Translate this page into:
Optimization of regeneration and Agrobacterium tumefaciens-mediated transient transformation systems for Australian native extremophile, Tripogon loliiformis
⁎Corresponding author. shukti.chowdhury@sau.edu.bd (Shukti Rani Chowdhury)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Tripogon loliiformis, an Australian native resurrection grass having an abundant gene pool for combating desiccation, can be the putative model system for functional characterization of stress tolerance genes due to its diploid genome and being a monocotyledonous plant and member of the grass family (Poaceae), like many important cereal crops. For developing callus mediated regeneration from mature grains of Tripogon, Murashige and Skoog medium containing growth regulator, 2,4-dichlorophenoxyacetic acid (2,4-D) at 1.0mgL−1 was the optimum concentration for induction and proliferation of healthy cream calli. Successful regeneration of shoots from callus clumps in MS medium supplemented with 2.0mgL−1 6-benzylaminopurine (BAP) and 0.5mgL−1 α-naphthalene acetic acid (NAA) was obtained from 2 consecutive rounds subculturing of the calli at 3 weeks interval. In addition, rooting needed another 2 rounds within the same media with 2.0mgL−1 BAP but with 0.25mgL−1 NAA. The transient expression of UidA gene at 3 days after Tripogon callus transformation, performed with Agrobacterium tumefaciens strains AGL1 and LBA4404 following rice and Brachypodium distachyon transformation protocols, indicates successful Agrobacterium infection and gene delivery in calli. A stable transformation system for Tripogon loliiformis can be developed near future following the protocols in this study.
Keywords
Tripogon loliiformis
Resurrection grass
Regeneration
Transient transformation
UidA gene
1 Introduction
Climate change is triggering more frequent and prolonged periods of water deficit by which global food security is extremely challenged. Exacerbating this issue, the world’s population is predicted to reach 9.8billions by 2050 (UNIDESA, 2017) with limited opportunity to expand global arable lands in the future. A 70% increase in crop productivity in agriculture can feed future populations satisfyingly though chronical malnutrition is predicted to cause sufferings to 870million people by 2050 (Friedrich, 2015; McGuire, 2013). Drought is the major environmental stress factor enhanced by global warming and increased evaporation, less precipitation, lowering of the ground water table and reduced soil water levels that constrains plant growth and crop productivity (Abdelaal et al., 2020; Hafez et al., 2020). Dehydration, associated with drought, results in major changes in gene expression, carbohydrate metabolism, photosynthetic-related processes and ultrastructures in plants (Abdelaal et al., 2018) leading to decreased yields in major cereal crops including 27.5% in wheat and 25.4% in rice (Zhang et al., 2018). A logical starting point is to look for natural genetic resources and unique drought tolerance mechanism in plants that can tolerate extreme drought conditions to bioengineer enhanced drought tolerant crops.
A unique group of 135 angiosperm species from 13 families, commonly stated as resurrection plants, can tolerate up to 95% water loss (desiccation) in vegetative tissues for prolonged periods without loss of viability (Challabathula and Bartels, 2013; Farrant et al., 2012; Gaff, 1977; Gaff and Oliver, 2013). The plant’s ability to tolerate desiccation is most probably due to a complex assortment of multigenic and multifactorial machinery, for instance, morphological and anatomical changes (Asami et al., 2018; Sherwin and Farrant, 1996, 1998), shutdown of photosynthesis (Aidar et al., 2010; Dinakar et al., 2012), instigation of antioxidant systems (Sherwin and Farrant, 1998), variations in carbohydrate levels (Benina et al., 2013) and cell wall properties (Moore et al., 2009; Vicré et al., 1999).
