Translate this page into:
Purification, characterization and studies of a novel cysteine protease inhibitor from Juglans regia: Implications as a potential biopesticide
⁎Corresponding author. moskhan@ksu.edu.sa (Mohd Shahnawaz Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
To isolate and characterize a novel phytocystatin from walnut and investigate it for biopesticide development.
Methods
A battery of methodology was employed. Initially, phytocystatin was extracted and purified from walnut using ammonium sulfate saturation (60–80%), followed by gel filtration chromatography on the Sephacryl S-100 HR column. Further characterization studies including pH and temperature stability, molecular weight, secondary structure, protease inhibitory assay and antimicrobial activity were carried using various techniques viz: spectroscopy, electrophoresis, and circular dichroism (CD) techniques.
Results
Thiol protease inhibitor from walnut (WCPI) was isolated and purified with high (71.4%) yield and 184-fold purification. The molecular weight of the purified inhibitor was found to be around 11.2 kDa. Kinetic tests revealed that the inhibitor competitively inhibited papain and other cysteine proteases such as ficin and bromelain. It also exhibited significant antimicrobial activity against bacterial species.
Conclusion
Walnut cysteine protease inhibitor (WCPI) from Juglans regia (Kashmiri walnut) was isolated to homogeneity and had features of other members of the phytocystatin family. It demonstrated potential antimicrobial activity and may serve as an initial step towards developing enhanced pest control methods based on natural molecules.
Keywords
Cystatin
SDS-PAGE
Chromatography
Antimicrobial activity
1 Introduction
Cystatins form a broad group of associated evolutionary proteins that function as protease inhibitors. Technically, cystatins form a single protein family due to significant similarities in their amino acid sequences. These inhibitors are reversible and have a particular inhibitory role in the enzymatic action of cysteine proteases (Otto and Schirmeister, 1997). Once detected in mammalian systems, they were later described in plants such as rice (Abe et al., 1992), kiwi fruit (Rassam and Laing, 2004), sugarcane (Soares-Costa et al., 2002) and cowpea (Fernandes et al., 1991). Such plant system cystatins are generally referred to as phytocystatins and have a broad range of molecular weights (5–87 kD) (Rawlings and Barrett, 1990). These phytocystatins include a consensus sequence [LVI]-[AGT]-[RKE]-[FY]-[AS]-[VI]-X-[EDQV]-[HYFQ]-N at N-terminal (Nt) which is an α-helix area missing from animal cystatins (Margis et al., 1998). Fundamental phytocystatin characteristic structural elements consist of four β sheets linked to alpha-helix in the Nt region and two hairpin loops (Nagata et al., 2000).
Phytocystatins perform diverse physiological roles in plants like storage, protein turnover, plant development and growth. Phytocystatins are also involved in plant defense processes such as regulators of proteolytic activity (Solommon et al., 1999), programmed cell death (Ceros and Carbonell, 1993) and abiotic stress conditions (Franco and Melo, 2000). Moreover, antifungal activity of sugarcane cystatins has been detected in vitro (Soares-Costa et al., 2002). Consequently, these phytocystatins are looked upon as suitable replacements for traditional pesticides, which in the last few years were banned in large numbers for their detrimental qualities like acute toxicity, accumulations as pesticide residues and contamination of the food chain. Owing to above facts, the aim of the present research was designed to purify and characterize walnut cysteine protease inhibitor (WCPI) and evaluate its bactericidal potency in-vitro.
2 Materials and methods
2.1 Materials
Walnuts were bought from Srinagar (Jammu and Kashmir) local market. Papain, ficin, bromelain, Sephacryl S-100 HR, p-nitrophenyl phosphate and anti-rabbit alkaline phosphatase (conjugate) were brought from Sigma (St. Louis, MO, USA). Molecular weight markers, adjuvants from Freund were purchased from Genie, India Ltd. Casein, L-cysteine and ethylenediamine tetra acetate (EDTA) were procured from SRL, India. All other chemicals used were of the highest degree of purity and was commercially available.
2.2 Methods
2.2.1 Extraction of walnut cysteine protease inhibitor (WCPI)
500 g of Juglans regia (Kashmiri walnuts) were washed, deshelled and further grind with a grinding machine to form a powder. The powdered sample was then defatted with n-hexane using the soxhlet apparatus (Soxhlet, 1879). Cysteine protease inhibitor subsequently was isolated as reported by Bhat et al. with slight modifications. Further, 40 gm of the defatted walnut was homogenized in ice-cold phosphate buffer (50 mM, pH 7.5) containing 0.15 M NaCl, 3 mM EDTA and thoroughly stirred for 1 h. The mixture was then filtered using clean muslin fabric. The filtrate was centrifuged for 10 min at 5000 rpm, and the supernatant was further processed for purification.
