Translate this page into:
Antifungal potential of Colchicum luteum and determination of colchicine content using HPLC for application as a fungicide
⁎Corresponding authors. rouf.haq@gmail.com (Rauoof Ahmad Rather), Nadeem.khan@mecw.ac.in (Nadeem Ahmad Khan),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Colchicum luteum L. is an economically important medicinal plant species of the North Western Himalaya. The corm extract of this plant is utilized in the treatment of many diseases. It is also used extensively in plant breeding program for the doubling of chromosomes. The antifungal activities of aqueous and ethanolic extracts of the corm of the medicinal plant Colchicum luteum were investigated in this study. These extracts were tested against the fungi, Colletotrichum sp. and Fusarium oxysporum. The ethanolic extract of corm (100 mg mL−1) inhibited Colletotrichum sp. and F. oxysporum with inhibition zones of 19.2 mm and 18.3 mm, respectively, whereas the aqueous extract of corm (100 mg mL−1) inhibited Colletotrichum sp. and F. oxysporum with inhibition zones of 17.1 mm and 15.2 mm, respectively. Colchicine was isolated from several plant components (corms, seeds, leaves, and flowers) and its concentration was determined using high-performance liquid chromatography (HPLC). The corm had the highest percentage of colchicine (0.191% ± 0.036) of all the plant parts examined, followed by the seeds (0.103% ± 0.021). As a result, it can be said that the corms and seeds of C. luteum could serve as a natural source of colchicine for the pharmaceutical industry.
Keywords
Colchicum luteum
Antifungal
Kirby-Bauer test
Colchicine
Sustainable environment
HPLC
1 Introduction
Medicinal herbs are generally used in the industrial production of antibiotics as well as in the preparation of traditional remedies by the local population in rural areas of Kashmir, India (Hamilton, 2004; Shinwari et al., 2014; Bano et al., 2018; Bao et al., 2021; Rather et al., 2022). The utilization of herbal plants in treatment of several diseases like burns, dermatophytes and infectious diseases has good healing results in the population. The most important weapons in fighting fungal and bacterial infections are the use of antibiotics in community (Matuska-Łyżwa et al., 2021; Wani et al., 2018b). However, the antimicrobial biopesticides have caused a dramatic change not only of the treatment of infectious diseases (Dafale et al. 2020; Lees et al., 2021: Villamizar et al., 2021). Scientists are increasingly concerned about the discovery of new antibiotic medicines against resistant infections (Spellberg et al., 2004; Uribe-Gutiérrez et al., 2021: Sala et al.,2021) The research for internal or online FDA databases has calculated the number of new antimicrobial agents authorized from 1980 till date (Wani et al., 2018a; Malik et al., 2018; Bano et al., 2021; Dullah et al., 2021). The identical analysis was carried out for newly licensed antivirals, antifungals and antibacterial from 1998 till date. Medicinal plants are increasingly reporting antimicrobial capabilities from many regions of the world (Senthilkumar and Reetha, 2009; Wani et al., 2018d; Wani et al., 2018c).
Colchicum luteum generally called as ‘Suranjan-e-Talkh’ in local language Urdu, belongs to the Liliaceae family (Shinwari and Gilani, 2003)”. The total of 31 different alkaloids have been isolated from this genus and colchicine being the major bioactive compound. The alkaloid colchicine is used to treat the Behçet’s disease and the Alzheimer’s disease, treatment of rheumatism, gout, antioxidants, doubling of chromosomes (Aisen et al., 2001; Ahmad et al., 2006; Ahmad, 2010). C. luteum is also used widely to treat gout, rheumatism, hepatitis and spleen illnesses and blood cleansers (Chopra et al., 1986; Shinwari et al., 2003). To alleviate the world’s dependency on pesticide, alternative such as biopesticide should be considered. Therefore, keeping in mind the enormous antimicrobial potential of C. luteum, this study was undertaken to find a natural source with antifungal potential against plant pathogenic fungi, Fusarium oxysporum and Colletotrichum sp. These pathogens cause wilt, rot, anthracnose, which affects sweet potatoes, tomatoes, legumes, melons and bananas. These fungi also cause red rot of coffee berry, sugar cane, coffee berry, crown rot of banana, and strawberry and brown blotch of cowpea (Hyde et al., 2009; Sumner, 2018; Cannon et al., 2012; Okungbowa and Shittu, 2012; Volcao et al., 2021). As far as the authors’ knowledge, the antimicrobial properties of C. luteum extracts have not been investigated before this study on these to fungal strains, i.e, Fusarium oxysporum and Colletotrichum sp. using this particular plant extract.