Tripogon loliiformis or five-minute grass belongs to the genus Tripogon, subfamily Chloridoideae and therefore the family Poaceae. It is annual to short-lived perennial grass native to Australia and New Guinea (Fabillo, 2015). T. loliiformis grows in rocky outcrops and nutrient poor soils with low water retention (Gaff and Latz, 1978) and can withstand drought stress to leaf relative water content below 10%. This extremophyte stimulates some structural, physiological and biochemical contrivances including leaf folding, cell wall folding and vacuole fragmentation, photosynthesis shut down at the initial stage of dehydration to safeguard the photosynthesis machinery, anthocyanin accumulation and maintaining membrane integrity during desiccation (Karbaschi et al., 2016). T. loliiformis also accumulates trehalose for the induction and maintenance of autophagy as a pro-survival mechanism for suppression of programmed cell death and senescence pathways in leaves (Williams et al., 2015) though the roots do not need autophagy for desiccation tolerance (Asami et al., 2019). Regulation of genes by miRNAs, including oxidoreductases and hydrolyases, as well as SPL, MYB and WRKY transcription factors may constitute a significant molecular mechanisms of desiccation tolerance in Tripogon loliiformis (Njaci et al., 2018). Additionally, transgenic rice plants expressing an osmotin protein from T. lolliformis, TlOsm, exhibited increased tolerance to osmotic stresses and maintained growth, higher water contents, membrane integrity and survival rates compared to the non-transgenic rice plants (Le et al., 2018). The extremophile has some exclusive characteristics to be considered as experimental model plant for desiccation tolerance studies denoted as (i) withstanding severe water loss in vegetative tissues, pigment accumulation during desiccation and being rehydrated quickly, (ii) large selection of habitats including Australian mainland, (iii) short life span, (iv) able to grow under greenhouse condition without much effort, (v) molecular work is straightforward because the plant is diploid and (vi) monocot and sharing the indentical family, Poaceae, with the most important staple cereal crops (Karbaschi, 2015).
Molecular characterization of genes associated with drought tolerance from this novel plant resource and engineering stress tolerance traits in staple crops such as rice, sorghum and wheat is highly imperative for continued global food security. Transformation protocols for dicotyledonous resurrection plants such as Craterostigma plantagineum (Toldi et al., 2002) and Ramonda myconi (Tóth et al., 2006) have been established though there has been no report of transformation system developed for monocotyledonous resurrection plants. Therefore, to our knowledge, this study is the first to describe a reliable regeneration and Agrobacterium tumefaciens-mediated transient transformation system in T. loliiformis which may lead to establish stable transformation system to open up the opportunities to study various stress tolerance genes from this unique resurrection plant.
2 Materials and methods
2.1 Plant materials
Mature grains of Tripogon loliiformis, used for callus induction in this research, were collected from the Centre for Agriculture and Bioeconomy (CAB), QUT. The 5 weeks old fresh calli, initiated from the grains, were employed for regeneration and transformation experiments. The friable callus was nodular in shape, healthy and cream white in colour.
2.2 Media and chemicals
Different media and chemicals used are given in Supporting Tables 1 and 2.
2.3 Reporter genes and vector backbones
The reporter gene UidA from pCAMBIA2301 (Supporting Fig. 1), pENTR/D-TOPO® entry vector (Supporting Fig. 2) and pCE104-EYFP plant expression vector (Supporting Fig. 3) were collected from the CAB, QUT.
2.4 Seed sterilization and callus induction
Dehusked mature grains of T. loliiformis were washed thrice with sterile MilliQ water followed by surface sterilization with 70% ethanol for 30s with vortexing and re-washing for 3 times with MilliQ water to get rid of traces of ethanol. The grains were then soaked in 6.5% sodium hypochlorite (w/v) for 5min and vortexed 3 times at 1min interval followed by washing with MilliQ water for 7 times. The clean grains were subjected for imbibation in 0.05% agarose for 2h at room temperature. The pre-sterilized 20 aseptic grains were inoculated on petri dishes (90 × 15mm size) containing suitable callus induction media with appropriate concentrations of 2, 4-D semi-solidified by using 2.5gL−1 of Gelzan.
2.5 Plant regeneration
After incubating the inoculated petri dishes at 27°C under dark condition inside growth cabinet for 5 weeks, the friable calli were selected and further multiplied by subculturing to the similar induction medium under dark for 3 weeks. These 8 weeks old calli clumps were then subcultured to the regeneration media for regenerating shoots and roots. 10 calli clumps were subcultured in each petri dish (90 × 14mm) containing 40mL of MS media supplemented with 30gL−1 sucrose, and 2.5gL−1 of solidifying agent Gelzan at pH 5.8 in addition to varying concentrations of BAP (0mgL−1, 1.0mgL−1; 2.0mgL−1 and 3.0mgL−1) and NAA (0mgL−1; 0.25mgL−1 and 0.5mgL−1). All the plates were incubated at 27°C under dark inside growth cabinet for a week. Finally, the calli were exposed to light at 70µmoless−1m−2 white fluorescent light inside growth cabinet with a 16h light/ 8h dark cycle.
