Translate this page into:
New flavane gallates from the aerial part of an African/Arabian medicinal plant Plicosepalus curviflorus by LC-MS and NMR based molecular characterization
⁎Corresponding author. shakhan@ksu.edu.sa (Shagufta Perveen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Plicosepalus curviflorus is distributed in Africa, Yemen and Saudi Arabia and applied to treat cancer, tonsillitis, otitis media and for curing diabetes. The objective of this study is to isolate chemical constituents from this medicinally important plant and it yielded one undescribed gallocatechin derivative 1 along with nine known compounds 2–10. In this study, the metabolite profiling of n-butanol fraction of P. curviflorus was also measured by using liquid chromatography-quadrupole time-of-flight mass spectrometry (LC-QTOf-MS). Our research provided the facts for the theory that this plant is a good source of flavane and flavonoids and can be used as medicine. This application allowed to unambiguously identify eight compounds 11–18 from n-butanol soluble fraction of P. curviflorus. We describe here complete characterization of one new compound isolated from the n-butanol soluble fraction of P. curviflorus by NMR and ESI-MS. To the best of our good grasp, compounds 12–15, represents the example of substituted catechin derivatives identified by LC-QTOf-MS method.
Keywords
Plicosepalus curviflorus
Flavane gallates
Catechin derivatives
1 Introduction
Traditional medicinal systems have relied upon the presence of herbs and spices and these systems have been set up from many centuries, and it supplied protected and powerful remedies and cures to humankind (Ameenah, 2006). At present, the significance of various plants for human health has increased like, Cynanchum paniculatum (Xirong et al., 2020), Ganoderma lucidum (Faruque, 2020), Thalictrum foliolosum (Nitin et al., 2020), Tagetes Patula (Muhammad, et al., 2020), Cissus rotundifolia (Jawaher et al., 2020) and many types of research have been done and proved herbal effects on health for curing incurable diseases. Kingdom of Saudi Arabia (KSA) is distinguished by the diversity of vegetation, and it confirmed that KSA flora has all over 837 genera include over 2253 spp., which distributed around 134 families, about 20% of these are novel and scarce plants (Collenette, 1998, Hanan et al., 2019). The parasitic flora of KSA includes at least 13 genera with eight different families. Loranthaceae family, popularly known as mistletoe and it is one of the famous parasitic family which comprises about seventy genera and thousand of sp. (Calvin and Wilson, 2006). Species of Loranthaceae family consists on hemi parasitic and epiphytic plants of the southwestern mountains of the KSA. Many species of Loranthaceae have ethnobotanical importance and used as medicinal plants in various regions of the world. Plicosepalus is a salient genus of the Loranthaceae family and it comprises about 11 species distributed throughout Middle East, Africa and Arabian Peninsula. In KSA, it is denoted by two species, P. acacia and P. curviflorus (Anthonyet al., 1996). This plant used worldwide as traditional medicine, like in Yemen the stem used for curing cancer, tonsillitis and otitis media (Mohammad, 1996), while in KSA plant leaves consumed for curing diabetes (Mossa, 1985). Phytochemical screening of P. curviflorus leaves extracts proved the presence of sterols, terpenoids and flavane gallates as well flavonoids (Badr et al., 2016). Previous investigations on genus Plicosepalus showed interesting biological activities such as antiviral, antimicrobial, cytotoxic, anti-diabetic and antihepatotoxic activities (Areej et al., 2012). Previously, our group carried out a research study on the isolation and structure elucidation of Saudi P. curviflorus extract, revealed to the isolation of two new flavane gallates from the polar fractions, namely; pentahydroxy flavane–5–O–gallate and hexahydroxy flavane 5–di–O–gallate. Consequently, to explore the pharmacological activity of P. curviflorus and to verify its traditional use, the previous isolated compounds were investigated for their hypoglycemic (Areej et al., 2012), cytotoxic (Ghada et al., 2014), peroxisome proliferator-activated receptors agnostic, antimicrobial and anti-inflammatory activities (Raha et al., 2019). Results of previous studies on the same plant showed potent hypoglycemic activity in Albino mice with the isolation of new flavane gallates from the polar fraction, which possessed the highest anticancer effect against colon and liver cancer cell lines (Ghada et al., 2014). On the other hand, catechin proved dual PPAR activation effect and quercetin showed promising anti-inflammatory activity. Interestingly, both P. curviflorus polar extract and the pure compounds showed strong antioxidant potential while weak antimicrobial outcome against tested microbes (Raha et al., 2019). Thus, polar fractions of this plant contain interesting biological active compounds which encourage us to continue the work on n-butanol fraction to identify more interesting compounds that could be used as starting materials for producing medicinally active and safe drugs for treating contemporary diseases like the same fraction of other medicinal plants (Shagufta et al., 2019a, 2019b).
From last two decades LC-MS technique has been commonly used for the chemical characterization of various medicinal plants extracts (Chunlian et al., 2020; Marco et al., 2020; Rachel et al., 2018). ESI-MS (Electrospray ionization mass spectrometry) method has proven to be a robust technique that ease the detection of polar, thermally labile and stable plants constituents. Several high-resolution mass instruments have been used for chemical characterization of plant secondary metabolites. Hybrid high resolution mass spectrometry instruments such as LC-ESI-MS, IT (ion-trap mass), FT (Fourier trasformation) and QTOF (quadrupole-time-of-flight) can provide high quality mass and MS/MS spectra for the identification of compounds. As far as we know, many other recent studies on this medicinal plant P. curviflorus have only described the isolation of few compounds from it without providing full information about it. The aim of our research is to report the proper analysis on the characterization of pure chemical constituents of the n-butanol fraction of this plant by using 1D and 2D NMR (for isolated compounds) and LC-MS/MS instruments-based structures elucidation by their fragmentation motif. These metabolomic approaches were concentrated on mass inspection in negative (-ve) ion mode, as this mode is more delicate than positive (+ve) mode for establishing the variance between fragments connectivity.