2.2.2 Purification of WCPI
Cysteine protease inhibitor was purified from the walnut extract by a two-step process including ammonium sulfate precipitation and gel filtration column (GFC) chromatography. Ammonium sulfate required to precipitate the protein was optimized by independently adding different salt concentrations (0–20%, 20–40%, 40–60%, 60–80%) to the crude extract. The precipitate was collected at 10,000 rpm for 15 min by centrifugation. The precipitate was then re-dissolved in buffer and dialyzed against a 50 mM phosphate buffer, pH 7.5 containing 0.15 M NaCl.
After dialysis, the crude protein extract was subjected to second step of the purification process by size exclusion chromatography on Sephacryl S-100 HR column (70 × 1.8 cm). The crude walnut extract was loaded on Sephacryl S-100 HR column with a flow rate of around 15 ml/hr, and pre-equilibrated with 50 mM NaPO4 buffer of pH 7.5. The protein was eluted and 20 fractions of 5 ml each were collected. Finally, protein concentration and inhibitory activity against proteases were conducted on each fraction. Fractions with maximum inhibitory activity were collected, pooled and subjected to further analyses.
2.2.3 Protein estimation
The concentration of protein was estimated using Lowry’s method (Lowry et al., 1951). Bovine serum albumin (BSA) was used to prepare the standard curve.
2.2.4 Assay of cysteine proteinase inhibitory (CPI) activity
Functional property of purified inhibitor was accomplished by its ability to inhibit Papain’s caseinolytic activity using the Kunitz method (Kunitz, 1947). In brief, papain was incubated with various amounts of purified inhibitor in a phosphate buffer (20 mM, pH 7.5, 0.14 M cysteine and 0.047 M EDTA) at 37 °C for 40 min. Further, 2% casein solution was added to the mixture and incubated again for 30 min. The reaction was stopped by adding 10% TCA. Finally, the supernatant was collected after centrifugation at 2500 rpm for 10 min. The protein concentration in the supernatant was measured at 280 nm absorbance. One unit of inhibitor activity has been defined as the decline by one unit of absorbance of trichloroacetic acid-soluble casein hydrolysis product released by protease action at 280 nm at 37 °C in a given volume of assay.
Percentage Inhibition, as shown below, has been determined by the following equation:
WCPI inhibitory activity has also been investigated against other proteases (ficin, bromelain, trypsin, and chymotrypsin)
2.2.5 Polyacrylamide gel electrophoresis (PAGE)
The Laemmli method (Laemmli, 1970) of electrophoresis was used to examine the homogeneity and purity of the isolated protease inhibitor. Native PAGE (7.5%) and SDS-PAGE (12.5%) under reducing and non-reducing conditions were tested.
2.2.6 Determination of the molecular weight
The molecular weight of the purified inhibitor (WCPI) was determined by two different methods. Sephacryl S-100 HR column was used to compare the elution profile or retention time of our purified protease inhibitor along with specific marker proteins of different molecular weight. SDS-PAGE under reducing conditions was also used for the electrophoretic calculation of molecular weight.
2.2.7 Estimation of Thiol group
Thiol groups were calculated by Ellman method, using Ellman's reagent {5,5′-dithiobis-(2-nitrobenzoic acid) or DTNB} and 13,600 M−1 cm−1 extinction coefficient (Ellman, 1959). L-Cysteine was used as a standard.
2.2.8 Carbohydrate content
The content of carbohydrates was measured employing glucose as standard by the Dubois et al. (Dubois et al., 1956) method.
2.2.9 Optimum temperature and stability
The optimum temperature for the activity of purified inhibitor (WCPI) was measured by incubating the reaction mixtures at different temperatures ranging from 10 to 100 °C with an increase of 10-units. Samples were then checked for residual protease inhibitory activity against papain after rapidly cooling them in ice-cold water bath. Moreover, its stability was checked after incubating enzyme at its optimum temperature for different time and their protease activity were measured as discussed above.
2.2.10 Optimum pH for the activity of WCPI
Pre-incubation of 50 μg of WCPI in variable pH buffers, 50 mM sodium acetate buffer (pH 3–5), sodium phosphate buffer (pH 6–8) and Tris-HCl buffer (pH 9–10) for 30 min at 37 °C was used to estimate the optimum pH. Residual activity for the purified inhibitor was then measured against papain.
