Translate this page into:
In vitro thrombolytic potential of fibrinolytic enzyme from Brevibacterium sp. isolated from the root of the plant, Aloe castellorum
⁎Corresponding author at: Prince Sultan Research Chair for Environment and Wildlife, Department of Botany and Microbiology, College of Sciences, King Saud University (KSU), Riyadh, Saudi Arabia. anazeer@ksu.edu.sa (N. Anis Ahamed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cardiovascular diseases (CVDs) like stroke and heart attack are leading causes of mortality worldwide, resulting in about 30% of deaths. The proportion in the Kingdom of Saudi Arabia was 46%. The commercial thrombolytic agents are thus associated with a range of side effects, while bacterially derived fibrinolytic enzymes have no or little side effects. Hence, there is an urgent need to identify novel fibrinolytic enzymes to treat or prevent CVDs. An endophytic bacterial strain producing fibrinolytic enzyme was isolated from the root of the plant Aloe castellorum. Enzyme production was enhanced through a conventional method and a statistical approach. The in vitro lytic activity of blood clots was also assessed. Endangered plant of Aloe castellorum roots, researchers isolated an endophytic strain producing fibrinolytic enzymes. It was identified as Brevibacterium sp. The culture medium was also optimized by a conventional method to screen the factors significant for successful culture. Fibrinolytic enzyme production peaked when Brevibacterium sp. was cultured in the presence of maltose (613 ± 12 U/mL), followed by starch (576 ± 13 U/mL). Among the selected nitrogen sources, yeast extract (642 ± 5.9 U/mL) and beef extract (610 ± 13 U/mL) enhanced enzyme yield relative to that in the control (487 ± 7.2 U/mL). Ionic sources such as Mg2+ (672 ± 10.3 U/mL) and Ca2+ (605 ± 12.3 U/mL) showed enhanced fibrinolytic enzyme production relative to the control (405 ± 13.2 U/mL). Upon employing a two-level full factorial design for examining the significant factors, it was revealed that maltose, yeast extract, and Mg2+ were important (p < 0.05). To enhance thrombolytic agent production using central composite design and response surface methodologies. The fibrinolytic enzyme obtained from Brevibacterium sp. has great potential to lyse blood clots in vitro.
Keywords
Blood clot
In vitro
Thrombolytic agent
Cardiovascular disease
Clot lysis
1 Introduction
Cardiovascular diseases (CVDs) cause more deaths worldwide than any other diseases. They cause about 30% of all deaths globally and 17.3 million people died of them in the year. Among the six Gulf Cooperation Council countries (GCC), CVDs were estimated to account for 45% of deaths, and according to the WHO, CVDs accounted for 46% of deaths in the Kingdom of Saudi Arabia (WHO, 2011). The overall number of deaths due to heart attack and stroke is expected to reach 23.3 million by 2002–2030 (Mathers et al., 2006).
Thrombosis occurs when blood clots appear in the blood vessels and is a major causes of Cardiovascular diseases, resulting in heart attack and thrombosis. Fibrin formed through the action of thrombin from fibrinogen is an important component of blood clots (Voet and Voet 1990). Naturally occurring plasminogen activator stimulates plasminogen and generates plasmin. This generated plasmin completely degrades insoluble fibrin (Dobrovolsky and Titaeva, 2002). The concept of fibrinolytic treatment involves the administration of plasminogen stimulator intravenously, which completely lyses thrombi that develop inside blood vessels and restores the bloodstream at sites of ischemia. Currently, three kinds of fibrinolytic agent are used for the treatment of CVDs: genetically engineered tissue plasminogen activator (t-PA), streptokinase, and urokinase. Though, these available thrombolytic agents are thermolabile, expensive and can cause number of side effects, especially susceptibility to allergic reactions and gastrointestinal bleeding (Turpie et al., 2002). Many fermented products have been used in recent years as sources for characterizing fibrinolytic enzymes, for example, doenjang (Kim and Choi, 2000), Korean cheonggukjang (Kim et al., 2009), skipjack shiokara (Sumi et al 1987), fermented rice (Vijayaraghavan et al., 2017; Vijayaraghavan et al., 2016) and douche (Peng et al., 2003). Bacillus subtilis in natto was shown to generate nattokinase as a fibrinolytic agent, Fibrin in blood clots is directly dissolved as well as t-PA is produced.
