Biological synthesis and characterization of Passiflora subpeltata Ortega aqueous leaf extract in silver nanoparticles and their evaluation of antibacterial, antioxidant, anti-cancer and larvicidal activities
⁎Corresponding author. selsarat@yahoo.com (Kuppusamy Selvam),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Passiflora subpeltata contain rich source of secondary metabolites and its plant materials mostly used for cancer and mosquito related problem. Therefore, in this study, green synthesis of silver nanoparticles using Passiflora subpeltata of aqueous extract (Ps-AgNPs) as reducing and stabilizing agent. The UV–Visible spectra of Ps-AgNPs showed absorption peak at 456 nm and formation of AgNPs. XRD pattern showed the crystalline nature and SEM images displayed predominantly spherical shape of NPs. FT-IR analysis exhibition capping of AgNPs by bioactive compounds from Ps-AgNPs. Antibacterial activity in PS-AgNPs shown good activity with best of B. cereus (27 mm) at higher dose of 75 μg/mL. Antioxidant activity was tested against DPPH, ABTS and H2O2 assays and the best scavenging assay were observed in DPPH with IC50 value of 108.92 μg/mL. The anti-proliferative activity in human colon cancer cell-line (HT-29) indicates that the significant result with IC50 value of 33.05 μg/mL. Larvicidal activity of PS-AgNPs showed good activity against Cx. quinquefasicatus larvae (LC50-4.99 units and LC90-22.19units). The green synthesized NPs could be potential activity and it used in different biomedical application.
Keywords
Silver Nanoparticles
FE-SEM
H2O2
Colon cancer
Cx. quinquefasciatus
1 Introduction
Nanoparticles stand for a particle with a nano size of 1–100 nm. These materials are latest, unique, and superior physical and chemical properties compared to its massiveness structure, due to an increase in the ratio of the surface area per volume of the material/particle (Aritonang et al., 2019). Hence, the nanoparticles find applications in human life for different fields (Rathnakumar et al., 2019). The nanoparticles such as gold, silver, titanium and platinum are commonly used in nanoparticle synthesis (Khandel et al., 2018). Metallic NPs, particularly, silver NP’s has various therapeutically applications due to low dose have enhanced bioavailability, target specificity and long-lasting activity. The synthesis of metal nanoparticles, can be achieved by various strategies for example, physical, chemical and biological. Among these methods, biological synthesis is fast, low cost, environmental-friendly and hence is a preferred method (Rajkumar et al., 2018). Silver nanoparticles (AgNPs) synthesized using different plants such as Rumex hastatus (Gul et al., 2021a,b), Galphimia glauca (Chakraborty et al., 2021), Ixora brachypoda (Bhat et al., 2021), Commiphora myrrh (Alwhibi et al., 2020). The plant derived silver nanoparticles (Ag-NPs) find extensive use in biological field such as antibacterial, anticancer and larvicidal activities (Divya et al., 2018). Mosquitoes are a vector to convey several tropical diseases as malaria, dengue fever, chikungunya, Rift Valley fever, filariasis, West Nile fever and Japanese encephalitis. Understanding the main concepts of plant extracts with AgNPs used in traditional medicine as therapeutic agents can help to identify the novel compounds for treating many diseases (Ashwini and Asha, 2017).
Plants are rich source of secondary metabolites and it used for various therapeutically application (Sowndarya et al., 2017). Nano-based drug delivery systems create a new way in pharmaceutical industry (Patra et al., 2018). Plant metabolites with metal nanoparticles combination could be capable of deliver the drugs. Passiflora subpeltata Ortega belongs to the Passifloraceae (Passion flower family) and its commonly known as “passion flower” as well as malaikovai (tamil name). It distributed in some Pacific islands. Ps plants an exotic fast-growing vine plant with two–three ornate flowers contain yellow-green fruits. Traditionally, the plant is utilized for its properties such antioxidant, analgesic, anti-inflammatory, antipyretic, anti-proliferative etc., (Chunchegowda et al., 2020). In the current work low cost synthesis of AgNPs by a one-step reduction of silver ions using Passiflora subpletata leaf extract was done to assess the nanoparticles synthesis. The physicochemical characteristics of the synthesized silver nanoparticles were analyzed using various techniques (UV–vis, FT-IR, FE-SEM with EDAX and XRD analysis). The antibacterial, antioxidant, anti-proliferative and larvicidal activities of the synthesized Ps-AgNPs were estimated.
2 Materials and methods
2.1 Collection and extraction
P. subpeltata was collected from Shevaroy hills (August-2019) in Salem district, Tamil Nadu, India. Ps-leaves were dried for two weeks and it’s powdered using steel blender. The powder of the plant leaves was weighed at 10 g and transfer into beaker (100 mL-D·H2O) than boiled in 30 min. The filter paper (Whatman no. 1) was used to filter the extract and the solutions were refrigerated (4 °C) for further study.
2.2 Synthesis of AgNPs
About 10 mL of Ps-aqueous leaf extract (ALE) was added into 90 mL of silver nitrate (Mercury Scientific, Salem) solution at room temperature and allowed them to mix properly. As a control AgNO3 was used. After, 24 h, the mix elucidation changed from yellow to dark brown colour. The solution was centrifuged at 5000 rpm (560 g force) for 12 min and the pellet was obtained. The pellets were washing out double distilled water than dried and analyzed.