2.6 Preparation of constructs for Agrobacterium mediated transformation
The construct pCE104-UidA-EYFP was prepared by using TOPO cloning and Gateway LR cloning system. The amplification of UidA gene from the pCAMBIA2301 vector was done by PCR reactions employing Q5 High Fidelity DNA polymerase kit. After gel electrophoresis, the amplified gene was purified from agarose gel using a Freeze ‘N Squeeze DNA gel extraction spin column (Bio-Rad). The purified PCR product was cloned into pENTR/D-TOPO® vector and therefore the resulting pENTR-UidA (Supporting Fig. 4) was transformed into electro-competent cells of E. coli by electroporation. The pENTR-UidA plasmid purification was done using Wizard Plus SV Minipreps DNA purification system (Promega) for Sanger sequencing using Big Dye Terminator Cycle Sequencing Kit TM v3.1 (Applied Biosystems) and Vector NTI Advance 11 software was applied to analyse the sequencing data.
The digestion of the pENTR-UidA was executed using Cutsmart buffer and PvuI restriction enzyme. UidA gene together with the attL1 and attL2 recombination sites was transferred from pENTR vector into pCE104 destination vector using the Gateway™ LR Clonase™ II Enzyme Mix (Invitrogen). The LR reaction mixture was used to transform 50µL of thawed E. coli XL1 Blue chemically-competent cells following the heat shock method described by Inoue et al. (1990) and the construct pCE104-UidA-EYFP was subjected to Sanger sequencing for confirmation after analysing throughVector NTI Advance 11 software. The ultimate cloned product of pCE104-UidA-EYFP (Supporting Fig. 5) was transformed into Agrobacterium strains AGL1 and LBA4404 by electroporation. The AGL1 colonies with construct pCAMBIA2301 were collected from CTCB lab. The colonies were grown in 50mL LB liquid media with 25mgL−1 rifampicin and 50mgL−1 kanamycin and incubated 48h at 28°C for subsequent use for transformation. The primers used in every step of cloning are enlisted in Supplementary Table 3.
2.7 Agrobacterium-mediated transformation and GUS expression analysis
Fresh 5 week-old Tripogon calli were transformed following Agrobacterium-mediated transformation method for rice described in Hoang (2014) (Supporting Fig. 6) and for Brachypodium distachyon as described by Alves and colleagues (Alves et al., 2009) (Supporting Fig. 7) with 2 Agrobacterium strains, AGL1 or LBA4404 harboring single appropriate construct- pCE104-UidA-EYFP or pCAMBIA2301, were used for transformation. A 100µL aliquot of fresh Agrobacterium culture or 200µL of bacterial glycerol stock was added into 10mL liquid LB media (Supporting Table 1) with 50mgL−1 kanamycin and 25mgL−1 rifampicin in a 50mL falcon tube and shaken on a shaking incubator for 72h at 28°C, 200rpm. After that, PCR reactions and agarose gel electrophoresis were performed to verify the presence of appropriate construct within the culture.
Agrobacterium mediated transformation method described in Hoang (2014) was employed for the transformation of 5 weeks-old Tripogon calli. 40mL of ATM2 media (Supporting Table 1) supplemented with 50mgL−1 kanamycin was mixed with 10mL growing Agrobacterium culture in liquid LB media in an autoclaved 250mL conical flask and kept shaking overnight at 28°C, 200rpm. On the day of transformation, following the transfer of each 25mL of Agrobacterium culture to a 50mL falcon tube, centrifugation of the cultures was done at 25°C, 3000rpm for 10min. The supernatant was discarded and the pellets were suspended in BRM media (Supporting Table 1) supplemented with 1µLmL−1 of 200μM acetosyringone. For facilitating vir gene induction of Agrobacterium, the culture was then incubated at room temperature at a speed of 100rpm in a shaking incubator for 5h. Following a 5h incubation, an aliquot of 1mL from each falcon tube was taken to quantify the optical density (OD) employing a spectrophotometer at a wavelength of 600nm (OD600) and the culture was centrifuged at 25°C, 3000rpm for 10min. The supernatant was discarded followed by suspending the pellets by vortexing in an appropriate amount of ATM4 media (Supporting Table 1) supplemented with 1µLmL−1 of 200μM acetosyringone to acquire the OD600 of 0.7. Simultaneously, 10mL of the preheated (45°C) ATM6 media (Supporting Table 1) was added to 20 calli clumps in a 50mL falcon tube. The calli were allowed to have heat shock by placing the falcon tube in water bath at 45°C for 5min followed by keeping in a 4°C fridge for 30min. After the cold shock, ATM6 media was decanted and 10mL of induced Agrobacterium culture was added. Before being centrifuged at 25°C, 1000rpm for 10min, the mixture was then shaken at 70rpm at room temperature for 5min. Following that, the calli in the Agrobacterium suspension left standing inside laminar hood at room temperature for 30min. The media was then discarded and the calli blotted dry on sterile Whatman filter paper. However, mock transformation was applied by placing some calli in ATM4 media without Agrobacterium. The inoculated calli were placed on co-cultivation media (Supporting Table 1) for 3 days at 28°C in dark growth cabinet.