The t-PA produced activates plasminogen to active plasmin to dissolve fibrin. The in vivo administration of fibrinolytic enzymes was shown to enhance endogenous plasminogen activator in animals and humans (Kim et al., 1996). Fibrinolytic enzymes using microbes, mainly food-grade organisms, have the potential to be used as drugs or additive in health foods for the treatment or prevention of CVDs. Fibrinolytic enzyme-producing organisms were isolated from traditional fermented food and the isolated enzymes showed potent activity as well as fermented shrimp paste, these enzymes were characterized from as an nattokinase (Wong and Mine, 2004) which digested blood clots in vivo (Sumi et al., 1990). The aim of the present study is to fibrinolytic enzyme obtained from Brevibacterium sp. has great potential to lyse blood clots in vitro.
2 Materials and methods
2.1 Collection of plant root and isolation of bacteria
The roots of Aloe castellorum were extracted from the Al Baha Region in Saudi Arabia using sterile plastic containers and transported to the laboratory at 4 °C. The root samples were washed in running water, surface sterilized with 70% alcohol for 1 min, washed with 2.5% sodium hypochlorite solution for 4 min, rinsed three times in sterile distilled water and finally dried. For the isolation of bacteria, a previously reported procedure was used (Jain et al., 2021).
2.2 Screening of bacterial isolates for proteolytic activity
After the bacterial culture was purified, it was transferred to nutrient broth and incubated at 37 °C for 24 h in an orbital shaking incubator. A cell-free sample was then centrifuged at 10,000 × g for 10 min and the results then loaded onto skimmed milk agar plates that which were used to determine proteolytic activity. Proteolytic enzyme-producing organisms showed a clear halo zone around the well. The proteolytic enzyme-producing bacterial isolates were selected for secondary screening. (Vijayaraghavan et al., 2016)
2.3 Screening of bacteria for fibrinolytic enzyme activity
The method of fibrin agarose followed that of Astrup and Müllertz (1952). The fibrin agarose prepared as 0.75% (w / v) with agarose and 0.15% fibrinogen (Hiemedia, Mumbai, India) and used thrombin (0.125 ml of 200 NIH U / mL) (Sigma-Aldrich, USA) and the plate was left to stand for 1 h for complete fibrin clot formation. The wells at the center of the plates were also prepared by loading cell-free culture supernatant (20 µl). The fibrinolytic enzyme-producing organisms showed a clear zone around the well (Stephani et al., 2017).
2.4 Characterization of fibrinolytic enzyme-producing strain
The isolated bacterial strain producing the maximum level of fibrinolytic enzyme was selected for characterization. This strain was studied morphologically and biochemically (Dubey et al., 2013). DNA was extracted from the potent strain as defined by Yang et al. (2008). Analysis of the 16S rDNA gene of this candidate strain was executed universal forward (27 F, 5′-AGAGTTTGATCMTGGCTAG-3′) and reverse primers (R, 5′-ACGGGCGGTGTGTRC-3′).
2.5 Fibrinolytic enzyme test
The degradation of Fibrin was qualitatively determined by the method of (Stephani et al., 2017). Briefly, an artificial blood clot was prepared using thrombin (10 µl) on fibrinogen (100 µl) (Sigma Aldrich, USA). Then, 50 µl of the fibrinolytic enzyme was added to the substrate (fibrin) and incubated for 30 min. Next, trichloroacetic acid (TCA) (0.2 M) was added and the fibrin degradation was measured at 275 nm against a reagent blank using a UV–visible spectrophotometer. For standard assay conditions, the fibrinolytic enzyme activity of resembles to the quantity of enzyme required to release 1 M L-tyrosine per minute.
2.6 Inoculum preparation and submerged fermentation
An Erlenmeyer flask of 100 ml was used to administer nutrient broth medium and the bacterial strains (Himedia, Mumbai, India). In a rotary shaker incubator with a temperature of 37 °C and a rotation speed of 150 rpm, the culture was inoculated into the medium for 18 h. Double distilled water was used for the preparation of culture medium. Submerged fermentation was performed using a production medium (Kumar et al., 2018). The fermentation took place in a 250 ml Erlenmeyer flask with incubation at 37° C. for 2 days. After 48 h, the supernatant was separated with centrifuge for 15 min at 10,000 rpm and examined for enzyme activity.
2.7 Enhanced production of fibrinolytic enzymes by conventional method
Fibrinolytic enzyme production was affected by nutrient factors rather than environmental parameters in the case of submerged fermentation. For growth and enzyme production, carbon and nitrogen sources are both important factors in nutrient balance. Carbon sources such as starch, galactose, sucrose, lactose, maltose, and xylose were selected at the 1% (w/v) level. Nitrogen sources such as beef extract, casein, yeast extract, gelatin, peptone and ammonium sulfate were also included in an amount of 1% (w / v). The ions Ca2+, Mg2+, Mn+, Co2+, and Na+ were added to the production medium at the 0.1% (w/v) level. (Vijayaraghavan et al., 2016).