2 Materials and methods
2.1Plant collection and extraction
Colchicum luteum plants were gathered from selected areas of Kashmir Himalaya located in the western Himalayas (33.27778° N, 75.3412°E), onset of March-April in the year 2018 and 2019. The identification of the collected plants was done by Dr. Haleema Bano, Assistant Professor at (Division of Environmental Sciences, SKUAST-Kashmir, Shalimar, J&K, and India). The plants were divided into different parts (corms, seeds, leaves, and flowers) and preserved at −2 °C. Corms of C. luteum were shade dried at 28 ± 3 °C. Then, they were milled by pestle and mortar until obtaining a fine powder with a particle size of 180 µm) and dissolved in ethanol and water (80:20). The extracts so obtained were filtered through four cloth layers of muslin for 20 min. The concentrated solid extracts were obtained by means of a rotary evaporator under reduced pressure. Total of five different concentrations viz., (10, 30, 50, 80 and 100 mg mL−1) of both concentrated solid extracts were prepared by dissolving them in 10% dimethyl sulfoxide (DMSO) in distilled water. Finally, the produced extracts were kept at 4 °C in a refrigerator for the further examination.
2.2 Test microorganisms
The two fungal species for the antifungal test examination includes Fusarium oxysporum and Colletotrichum sp. which were obtained from the Division of Plant Pathology, SKUAST-K, Shalimar, J&K, India. The fungal species were preserved on petri plates containing potato dextrose agar (PDA) at 2 °C and sub-cultured every after 2 weeks in biocontrol plant pathology lab SKUAST-K, Shalimar. ‘The fungicide Hexaconazole was used as the (positive control) for antifungal evaluation and DMSO as the negative control’.
2.3 Antifungal assessment
The antifungal assessment for ethanolic as well as aqueous extracts were done by the agar well diffusion procedure as defined by Bauer et al. (1966) and Klančnik et al. (2010). An aliquot of 100 μL of standardized inoculum of each tested fungal species was inoculated in sterile molten PDA, homogenized and poured into sterile petri dishes to provide a uniform depth of 4 mm. Inside the laminar hood, petri plates were allowed to solidify and provide uniform structure for wells. So, five wells on the periphery and one well at the center of each petri plate were made using sterile 5 mm diameter cork borers. The wells were filled with 100 μL (10, 30, 50, 80 and 100 mg mL−1) of plant corm extract prepared in 10% (DMSO). The fungicide Hexaconazole (0.5 mg mL−1) was loaded into the middle well (positive control) whereas 10% (DMSO) act as −ve control in an aside Petri dish (Dar et al., 2017). Thereafter, these dishes were incubated at (32 °C for 24–36 h). Finally, inhibition zones (mm) were recorded for each petri plate to determine the biopesticide activity of the of C. luteum plant.
2.4 Isolation and extraction of colchicine
Using a Soxhlet instrument, all dried plant parts corms, seeds, leaves and flowers were extracted with ethanol. The plant parts, before extraction, were defatted in petroleum ether before being removed with ethanol in a rotary evaporator. The plant material was extracted in a Soxhlet extractor using an ultrasonic bath for 6 h. The extract was diluted by the distilled water and afterwards partitioned using a conical 500 mL flask into petroleum ether. The chloroform extract was evaporated and the residues re-dissolved and acidified with 3% H2SO4 (pH 3–4) and lastly filtered through 0.45 μm filter (Hayashi et al., 1988). The resulting filtrate was the test sample ready for HPLC analysis. To avoid compound oxidation, all extracts were kept out of direct sunlight during the assay.