2.3 Characterization of AgNPs
Ps-AgNPs in reduction was observed using UV–Vis spectroscopy (Shimadzu UV-1800) in the range of 400 to 700 nm wavelength. Crystalline property was analyzed by the X-Ray Diffraction (Rigaku Mini Flex,) operating at 40 kV with 30 mA using CuKα (λ = 0.154 Å) radiation with the crystallite sizes of silver nanoparticles and were calculated by the equation of Debye-Scherrer formula. Functional groups were analyzed through FT-IR (Perkin Elmer-Tensor 27, Bruker, Germany) and wavelength of 400–4000 cm−1. Scanning electron microscopy (FE-SEM-EVO 18, ZEISS, UK) was used in shape and size were analyzing for NPs with elemental was analyzed (EDAX-Quorum Technologies, U.K).
2.4 Antioxidant assay
2.4.1 2,2-Diphenyl-1-picrylhydrazyl assay (DPPH)
DPPH radical scavenging activity of Ps-AgNPs was described by earlier, Loganathan et al. (2021a,b). In briefly, 1.0 mL of DPPH solution (0.2 mM) was taken having sample various dose (20, 40, 60, 80, and 100 µg/mL) were used and stand for 30 min beneath dark conditions. After 30 min the absorbance was read at 517 nm (UV–visible Spectrophotometer). Ascorbic acid was used as control. The percentage of inhibition in DPPH radical scavenging activity was calculated as follows;
2.4.2 Hydroxyl scavenging
The assay was described by earlier, Loganathan et al. (2021a,b). 1 mL of various concentration of sample (20–100 µg/mL) was mixed into 3 mL H2O2 (1.0 mL of 1.5 mM FeSO4, 0.7 mL of 6 mM H2O2, 0.3 mL of 20 mM sodium salicylate). The reaction mixture was incubated for 37 °C and the absorbance was measured at 562 nm. The percentage scavenging effect was calculated as,
2.4.3 2,2′-Azino-bis-3-ethylbenzothiazoline-6-Sulfonic acid (ABTS)
This assay was followed by, Loganathan et al. (2021a,b). The reaction was began via the addition of 1.0 mL of diluted ABTS to 10 µl of different concentrations (20, 40, 60, 80 and 100 µg/mL) of sample and also 10 µl of ethanol as a control. The absorbance was read at 734 nm after 6 min and the percentage inhibitions were calculated. The inhibition was calculated according to the equation;
2.5 Antibacterial activity
The antibacterial efficacy of AgNPs was examined by the agar well diffusion method (Anonymous, 1996). The selected human pathogens like as, Bacillus cereus and Escherichia coli were obtained from Department of Microbiology, Periyar University, Salem. They were maintained in nutrient agar slants as well as sub-cultured from time to time in the laboratory. The inoculums were prepared by growing a single colony for overnight in nutrient broth and by adjusting the turbidity to 0.5 McFarland standards Kora et al., (2009). B. cereus and E. coli were tested using of Ps-AgNPs. The test cultures were swabbed on nutrient agar (NA) Plates and permissible to dry for 10 min. A total of three concentrations used like as 25, 50 and 75 µg. Chloramphenicol was used as positive controls (30 μg/disc). The culture plates were incubated for 12 h at 37 °C. Inhibition zones were measured in millimeters (mm).
2.6 Anti-proliferative activity
2.6.1 Cell culture
Colon cancer cell line (HT-29) was obtained from National Centre for Cell Science. The cell lines were grown in Dulbecco with modified eagle medium (DMEM) elevated glucose medium (Sigma Aldrich, USA), as increase with 10% fetal bovine serum, as well as antibiotics (20 mL of penicillin) (Hi Media, India). This cell line was kept in culture at 37 °C and 5% of humidified CO2 atmosphere.
2.6.2 Cell treatment procedure
This assay was followed by earlier, Mosmann, (1983). The monolayer cells were detached with trypsin-ethylene diamine tetra acetic acid (EDTA) to make single cell suspensions and viable cells were counted using a hemocytometer and diluted with medium containing 5% FBS to give final density of 1 × 105 cells/mL. One hundred microlitres per well of cell suspension were seeded into 96-well plates at plating density of 10,000 cells/well and incubated to allow for cell attachment at 37 °C, 5% CO2, 95% air and 100% relative humidity. After 24 h the cells were treated with serial concentrations of the Ps-AgNPs. They were initially dissolved in dimethylsulfoxide (DMSO) and an aliquot of the sample solution was diluted to twice the desired final maximum test concentration with serum free medium. Additional four serial dilutions were made to provide a total of five concentrations of AgNPs (6.5, 12.5, 25, 50 and 100 µg/mL). Aliquots of 100 µl of these different sample dilutions were added to the appropriate wells already containing 100 µl of medium, resulting in the required final sample concentrations. Following sample addition, the plates were incubated for an additional 48 h at 37 °C, 5% CO2, 95% air and 100% relative humidity. The medium containing without samples were served as control and triplicate was maintained for all concentrations.
2.6.3 MTT assay
3-[4,5-Dimethylthiazol-2-yl] 2,5-diphenyltetrazolium bromide (MTT) is a yellow water soluble tetrazolium salt. A mitochondrial enzyme in living cells, succinate-dehydrogenase, cleaves the tetrazolium ring, converting the MTT to an insoluble purple formazan. Therefore, the amount of formazan produced is directly proportional to the number of viable cells. After 48 h of incubation, 15 µl of MTT (5 mg/ml) in phosphate buffered saline (PBS) was added to each well and incubated at 37 °C for 4 h. The medium with MTT was then flicked off and the formed formazan crystals were solubilized in 100 µl of DMSO and then measured the absorbance at 570 nm using micro plate reader. Cell morphology was taken from Confocal microscope (Olympus, Japan). The living cells percentage was calculated below.