2 Materials and methods
2.1 General
Optical rotations were measured in LC grade methanol (CH3OH) using a polarimeter (JASCO P-2000, 2967–5, Japan). The one-dimensional (1D) and two-dimensional (2D) NMR spectra were recorded by using a Bruker AVANCE spectrometer (Bruker, MA, USA) (700 and 175 MHz for 1H, 13C; respectively). Chemical shift δ calculated in ppm, tetramethyl silane TMS used as standard, were calculated basing on the residual solvent signal, and J coupling constants are reported in Hertz (Hz). The ESI-MS experiments for the pure constituents (1–10) were measured on an Triple Quadrupole 6410 QQQ LC/MS mass spectrometer (Agilent, Santa Clara, CA, USA) with ESI ion source (gas temperature was 350 °C, nebulizer pressure was 60 psi, and gas flow rate was 10 L/min), operating in the -ve/+ve ions scan modes of ionization through direct infusion method using CH3OHnH2O (1:1 v/v) at a flow rate of 0.6 ml/min. Column chromatography (CC) procedures were performed using silica gel (230–400 mesh, Merck, Germany), RP-18 (LiChroprep 25–40 µm, Merck, Germany), Sephadex LH-20 (25–100 µm, Merck, Germany). TLC method was performed by using aluminum precoated silica gel 60 F254 and RP-18 (Merck, Germany) TLC plates, and spots were visualized on exposure under UV light (short 254/ long 365 nm) and by spraying with ceric sulphate and thymol spraying reagents. Analytical grade solvents and reagents were purchased from Sigma-Aldrich (St. Louis, USA). Deuterated dimethyl sulfoxide (DMSO‑d6) was purxhased from Cambridge Isotope Laboratories (Tewksbury, USA). Methanol, dichloromethane, ethanol and n-butanol was purchased from Thermo Scientific (Rockford, USA). Formic acid, catechin, epicatechin and gallic acid was purchased from Sigma-Aldrich (USA). Millipore water (30 ml) was obtained from Milli-Q Direct Water Purification System (Merck, Germany).
2.2 Plant material
The aerial parts of P. curviflorus (900 gm) were collected in February 2019 from the South of Hijaz region of Saudi Arabia and identified by Dr. M. Atiqur Rahman (plant taxonomist) College of Pharmacy, King Saud University. A voucher specimen (No. #127) is kept in the herbarium of College of Pharmacy, King Saud University.
2.3 Extraction and isolation
Finely chopped dried aerial plant parts (0.9 kg) were extracted with ethanol:water (8:2) mixture at room temperature 28 °C (3 × 2.5 L). The hydroalcoholic extract was evaporated by using Buchi (Flawil, Switzerland) rotary evaporator system. Concentrated extract (70 g) was dissolved in half liter of millipore H2O and then successively extracted with dichloromethane and n-butanol, remaining water-soluble fraction was freeze dried in powder form. The n-butanol fraction (20 g) was then subjected to an open silica (Si) gel column chromatography (CC 400 mm × 50 mm) by using dichloromethane and methanol as a gradient solvent system (v/v, 100: 50–50: 100) to give seven (1–7) major sub-fractions. Sub-fraction 2 (200 mg) was loaded to an open Si gel CC which developed with dichloromethane (DCM) and methanol ((v/v, 8:2) to give 1 (6 mg) and 2 (10 mg). Fraction 3 (100 mg) was further subjected to C-18Si gel CC, eluted with a gradient mixture of water:methanol (8.0:2.0 → 3.0:7.0), which gave in compounds 8 (10 mg) and 9 (12 mg). Fraction 4 (100 mg) was loaded to C-18Si gel CC using solvent mixture water:methanol (8.0:2.0 → 1.0:1.0) to provided compound 5 (12 mg). Fraction 5 (200 mg) was loaded to Si gel CC eluting with DCM and methanol (v/v, 8: 2), and further purified by using HPLC (RP-18, 9.4 × 250 mm, 7 μm, 1.5 ml/min) eluted with H2O-MeOH (7: 3) to obtain pure isolate 4 (10 mg) and 6 (15 mg). Fraction 6 (100 mg) was separated on LH-20 Sephadex CC by using mixture of water:methanol mixture (9.0:1.0 → 6.0:4.0), to give in compound 3 (25 mg). Fraction 7 (300 mg) was loaded to C-18Si gel CC using mixture of water:methanol (8.0:2.0 → 3.0:7.0) to give in compounds 7 (20 mg) and 10 (25 mg) from top and tail fraction, respectively.
2.3.1 Compound 1
Red amorphous solid; [α]D27 −15 (c 0.10, analytical grade methanol); 1H and 13C NMR data: see Table 1. HR-ESIMS: m/z 457.1855 [M‐H]-, 457.3574 calcd. for C22H17O11, m/z 459.2036 [M + H]+, 459.3730 calcd. for C22H19O11. (DMSO, J in Hz, δ in ppm).
No.