2.5 Quantification of colchicine by HPLC
The HPLC analysis was performed on an Agilent (1200 series) instrument with a [Sepax C18 column particle size of 5 m (4.6 mm × 250 mm)] and Empower 3 chromatography data software (Waters, Germany), which was equipped with quaternary pumps, a degasser, and a photo-diode-array detector. An Agilent (1200 series) instrument with a [Sepax C18 column particle size of 5 µm (4.6 mm × 250 mm)]. An acetonitrile (solvent A) was used as the mobile phase and acetic acid in water (solvent B) as the stationary phase. The elution gradient was as follows: (0–7.5 min 10–60% solvent A, 7.5–10 min 60% solvent B, 10–12.5 min 60–100% and 12.5–25 min 10% solvent A). The USP (Standard of drug reference) was used to provide a colchicine reference norm. ‘The flow rate was set to 1 mL/minute and the injection volume and column temperature was 40 °C µL and 40 °C, respectively’. The absorbance was monitored at 352 nm represented in Table 1. The quantitative measurements are presented in percent (%) of colchicine and are based on the relative peak positions of standards at a given retention time versus the concentration.
Compound
Mobile Phase
Gradient
λ max
Colchicine
(Solvent A) Acetonitrile
10–60% A (0–7.5 min)
(Solvent B) Acetic acid in water
60% B (7.5–10 min)
60–100% A (10–12.5 min)
352 nm
10% A (12.5–25 min)
2.6 Statistical analyses
The data was evaluated and statistical tests were performed, by using IBM-SPSS- Statistics (Version 20) software. Three-way analysis of variance (ANOVA) was used to compare the variation in between the treatments).
3 Results
From all the tested concentrations of the corm aqueous extracts of C. luetum, the 100 mg mL−1 extract exhibited the highest antifungal activity towards Colletotrichum sp. with zone inhibition of 17 ± 0.12 mm followed by F. oxysporum with an inhibition zone of 15 ± 0.23 mm. Likewise, the corm ethanolic extracts also showed the highest antifungal activity against Colletotrichum sp. and F. oxysporum at 100 mg mL−1 with inhibition zones of 19 ± 0.29 mm and 18 ± 0.39 mm, respectively. Hexaconazole (positive control; 0.5 mg mL−1) showed an inhibition zone of 32 ± 0.14 mm against Colletotrichum sp. and of 20 ± 0.40 mm against F. oxysporum. While the negative control showed zero inhibition (10% DMSO), exhibited no antifungal action towards selected tested fungal species in the separate pertiplate (Tables 2 and 3 and Figs. 1–4). At a concentration of 80 mg mL−1 of plant corm extract, the aqueous extracts of C. luetum showed the antifungal activity towards Colletotrichum sp. with an inhibition zone of 15 ± 0.13 mm followed by F. oxysporum with an inhibition zone of 12 ± 0.28 mm. Hexaconazole (positive control) on the other hand, showed an inhibition zone of 32 ± 0.26 mm against Colletotrichum sp and 20 ± 0.40 mm against F. oxysporum. While as ethanolic extracts of C. luetum showed the antifungal activity towards Colletotrichum sp. with an inhibition zone of 17 ± 0.1 mm followed by F. oxysporum with an inhibition zone of 15 ± 0.10 mm at a concentration of 80 mg mL−1 of plant corm extract. While, Hexaconazole (positive control) showed an inhibition zone of 31 ± 0.26 mm against Colletotrichum sp. and 20 ± 0.40 mm against F. oxysporum. The negative control (10% DMSO) showed no fungal activity against any of the tested fungal strains (Tables 2 and 3 and Figs. 1-4). In general, the ethanolic extract of corms (100 mg mL−1) of C. luteum was noted to be more effective than its aqueous extract against all tested fungi (Tables 2 and 3). Whereas, C.D. (≤0.05). S1, S2: fungal strains; C1, C2, C3, C4, and C5: Different concentrations; Strain: 0.18128; Solvent: 0.18128; Concentration: 0.28662; Strain × Solvent: 0.25636; Strain × Concentration: 0.40534; Solvent × Concentration: 0.40534; Strain × Solvent × Concentration: 0.57324. Negative Control: 10% DMSO; Positive Control: The fungicide Hexaconazole (0.5 mg mL−1).