2.7 Larvicidal activity
2.7.1 Collection and rearing of mosquito larvae
Ae. aegypti, An. stephensi and Cx. Quinquefasciatus were collected from Indian Council of Medical Research (ICMR)-Vector Control Research Centre, Madurai and then it kept during 14:10 h photoperiod, 27 °C, 70 ± 5%- Relative Humidity (RH) with provided the fed.
2.7.2 Larvicidal bioassay
Bio-efficacy was performed as per method of WHO (2005) standard with several changes as per the protocol of Thandapani et al. (2018). Larvae of 4th instrar in 20 number were added each plastic cup (200 mL - D.H20). Ps-ALE and Ps-AgNPs concentrations such as, 5, 10, 15, 20 and 25 mg/L were supplemented to the cups. The control treatment was also maintained (distilled water). Larval death was counted after 12 and 24 h. Mortality percentage (larvae) was labeled to average of three replicates. In earlier, method of Abbott, (1925) was used for calculated in larval mortality and death.
2.8 Statistical analysis
The results were analyzed a Mean ± SD. Biological activity data was subjected to One and Two-Way ANOVA, with Dunnett’s Multiple Comparison tests using PRISM software version 5.2 (Graph Pad Software Inc, USA). The larval mortality was calculated by Probit analysis for finding out LC50, and LC90 values. The chi square values were also calculated by SPSS software version 20.0 (SPSS., USA).
3 Results and discussion
3.1 UV-Vis spectra analysis
Ps-AgNPs in UV absorption peak at wavelength of 456 nm (Fig. 1). The same results were reported by Patra et al. (2019). The Surface Plasmon Resonance (SPR) is a confirmation of the synthesis of Ps-AgNPs. In recent report, AgNPs in Garcinia kola was showed UV absorbance peak at 445 nm (Akintelu et al., 2021). Mtambo et al. (2019) was studied the Bidens pilosa extract of AgNPs showed uv absorption peak at 410 nm.
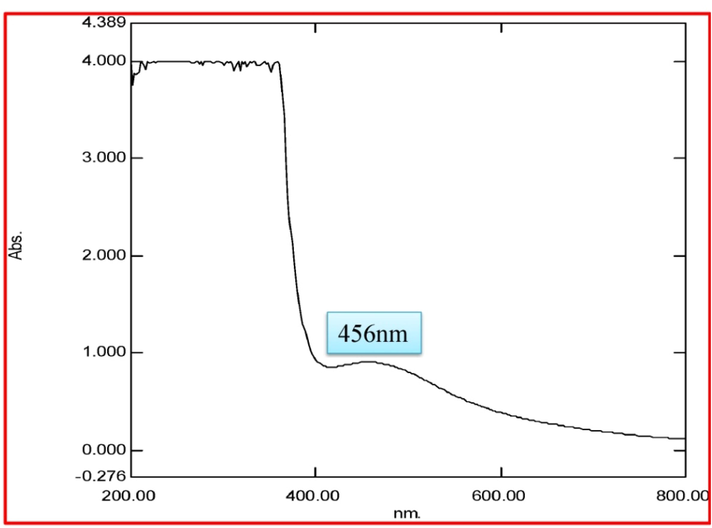
- UV–visible absorbance spectra obtained from silver nanoparticles using P. subpeltata aqueous leaf extracts (Ps-ALE).
3.2 FT-IR study
Ps-AgNPs of FT-IR spectra shown in Fig. 2. The intense peaks range at 1050 cm−1 for S=O (sulfoxides), 1347.41 cm−1 for C-X (Fluoride), 1605.44 cm−1 for N–H (amines and amides) 2836.42 cm−1 for C–H (Aldehyde) and 3078.00 cm−1 for C–H (Alkenes) are represented in Table 1. The essential functional groups such as, alcohol, amides, alkanes, methyl, aliphatic and halides confirmed their presence of NPs. It was stabilizing, capping and dipping agents of the AgNPs (Vijayaraghavan et al., 2012). Gul et al. (2021a,b) have reported that the Rumex hastatus derived AgNPs showed the presence of various functional groups. Malva verticillata extracts of AgNPs displayed the presence of different bioactive compounds (Sk et al., 2020).
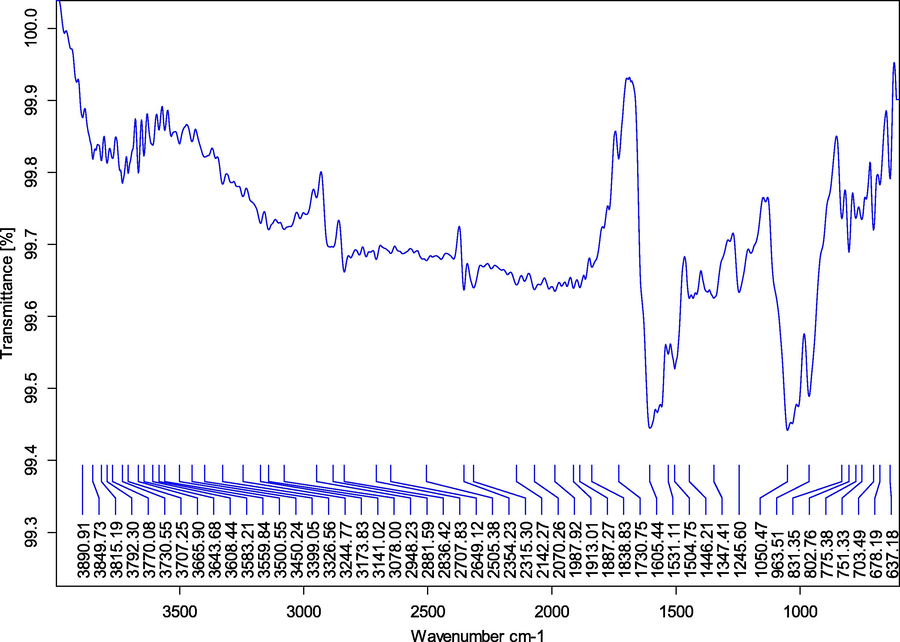
- FT-IR studied from silver nanoparticles synthesized in P. subpeltata aqueous leaf extracts (Ps-ALE).