2.8 Optimization of fibrinolytic enzyme production by statistical method
In order to screen the variables that were most important for enzyme production, a two-level full factorial design was applied. Five variables (fermentation period, pH, yeast extract, maltose, and MgSO4) were selected for 32 experimental runs. In this experimental design, two levels (low and high) of values were selected and the yeast extract, maltose, and MgSO4 variables were found to enhance enzyme production. Therefore, these three variables were chosen to be further optimized. To analyze the optimal concentrations of these variables, central composite design (CCD) and response surface methodology were employed. CCD at five levels (−α, −, 0, +, +α) is described in Table 1. To design and validate the experimental results, Design Expert statistical software was used.
Run
A:Yeast extract
B:Maltose
C:MgCl2
Enzyme activity
%
%
(U/mL)
1
1.170448
0.55
0.055
959
2
0.75
0.55
0.055
1810
3
0.75
1.306807
0.055
1749
4
1
1
0.1
2019
5
0.75
0.55
−0.02068
1598
6
1
0.1
0.1
12
7
0.75
0.55
0.130681
2559
8
0.5
1
0.1
2489
9
0.5
0.1
0.1
2092
10
1
1
0.01
2271
11
0.5
0.1
0.01
712
12
0.75
0.55
0.055
1650
13
0.329552
0.55
0.055
951
14
0.75
−0.20681
0.055
539
15
0.75
0.55
0.055
1672
16
0.75
0.55
0.055
1790
17
0.5
1
0.01
181
18
1
0.1
0.01
1179
19
0.75
0.55
0.055
1740
20
0.75
0.55
0.055
1781
Twenty experiments were performed for the selected three variables, which included six center points. The results were used to produce a 3D response surface plot and the optimal point was analyzed. All experiments were performed in triplicate, with the mean value being measured as the response (Y). ANOVA used to analyze the results. The experimental model was suited for second order the polynomial equation. (Vijayaraghavan et al., 2017).
Y = α0 + α1A + α2B + α3C + α1α2AB + α1α3AC + α2α3BC + α1α1A2 + α2α2B2 + α3α3C2
Where a represents yeast extract, b represents maltose, and c represents MgSO4.
2.9 Fibrinolytic enzymes as thrombolytic agents: In vitro analysis
The thrombolytic potential of fibrinolytic enzymes was analyzed via an in vitro experiment as described previously (Prasad et al., 2006). The blood clot was washed with PBS (pH 7.2) and the sample was added. The Streptokinase and buffer was used as positive and negative control. An hourly determination of the lytic activity of blood clot was carried out (Vijayaraghavan et al., 2016).
3 Results and discussion
3.1 Screening and characterization of fibrinolytic enzyme-producing bacteria
A root sample from a plant (Aloe castellorum) was used for the isolation of roots-associated endophytes. A total of around 17 bacterial isolates were obtained from the root sample and subjected to primary screening (protease screening). Out of these 17 bacterial isolates, the majority were found to be effective protease producers. Among the isolates, Brevibacterium sp. showed a 22 mm zone of inhibition around the bacterial colony on skim milk agar plates. From these protease-producing bacteria, the strain Brevibacterium sp hydrolyzed fibrin on fibrin-agarose plates (17 mm zone of hydrolysis). This strain was thus selected for characterization. The bacterium is gram-positive, not motile, nonspore-forming, and almost rod-shaped.
Its colonies are low-convex, circular with whole margins, methyl-red-positive, indole-negative and citrate utilization-positive. Molecular characterization was performed using 16S rDNA sequencing, as shown in Fig. 1a. The 16S rDNA sequence showed similarity to Brevibacterium sp. Generally, Brevibacterium sp. is an opportunistic pathogen and the proteases produced by it interact with the host during infection. This organism is involved in nosocomial and other clinical infections (Funke et al., 1997). Brevibacterium species have been identified in a variety of environments, including brown algae (Ivanova et al., 2004), moth caterpillars (Katı et al., 2010), and activated sludge (Kim et al., 2013). Recently, Renganath Rao et al. (2017) applied Brevibacterium luteolum (MTCC 5982) for the production of alkaline proteases for eco-friendly applications.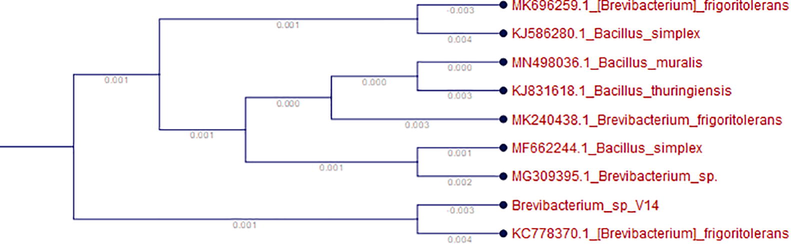
Phylogenetic assessment of 16S rDNA Brevibacterium sp. isolated from the root of a plant, Aloe castellorum, with other bacterial strains obtained by BLASTN. The sequences of the bacteria were aligned for the construction of a phylogenetic tree.