Strain solvent
Concentration
Solvent
C1
C2
C3
C4
C5
Mean
Factor mean of solvent
S1
Aqueous
Ethanol06.36
06.5208.39
10.3810.29
11.5512.40
15.4515.44
18.5510.58
12.48
Aqueous = 10.91
Ethanolic = 13.14
Sub mean
06.44
09.39
10.92
13.93
16.97
12.53
S2
Aqueous
Ethanol02.34
04.3407.45
11.1813.59
16.3915.42
17.7017.44
19.4211.25
13.81
Sub mean
03.34
09.31
14.99
16.56
17.70
11.32
Mean
04.89
09.35
12.95
15.24
17.70
Fungi
Solvent
Plant extract Concentration (mg mL−1)
Positive control
Negative control
10
30
50
80
100
Zone of inhibition (mm)
Fusarium oxysporum
Aqueous
06 ± 0.29
08 ± 0.27
10 ± 0.36
12 ± 0.28
15 ± 0.23
20 ± 0.40
0.0
Ethanolic
06 ± 0.21
10 ± 0.33
11 ± 0.39
15 ± 0.10
18 ± 0.39
20 ± 0.40
0.0
Colletotrichum Sp.
Aqueous
02 ± 0.28
07 ± 0.28
13 ± 0.31
15 ± 0.13
17 ± 0.12
32 ± 0.26
0.0
Ethanolic
04 ± 0.16
11 ± 0.36
16 ± 0.82
17 ± 0.1
19 ± 0.29
31 ± 0.26
0.0
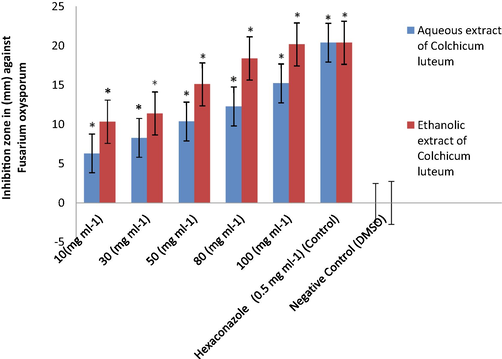
Antifungal properties of Colchicum luteum against Fusarium oxysporum. Error bar indicates SD mean, Values with * are significantly different compared to control (P-value < 0.05).
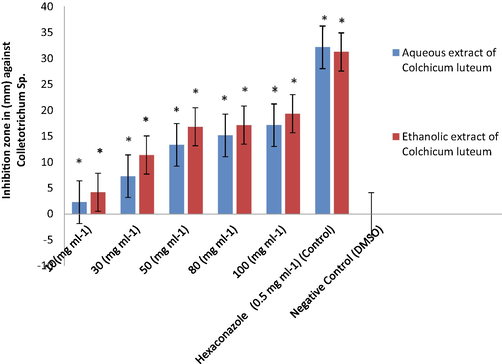
Antifungal properties of Colchicum luteum against Colletotrichum sp. Error bar indicates SD mean, Values with * are significantly different compared to control (P-value < 0.05).
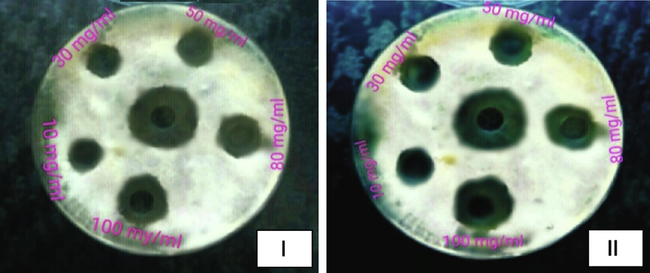
Inhibition zones around the agar wells against Fusarium oxysporum. Aqueous extracts (I) and Ethanolic extracts (II) and Middle well indicates Positive control (Fungicide).