Tripogon calli were also transformed using Agrobacterium-mediated transformation method for Brachypodium distachyon as described by Alves and colleagues (Alves et al., 2009) when 5 weeks-old. Before the day of transformation, 10mL liquid LB media with Agrobacterium strain harboring the genes of interest was transferred to a 250mL sterile conical flask and cultured overnight in 90mL MSB media (Supporting Table 1) supplemented with 45mgL−1 of acetosyringone and 100mgL−1 kanamycin at 28°C with a speed of 200rpm in a rotary shaker. On the following day, the overnight culture was transferred to 50mL falcon tube with 25mL in each tube and was centrifuged at 25°C, 3000rpm for 10min. The supernatant was discarded and the pellets in each falcon tube were suspended by vortexing in 25mL of MSB media supplemented with 45mgL−1 of acetosyringone. The culture was kept in a shaker incubator at room temperature for 45min to urge the acceptable density. The optical density of 1mL of culture from each falcon tube was measured by spectrophotometer at a wavelength of 600nm (OD600). The falcon tubes with culture were centrifuged at 3000rpm for 10min and the supernatant was decanted. The pellet was vortexed again in appropriate amount of MSB media supplemented with 45mgL−1 of acetosyringone to prepare the OD600 of the Agrobacterium suspension to 1.0. Approximately 20 Tripogon calli pieces were transferred into each 90 × 15mm petri plate and flooded with 15mL of Agrobacterium culture (OD600 of 1.0) for 5min at room temperature. After inoculation, the calli were desiccated on sterile Whatman filter paper for 7min in laminar flow and then co-cultured on MSB3 media (Supporting Table 1) supplemented with 60mgL−1 of acetosyringone for 3 days. Beside this, some calli were subjected to be mock-transformed in MSB media without any Agrobacterium strain.
Transient expression of UidA gene was analysed after three days of transformation following the strategy described by Jefferson et al. (1987). The calli were observed under a Zeiss Steri-2000-C stereomicroscope and pictures were captured with a digital microscope camera Progress®C5 (Jenoptic, Germany).
2.8 Data analysis
All the data were analysed by using the one-way ANOVA at P ≤ 0.05 in Minitab statistical software version 19.1.1. Tukey’s HSD tests were used to analyse the significant differences among the data. Data within the same parameter category followed by different letters are significantly different at P ≤ 0.05.
3 Results
3.1 Establishment of regeneration system for Tripogon loliiformis