| Wave number (cm−1) | Intensity | Group Compound |
Functional group |
|---|---|---|---|
| 1050.47/Bending | Strong | S=O | Sulfoxides |
| 1347.41/Stretch | Strong | C–X | Fluoride |
| 1605.44/Bending 2836.42/Stretch 3078.00/Stretch |
Medium-Strong Weak Medium |
N–H C–H C–H |
Primary and secondary amines and amides Aldehyde Alkenes |
3.3 XRD
XRD results from Ps-AgNPs shown 9 peaks at 2θ ranges of 27.87°, 32.14°, 38.77°, 44.20°, 46.10°, 54.40°, 57.34°, 64.34° and 77.72° can be corresponds values to 210, 101, 111, 200, 231, 142, 241, 220 and 311 are displayed in Fig. 3. In, similar result was finding in AgNPs of Sargassum myriocystum (Balaraman et al., 2020). Asphodelus aestivus aerial part extract of AgNPS displayed presence of four diffraction values (Fafal et al., 2017). The lattice planes of pure silver based on the face-centre cubic structure (JCPDS No. 4-0783) and the XRD results was clearly indicate that the crystalline in nature of AgNPs. In earlier report, the AgNPs from Carmona retusa leaf extract showed 4 diffraction peaks by XRD (Rajkumar et al., 2018). In this study, the average crystal size of nanoparticles measure by Debye Scherrer’s formula and its size of 22.6 nm.
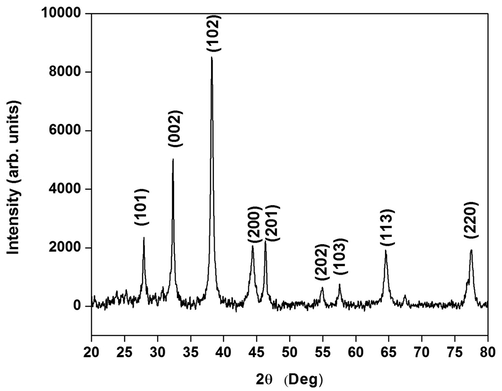
- XRD pattern of silver nanoparticles synthesized in P. subpeltata aqueous leaf extracts (Ps-ALE).
3.4 FE-SEM
Ps-AgNPs nanoparticles morphological structure in spherical with size ranging between 20 and 40 nm (Fig. 4a). Similarly were observed from, AgNPs of Ajug abracteosa; Cinnamomum tamala (Afreen et al., 2020; Nahar et al., 2020). EDAX spectra indicated that the strong Ag signal and other elemental such as Carbon and Silver oxide (Fig. 4b). Huong and Nguyen (2021) have analysed the AgNPs by Brassica oleracea leaves extract of EDAX spectrum showed the presence of Ag (Senthil-Nathan, 2013,2015, 2020).
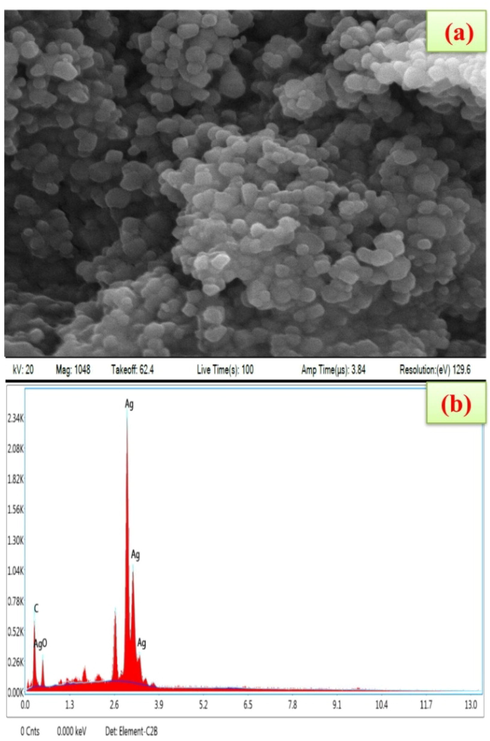
- (a) Scanning Electron Microscope image of silver nanoparticles synthesized in P. subpeltata aqueous leaf extracts (Ps-ALE), (b) Edax spectrum showed the presence of strong Ag signals.
3.5 Antibacterial potential
Fig. 5 displayed the potential antibacterial activity in Ps-AgNPs. The high level zone of inhibition were recorded in B. cereus (27.5 mm) and E. coli (23.0 mm) at 75 µg/mL (Table 2). Biosynthesized in CuO-NPs contain good activity in lower concentrations (Kasi et al., 2021). Gul et al. (2021a,b) have stated that the Rumex hastatus derived AgNPs showed promising activities. The three most common mechanisms proposed to date are (1) the disruption of ATP production and DNA replication by uptake of free silver ions, (2) ROS generation by silver and silver nanoparticles and (3) direct damage of silver nanoparticles to the cell membranes (Marambio-Jones and Hoek, 2010). The study indicated that the AgNPs were acted efficiently against the tested microbes and than it used for another source of antibiotics.