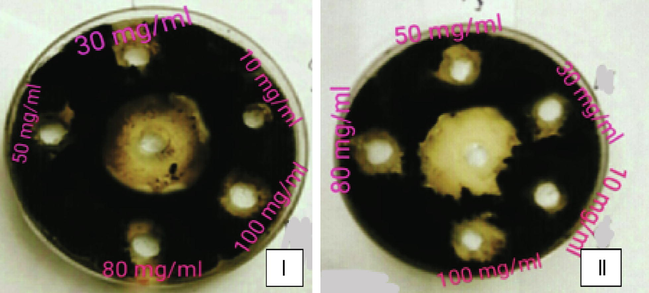
Inhibition zones around the agar wells against Colletotrichum sp. Aqueous extracts (I) and Ethanolic extracts (II) and Middle well indicates Positive control (Fungicide).
Colchicine concentrations in the ethanolic extract from each part of the C. luteum (i.e. corm, seed, leaves and flowers) was determined by HPLC (Table 4). The concentration of colchicine concentrations varies among the different plant parts and was found the highest in corms (0.191% ± 0.036) followed by the seeds (0.103% ±0.021) (Fig. 5).
Plant
Plant parts
% Value of colchicine (w/w)
Colchicum luetum
Corm
0.191 ± 0.036
Seeds
0.103 ± 0.021
Leaves
0.084 ± 0.030
Flowers
0.056 ± 0.010
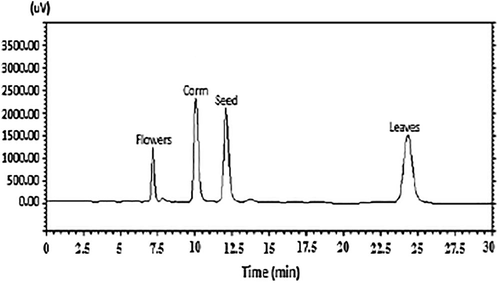
HPLC chromatogram comparison of colchicine extracted from different parts of C. luteum at λ 352 nm.
4 Discussion
Antifungal activities of different concentrations of ethanolic and aqueous extracts of C. luteum corm were screened for its antifungal behavior by Ahmad et al., 2006 against different fungal strains (Trichophyton longifusus and Microsporum canis) Observations recorded by these investigators revealed that the crude extract at all the concentrations were fungistatic in nature. In present investigation it was observed that the ethanolic and aqueous corm extracts seemed to have the best antifungal activity against F. oxysporum and Colletotrichum sp. The extracts in the present investigation were far superior in contrast with the tested extracts against Fusarium oxysporum by other authors (Rongai et al., 2015). Mammadov et al., 2009; Theer et al., 2021 while investigating the antimicrobial activity of Colchicum species (ethanol extract) observed that the ethanol extract had a weak inhibitory effect against tested bacteria. S. aureus but the same extract showed higher efficacy (58%) against the bacterium Bacillus subtilis. The plant extracts obtained from Ribes nigrum showed a good antifungal activity against F. oxysporum with the efficacy of 78.6 % as reported by Şesan et al., (2017). Another investigation revealed the better antifungal activity of ethanol and acetone extract of leaves of few medicinal plants against F. oxysporum (Neela et al., 2014). Butanoic and ethanolic extracts of the tested plant Ocimum basilicum inhibited the growth of F. oxysporum to a higher extent against (Isaac and Abu-Tahon, 2014).
Our findings show that C. luetum extracts are extremely efficient in preventing and fungal growth of F. oxysporum and Colletotrichum sp. This suggests that the extract derived from C. luetum corms might be used to prevent pathogen spread, infectivity, and persistence in the host. These findings are in line with the findings of Bhutia et al. (2016). The antifungal activity of plant extracts against the Colletotrichum sp. has been carried out by various authors (Bussaman et al., 2012; Silva et al., 2008.; Maqbool et al., 2010; Sangeetha et al., 2013; Cruz et al., 2013; Padder et al., 2021; Bhat et al., 2021) and findings from their investigations revealed a broader variation of sensitivity of the various strains of Colletotrichum sp. to different concentrations of the both ethanolic and crude extracts of different plant parts. Our findings showed that C. luetum extracts are extremely efficient in preventing the fungal growth of F. oxysporum and Colletotrichum sp. This suggests that the extract derived from C. luetum corms could be used to prevent pathogen spread, infectivity, and persistence in the host. Furthermore, our findings showed the best antifungal potentional of plant extract against the selected fungal species with a foundation to a larger investigation field highlighting the importance of Colchicum members as producers of fungicidal or fungistatic ingredients against various phytopathogenic entities like F. oxysporum and Colletotrichum sp.