1δH
δC
2
4.76 d 7.0
81.1
3
3.99 brs
66.2
4a
2.56 dd, J = 8.4, 16.8
28.1
4b
2.80 dd, J = 4.9, 16.8
5
–
150.2
6
6.23 d, J = 2.1
101.3
7
–
156.6
8
6.18 d, J = 2.1
100.9
9
–
156
10
–
106.1
1′
–
130.6
2′& 6′
7.05 s
109.4
3′& 5′
–
146.1
4′
–
139.4
1′′
–
164.9
2′′
118.9
3′′& 7′′
7.09 s
109.6
4′′& 6′′
–
146.2
5′′
–
139.6
2.4 LC-MS metabolomic analysis
Powdered n-butanol fraction of P. curviflorus were dissolved in water:methanol mixture (3:7, 10 ml) at 5 mg/mL of final concentration and then filtered by a 0.45 μm cellulose filter (Millipore, MA) and then subjected to Agilent 1260 LC analysis. Analyses were performed on Agilent 1260 Infinity HPLC chromatographic system (Agilent, Germany) hyphenated with Agilent 6530 Quadrupole Time of Flight (Agilent QTOf, Singapore), fitted with degasser, an automatic injector, a column compartment (40 °C) and a quaternary pump, incorporated with Quadrupole and Time of Flight mass spectrometer along with an orthogonal ESI source with Jet Stream thermo focusing technique. 2 µl of the plant sample was injected into the Agilent Zorbax SB-Carbon 18 column (4.6 mm × 150 mm, 1.8 μm) at 40 °C temperature. Compounds separation was accomplished by using following elution gradient; 0–5 min, 10% B; 5–25 min, 10–100% B; 26–30 min, 100% B; 30–31 min, 100–5% B; 31–35 min, 5% B using mobile phase A (0.1% HCOOH in Millipore water) and mobile phase B (0.1% Formic acid HCOOH in Acetonitrile, CH3CN) and the flow rate was as 250 µl/min. MS1 (full scan) was used as acquisition mode and the mass was ranged from 100 to 1000 m/z with scan rate 1 (Spectra/sec). The mass spectrometer frameworks were set as following: gas temperature 300 °C, gas flow l/min, sheath gas temperature 350 °C, nebulizer 35 psi and sheath gas flow was 11 L/min. Mass chromatograms were obtained in negative ion (-ve) mode.
3 Results and discussion
Previous investigations on P. curviflorus leaves selected the methanol soluble fraction as the richest in polar flavane gallates and phenolic derivatives. In continuation of our work on this medicinal plant, the chromatographic separation was achieved on its n-butanol fraction by using a sequence of Sephadex LH-20, Si gel and RP-18 column chromatography (CC), which yielded one undescribed compound 1 and nine previously reported constituents 2–10. The structures of pure compounds (Fig. 1) were established by 1D (one dimensional) and 2D (two dimensional) NMR and ESIMS. Compounds 2–10 were identified as 3,3′,4′,5,7-pentahydroxyflavane-5-O-gallate 2, catechin 3 (Areej et al., 2012), caffeic acid 4, caffeoyl-β-D-glucose 5, gallic acid 6, chlorogenic acid 7 (Shagufta et al., 2020), querecetin-3-O-glucoside 8, kaempferol-3-O-glucoside 9, and protocatechuic acid 10 (Nawal et al., 2011) by a collation of their spectroscopic facts with those published earlier. To the best of our expertise compounds 4–10 are first time purified from P. curviflorus.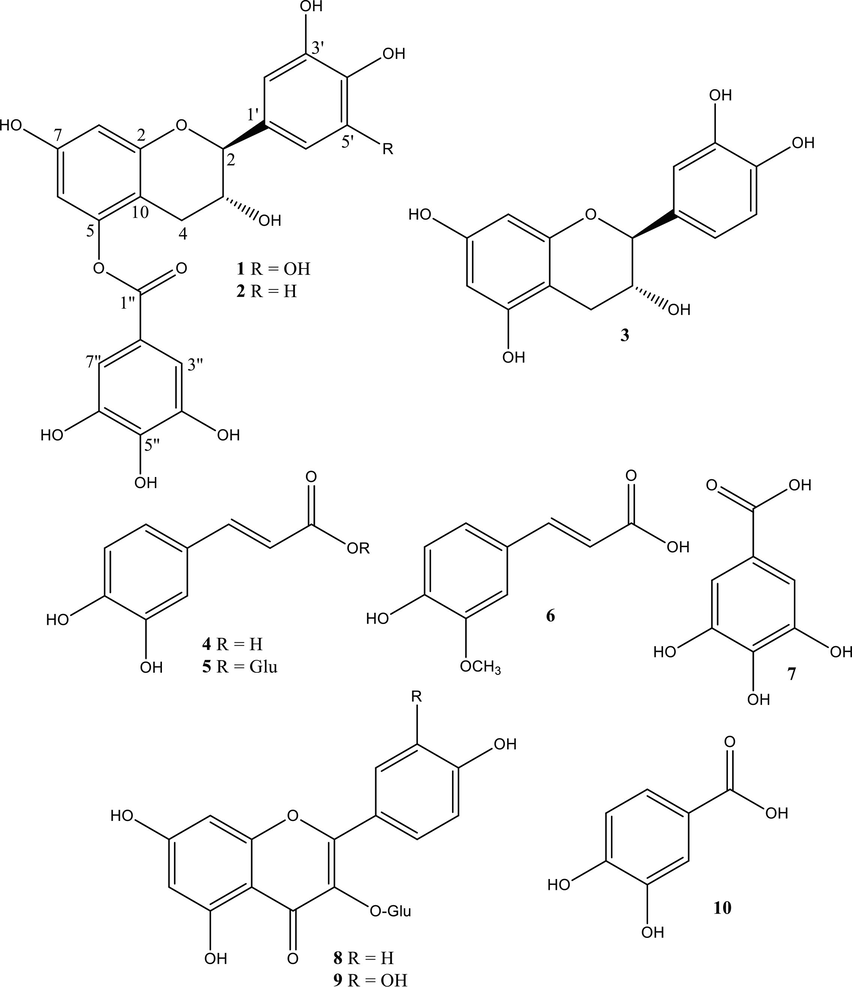
Structures of isolated compounds 1–10.