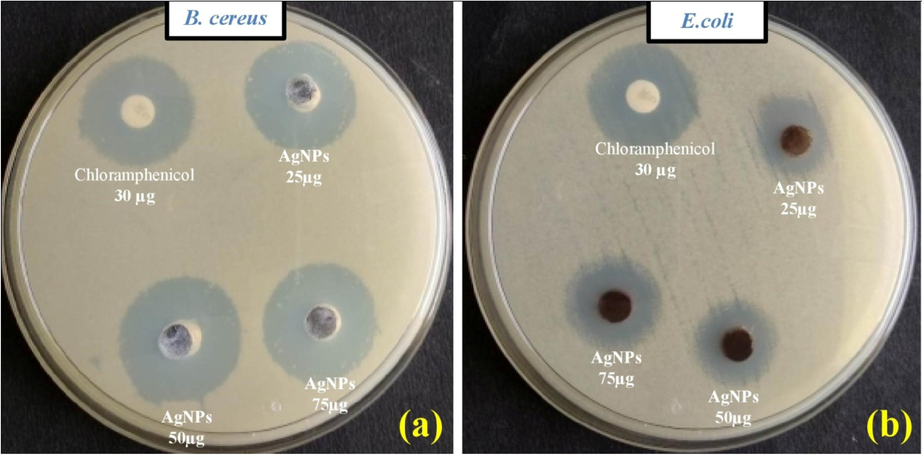
- Antibacterial study of silver nanoparticles synthesized in P. subpeltata aqueous leaf extracts (Ps-ALE), (a) B. cereus, (b) E. coli.
| Microorganisms | Zone of inhibition (mm) | |||
|---|---|---|---|---|
| Positive Control (Chloramphenicol) |
25 (µg/mL) | AgNPs | ||
| 50 (µg/mL) | 75 (µg/mL) | |||
| B. cereus | 29.0 ± 1.5 | 22.5 ± 1.1 | 24.5 ± 0.5 | 27.0 ± 1.1 |
| E. coli | 25.5 ± 0.5 | 17.5 ± 0.5 | 21.0 ± 1.1 | 23.0 ± 1.0 |
Value are given as Data (Mean ± SD) (n = 3) represent the zone of bacterial growth inhibition (mm).
3.6 Antioxidant activity
The scavenging ability of Ps-AgNPs was determined using DPPH, ABTS and H2O2. DPPH assay, the highest percentage of inhibition was seen in 55.30 at 125 µg/mL. The percentage of inhibition was increased with increasing the concentration of the sample (F-16.12; df-4; P < 0.01, P < 0.001) (Fig. 6(a). Similar report were obtained from, AgNPs in stem bark extract of Diospyros montana (Bharathi et al., 2018). DPPH-IC50 value of Ps-AgNPs was found to be 108.92 μg/mL while IC50 values for standard (ascorbic acid) 108.60 μg/mL. In earlier studied from, AgNPs in Erythrina suberosa leaf extract showed the DPPH good activity with IC50 value of 30.04 μg/mL (Mohanta et al. (2017). The ABTS of Ps-AgNPs were displayed the standard (ascorbic acid) IC50value of 102.39 μg/mL and also 109.94 μg/mL where estimated by Ps-AgNPs (F-47.53; df-4; P < 0.001) (Fig. 6(b). In earlier report, the IC50value of 78.10 μg/mL was obtained to Cassia angustifolia flower extract of AgNPs (Bharathi and Bhuvaneshwari, 2019). In recent study, green synthesis of AgNPs using various plants extract showed the significant ABTS activities (Viskelis et al. 2021). The H2O2 of Ps-AgNPs and ascorbic acid displayed the scavenging percentage of 57.30% as well as 51.69% at higher dose of 125 µg/mL with IC50 value of Ps-AgNPs as well as standard (ascorbic acid) 121.35; 108.57 µg/mL (F-16.11; df-4; P < 0.001). (Fig. 6c). Guntur et al. (2018) were reported that the silver NPs synthesis of Desmostachhya bipinnata leaf extract showed the good activity with IC50 assessment of 259.14 μg/mL. In another report, AgNPs in Carmona retusa leaf extract were showed the H2O2 IC50 value of 47 µg/mL. The antioxidant results indicate that the usage of Ps-AgNPs as having antioxidant toward protect health against oxidative stresses with degenerative sicknesses.