3.2 Improved fibrinolytic enzyme production by conventional method
Fibrinolytic enzyme production peaked when the Brevibacterium sp. was cultured in the presence of maltose (613 ± 12 U/mL), followed by starch (576 ± 13 U/mL) (Fig. 1b). Carbon sources such as sucrose, galactose, and starch had the least influence on fibrinolytic enzyme production. The carbon sources in the culture medium influenced bacterial cell growth and also the production of various enzymes and metabolites (Singh et al., 2016). Enzyme production peaked when the bacteria were cultured in the production medium containing 1.5% maltose (653 ± 7.6 U/mL) (Fig. 1c). At maltose concentrations of 2% and 2.5%, enzyme production was affected due to catabolic repression. In one study, (Pan et al., 2019) reported the enhanced production of fibrinolytic enzymes when the marine Bacillus subtilis was cultured in production medium containing maltose at a concentration of 1.5%. Among the selected nitrogen sources, yeast extract (642 ± 5.9 U/mL) and beef extract (610 ± 13 U/mL) enhanced enzyme yield relative to the control (487 ± 7.2 U/mL; Fig. 2a). Recently, Pan et al. (2019) reported yeast extract and tryptone as optimal medium components for fibrinolytic enzyme production. Yeast extract was supplemented at various concentrations and 1% was found to be optimal for enzyme production (655 ± 19 U/mL; Fig. 2b). Casein also stimulated the production of fibrinolytic enzymes (587 ± 19 U/mL). In Pseudomonas aeruginosa KU1, 0.1% skimmed milk enhanced the production of fibrinolytic enzymes (Kumar et al., 2018). Fibrinolytic enzymes are inducible enzymes, so the incorporation of protease-rich substances induced enzyme production. Similar results have been reported previously by (Quadar et al., (2009)).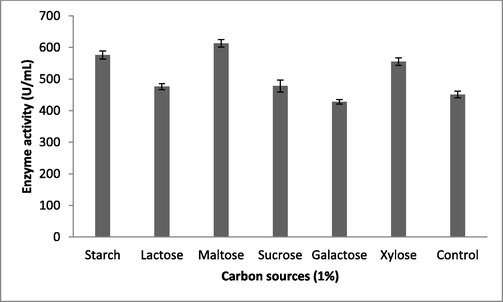
Production of fibrinolytic enzymes by carbon sources in submerged fermentation. About 1% carbon sources were incorporated into the production medium, which was incubated for 24 h. Error bar represents standard deviation.
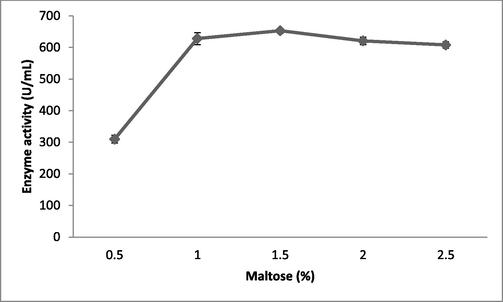
Effect of various concentrations of maltose on fibrinolytic enzyme production in submerged fermentation. Maltose was incorporated into the production medium, which was incubated for 24 h. Error bar represents standard deviation.
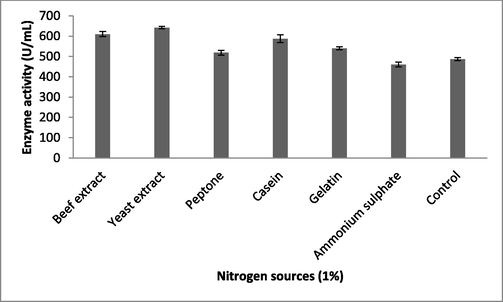
Effect of nitrogen sources on fibrinolytic enzyme production in submerged fermentation. About 1% nitrogen sources were incorporated into the production medium, which was incubated for 24 h. Error bar represents standard deviation.