n-Butanol extract of aerial part of P. curviflorus were analyzed by LC-ESI mass instrument to detect its chemical composition based on mass fragmentation patterns. In general, we observed -ve ion mode deprotonated molecule [M−H] - and its typical fragment ions by MS/MS experimental analysis. Eight compounds are listed in Table 2 along with their retention time, molecular formula and experimental mass and the theoretical mass of each investigated compound. Thus, owing to the importance of catechin based compounds as chemo taxonomical markers, an unambiguous chemical characterization of P. curviflorus was obtained. a Elemental composition refers to the deprotonated ion of molecules observed by mass spectrometry.
Signal
Rt min
Assignedidentity
Mol. ion[M−H] -
Fragmentm/z
Elemental composition
Theoretical mass
Ref.
1
0.55
Procyanidin trimer 11
865.2010
577
C45H37O18
865.2715
14
2
1.03
Pentahydroxy flavane di-O-gallate-mono protocatechuate 12
729.1609
593, 441, 289, 245, 197, 125
C36H25O17
729.1786
15
3
1.60
Catechin mono gallate caffeic/cinnamic acid derivative 13
725.4118
793 [M + 3Na], 441, 289
C38H29O15
725.6185
8
4
2.03
Catechin monogallate di-O-methyl inositol 14
631b
699 [M + 3Na], 571, 767 [M + 6Na]-, 441, 289
C30H31O15
631.5485
16
5
2.23
Catechin monogallate amide derivative 15
540.3432
608 [M + 3Na], 441, 289, 197
C27H26NO11
540.4880
8
6
3.12
Diosmetin glucoside 16
461.2809
529 [M + 3Na]
C22H21O11
461.3887
17
7
5.01
Catechin 17
289.0735
245
C15H13O6
289.2622
8
8
8.25
Gallic acid 18
169.0970
C7H5O5
169.1090
13
3.1 Structure elucidation of isolated new compound 1
Compound 1 was segregated as red amorphous talc with the formula C22H18O11 (4 5 8), determined by positive/negative ion mode ESIMS (m/z 459.2036 [M + H]+, m/z 457.1855 [M−H] -) (Figs. 2 and 3).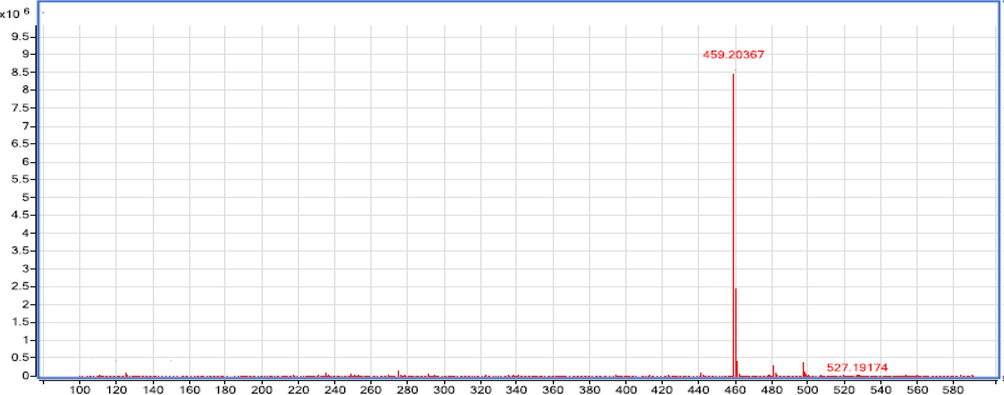
The MS product positive ion mode scan of compound 1.
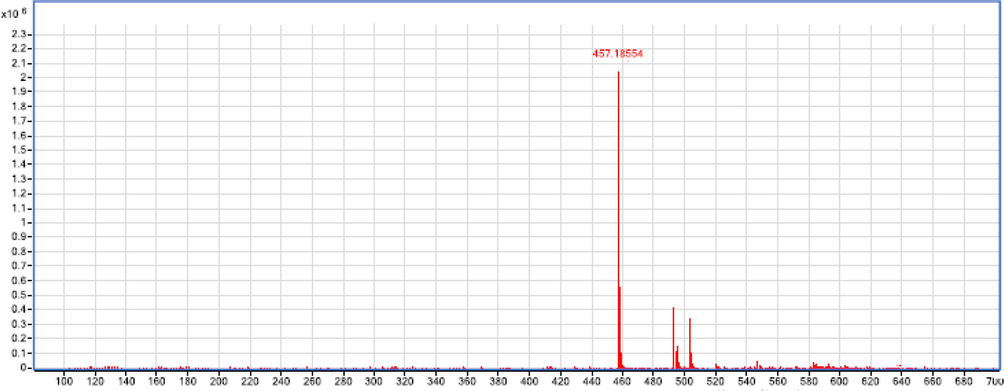
The MS product negative ion mode scan of compound 1.