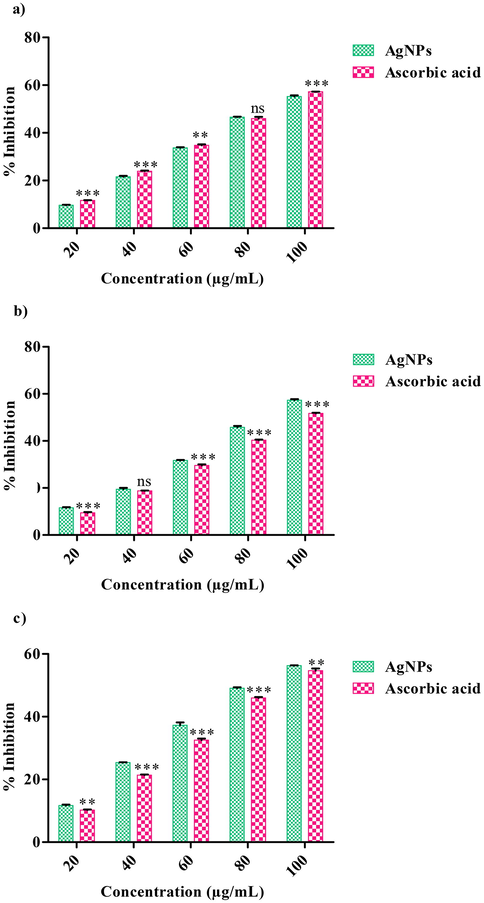
- Antioxidant activity of AgNPs using P. subpeltata aqueous leaf extract (Ps-ALE), (a) DPPH radical scavenging activity, The values are expressed as mean ± SD values and analyzed by Two-Way analysis of variance (ANOVA), Not Significant (ns), Asterisk (**, ***) indicates significant different among treatments with respect to control (P < 0.01 and P < 0.001). (b) ABTS radical scavenging activity, The values are expressed as mean ± SD and analyzed by Two-Way analysis of variance (ANOVA), Not Significant (ns), Asterisk (***) indicates significant different among treatments with respect to control (P < 0.001). (c) Hydroxyl scavenging activity, The values are expressed as mean ± SD and analyzed by Two-Way analysis of variance (ANOVA), Asterisk (**, ***) indicates significant different among treatments with respect to control (P < 0.01 and P < 0.001).
3.7 Anti-cancer study
In this study, Ps-AgNPs were examined in Human colon cancer cell-line (HT-29) by MTT assay at 24 h. The cell inhibition percentages as well as treated images are displayed in (F-12.27; df-5; P < 0.001) Figs. 7 and 8. In this study, was dose depended activity (HT-29). Similar observations were studied from Murraya koenigii leaf extract of Ag nanoparticles (Roshni et al., 2018). Ps-AgNPs IC50 value was obtained and it found to be 33.05 μg/mL when the results was compare to earlier report the cytotoxicity studied against HT-29 showed the IC50 value of 150.8 μg/mL by AgNPs (Venjatadri et al., 2020). Dehghanizade et al. (2018) have exerted that the AgNPs from Anthemisa tropatana leaves extract shown better activity against HT-29 with IC50 value of 4.88 μg/mL.
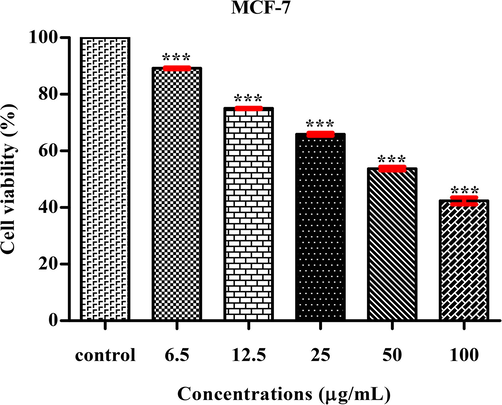
- MTT assay confirming the anti-cancer activity of Ps-AgNPs against HT-29 cell line, The values are expressed as mean ± SD values and analyzed by One-Way analysis of variance (ANOVA), Asterisk (***) indicates significant different among treatments with respect to control (P < 0.001).

- Anti-cancer observed from colon cancer cell line (HT-29) in confocal microscope (340 pixel); Control and various concentrations (6.5, 12.5, 25, 50 and 100 µg/mL) of AgNPs using P. subpeltata aqueous leaf extract (Ps-ALE).
3.8 Larvicidal activity
P. subpeltata aqueous leaf extract and AgNPs showed strong larvicidal potential are represented in Tables 3–5. In this study, 100% mortality of larva was identified for Cx. quinquefasciatus by Ps-AgNPs. The same work where studied from plant mediated from AgNPs have declared the better larivicidal activity (Sultana et al., 2020; Rajkumar et al., 2019). ZnO-NPs of K. sumatrensis shown better activity with LC50 0.08, mg/mL and LC9019.46 mg/mL against Cx. quinquefasciatus (Loganathan et al., 2021a,b). Loganathan et al. (2021a,b) reported that the Knoxia sumatrensis of the methanolic extract shown strong larvicidal activity on 3 mosquito species with highest toxicity on Cx. quinquefasciatus larvae with LC50 4.84 mg/mL and LC90 19.33 mg/mL. The plant mediated AgNPs has effectively controlled the fourth instar larvae of Cx. quinquefasciatus.
| Time (Hour) | Samples | LC50 (mg/mL) (LCL-UCL) |
LC90 (mg/mL) (LCL-UCL) |
χ2 | Df |
|---|---|---|---|---|---|
| 12 | Plant extract |
25.68(22.21–32.45) | 47.23(38.32–67.07) | 2.35 | 13 |
| AgNPs | 20.88(19.30–28.11) | 46.31(36.21–68.15) | 1.87 | 13 | |
| 24 | Plant extract | 13.88(11.72–15.87) | 30.72(26.76–37.58) | 1.28 | 13 |
| AgNPs | 10.25(07.67–12.19) | 25.94(22.81–31.12) | 1.05 | 13 | |
LC50-Lethal concentration kills 50% of the exposed larvae, LC90-Lethal concentration kills 90% of the exposed larvae. LCL-Lower confidence limit, UCL- Upper confidence limit, χ2 Chi-square value, df = degrees of freedom.