The chemical-based fungicide uses for the control of phytopathogens have raised the concerns of fungicide resistance and bioaccumulation of these xenobiotic compounds in the environment (Padder et al., 2021). Therefore, the ecologically sound strategies need to be expounded for the control of phytopathogenic fungi and bacteria for a sustained agriculture, plant based organic extracts the slow but prolonged activity during their application (Rongai et al., 2015)
Colchicine levels in Anatolian colchicum and other species of Colchicum, were observed to be in the range from 0.039% to 0.3% (w/w) in previous studies (Toplan et al., 2016; Felipe et al., 2014; Ahmad, 2010. Other investigators like Alali et al. (2006) found that the underground parts of C. tunicatum, other species of Colchicum contain the colchicine content of 0.12% (w/w) as well as 0.13% (w/w) during their and vegetating and flowering stages, respectively, while that of aerial parts was only about 0.04% (w/w) and 0.02% (w/w), respectively. Our findings demonstrated the occurrence of the alkaloid colchicine at higher concentrations in various parts of C. luteum compared to previous authors (Siddiqui et al., 2019). The colchicine concentration in different parts of plant sections ranged from 0.051 percent to 0.695 percent which largely deferred from the findings of other investigators (Sharma et al., 2019; Saleem et al., 2020; Salehi et al., 2021). Therefore, the present investigation confirmed the presence of a potential alkaloid colchicine in the plant extracts of was C. luteum, furthermore this study supports the use of plant extracts in the treatment of infectious diseases and has provided important insights for the identification of new plant-based antifungal medicines.
5 Conclusion
The C. luteum corm extract showed significantly positive actions against the F. oxysporum and Colletotrichum sp. Ethanolic extracts demonstrated the best inhibition potential against fungal species, thus the plant extract has lot of fungicidal potential. Hence, C. luteum could be useful to fight against several fungal diseases in agriculturally important crops such as Fusarium wilt. This study recommends the antifungal potential of C. luteum extract as a source of effective bio -fungicide as an organic remedy to various phytopathogens. More research is needed to describe the antifungal effects of C. luteum against F. oxysporum and Colletotrichum sp., as well as the other potential pathogenic fungi. The method for identifying bio-fungicides in higher plants reported in this study is simple, quick, and allows for simultaneous examination of a large number of plant species.
Acknowledgement
The authors would like to acknowledge the support provided by Researchers Supporting Project Number (RSP-2021/358), King Saud University, Riyadh, Saudi Arabia.
The authors would also like to acknowledge the Division of Environmental Sciences and Division of Plant Pathology, FoH, SKUAST-K, J & K, India, for help provided in carrying out this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antioxidant activity and phenolic compounds from Colchicum luteum Baker (Liliaceae) J. Biotechnol.. 2010;9:5762-5766.
- [Google Scholar]
- Antimicrobial bioassay of Colchicum luteum Baker. J. Enzyme Inhib. Med. Chem.. 2006;21(6):765-769.
- [Google Scholar]
- Pilot tolerability studies of hydroxychloroquine and colchicine in Alzheimer disease. Alzheimer Dis. Assoc. Disord.. 2001;15(2):96-101.
- [Google Scholar]
- Seasonal variation of colchicine content in Colchicum brachyphyllum and Colchicum tunicatum (Colchicaceae) Nat. Prod. Res.. 2006;20(12):1121-1128.
- [Google Scholar]
- Hokersar Wet Land of Kashmir: its utility and factors responsible for its degradation. Plant Arch.. 2018;18:1905-1910.
- [Google Scholar]
- Effect of pre-sowing treatments using phytohormones and other dormancy breaking chemicals on seed germination of Dioscorea deltoidea Wall. Ex Griseb.: an Endangered Medicinal Plant Species of North Western Himalaya. Eco. Environ. Conserv.. 2021;27:253-260.
- [Google Scholar]
- Potential applicability of a cyanobacterium as a biofertilizer and biopesticide in rice fields. Plant Soil. 2021;463(1):97-112.