3.1.1 Friable callus induction of Tripogon lolliformis from mature grains
We chose to investigate the suitable concentration of 2,4-D for embryogenic callus induction from mature grains of Tripogon among the 4 concentrations (0.5, 1.0, 2.0 and 3.0mgL−1) in MS media supplemented with 30gL−1. The embryogenic callus was friable, globular in shape and cream white in colour (Fig. 1a). We observed that embryogenic callus induced in 1.0mgL−1 2,4-D after 5 weeks was the highest in percentage (73%) significantly compared to other concentrations (Fig. 1b). However, at 2.0mgL−1 2,4-D concentration, the percentage of callus induction (defined as percentage of seeds developing at least 1 callus) was not statistically different with 1.0mgL−1 2,4-D. Not enough amount of calli formed at low concentration (0.5mgL−1) and high concentration (3.0mgL−1) to proceed with further experiments of regeneration. As 2,4-D is a strong growth regulator, We hypothesized that the higher concentrations (2.0 and 3.0mgL−1) might inhibit plant regeneration from calli masses and considered 1.0mgL−1 as the optimum concentration for callus induction in Tripogon.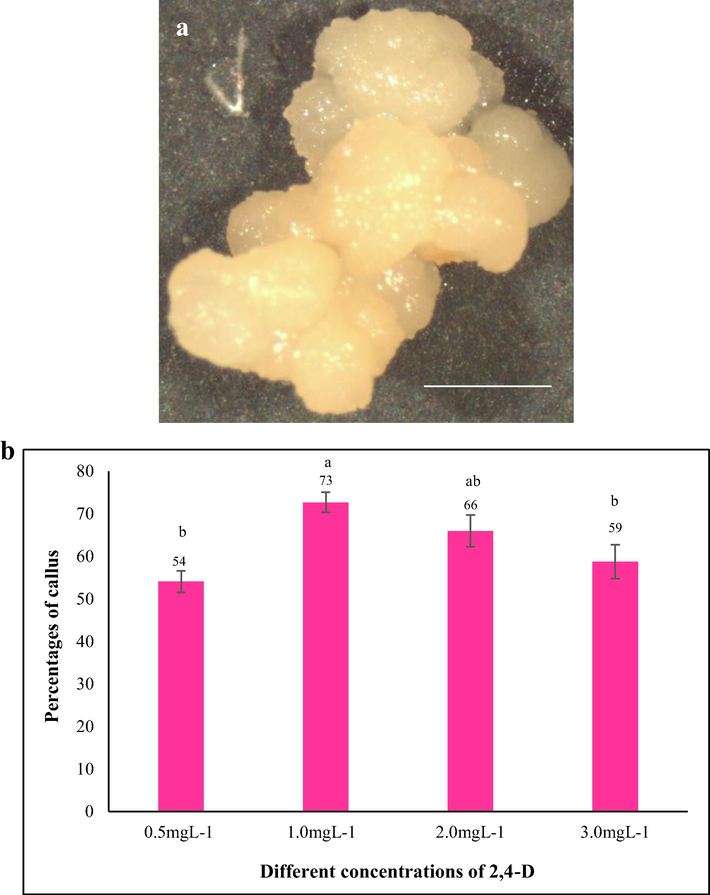
The initiation of callus on MS media supplemented with different concentrations of 2,4-D. a) Morphological features of embryogenic callus of Tripogon loliiformis. b) Mean percentage of callus induced in MS media with different concentrations of 2,4D. n = 10 (corresponding to 10 independent biological replications with 20 seeds each). Error bars represent standard error. Scale bar = 1 mm.
3.1.2 Effect of different media in combination with optimised 2,4-D for callus induction
We investigated whether MS medium supplemented with 1.0mg L−1 2,4-D is the optimum concentration for callus induction. For this, an experiment was conducted with another medium that was used successfully for rice callus induction/transformation namely 2N6 - a modification of N6 medium (Hiei and Komari, 2008) supplemented with 1.0 and 2.0mgL−1 2,4-D (optimum concentration for higher percentage of callus induction inferred from previous experiment). The grains produced 72% of embryogenic callus after 5 weeks in MS medium supplemented with 1.0mgL−1 of 2,4-D which is significantly higher compared to the percentages of calli obtained in 2 N6 medium supplemented with 1.0 (56%) and 2.0mgL−1 2,4D (51%), respectively (Fig. 2). We theorized that MS medium with 1.0mgL−1 2,4-D was most suitable for callus induction than 2N6 medium.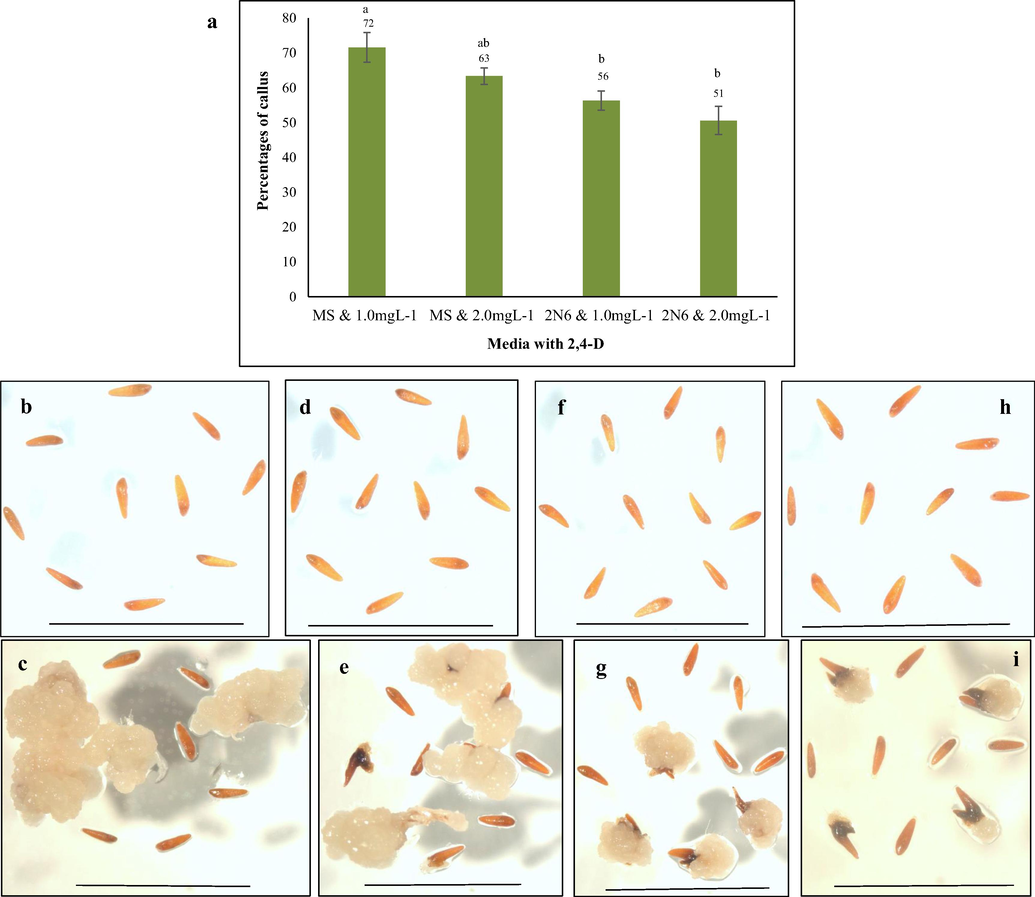