2.2.11 Structural characterization
UV–Vis absorption spectroscopy: A UV–Visible absorption difference spectrum was recorded for WCPI (20 μM) along with activated papain at 25 °C with a molar ratio of 1:1. All Spectra were measured between 200 and 300 nm on a Shimadzu UV–Visible Spectrophotometer UV-1700, using a 1 cm path length cuvette. All of the measured spectra were typical of minimum 4 scans.
Intrinsic fluorescence spectroscopy: Fluorescence measurements were performed on Shimadzu spectrofluorophotometer (RF-540) for papain, WCPI and WCPI-papain complexes. The wavelength of excitation was 280 nm, and the wavelength range of emissions was 300–400 nm. The concentration of WCPI was 5 μM. Each spectrum was typical of 3 minimum scans.
Circular dichroism (CD) spectroscopy: The CD spectra were measured on a Jasco spectropolarimeter (Jasco-815 model) in the far-ultraviolet region between 190 and 250 nm with a path length of 0.1 cm at 25 °C. The concentration of WCPI was kept to 0.2 mg/ml in sodium phosphate buffer (20 mM, pH 7.5).
2.2.12 Antibacterial activity
In order to determine the antibacterial activity, Agrobacterium tumefaciens LBA 4404, and Erwinia carotovora bacterial strains were provided from the culture collection of botany and microbiology departments, Aligarh Muslim University, India. Muller Hinton liquid medium was prepared, and the bacterial test organism was grown for 24 h at 28 °C. Overnight cultures were diluted to approximately 104 colony-forming units (CFU) with a fresh Muller Hinton liquid medium and incubated with the increasing WCPI concentrations (0, 2, 5, 10 and 15 µM) at 28 °C for 20 h. We prepared the control samples using the same protocol.
3 Results and discussion
3.1 Purification of walnut cysteine protease inhibitor (WCPI)
Cysteine protease inhibitor (CPI) was purified from Kashmiri walnut kernels using a simple two-step procedure. Crude extract of walnuts was subjected to different concentration of ammonium sulfate and the highest inhibitory activity and protein content were detected at 60–80% saturation of (NH4)2SO4. Dialyzed protein loaded on size exclusion chromatography (Sephacryl S-100 HR) column resulted into its fractionation and single peak having maximum anti-papain activity was obtained (Fig. 1). This single peak corresponded to fractions 12, 13, 14, and 15 showed maximum anti-papain activity and high protein content (Fig. 1). These fractions showed protease inhibitory activity of approximately 65% (fraction-12) to about 88% (fraction-14), were pooled and named as WCPI (walnut cysteine proteinase inhibitor). This procedure of gel filtration chromatography after ammonium sulfate fractionation lead to a high purification fold of 183.89 and protein yield of 71.43% (Table 1). Purification of CPI from other sources has been reported earlier using ion exchange and affinity chromatography, chromatofocusing and gel filtration (Rassam and Laing, 2004; Bhat et al., 2017). One unit of enzyme inhibitory activity is defined as the amount of inhibitor bringing about 0.001 change in O.D./ml/min.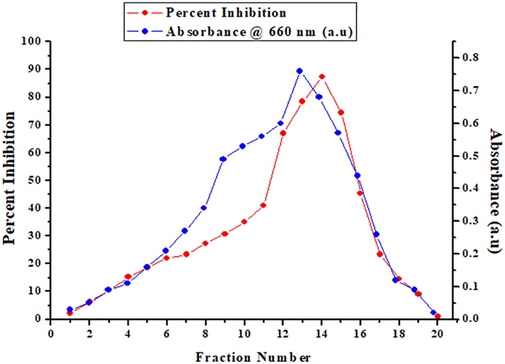
Elution profile of WCPI on Sephacryl S-100 HR gel filtration chromatography. Dialyzed sample (3 ml) was applied in column, equilibrated with 50 mM NaPO4 buffer (pH 7.5). The inhibitor was eluted in the same buffer at a flow rate of 15 ml/hour. Fractions (5 ml) were monitored for activity and protein. The single inhibitory peak corresponding to WCPI including fractions 12, 13, 14 and 15, having good amount of protein was proceeded for further studies.
S. no. Step of purification
Total Volume (ml)
Total Protein (mg)
Total Activity (units)
Specific Activity (units/mg
Fold Purification
% Yield
1
Crude homogenate
100
3100
58
0.018
1
100
2.
Ammonium sulphate fractionation (60–80%)
12
276.3
49.2
0.18
10
84.83
3.