The 1H NMR spectrum of 1 showed the existence of two meta coupled doublets at δ 6.23 (1H, d, J = 2.1) and 6.18 (1H, d, J = 2.1) which were congruous with a 5,7-dioxygenated ring A of flavonoid class of compounds. One singlet with two protons integration at δ 7.05 (2H, s) was evident for 1′,3′,4′,5′-tetrasubstituted aromatic ring B. The 1H NMR spectrum further showed resonances in conjunction with δ 2.56 (1H, dd, J = 16.8, 8.4 Hz), 2.80 (1H, dd, J = 16.8, 4.9 Hz), 3.99 (1H, brs) and 4.76 (1H, d, J = 7.0 Hz) revealed that 1 was hexahydroxy flavane. Further exploration of the 1H NMR spectrum of 1 manifested one more deshielded signal integrated for two protons at δH 7.09 (2H, s) and one downfield shifted carbonyl carbon signal at δc 164.9 in 13C NMR attesting the existence of galloyl group in 1. It was validated by upfield C-5 carbon shift at δ 150.0 for and downfield shift at δ 101.3 for C-6, δ 100.9 for C-8 and δ 106.1 for C-10 signals, in conformation of the presence of galloyl moiety at C-5 carbon atom. All of the explained experimental data were firmly related to the formerly isolated compound pentahydroxy flavane-5-O-gallate (Areej et al., 2012) excluding the occupancy of additional hydroxyl moiety at C-5′ carbon atom at ring B. The point of attachment of hydroxy moiety at C-5′ was firm by the HMBC long range intercorrelations. The oxygen carriage carbons of the rings A and B revealed signals at δc 156.0, 150.0, 146.1 × 2, and 139.4 indicating the presence of five hydroxy groups in compound 1. The flavane gallate frame of 1 was deduced on the strong proofs of NMR and mass spectroscopy. Acid hydrolysis of compound 1 yielded gallic acid and (−)-gallocatechin (Areej et al., 2012), which were identified by keen comparison of their TLC, optical rotations and 1H NMR with standard sample. A considerable analysis of the 1D and 2D NMR spectrum supported the structural assignment of compound 1 as gallocatechin 5-O-gallate.
3.2 Acid hydrolysis of compound 1
Compound 1 (2.0 mg) was fully liquefy in 0.5 ml of 1 N methanol-HCl (1/1) solution. Soluble material was warmed up at 60° C for 1 h and evaporated under Buchi, then water was poured and the mixture was treated with EtOAc (ethyl acetate). The EtOAc soluble part was filtered and the filtrate was concentrated by using Buchi rotavapor, and chromatographed over C-18 silica by using methanol:water which afforded (−)-gallocatechin and gallic acid from top and tail fraction, respectively. The experimental data were recognized by contrast of optical rotations and NMR resemblances with standard samples.
3.3 Structural identification of compounds by LC-ESI-MS/MS method
The goal of metabolomic study is to acquire a chemical constituent fingerprint by detecting all secondary metabolites exist in the n-butanol fraction of P. curviflorus (Fig. 4). Total eight compounds were identified in the n-butanol fraction of aerial parts of P. curviflorus by LC-ESI-MS, which were mainly characterized as catechin and its derivatives and flavonoid and its glycosides. Identification of metabolites was achieved by comparison to the available mass spectral (MS) data in available literature presented in Table 1. Most importantly, this method detected almost all catechin derivatives present in n-butanol fraction of this plant. In addition, our analysis revealed the presence of compounds 11–18 for the first time in P. curviflorus. New compounds detected by this approach are complexed catechin derivatives, which were described for the first time as natural products.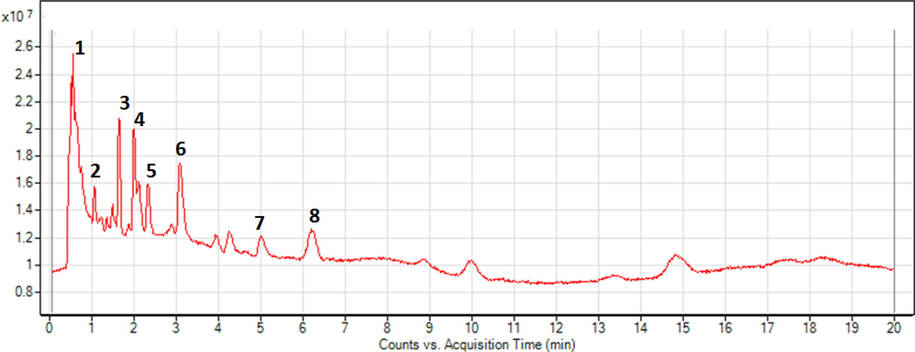
Typical HPLC-ESI-MS chromatogram from of P. curviflorus n-butanol fraction analyzed in negative ion mode.
Compound 11 displayed a molecular ion signal at m/z 865.2010 ([M−H] -, [C45H37O18] -) for procyanidin trimer and it further showed precursor ion at m/z 577 indicative of type B procyanidin dimers, so these two important signals confirmed compound 11 as catechin trimer (Emily et al., 2018).