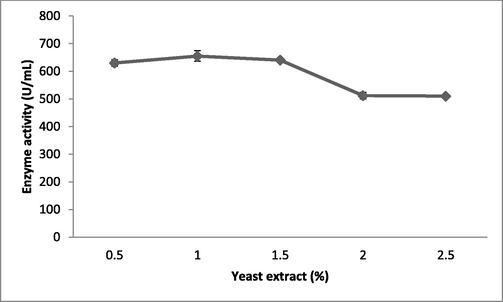
The effect of yeast extract concentrations on fibrinolysis enzyme production in submerged fermentation. Yeast extract was incorporated into the production medium, which was incubated for 24 h. Error bar represents standard deviation.
The ions such as Mg2+ (672 ± 10.3 U/mL) and Ca2+ (605 ± 12.3 U/mL) showed enhanced fibrinolytic enzyme production relative to the control (405 ± 13.2 U/mL) (Fig. 3a). Among the various concentrations of Mg2+ used, 0.03% Mg2+ enhanced fibrinolytic enzyme production, but this was depleted at higher concentrations (Fig. 3b). These ions also maintain enzyme stability. The requirement for ionic substances varies widely. In a study, (Jhample et al., 2015) reported enhanced production of fibrinolytic enzymes in Proteus penneri SP-20 supplemented with culture medium containing MnCl2·4H2O The perfect concentration of MgSO4 for the production of fibrinolytic enzymes by Bacillus sp. according to recent study to be 0.093%. IND12 (Vijayaraghavan et al., 2017).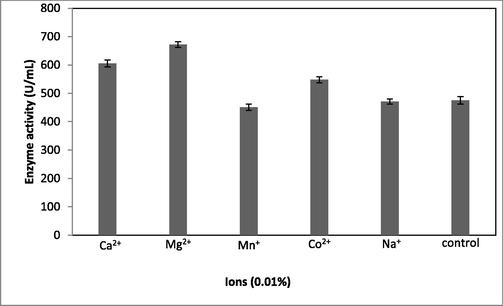
Effect of ionic sources on fibrinolytic enzyme production in submerged fermentation. About 0.01% ions were incorporated into the production medium, which was incubated for 24 h. Error bar represents standard deviation.
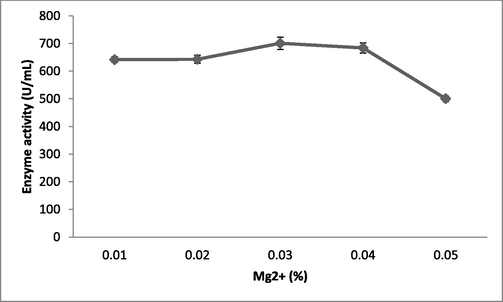
Effect of various concentrations of MgCl2 on fibrinolytic enzyme production in submerged fermentation. MgCl2 was incorporated into the production medium, which was incubated for 24 h. Error bar represents standard deviation.
3.3 Optimization of fibrinolytic enzyme production by statistical method
To optimize medium components, CCD and response surface methodology (RSM) are commonly utilized. This method has several advantages over conventional optimization methods (Chen et al., 2007; Liu et al., 2005). Recently, fibrinolytic enzymes have been optimized using various statistical approaches. In addition to the nutrient sources, minerals, temperature, and pH also critically affect the production of fibrinolytic enzymes (Mahajan et al., 2012). Optimization of enzyme production through the conventional method is strenuous and tedious (Deepak et al., 2010). RSM is a classical statistical tool used for analyzing more than one factor simultaneously and the interaction between selected variables (Box and Draper 1959). In the present investigation, optimized medium showed an enhanced enzyme yield compared to previous reports from Bacillus subtilis A26 (Agrebi et al., 2009) and Bacillus sp. strain AS-S20-I (Mukherjee and Rai, 2011). Two-level full factorial experimental designs have been widely used to screen the important variables and CCD has been widely used to identify the optimal concentrations of the selected variables. Initial screening revealed that components such as yeast extract, maltose, and MgSO4 were significant (p < 0.05). Further optimization of medium components was performed using CCD. The fibrinolytic enzyme yield varied widely and enzyme production peaked in run 7 (Table 1). The predicted R2 value was 0.9965, which strongly agreed with the adjusted R2 (0.9957); the difference was less than 0.2, indicating the accuracy of the designed model. Given its adequate precision, the model has been widely used to measure the signal-to-noise ratio. The obtained signal-to-noise ratio was more than 4 (76.107), indicating an adequate signal. The F-value of the designed model (CCD) was 495.55, which clearly implies that the designed model was significant (Table 2). The p value of all terms was significant (p < 0.05), indicating that all factors interacted and stimulated enzyme production. The lack of fit F-value was more than 0.05, indicating the robustness of the designed model.