Gel filtration S-100 HR
20
12.5
41.4
3.31
183.89
71.4
3.2 Specificity of the purified protease inhibitor (WCPI)
The homogeneity and purity of WCPI were also examined. Functional analysis was conducted using casein as a substrate that revealed the inhibitor to be significantly inhibiting the activity of papain protease. The inhibitor’s specificity was also investigated by examining its inhibitory behaviour against various proteases including cysteine proteases (papain, ficin, bromelain), and serine proteases (chymotrypsin and trypsin). It was found that the inhibitor inhibits only cysteine proteases rather than serine proteases suggesting homogeneity of the isolated preparation (Fig. 2). Similarly, different cystatins have been isolated, and their functional parameters were elucidated via employing the same procedures (Bhat et al., 2017; Khaki et al., 2017).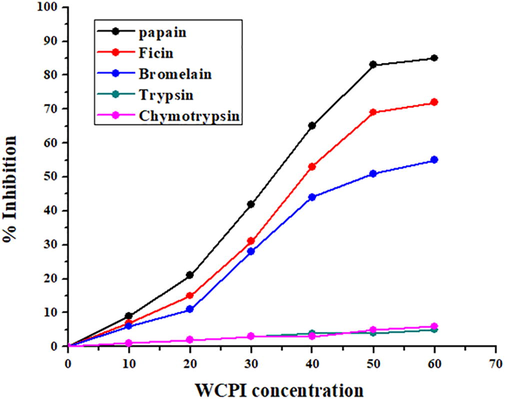
Differential inhibitory activity against different proteases. The inhibitory activity of WCPI towards thiol proteinases, papain, ficin and bromelain and serine proteinases, trypsin and chymotrypsin was examined using casein as substrate.
3.3 Biochemical characterizations of WCPI
Determination of molecular weight: The molecular weight of WCPI in native state was determined by gel-filtration chromatography on Sephacryl S-100 HR column by passing marker proteins through the column. The elution volume (Ve) of each protein was determined and the values of which were divided by void volume (Vo) to obtain (Ve/Vo). The ratio Ve/Vo was plotted against log of molecular weight of different marker proteins which gave a satisfactory linear relationship between Ve/Vo and log M. The Ve/Vo of WCPI was also determined from the same column and then used to estimate its molecular weight from the standard calibration plot. As depicted in the Fig. 3a, the molecular weight of the inhibitor isolated from walnut kernels came out to be 11.43 kDa.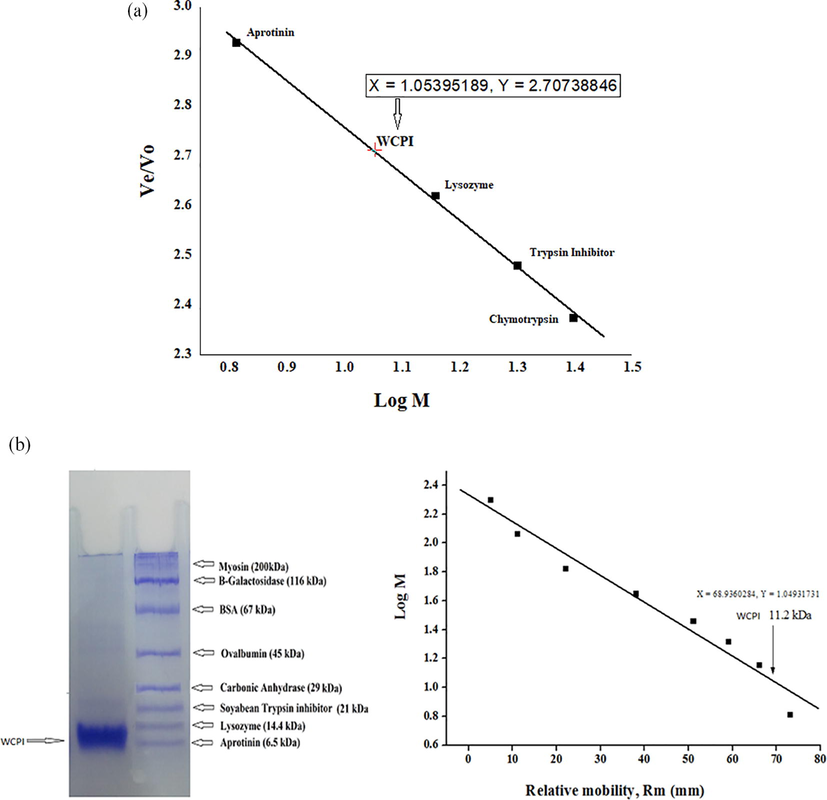
Molecular weight determination of WCPI. Different standard protein of known molecular weight were run on gel filtration column and their Ve/V0 vs log M was used to calculate the molecular weight of WCPI (Fig. 3a). The gel pattern of the inhibitor fraction yielded a single polypeptide band with a molecular weight of 11.2 kDa. Plot of log M vs relative mobility (Rm) of markers for determination of molecular weight of purified inhibitor. The arrow indicates position of WCPI (Fig. 3b).
