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Insecticidal, antimicrobial and antioxidant activities of essential oil from Lavandula latifolia L. and its deterrent effects on Euphoria leucographa. Ind. Crop. Prod.. 2021;170:113740.
- [Google Scholar]
- Probiotic and antioxidant potential of Lactobacillus reuteri LR12 and Lactobacillus lactis ll10 isolated from pineapple puree and quality analysis of pineapple-flavored goat milk yoghurt during storage. Microorganisms. 2020;8(10):1461.
- [Google Scholar]
- Green synthesis of magnetite iron oxide nanoparticles by using Al-Abbas’s (AS) hund fruit (Citrus medica) var. sarcodactylis swingle extract and used in Al-’alqami river water treatment. J. Nat. Sci. Res.. 2015;5(20):125-135.
- [Google Scholar]
- Structural and optical properties of nickel oxide nanoparticles: investigation of antimicrobial applications. Surface. Interface. 2020;18:100460.
- [Google Scholar]
- The biotechnological potential of secondary metabolites from marine bacteria. J. Mar. Sci. Eng.. 2019;7:176.
- [Google Scholar]
- One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol. B: Biol.. 2019;190:154-162.
- [Google Scholar]
- Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L and its antibacterial and cytotoxic effects: an in vitro study. Int. J. Nanomed.. 2014;9:379.
- [Google Scholar]
- Preparation, characterization, and antimicrobial activities of ZnO nanoparticles/cellulose nanocrystal nanocomposites. BioResources. 2013;8(2):1841-1851.
- [Google Scholar]
- In vitro antibacterial, antifungal, nematocidal and growth promoting activities of Trichoderma hamatum FB10 and its secondary metabolites. J. Fungi.. 2021;7(5):331.
- [Google Scholar]
- Effect of PVA/PVP protective agent on the formation of silver nanoparticles and its photocatalytic and antimicrobial activity. Mat. Today: Proc.. 2021;36:121-125.
- [Google Scholar]
- Synthesis of silver nanoparticles using marine macroalgae Padina sp. and its antibacterial activity towards pathogenic bacteria. Beni-Suef Uni. J. Basic Appl. Sci.. 2020;9(1):1-15.
- [Google Scholar]
- Plant-mediated synthesis of iron oxide nanoparticles and evaluation of the antimicrobial activity: a review. Mini-Rev. Organic Chem.. 2021;18(6):725-734.
- [Google Scholar]
- Bio synthesis of iron nanoparticles using the brown seaweed, Dictyota dicotoma. Biotechnol. Indian J. 2016;12(12):112.
- [Google Scholar]
- Photo-mediated biosynthesis and characterization of silver nanoparticles using bacterial xylanases as reductant: Role of synthesized product (Xyl-AgNPs) in fruits juice clarification. Surface. Interface.. 2020;21:100747.
- [Google Scholar]
- Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: cytotoxicity effect of nanoparticles against HT-29 cancer cells. J. Photochem. Photobiol. B: Biol.. 2016;164:352-360.
- [Google Scholar]
- Phytochemical screening and antioxidant and cytotoxic effects of acacia macrostachya. Plants. 2021;10(7):1353.
- [Google Scholar]
- Ilavenil, S., Kim, D.H., Vijayakumar, M., Srigopalram, S., Roh, S.G., Arasu, M.V., Lee, J.S., Choi, K.C., 2016. Potential role of marine algae extract on 3T3-L1 cell proliferation and differentiation: an in vitro approach. Biol. Res. 49(1), 1-1.
- Emerging paradigms of viral diseases and paramount role of natural resources as antiviral agents. Science Total Environ.. 2021;759:143539.
- [Google Scholar]
- Green synthesis of Ag nanoparticles using Tamarind fruit extract for the antibacterial studies. J. Photochem. Photobiol. B: Biol.. 2017;169:178-185.
- [Google Scholar]
- Enhanced production antibiotics using green gram husk medium by Streptomyces sp. SD1 using response surface methodology. J. King Saud Uni. Sci.. 2020;32(3):2134-2141.
- [Google Scholar]
- Photodegradation of organic pollutants RhB dye using UV simulated sunlight on ceria based TiO 2 nanomaterials for antibacterial applications. Sci. Rep.. 2016;6(1):1-12.
- [Google Scholar]
- Antiproliferative effects on human lung cell lines A549 activity of cadmium selenide nanoparticles extracted from cytotoxic effects: investigation of bio-electronic application. Mat. Sci. Eng. C.. 2017;76:1012-1025.