The ESI-MS spectrum of P. curviflorus n-butanol extract displayed compound 12 molecular ion signals in negative ion mode at m/z 729.1609 ([M−H] -, [C36H25O17] -) and MS/MS spectrum of its precursor ions at m/z 593.1070 [C29H21O14] - → m/z 441.0941 [C22H17O10] - → m/z 289.0811 [C15H13O6] - allowed us to identify 12 as pentahydroxy flavane-di-O-gallate-mono protocatechuate (Fig. 5). In addition, further detected ions in MS/MS spectrum showed more [M−H] - fragmentations at m/z 245.0905 [C14H13O4] -, m/z 197.0534 [C9H9O5] - and 125.0306 [C6H5O3] -, which were ascribed to catechin derivatives (Rosa, 2014). On completion of full literature survey, we tentatively identified compound 12 as the new compound which was fixed on the evidences of MS fragmentation sequences listed in Table 2.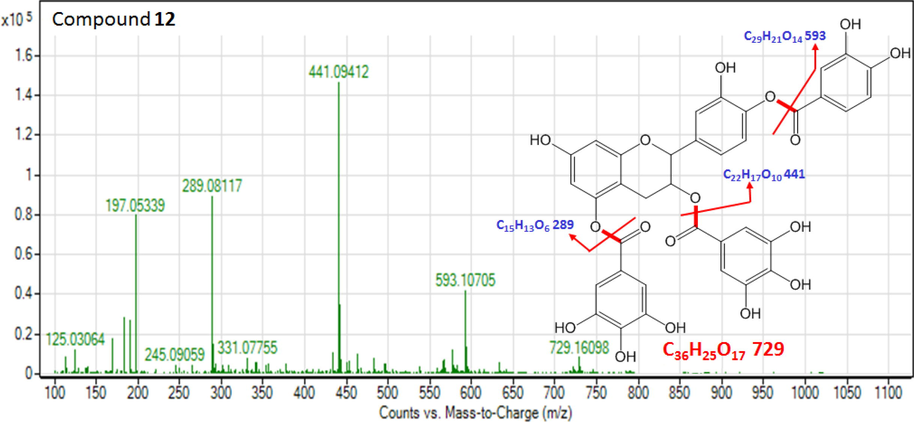
The MS product negative ion mode scan of compound 12.
Compound 13 has [M−H] - molecular ion at m/z 725.4118 and [M−H + 3Na]− at m/z 793 with the fragment pattern m/z 441 → m/z 289 and → m/z 197. The major fragment was at m/z 441 and 289 indicated that this compound is a derivative of catechin gallate which has phenolic constituent attached at free hydroxyl groups. It indicating that carboxylation process has occurred at the hydroxy group which provide enough space for occupying other phenolics. On the basis of mass spectral data and previously published data (Areej et al., 2012), compound 13 was tentatively identified as catechin mono gallate caffeic/cinnamic acid derivative. (Fig. 6).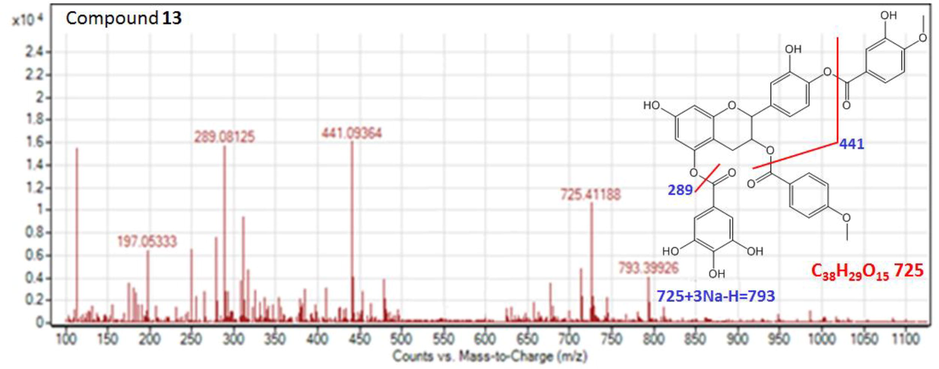
The MS product negative ion mode scan of compound 13.
Compound 14 displayed the deprotonated molecular ion signal at m/z 631 and exhibited fragment ions at 571 (after loss of two methoxy groups), 441 (catechin gallate) and, 289 (catechin) and 699 [631 + 3Na-H = 699]. This fragmentation pattern is consistent with a catechin gallate derivative, condensed product of catechin with methyl inositol. From these evidences, compound 14 was found a catechin monogallate di-O-methyl inositol derivative, recently isolated from the Saudi P. curviflorus in 2016 (Giuseppe et al., 2016). The structural assignment of compound 14 was performed on the basis of the ESI-MS/MS fragment ions (Fig. 7). The described HPLC-ESI-MS course of actions offer the capability to obtain different flavane gallates and flavonoids from P. curviflorus n-butanol extract in an expeditious, inexpensive and effectual manner. Moreover, the use of the proposed HPLC-ESI-MS and negative ion MS/MS profiling allows the identification of its almost all metabolites in mixture with high assurance.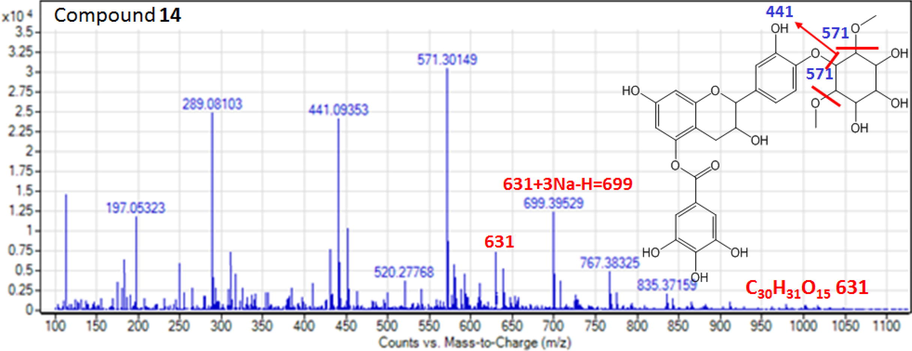
The MS product negative ion mode scan of compound 14.