SDS-PAGE further examined the purified WCPI for its molecular weight by plotting log M against mobility as illustrated in Fig. 3b. It was found that molecular weight was 11.2 kDa, under reduction conditions (with βME) and non-reduction conditions (without β-ME). Thus, from these two independent methods a significantly satisfactory reproducible molecular weight of WCPI was obtained. As we can see, the protein migrated as a single band on SDS-PAGE in both reductive and non-reductive environments suggesting that WCPI is constituted of a single polypeptide chain with no subunit structure. Earlier, cysteine protease inhibitor from black gram (Vigna mungo Hepper) and rice bean (Vigna umbellata Thunb) reported a molecular weight of 12 kDa by Tricine-SDS-PAGE method (Benjakul et al., 1998). Cysteine protease inhibitor having molecular weight between 10 and 15 kDa is widely distributed in plant seeds (Hines et al., 1991). Cystatins were categorized in 3 distinct forms based on the complexity of their molecules. Type 1 and 2 are having a lower molecular weight of approximately 11–14 kDa, the occurrence or non-occurrence of disulphide bonds and carbohydrate content (Abrahamson et al., 2003). Phytocystatins are known to be the intermediate family between group 1 and 2. Type 3 the Kininogens are more complex members with high molecular weight, and their distinctive property is the presence of carbohydrates (Ohkubo et al., 1984). WCPI in the present study can be placed in-between type 1 and type 2 families, which is a distinctive feature of the phytocystatins.
Thiol group and carbohydrate content: It was found that newly isolated WCPI was lacking the content of carbohydrate and sulfhydryl groups similar to the distinctive features of both Type I/II cystatin families. This again confirms that the WCPI finds its place in the family phytocystatins, as both cystatins of type 1 and type 2 and phytocystatins, generally lack carbohydrate content.
4 Optimum pH and temperature
The inhibitor (WCPI) has an optimal pH of about 7.6 (Fig. 4a) and is established to be inactive in extreme acidic (pH 3.0) and alkaline (pH 10.0) conditions. The isolated WCPI was stable in the neutral and somewhat at alkaline pH range. This indicates that extreme pH conditions may modify the electrostatic interactions between charged amino acids such as aspartate, lysine, glutamate and arginine, thereby disturbing the cysteine protease inhibitor’s structure and rendering it completely or partly inactive with respect to its inhibition activity.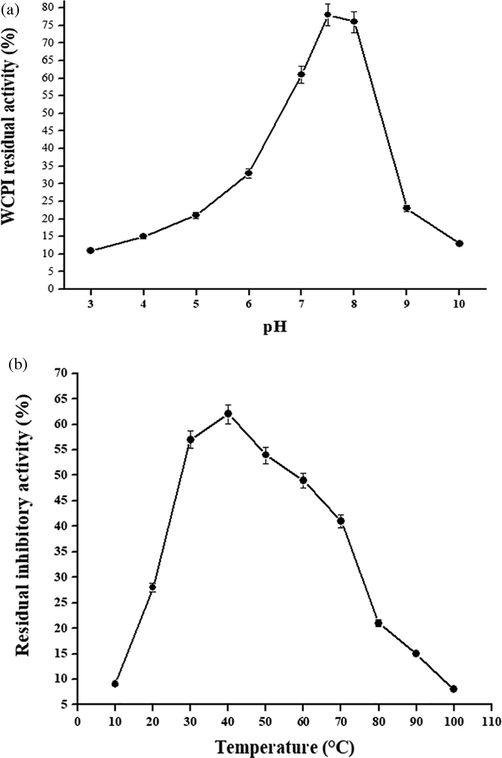
Optimum pH and temperature of purified WCPI. (A) The optimum pH of purified cysteine protease inhibitor was checked over a pH range of 2–10. The residual activity was measured against papain. (B) The optimum temperature for the activity of WCPI was checked after incubating purified inhibitor at temperature range of 30–70 °C followed by their activity measurement.