Source
Sum of
df
Mean
F-value
p-value
Model
9,924,508
9
1,102,723
495.552
<0.0001
A-Yeast extract
30.63518
1
30.63518
0.013767
0.908919
B-Maltose
1,830,560
1
1,830,560
822.6343
6.18E-11
C-MgCl2
1,105,291
1
1,105,291
496.7061
7.43E-10
AB
1,306,536
1
1,306,536
587.1434
3.26E-10
AC
3,260,181
1
3,260,181
1465.091
3.53E-12
BC
424581.1
1
424581.1
190.8022
7.7E-08
AÂ2
1,114,896
1
1,114,896
501.0224
7.12E-10
BÂ2
643551.8
1
643551.8
289.2053
1.04E-08
CÂ2
204338.3
1
204338.3
91.82745
2.35E-06
Residual
22252.42
10
2225.242
Lack of Fit
448.9182
5
89.78365
0.020589
0.999693
Pure Error
21803.5
5
4360.7
Cor Total
9,946,761
19
3D response surface plots are produced as graphical illustrations of the interactions between two selected variables and also used to detect the optimal concentration for the enhanced production of enzymes. 3D graphs showed the interaction among the selected three variables (Fig. 4a–c). A 3D RSM graph provides a very simple way to understand interactions between variables, as well as the optimal levels between them for optimum enzyme production. (Surwase et al., 2012). Elliptical or circular order RSM plots imply interactions between variables. In this study, the interactions between yeast extract and MgCl2 were found to be more significant than those of the other tested variables. In this study, enzyme yield increased over three-fold than unoptimization. Threefold relative to that without optimization. As a result of this study, the yield of fibrinolytic enzymes was higher than that of Bacillus sp. enzymes. Bacillus subtilis AS-S20-I (Agrebi et al., 2009) and AS-S20-I (Mukherjee and Rai, 2011). The selected factors remarkably enhanced the production of fibrinolytic enzymes in submerged fermentation. Our results show that optimizing the medium composition using two-level full factorial design and CCD of RSM increased the enzyme yield. The enzyme yield increased from 451 to 2559 U/mL using the optimized medium.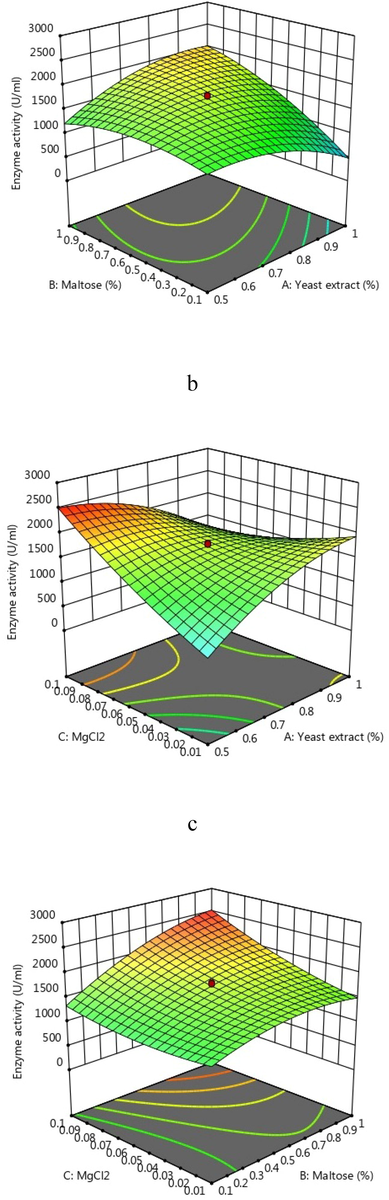
3D surface revealed the interactions between maltose and yeast extract (a), yeast extract and MgCl2 (b), and maltose and MgCl2 (c).