One minor signal of compound 15 showed [M−H] - signal at m/z 540.3432 with the fragment ions at m/z 441, 289 and 197. The presence of nitrogen atoms in the molecule was proved by presence of even molecular weight at m/z 540. The m/z 441 ion and its conjugate ion at 289 were produced after cleavage of the inter flavan bond. On the basis of these evidences, compound 15 conditionally identified as amine derivative of catechin gallate which is a new natural product and to our best knowledge, it is reported here for the first time (Fig. 8).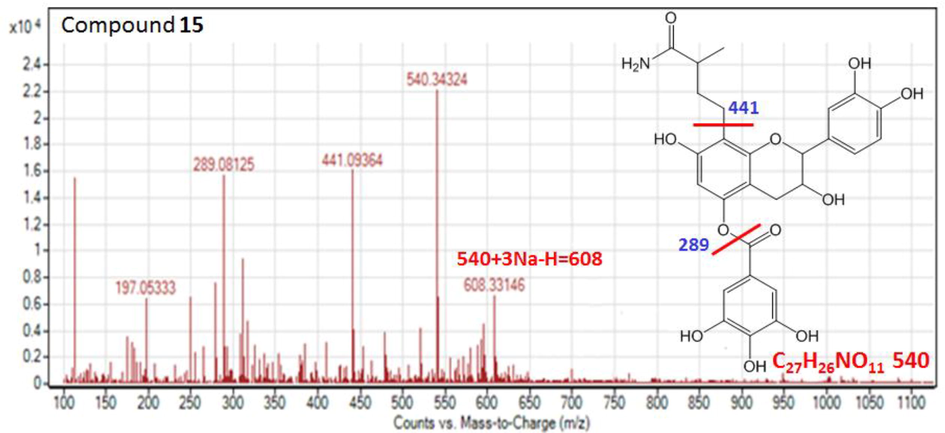
The MS product negative ion mode scan of compound 15.
Compound 16 was identified as diosmetin glucoside 16 and it showed [M‐H] - ion at m/z 461.2809. It further showed the signal at 529 after the addition of three sodium ions. It is a known compound diosmetin glucoside and previously isolated from many plants specially from Caucasian vetch (Andreeva et al., 1998). Compound 17 and 18 was characterized as catechin and gallic acid with the molecular ion signals at 289 and 169, respectively. If liquid chromatography coupled with ESI/MS, positive ion mode is generally more favorable as many compounds are expected to ionized by using this mode. Although, the major dominance of negative ion mode is the lesser background noise. Up to now, positive ion mode ESI technique has generally been preferred among researchers; though, it can be seen in many instances that, negative ion mode ESI is much better choice owing to its finer sensitivity and potential for lower limits detection. Our results showed that the negative ion mode was more efficient and sensitive for the analyzed compounds present in this plant (Piia et al., 2017). The described LC-ESI-MS course of actions offered the potential to acquire different flavane gallates from P. curviflorus n-butanol extract in an expeditious, inexpensive and effectual manner. Moreover, the use of the proposed LC-ESI-MS and negative ion MS/MS profiling allows the identification of almost all metabolites in mixture with high assurance/confirmation
4 Conclusions
This phytochemical investigation on the Arabian/Africa medicinal plant P. curviflorus yielded one new 1 and nine 2–10 known secondary metabolite, elucidated by 1D and 2D NMR techniques. The potential of an untargeted metabolomic experimental procedure was illustrated for the first time as a meaningful tool for evaluating P. curviflorus n-butanol fraction, which provided the data of eight compounds 11–18 related to flavane gallets and flavonoid glycosides. These compounds were identified by ESI-MS spectra acquired by using LC-ESI-MS. QTOf-MS coupled with LC provides a swift and tactful method for the identification of flavane gallets, by using this method, new structures were deduced for the first time. The diversity of the structures of catechin gallate derivatives was divulged by MS chromatograms. Arabian folk medicinal plants have not yet achieved the popularity it deserves in global arena; therefore, this study distinctly provided the potential evidences of P. curviflorus to bestow a long list of polyphenols of the Saudi Arabian flora to enhance the worldwide phytochemical attempts. The facts obtained will reinforce the exploitation of this plant as a potential source of natural antioxidant.
5 Ethical approval and consent to participate
Not applicable
6 Consent for publication
Not applicable
7 Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Funding
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no. RGP-221.
CRediT authorship contribution statement
Areej Mohammad Al-Taweel: Investigation, Writing - original draft, Writing - review & editing, Funding acquisition. Shagufta Perveen: Investigation, Writing - original draft, Writing - review & editing, Funding acquisition. Saleh Ibrahim Alqasoumi: Writing - review & editing. Raha Orfali: Investigation. Hanan Y. Aati: Investigation. Shahnaz: . Ebtesam Nasser Alsultan: . Bandar Alghanem: Writing - original draft. Hayat Shaibah: Funding acquisition.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no. RGP-221.