The changes in the protease inhibitory activity were measured in purified WCPI at various temperatures ranging from 10 to 100 °C. The inhibitory activity was relatively unaffected by heating below 40 °C, although it shows detectable activity upto 60 °C. However, when heated above 80 °C, a sharp decrease in activity was observed. Also, anti-papain activity was less when measured at 10–25 °C (Fig. 4b). Further, inhibitory activity of WCPI decreased significantly below and above its optimum temperature (50 °C). This result shows that WCPI might undergo thermal denaturation when exposed to temperatures higher than 60 °C. Likewise, at low temperatures the inhibitor is rendered inactive possibly due to rigidity and lesser collisions between cysteine protease and the inhibitor.
The optimal pH and temperature of WCPI is consistent with previous reports from different sources such as Phaseolus mungo, black gram, rice bean and Moringa oleifera leaves (Sharma et al., 2006; Balakrishnan et al., 2011).
Structural characteristics: Various spectroscopic techniques were used for carrying out structural investigations of WCPI alone and their complex formation with protease such as papain. At tertiary level, structural changes were examined using sensitive UV–Vis absorption techniques and intrinsic fluorescence spectroscopy.
The absorption difference spectra of WCPI-papain complex had a peak approximately at 210 nm and a small secondary peak at 280 nm. The peak witnessed at 210 nm could be partially caused by phenylalanine residue disruption and the role of other aromatic amino acid residues, as shown in Fig. 5. The increased absorbance around 280 nm spectral region is suggestive of the change of conformation in either inhibitor or protease or both caused by interaction between the two leading to protease-inhibitor complex formation.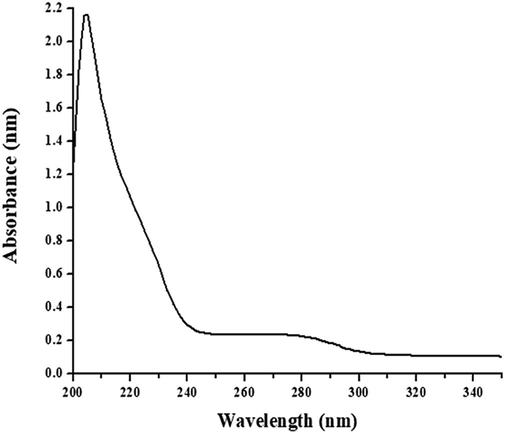
Absorbance difference spectra measured for WCPI-papain complex. WCPI was incubated with activated papain for 30 min, and an absorbance difference spectrum was calculated between 200 and 300 nm. The inhibitor and papain were in a molar ratio of 1:1.
Another sensitive tool for accessing tertiary proteins structure is intrinsic fluorescence. This fluorescence is contributed by specific fluorophores such as aromatic amino acids (W, Y, and F) present in the protein, and any changes in the microenvironment of these fluorophores are reflected in changes in the strength and/or shift of the λmax. The native WCPI showed λmax at around 335 nm, while as for proteinase, it was around 350 nm. Upon binding, the complex formation took place as evidenced by the increase in intrinsic fluorescence intensity relative to the both protein intensities as shown in Fig. 6. There was a little blue shift also with respect to the WCPI, suggesting that some unfolding of WCPI might have accompanied its complexation with the proteinase. Overall, the increase in UV–Vis absorbance as well as the intensity of fluorescence could be attributable to the fact that cystatins act as pseudo-substrates to go in the active-site cleft of cysteine proteinases inducing the conformational change. The above two studies are coherent with the previous studies related to cystatins in the context of structural changes monitored via UV–Vis absorption and intrinsic fluorescence spectroscopies (Bhat et al., 2017; Khaki et al., 2017). The structural features of the purified inhibitor were also investigated by CD spectroscopy. The CD spectra in far UV region reveal the content and contribution of protein secondary structures. Alpha helical structure displays typical negative ellipticity peaks at 207–210 nm and at around 222 nm and positive peaks at 190–192 nm (Chen et al., 1972). In this analysis, we have measured the far UV-CD spectra of WCPI as shown in Fig. 7. The ellipticities (in mdeg) were then converted to mean residue ellipticity (MRE) for the calculation of secondary structural contents by the method of Chen et al. (1972). The CD analyses of WCPI shows 17.11% alpha-helical content. Our result is well supported by previous finding where they reported 25% alpha helical content from the cystatin isolated from the plant (Bhat et al., 2017).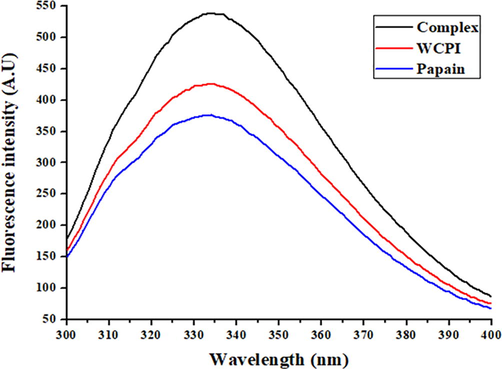
Fluorescence spectra of WCPI alone, papain, and WCPI in complex with papain. Fluorescence spectra were measured at excitation wavelength of 280 nm and emission recorded in the range of 300–400 nm. The concentration of phytocystatin was 2 μM. The fluorescence of WCPI-papain complex was measured at a molar ratio of 1:1. The slit width was 5 nm for excitation and 5 nm for emission beams.