- [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol.. 1966;45(4):493-496.
- [Google Scholar]
- Influence of Growing Media on Vegetative, Floral and Bulb Parameters of Crown Lily (Fritillaria Imperialis L.). Acta Scientific. Agriculture. 2021;5(4):56-60.
- [Google Scholar]
- Bhutia, D.D., Zhimo, Y., Kole, R., Saha, J., 2016. Antifungal activity of plant extracts against Colletotrichum musae, the post harvest anthracnose pathogen of banana cv. Martaman. Nutr. Food Sci.
- Effect of Crude Leaf Extracts on Colletotrichum gloeosporioides (Penz.) Sacc. Psyche: J. Entomol.. 2012;2012:1-6.
- [Google Scholar]
- Colletotrichum–current status and future directions. Stud. Mycol.. 2012;73:181-213.
- [Google Scholar]
- Glossary of Indian Medicinal Plants (including the Supplement). New Delhi: Council of Scientific and Industrial Research; 1986.
- Plant extracts for controlling the post-harvest anthracnose of banana fruit. Rev. Bras. Plantas Med.. 2013;15(4 suppl 1):727-733.
- [Google Scholar]
- Zoonosis: an emerging link to antibiotic resistance under “One health approach”. Indian J. Microbiol.. 2020;60(2):139-152.
- [Google Scholar]
- Fungal interactions induce changes in hyphal morphology and enzyme production. Mycology. 2021;12(4):279-295.
- [Google Scholar]
- Phytochemical analysis of Pfaffia glomerata inflorescences by LC-ESI-MS/MS. Molecules. 2014;19(10):15720-15734.
- [Google Scholar]
- Medicinal plants, conservation and livelihoods. Biodiver. Conserv.. 2004;13(8):1477-1517.
- [Google Scholar]
- Formation of alkaloids in suspension-cultured Colchicum autumnale. Phytochemistry. 1988;27(5):1371-1374.
- [Google Scholar]
- In vitro antifugal activity of medicinal plant extract against Fusarium oxysporum f. sp. lycopersici race 3 the causal agent of tomato wilt. Acta Biol. Hung.. 2014;65(1):107-118.
- [Google Scholar]
- Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods. 2010;81(2):121-126.
- [Google Scholar]
- A history of antimicrobial drugs in animals: Evolution and revolution. J. Vet. Pharmacol. Ther.. 2021;44(2):137-171.
- [Google Scholar]
- Cloud seeding; its prospects and concerns in the modern world-A review. Int. J. Pure App. Biosci.. 2018;6(5):791-796.
- [Google Scholar]
- Mammadov, R., Düşen, O., Uysal Demir, D., Köse, E., 2009. Antioxidant and antimicrobial activities of extracts from tubers and leaves of Colchicum balansae Planchon.
- Control of postharvest anthracnose of banana using a new edible composite coating. Crop Prot.. 2010;29(1):1136-1141.
- [Google Scholar]
- Comparison of biological activity of field isolates of Steinernema feltiae with a commercial S. feltiae biopesticide product. Insects. 2021;12(9):816.
- [Google Scholar]
- Neela, F.A., Sonia, I.A., Shamsi, S., 2014. Antifungal activity of selected medicinal plant extract on Fusarium oxysporum Schlechtthe causal agent of fusarium wilt disease in tomato. Am. J. Plant Sci., 2014.
- Bacterial endophyte community dynamics in apple (Malus domestica borkh.) germplasm and their evaluation for scab management strategies. J. Fungi. 2021;7(11):923.
- [Google Scholar]
- Bioenergy: a foundation to environmental sustainability in a changing global climate scenario. J. King Saud Univ.-Sci.. 2022;34:101734 https://doi.org/10.1016/j.jksus.2021.101734
- [Google Scholar]
- Antifungal activity of some botanical extracts on Fusarium oxysporum. Open Life Sciences. 2015;10(1)
- [Google Scholar]
- Scanning agro-industrial wastes as substrates for fungal biopesticide production: Use of Beauveria bassiana and Trichoderma harzianum in solid-state fermentation. J. Environ. Manage.. 2021;295:113113
- [Google Scholar]
- HPLC–PDA polyphenolic quantification, UHPLC–MS secondary metabolite composition, and in vitro enzyme inhibition potential of Bougainvillea glabra. Plants. 2020;9(3):388.