Declaration of Competing Interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Medicinal plants: Traditions of yesterday and drugs of tomorrow. Molecular Aspects of Medicine. 2006;27(1):1-93.
- [CrossRef] [Google Scholar]
- Diosmetin glycosides from caucasian vetch: Isolation and study of biological activity. Pharm Chem J. 1998;32(11):595-597.
- [CrossRef] [Google Scholar]
- Flora of the Arabian Peninsula and Socotra. Royal Botanic Gardens, Kew, 1.: Edinburgh University Press; 1996. p. :121-122.
- New flavane gallates isolated from the leaves of Pilcosepalus curviflorus and their hypoglycemic activity. Fitoterapia. 2012;83:1610-1615.
- [Google Scholar]
- Plicosepalin A, a new antioxidant catechin-gallic acid derivative of inositol from the mistletoe Plicosepalus curviflorus. Z. Naturforsch C. 2015;71:375-380.
- [Google Scholar]
- Comparative morphology of epicortical roots in Old and New World Loranthaceae with reference to root types, origin, patterns of longitudinal extension and potential for clonal growth. Flora - Morphology, Distribution, Functional Ecology of Plants. 2006;201(1):51-64.
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity in vitro, extractive process and HPLC-MS characterization of total saponins extract from Tribulus terrestris L. fruits. Industrial Crops and Products. 2020;150:112343.
- [CrossRef] [Google Scholar]
- Checklist of botanical species in Saudi Arabia. UK: International asclepiad society; 1998. p. :1-80.
- Procyanidins: a comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem Rev. 2018;17(1):1-16.
- [CrossRef] [Google Scholar]
- Ganoderma lucidum: A rational pharmacological approach to surmount cancer. Journal of Ethnopharmacology. 2020;260:113047.
- [CrossRef] [Google Scholar]
- Anticancer activity of flavane gallates isolated from Plicosepalus curviflorus. Pharmacogn. Mag.. 2014;10:519-523.
- [Google Scholar]
- Plicosepalin A, a new antioxidant catechin–gallic acid derivative of inositol from the mistletoe Plicosepalus curviflorus. Z. Naturforsch. C.. 2016;71:375-380.
- [Google Scholar]
- Traditional use of ethnomedicinal native plants in the Kingdom of Saudi Arabia. J. Ethnobiol. Ethnomed.. 2019;15:1-9.
- [Google Scholar]
- Glycosylated Phenols and an Unprecedented Diacid from the Saudi Plant Cissus rotundifolia. J. Nat. Prod.. 2020;83(11):3298-3304.
- [CrossRef] [Google Scholar]
- Current developments in LC-MS for pharmaceutical analysis. Analyst. 2020;145(4):1129-1157.
- [CrossRef] [Google Scholar]
- Plants used in Saudi folk-medicine. King Abdulaziz City for science and Technology: KACST Publishing, Riyadh, Saudi Arabia; 1996. p. :236.
- A Study on the Crude Antidiabetic Drugs Used in Arabian Folk Medicine. International Journal of Crude Drug Research. 1985;23(3):137-145.
- [CrossRef] [Google Scholar]
- Traditional uses, Phyto-chemistry and pharmacological activities of Tagetes Patula L. J. Ethnopharmacol.. 2020;255:112718
- [Google Scholar]
- Antioxidant, Anti-Glycation and Anti-Inflammatory Activities of Phenolic Constituents from Cordia sinensis. Molecules. 2011;16:10214-10226.
- [Google Scholar]
- Thalictrum foliolosum: A lesser unexplored medicinal herb from the Himalayan region as a source of valuable benzyl isoquinoline alkaloids. Journal of Ethnopharmacology. 2020;255:112736.
- [CrossRef] [Google Scholar]
- Think Negative: Finding the Best Electrospray Ionization/MS Mode for Your Analyte. Anal. Chem.. 2017;89:5665-5668.
- [Google Scholar]
- Production, separation, and characterization of apo-luteinoids by LC-MS/MS. Journal of Chromatography B. 2018;1102-1103:45-51.
- [CrossRef] [Google Scholar]
- Pharmacological Evaluation of Secondary Metabolites and Their Simultaneous Determination in the Arabian Medicinal Plant Plicosepalus curviflorus Using HPTLC Validated Method. J. Anal. Methods Chem.. 2019;7435909:1-8.
- [Google Scholar]
- Antibacterial and Antifungal Sesquiterpenoids from Aerial Parts of Anvillea garcinia. Molecules. 2020;25:1730.
- [Google Scholar]
- Antimicrobial guaianolide sesquiterpenoids from leaves of the Saudi Arabian plant Anvillea garcinii. Fitoterapia. 2019;134:129-134.
- [CrossRef] [Google Scholar]
- Montbresides A–D: antibacterial p-coumaroyl esters of a new sucrose-based tetrasaccharide from Crocosmia × crocosmiiflora (montbretia) flowers. Fitoterapia. 2019;139:104377.
- [CrossRef] [Google Scholar]
- Phenolic profiling of the skin, pulp and seeds of Albarino grapes using hybrid quadrupole time-of-flight and triple-quadrupole mass spectrometry. Food Chem.. 2014;145:874-882.
- [Google Scholar]
- Cynanchum paniculatum (Bunge) Kitag. ex H. Hara: A review of its ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol.. 2020;260:112994
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.101289.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