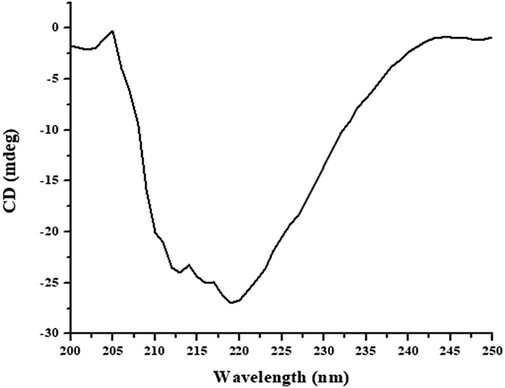
Far-UV-CD spectra of native WCPI. The concentration of inhibitor for far-UV-CD analysis was 25 µM and the path length was 0.1 cm.
4.1 Bactericidal activity of WCPI: A potential biopesticide
WCPI was investigated to evaluate the antibacterial activity against Erwinia carotovora, a causative infectious agent in vegetables and plants. The bacterium contaminates lettuce, cucumbers, tomatoes, carrots, potatoes, onions, and ornamental plants like iris (Wood. 1998). Typically present in soil, water, insect guts, and aerosols suspended in the air. It also infects several other hosts of plants, including potatoes. Bacterium Agrobacterium. tumefaciens is another economically significant pathogen of nut trees, walnuts, grape vineyards, sugar beets, and rhubarb. The large range of Agrobacterium-affected plants is of great concern to the agricultural industry (Moore et al., 1997). Evaluation of the antibacterial activity of WCPI against A. tumefaciens was also done and recorded in Fig. 8. The results revealed that increasing concentrations of WCPI were potentially effective in suppressing microbial growth of the E. carotovora and A. tumefaciens with variable potency. To evaluate the antibacterial activity of WCPI approximately 104 CFU of E. carotovora as well as A. tumefaciens were grown separately in Muller Hinton liquid medium with increasing WCPI concentrations (2, 5, 10 and 20 µM) relative to growth of the control culture which lacked WCPI. As expected 20 µM dose of WCPI resulted in the maximal inhibition of cell growth in both the bacterial species. The effect of WCPI differed remarkably with respect to the type of bacteria suggesting the variable potencies against each. Comparatively the growth rate for A. tumefaciens was 35 %, and for E. carotovora was 50.5 %, in the presence of 20 µM WCPI which indicated the higher bactericidal activity of the phytocystatin against A. tumefaciens.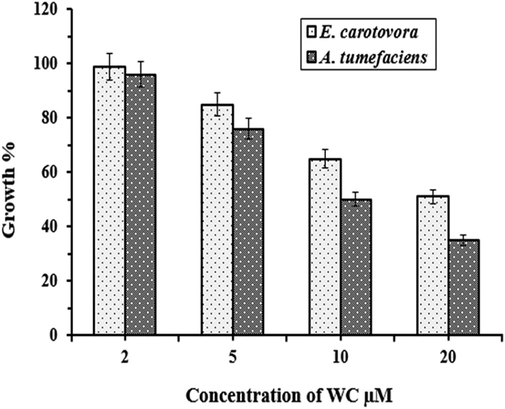
Effects of WCPI on the growth inhibition of E. Carotovora and A. tumefaciens. For the bioassay, 104 cells were incubated in the presence of increasing concentrations of inhibitor. The errors bars indicate standard deviations for triplicate experiments.
The defensive part of cystatin has also been revealed for phytopathogenic fungi for its inhibitory activity against fungal proteases that could demonstrate its role in plant protection from fungal pathogens. Antimicrobial activity of certain cystatins has been observed (Wesierska et al., 2005; Szpak et al., 2014), but study about cysteine protease inhibitors with antibacterial activity is rare. The antibacterial properties demonstrated in this study make WCPI a significant target for further analyses with respect to its usage as a non-chemical pest control agent.