3.4 Human blood clot lytic activity of fibrinolytic enzyme
The crude fibrinolytic enzyme completely hydrolyzed the fibrin blood clot within 7 h at 32 ± 1 °C. Bacillus subtilis ICTF-1 has been demonstrated to break up blood clots in vitro with fibrinolytic enzyme (Mahajan et al., 2012). In addition, according to (Yuan et al., 2012). In vitro and in vivo a fibrinolytic enzyme from Bacillus subtilis LD-8547 hydrolyzed blood clots. Recent studies have also purified and characterized the fibrinolytic enzyme from Bacillus sp. IND7. The purified enzyme completely digested a blood clot in vitro (Vijayaraghavan et al., 2016). It is widely accepted that in vitro blood clot lysis is a reliable method of evaluating fibrinolytic agents' thrombolytic activities (Prasad et al., 2006).
4 Conclusion
An enzyme-producing bacterium that produces fibrinolytic enzymes was characterized in the present study, and it was identified as Brevibacterium sp. In the early stages, the variables were screened through one variable at a time; then, the central composite design was used to improve yield and determine maximum enzyme production. The fibrinolytic enzyme obtained from Brevibacterium sp. has great potential to lyse blood clots in vitro. The fibrinolytic enzymes from bacteria could be effectively used as alternative thrombolytic agents because commercially available thrombolytic agents have various side effects.
Acknowledgements
This project was supported by King Saud University’s Deanship of Scientific Research Chair. We are very grateful to the Prince Sultan Research Chair for Environment and Wildlife & Saudi Biological Society. We also thank the Department of Botany & Microbiology, College of Sciences, King Saud University (KSU), Riyadh, Saudi Arabia, for encouragement and support by funding this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Fibrinolytic enzymes from a newly isolated marine bacterium Bacillus subtilis A26: characterization and statistical media optimization. Can. J. Microbiol.. 2009;55(9):1049-1061.
- [CrossRef] [Google Scholar]
- The fibrin plate method for estimating fibrinolytic activity. Arch. Biochem. Biophys.. 1952;40(2):346-351.
- [CrossRef] [Google Scholar]
- A basis for the selection of a response surface design. Ann. Stat. Asso.. 1959;54(287):622-654.
- [Google Scholar]
- Medium optimization for the production of recombinant nattokinase by Bacillus subtilis using response surface methodology. Biotechnol. Prog.. 2007;23(6):1327-1332.
- [CrossRef] [Google Scholar]
- Medium optimization and immobilization of purified fibrinolytic URAK from Bacillus cereus NK1 on PHB nanoparticles. Enzy. Microb. Technol.. 2010;47(6):297-304.
- [CrossRef] [Google Scholar]
- The fibrinolysis system: regulation of activity and physiologic functions of its main components. Biochem. Biokhimiia. 2002;67:99-108.
- [CrossRef] [Google Scholar]
- Isolation, production, purification, assay and characterization of fibrinolytic enzymes (Nattokinase, Streptokinase and Urokinase) from bacterial sources. Afr. J. Biotechnol.. 2013;10:1408-1420.
- [CrossRef] [Google Scholar]
- Clinical microbiology of Coryneform bacteria. Clin. Microbiol. Rev.. 1997;10(1):125-159.
- [Google Scholar]
- Brevibacterium celere sp. nov., isolated from degraded thallus of a brown alga. Int. J. Syst. Evol. Microbiol.. 2004;54:2107-2111.
- [CrossRef] [Google Scholar]
- Arnebia euchroma, a plant species of cold desert in the himalayas, harbors beneficial cultivable endophytes in roots and leaves. Front. Microbiol.. 2021;12
- [CrossRef] [Google Scholar]
- Statistical media optimization for enhanced production of fibrinolytic enzyme from newly isolated Proteus penneri SP-20. Biocatal. Agric. Biotechnol.. 2015;4(3):370-379.
- [CrossRef] [Google Scholar]
- Brevibacterium pityocampae sp. nov., isolated from caterpillars of Thaumetopoea pityocampa (Lepidoptera, Thaumetopoeidae) Int. J. Syst. Evol. Microbiol.. 2010;60:312-316.
- [CrossRef] [Google Scholar]
- Characterization of a 27 kDa fibrinolytic enzyme from Bacillus amyloliquefaciens CH51 isolated from cheonggukjang. J. Microbiol. Biotechnol.. 2009;19:997-1004.
- [CrossRef] [Google Scholar]
- Brevibacterium ammoniilyticum sp. nov., an ammonia-degrading bacterium isolated from sludge of a wastewater treatment plant. Int. J. Syst. Evol. Microbiol.. 2013;63:1111-1118.
- [CrossRef] [Google Scholar]
- Purification and characterization of subtilisin DJ-4 secreted by Bacillus sp. strain DJ-4 screened from Doen-Jang. Biosci. Biotechnol. Biochem.. 2000;64(8):1722-1725.