| Time (Hour) | Samples | LC50 (mg/mL) (LCL-UCL) |
LC90 (mg/mL) (LCL-UCL) |
χ2 | Df |
|---|---|---|---|---|---|
| 12 | Plant extract |
27.61(23.68–35.80) | 49.31 (39.65–71.59) | 2.59 | 13 |
| AgNPs | 23.40(19.98–30.17) | 48.45 (38. 42–72.81) | 2.41 | 13 | |
| 24 | Plant extract | 15.43(13.31–17.59) | 33.02 (28.53–41.03) | 2.89 | 13 |
| AgNPs | 11.55(08.99–13.58) | 28.66 (24.95–35.11) | 2.08 | 13 | |
LC50-Lethal concentration kills 50% of the exposed larvae, LC90-Lethal concentration kills 90% of the exposed larvae. LCL-Lower confidence limit, UCL-Upper confidence limit, χ2 Chi-square value, df = degrees of freedom.
| Time (Hour) | Samples | LC50 (mg/mL) (LCL-UCL) |
LC90 (mg/mL) (LCL-UCL) |
χ2 | Df |
|---|---|---|---|---|---|
| 12 | Plant extract |
20.04 (17.58–23.69) | 40.45 (33.76–53.89) | 1.12 | 13 |
| AgNPs | 15.60 (13.05–18.31) | 37.06 (30.92–49.55) | 1.04 | 13 | |
| 24 | Plant extract | 08.45 (4.11–11.12) | 29.31 (24.79–38.27) | 1.39 | 13 |
| AgNPs | 04.99 (1.26–7.78) | 22.19 (19.21–27.38) | 2.05 | 13 | |
LC50-Lethal concentration kills 50% of the exposed larvae, LC90-Lethal concentration kills 90% of the exposed larvae. LCL-Lower confidence limit, UCL-Upper confidence limit, χ2 Chi-square value, df = degrees of freedom.
4 Conclusion
AgNPs from Passiflora subpeltata was successfully synthesized and characterized. UV–Vis spectral analyses confirm the AgNPs peak at 456 nm. Larvicidal activity of AgNPs exhibited significant toxic effects on Cx. quinquefascitatus. In-vitro antioxidant study displayed a significant activity on scavenging of free radicals activity. The anti-cancer potential of AgNPs was showed dose dependent activity and IC50 value of 33.05 μg/mL against colon cancer cell line. Antibacterial activity of Ps-AgNPs of tested microbes showed good activity at higher concentrations. Overall, the outcome of the study clearly stated the Ps-AgNPs act as simple, eco-friendly and low cost approach could be useful for the control of vector bone diseases and biomedical applications.
Acknowledgement
The authors Acknowledge to Periyar University, Salem to providing University Research Fellowship (Ref. No. PU/AD-3/URF/2016). The authors also thank to Department of Botany, School of Life Sciences, Periyar University, Salem, Tamil Nadu-636 011, India, for providing infrastructural facility. The authors extend their appreciation to the Researchers supporting project number (RSP2022R470) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abbott, W. S., 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18, 265-267.
- Synthesis of silver nanoparticles using aqueous extract of medicinal plants’ (Impatiens balsamina and Lantana camara) fresh leaves and analysis of antimicrobial activity. Int. J. Microbiol.. 2019;2019:1-8.
- [Google Scholar]
- Green biosynthesis of silver nanoparticle using Commiphora myrrh extract and evaluation of their antimicrobial activity and colon cancer cells viability. J. King Saud Univ.-Sci.. 2020;32(8):3372-3379.
- [Google Scholar]
- Anonymous, (1996). Pharmacopiea of India (The Indian Pharmacopiea), 3rdEdn., Govt. of India, New Delhi, Ministry of Health and Family Welfare.
- Larvicidal activity of natural products against mosquito species. Int. J. Chem. Technol. Res.. 2017;10:875-878.
- [Google Scholar]
- Phyco-synthesis of silver nanoparticles mediated from marine algae Sargassum myriocystum and its potential biological and environmental applications. Waste Biomass Valor.. 2020;11(10):5255-5271.
- [Google Scholar]
- Evaluation of the cytotoxic and antioxidant activity of phyto-synthesized silver nanoparticles using Cassia angustifolia flowers. BioNano Sci.. 2019;9(1):155-163.
- [Google Scholar]
- Biosynthesis of silver nanoparticles using stem bark extracts of Diospyros montana and their antioxidant and antibacterial activities. J. Nanostruct. Chem.. 2018;8(1):83-92.
- [Google Scholar]
- Biogenic synthesis, characterization and antimicrobial activity of Ixora brachypoda (DC) leaf extract mediated silver nanoparticles. J. King Saud Univ.-Sci.. 2021;33(2):101296.
- [CrossRef] [Google Scholar]
- Evaluation of antioxidant, antimicrobial and antiproliferative activity of silver nanoparticles derived from Galphimia glauca leaf extract. J. King Saud Univ.-Sci. 2021:101660.
- [Google Scholar]
- Biosynthesis of zinc oxide nanoparticles using leaf extract of Passiflora subpeltata: characterization and antibacterial activity against Escherichia coli isolated from poultry faces. J. Cluster Sci. 2020:1-10.
- [Google Scholar]
- Green synthesis of silver nanoparticles using Anthemisa tropatana extract: characterization and in vitro biological activities. Artif. Cells Nanomed. Biotechnol.. 2018;46(1):160-168.
- [Google Scholar]
- Biopolymer gelatin-coated zinc oxide nanoparticles showed high antibacterial, antibiofilm and anti-angiogenic activity. J. Photochem. Photobiol., B. 2018;178:211-218.