5 Conclusion
The cysteine protease inhibitor (WCPI) was isolated from walnut by ammonium sulfate fractionation and gel filtration chromatography. SDS-PAGE analysis assessed homogeneity and identity of purified inhibitor. Further, kinetic studies showed specificity of WCPI against cysteine proteases. The study established the WCPI’s effectiveness against the bacterial species and confirmed its antibacterial efficacy. WCPI exerted antibacterial activity against phytopathogenic A. tumefaciens and E. carotovora pathogens that could pave way for further research addressing the possible applications of this substance as a potential target for the development as an alternate pesticide for controlling plant diseases and maintaining food quality. The biopesticidal potential of WCPI might be the answer to the much harmful and toxic chemical pesticides used for handling agricultural pathogens.
Acknowledgements
MSK acknowledge the generous support from Research Supporting Project (RSP-2021/352) by King Saud University, Riyadh, Kingdom of Saudi Arabia.
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Corn kernel cysteine proteinase inhibitor as a novel cystatin superfamily member of plant origin. Molecular cloning and expression studies. Eur. J. Biochem.. 1992;209(3):933-937.
- [Google Scholar]
- Characterization of proteinase recovered from Pacific whiting surimi wash water. J. Food Biochem.. 1998;22(1):1-16.
- [Google Scholar]
- Purification and characterization of thiol-protease induced during senescence of unpollinated ovaries of Pisum sativum. Physiology Plant. 1993;88:267-274.
- [Google Scholar]
- Determination of the secondary structure of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972;11:4120-4131.
- [Google Scholar]
- Colorimetric method for determination of sugars and related substances. Anal. Chem.. 1956;28(3):350-356.
- [Google Scholar]
- The expression of papain inhibitors during development of cowpea seeds. Plant Sci.. 1991;74(2):179-184.
- [Google Scholar]
- Osmoprotectants-A plant strategy in response to osmotic stress. Russ. J. Plant Physiol.. 2000;47:137-144.
- [Google Scholar]
- Isolation and partial characterization of a soybean cystatin cysteine proteinase inhibitor of coleopteran digestive proteolytic activity. J. Agric. Food Chem.. 1991;39(8):1515-1520.
- [Google Scholar]
- Structural and functional studies on a variant of cystatin purified from brain of Capra hircus. J. Biomol. Struct. Dyn.. 2017;35(8):1693-1709.
- [Google Scholar]
- Crystalline soya bean trypsin inhibitor, general properties. J. Physiol.. 1947;30:291-310.
- [Google Scholar]
- Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685.
- [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193(1):265-275.
- [Google Scholar]
- Structural and phylogenetic relationships among plant and animal cystatins. Arch. Biochem. Biophys.. 1998;359(1):24-30.
- [Google Scholar]
- Diversity of opines and opine-catabolizing bacteria isolated from naturally occurring crown gall tumors. Appl. Environ. Microbiol.. 1997;63(1):201-207.
- [Google Scholar]
- Three-dimensional solution structure of oryzacystatin-I, a cysteine proteinase inhibitor of the rice, Oryza sativa L. japonica. Biochemistry. 2000;39(48):14753-14760.
- [Google Scholar]
- Isolation of a human cDNA for alpha 2-thiol proteinase inhibitor and its identity with low molecular weight kininogen. Biochemistry. 1984;23(24):5691-5697.
- [Google Scholar]
- Purification and characterization of phytocystatins from kiwifruit cortex and seeds. Phytochemistry. 2004;65(1):19-30.
- [Google Scholar]
- Studies on low molecular mass phytocystatins purified from Phaseolus mungo (Urd) Biochemistry (Mosc). 2006;71(4):406-413.
- [Google Scholar]
- Purification and Biochemical Characterization of a Cystatin-Like Thiol Proteinase Inhibitor from Cicer arietinum (Chickpea) Journal of Chromatography Separation Techniques.. 2017;8:6.
- [Google Scholar]
- A sugarcane cystatin: recombinant expression, purification, and anti-fungal activity. Biochem. Biophys. Res. Commun.. 2002;296(5):1194-1199.
- [Google Scholar]
- The involvement of cysteine proteinase and proteinases inhibitor genes in the regulation of programmed cell death in plants. Plant Cell. 1999;11:431-444.
- [Google Scholar]
- Die gewichtsanalytische Bestimmung des Milchfettes. Dinglers Polytechnisches J.. 1879;232:461-465.
- [Google Scholar]
- Evaluation of the antibacterial activity of cystatin against selected strains of Escherichia coli. Folia Biol. (Krakow). 2014;62(3):187-192.
- [Google Scholar]
- Antimicrobial activity of chicken egg white cystatin. World J. Microbiol. Biotechnol.. 2005;21(1):59-64.
- [Google Scholar]