- [Google Scholar]
- Investigation of structural and photoluminescence properties of gas and metal ions doped zinc oxide single crystals. J. Alloy. Comp.. 2014;616:614-617.
- [Google Scholar]
- Screening and characterization of phytochemical content of methanolic extract of Rhizome of Curcuma amada and their antibacterial activity against MRSA. Appl. Nanosci. 2021:1-11.
- [Google Scholar]
- In-vitro phytochemical and pharmacological bio-efficacy studies on Azadirachta indica A. Juss and Melia azedarach Linn for anticancer activity. Saud. J. Biol. Sci.. 2020;27(2):682-688.
- [Google Scholar]
- Systematic green synthesis of silver oxide nanoparticles for antimicrobial activity. Environ. Res.. 2021;202:111627.
- [Google Scholar]
- A novel biogenic Allium cepa leaf mediated silver nanoparticles for antimicrobial, antioxidant, and anticancer effects on MCF-7 cell line. Env. Res.. 2021;198:111199
- [Google Scholar]
- Organic and inorganic nano-Fe3O4: Alga Ulva flexuosa-based synthesis, antimicrobial effects and acute toxicity to briny water rotifer Brachionus rotundiformis. Environ. Poll.. 2018;237:50-64.
- [Google Scholar]
- Green synthesis and environmental application of iron-based nanomaterials and nanocomposite: a review. Chemosphere. 2020;259:127509.
- [Google Scholar]
- Formation of magnetic nanoparticles by low energy dual implantation of Ni and Fe into SiO2. J. Alloy. Comp.. 2016;667:255-261.
- [Google Scholar]
- Effect of Ag doped MnO2 nanostructures suitable for wastewater treatment and other environmental pollutant applications. Env. Res.. 2022;205:112560.
- [Google Scholar]
- Eco-friendly preparation of zinc oxide nanoparticles using Tabernaemontana divaricata and its photocatalytic and antimicrobial activity. J. Photochem. Photobiol. B: Biol.. 2018;181:53-58.
- [Google Scholar]
- Bioactive compounds in seaweeds: an overview of their biological properties and safety. Food Chem. Toxicol.. 2019;135:111013.
- [Google Scholar]
- Biosynthesis of silver nanoparticles using Phyllanthus emblica fruit extract for antimicrobial application. Biocat. Agric. Biotechnol.. 2020;24:101567.
- [Google Scholar]
- Green synthesis of silver nanoparticles using dry leaf aqueous extract of Pongamia glabra Vent (Fab.), characterization and phytofungicidal activity. Environ. Nanotechnol. Monitor Manage.. 2020;14:100349.
- [Google Scholar]
- Biosynthesis, characterization, and antibacterial activity of gold nanoparticles. J. Infect. Pub. Health. 2021
- [CrossRef] [Google Scholar]
- Overview on the antihypertensive and anti-obesity effects of secondary metabolites from seaweeds. Mar. Drug.. 2018;16(7):237.
- [Google Scholar]
- Extraction and bioautographic-guided separation of antibacterial compounds from Ulva lactuca. J. Appl. Phycol. 2012;24(3):513-523.
- [Google Scholar]
- Antioxidant and photocatalytic activity of aqueous leaf extract mediated green synthesis of silver nanoparticles using Passiflora edulis f. flavicarpa. J. Nanosci. Nanotechnol.. 2019;19(5):2640-2648.
- [Google Scholar]
- Metabolites with antioxidant activity from marine macroalgae. Antioxidants. 2021;10(9):1431.
- [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B: Biol.. 2019;191:65-74.
- [Google Scholar]
- Bioreduction potentials of dried root of Zingiber officinale for a simple green synthesis of silver nanoparticles: antibacterial studies. J. Photochem. Photobiol. B: Biol.. 2017;177:62-68.
- [Google Scholar]
- Generation of metal nanoparticles in a microchannel reactor. Chem. Eng. J.. 2004;101(1-3):251-260.
- [Google Scholar]
- Host associated mixed probiotic bacteria induced digestive enzymes in the gut of tiger shrimp Penaeus monodon. Saud. J. Biol. Sci.. 2020;27(9):2479-2484.
- [Google Scholar]
- Characterization of biofilm formed by multidrug resistant Pseudomonas aeruginosa DC-17 isolated from dental caries. Saud. J. Biol. Sci.. 2020;27(11):2955-2960.
- [Google Scholar]
- Preliminary phytochemical screening of six medicinal plants used in traditional medicine. Int. J. Pharm. Pharm. Sci.. 2014;6(5):539-542.
- [Google Scholar]