- [Google Scholar]
- Ethnopharmacology, phytochemistry and biological activities of native chilean plants. Curr. Pharm. Des.. 2021;27(7):953-970.
- [Google Scholar]
- Antimicrobial activity of medicinal plants and induction of defense related compounds in banana fruits cv: robusta against crown rot pathogens. Biol. Control. 2013;64(1):16-25.
- [Google Scholar]
- Screening of antimicrobial properties of certain Indian medicinal plants. J. Phytol.. 2009;1(3)
- [Google Scholar]
- In vitro antifungal activity of some plant extracts against Fusarium oxysporum in blackcurrant (Ribes nigrum L.) Acta Sci. Pol. Hortorum Cultus. 2017;16(6):167-176.
- [Google Scholar]
- Medicinal and Other Useful Plants of District Swat Pakistan. Peshawar, Pakistan: Al Aziz Communications; 2003. p. :55.
- Sustainable harvest of medicinal plants at Bulashbar Nullah, Astore (nortern Pakistan) J. Ethnopharmacol.. 2003;84(2):289-298.
- [Google Scholar]
- Shinwari, Z.K., Jamil, Khan. S.A., Zahra, N.B. 2014. Molecular systematics of selected genera of subfamily Mimosoideae-Fabaceae. Pak. J. Bot. 46(2): 591-598.
- HPLC profiling conclusively distinguished two important Unani drugs, namely, Suranjan Shirin (Colchicum autumnale) and Suranjan Talkh (Colchicum luteum) Indian J. Tradit. Knowl. (IJTK). 2019;19(1):170-173.
- [Google Scholar]
- Evaluation of the antifungal activity by plant extracts against Colletotrichum gloeosporioides PENZ. Cien. Agrotecnol.. 2008;32:420-428.
- [Google Scholar]
- Trends in antimicrobial drug development: implications for the future. Clin. Infect. Dis.. 2004;38(9):1279-1286.
- [Google Scholar]
- Sumner, D.R., 2018. Crop rotation and plant productivity. In CRC Handbook of Agricultural Productivity (pp. 273-314). CRC Press.
- Theer, R.M., Alheeti, A.A., Alfalahi, A.O., 2021. A novel and rapid method for detecting bio-fungicide properties in plant species. IOP Conf. Series: Earth Environ. Sci.. 761, (1), 012024).
- Importance of Colchicum species in modern therapy and its significance in Turkey. J. Pharm. Istanbul. 2016;46(2):129-144.
- [Google Scholar]
- Compatibility of a biopesticide based on the yeast Rhodotorula mucilaginosa (Lv316) with chemical fungicides used in blackberry crops. Biocontrol 2021:1-12.
- [Google Scholar]
- Characterization of a new strain of Metarhizium novozealandicum with potential to be developed as a biopesticide. Mycology 2021:1-18.
- [Google Scholar]
- Mushroom extract of Lactarius deliciosus (L.) Sf. Gray as biopesticide: Antifungal activity and toxicological analysis. J. Toxicol. Environ. Health, Part A 2021:1-13.
- [Google Scholar]
- Seri biodiversity: An important approach for improving quality of life. J. Ent. Zool. Study. 2018;6(1):100-105.
- [Google Scholar]
- Systemic acquired resistance (SAR): A novel strategy for plant protection with reference to mulberry. Int. J. Chem. Stud.. 2018;2:1184-1192.
- [Google Scholar]
- Wani, M.Y., Mir, M.R., Mehraj, S., Rather, R.A., Ganie, N.A., Baqual, M.F., Sahaf, K.A., Hussain, A., 2018c. Effect of different types of mulches on the germination and seedling growth of mulberry (Morus SP.). Int. J. Chem. Stud. 6,1364.
- Effect of zinc on the larval growth and quality cocoon parameters of silkworm (Bombyx mori L.): a review. Int. J. Fauna and Biol. Stud.. 2018;5(4):31-36.
- [Google Scholar]