- [Google Scholar]
- Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Asphodelus aestivus Brot. aerial part extract. S. Afr. J. Bot.. 2017;112:346-353.
- [Google Scholar]
- Rumex hastatus derived silver nanoparticles development and their potential applications as hepatic-protection agent along with antimicrobial activity. J. King Saud Univ.-Sci.. 2021;33(7):101587.
- [CrossRef] [Google Scholar]
- Rumex hastatus derived silver nanoparticles development and their potential applications as hepatic-protection agent along with antimicrobial activity. J. King Saud Univ.-Sci.. 2021;33:101587
- [Google Scholar]
- In vitro studies of the antimicrobial and free-radical scavenging potentials of silver nanoparticles biosynthesized from the extract of Desmostachya bipinnata. Anal. Chem. Insights. 2018;13
- [CrossRef] [Google Scholar]
- Green synthesis, characterization and antibacterial activity of silver nanoparticles using Sapindus mukorossi fruit pericarp extract. Mater. Today:. Proc.. 2021;42:88-93.
- [Google Scholar]
- Biogenic synthesis of copper oxide nanoparticles using leaf extracts of Cissus quadrangularis and Piper betle and its antibacterial effects. Micro Nano Lett.. 2021;16(8):419-424.
- [Google Scholar]
- Biogenesis of metal nanoparticles and their pharmacological applications: present status and application prospects. J. Nanostruct. Chem.. 2018;8:217-254.
- [Google Scholar]
- Superior bactericidal activity of SDS capped silver nanoparticles: synthesis and characterization. Mater. Sci. Eng., C. 2009;29:2104-2109.
- [Google Scholar]
- Phytochemical and pharmacological evaluation of methanolic extract of Knoxia sumatrensis leaves. J. Herbs Spices Med Plants. 2021;27(2):200-217.
- [Google Scholar]
- Metal oxide nanoparticle synthesis (ZnO-NPs) of Knoxia sumatrensis (Retz.) DC. Aqueous leaf extract and It’s evaluation of their antioxidant, anti-proliferative and larvicidal activities. Toxicol. Rep.. 2021;8:64-72.
- [Google Scholar]
- A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res.. 2010;12(5):1531-1551.
- [Google Scholar]
- Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.) Front. Mol. Biosci.. 2017;4:14.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1-2):55-63.
- [Google Scholar]
- Physico-chemical, antimicrobial and anticancer properties of silver nanoparticles synthesised from organ-specific extracts of Bidens pilosa L. S. Afr. J. Bot.. 2019;126:196-206.
- [Google Scholar]
- Synthesis and characterization of Silver nanoparticles from Cinnamomum tamala leaf extract and its antibacterial potential. Int. J. Nano Dimens.. 2020;11:88-98.
- [Google Scholar]
- Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol.. 2018;16(1)
- [CrossRef] [Google Scholar]
- Facile green biosynthesis of silver nanoparticles using Pisum sativum L. outer peel aqueous extract and its antidiabetic, cytotoxicity, antioxidant, and antibacterial activity. Int. J. Nanomed.. 2019;14:6679.
- [Google Scholar]
- Pharmacological and larvicidal potential of green synthesized silver nanoparticles using Carmona retusa (Vahl) Masam leaf extract. J. Cluster Sci.. 2018;29(6):1243-1253.
- [Google Scholar]
- Stalling behaviour of chloride ions: a non-enzymatic electrochemical detection of α-Endosulfan using CuO interface. Sens. Actuators, B. 2019;293:100-106.
- [Google Scholar]
- Anticancer activity of biosynthesized silver nanoparticles using Murraya koenigii leaf extract against HT-29 colon cancer cell line. Sci. World J. Cancer Sci. Ther.. 2018;10:72-75.
- [Google Scholar]
- Physiological and biochemical effect of neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front. Physiol.. 2013;4:359.
- [Google Scholar]
- A review of biopesticides and their mode of action against insect pests. In: Thangavel P., Sridevi G., eds. Environmental Sustainability. New Delhi: Springer India; 2015. p. :49-63.
- [CrossRef] [Google Scholar]
- A review of resistance mechanisms of synthetic insecticides and botanicals, phytochemicals and essential oils as alternative larvicidal agents against mosquitoes. Front. Physiol.. 2020;10:1591.
- [Google Scholar]
- Synthesis of gold and silver nanoparticles using Malva verticillata leaves extract: Study of gold nanoparticles catalysed reduction of nitro-Schiff bases and antibacterial activities of silver nanoparticles. Curr. Opin. Green Sustainable Chem.. 2020;3:100006.
- [CrossRef] [Google Scholar]
- Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif. Cells Nanomed. Biotechnol.. 2017;45(8):1490-1495.
- [Google Scholar]
- Bio-nanoparticle assembly: a potent on-site biolarvicidal agent against mosquito vectors. RSC Adv.. 2020;10:9356-9368.
- [Google Scholar]
- Enhanced larvicidal, antibacterial, and photocatalytic efficacy of TiO2 nanohybrids green synthesized using the aqueous leaf extract of Parthenium hysterophorus. Environ. Sci. Pollut. Res.. 2018;25:10328-10339.
- [Google Scholar]
- Biomimetic synthesis of silver nanoparticles by aqueous extract of Syzygium aromaticum. Mater. Lett.. 2012;75:33-35.
- [Google Scholar]
- World Health Organization (WHO) (2005). Geneva. www.WHO/CDS/WHOPES/GCDPP/13.