- [CrossRef] [Google Scholar]
- Purification and characterization of a fibrinolytic enzyme produced from Bacillus sp. strain CK 11–4 screened from Chungkook-Jang. Appl. Environ. Microbiol.. 1996;62(7):2482-2488.
- [Google Scholar]
- Process optimization for production of a fibrinolytic enzyme from newly isolated marine bacterium Pseudomonas aeruginosa KU1. Biocatal. Agric. Biotechnol.. 2018;14:33-39.
- [CrossRef] [Google Scholar]
- Optimization of nutritional conditions for nattokinase production by Bacillus natto NLSSE using statistical experimental methods. Process Biochem.. 2005;40(8):2757-2762.
- [Google Scholar]
- Fibrinolytic enzyme from newly isolated marine bacterium Bacillus subtilis ICTF-1: media optimization, purification and characterization. J. Biosci. Bioeng.. 2012;113(3):307-314.
- [CrossRef] [Google Scholar]
- Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med.. 2006;3(11):e442.
- [CrossRef] [Google Scholar]
- A statistical approach for the enhanced production of alkaline protease showing fibrinolytic activity from a newly isolated Gram-negative Bacillus sp. strain AS-S20-I. New Biotechnol.. 2011;28(2):182-189.
- [CrossRef] [Google Scholar]
- Non-sterile submerged fermentation of fibrinolytic enzyme by marine Bacillus subtilis harboring antibacterial activity with starvation strategy. Front. Microbiol.. 2019;10:1025.
- [CrossRef] [Google Scholar]
- Purification and characterization of a fibrinolytic enzyme produced by Bacillus amyloliquefaciens DC-4 screened from douchi, a traditional Chinese soybean food. Comp. Biochem. Physiol. B: Biochem. Mol. Biol.. 2003;134(1):45-52.
- [CrossRef] [Google Scholar]
- Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb. J.. 2006;4:14.
- [CrossRef] [Google Scholar]
- Opimization of protease production from newly isolated strain Bacillus sp. PCSIR EA-3. Ind. J. Biotechnol.. 2009;8:286-483.
- [Google Scholar]
- Alkaline protease production from Brevibacterium luteolum (MTCC 5982) under solid-state fermentation and its application for sulfide-free unhairing of cowhides. Appl. Biochem. Biotechnol.. 2017;182(2):511-528.
- [CrossRef] [Google Scholar]
- Strategies for fermentation medium optimization: an in-depth review. Front. Microbiol.. 2016;7:2087.
- [CrossRef] [Google Scholar]
- Food origin fibrinolytic enzyme with multiple actions. HAYATI J. Biosci.. 2017;24(3):124-130.
- [CrossRef] [Google Scholar]
- Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol.. 1990;84:139-143.
- [CrossRef] [Google Scholar]
- A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia. 1987;43(10):1110-1111.
- [CrossRef] [Google Scholar]
- Optimization of l-DOPA production by Brevundimonas sp. SGJ using response surface methodology. Microb. Biotechnol.. 2012;5:731-737.
- [CrossRef] [Google Scholar]
- ABC of antithrombotic therapy: Venous thromboembolism: treatment strategies. BMJ. 2002;325:948-950.
- [CrossRef] [Google Scholar]
- Cow dung is a novel feedstock for fibrinolytic enzyme production from newly isolated Bacillus sp. IND7 and its application in in vitro clot lysis. Front. Microbiol.. 2016;7:361.
- [CrossRef] [Google Scholar]
- Novel sequential screening and enhanced production of fibrinolytic enzyme by Bacillus sp. IND12 using response surface methodology in solid-state fermentation. Biomed Res. Int.. 2017;2017:1-13.
- [Google Scholar]
- Biochemistry (2nd ed.). USA: John Wiley & Sons; NY; 1990. p. :1087-1095.
- Global Status Report on Non-communicable Diseases 2010. Geneva, Switzerland: World Health Organization; 2011. p. :9-31.
- Novel fibrinolytic enzyme in fermented shrimp paste, a traditional Asian fermented seasoning. J. Agric. Food Chem.. 2004;52(4):980-986.
- [CrossRef] [Google Scholar]
- A simple and rapid method for extracting bacterial DNA from intestinal microflora for ERIC-PCR detection. World J. Gastroenterol.. 2008;14:2872-2876.
- [CrossRef] [Google Scholar]
- Thrombolytic effects of Douchi Fibrinolytic enzyme from Bacillus subtilis LD-8547 in vitro and in vivo. BMC Biotech.. 2012;12(1)
- [CrossRef] [Google Scholar]