Callus induction from Tripogon grains on MS and 2 N6 media. (a) Mean percentage of callus induction in MS and 2 N6 media, n = 10 (corresponding to 10 independent biological replications with 20 seeds each). Error bars represent standard error. (b and c) MS media with 1.0mgL−1 2,4-D on 0 day and after 5 weeks, (d and e) MS media with 2.0mgL−1 2,4-D on 0 day and after 5 weeks, (f and g) 2 N6 media with 1.0mgL−1 2,4-D on 0 day and after 5 weeks and (h and i) 2 N6 media with 2mgL−1 2,4-D on 0 day and 5 weeks, Scale bar = 1 mm.
3.1.3 BAP and NAA for green point emergence and regenerating miniature plantlets of Tripogon
Green points emerge from the creamy white calli when exposed to light and later form healthy shoots. We subcultured the calli induced in optimised media to MS medium supplemented with shoot and root inducing growth regulators (BAP and NAA) under the effect of light. The percentage of the green points (defined as percentage of calli developing at least 1 green point scored by eye) was recorded after 14 days of exposure to light (Fig. 3a). Almost all the calli obtained green points (100%) in MS media supplemented with (1) 2.0mgL−1 BAP and 0.5mgL−1 NAA combinations (Fig. 3b); (2) 3.0mgL−1 BAP and 0.25mgL−1 NAA and (3) 3.0mgL−1 BAP and 0.5mgL−1 NAA combinations when transferred to light for 14 days. The callus in control MS media without BAP and NAA fail to form any green points at the same time point. Green points also emerged in calli clumps when kept in media supplemented with other combinations of BAP and NAA but in lower percentages. Calli with green points failed to regenerate shoots and eventually died when subcultured on MS control and on MS medium with their respective BAP and NAA combinations for 3 weeks interval. However, the initiation of shoots ensued on medium with 2.0mgL−1 BAP and 0.5mgL−1 NAA after 3 weeks (Fig. 3c). This result reveals that higher concentrations of growth regulators in subsequent subculturing of calli was detrimental for regeneration for sensitive plant like Tripogon. We subcultured the callus with initiated shoots, obtained from 2.0mgL−1 BAP and 0.5mgL−1 NAA, for another 3 weeks on media with reducing amount of NAA into half though the amount of BAP was the same. Shoots emerged from each callus clumps in MS medium supplemented with 2.0mgL−1 BAP and 0.25mgL−1 NAA (Fig. 3d). Rooting was obtained from the base of each shoot clumps by subculturing the regenerated shoots in MS medium supplemented with 2.0mgL−1 BAP and 0.25mgL−1 NAA within 3 weeks (Fig. 3e). The procedure for regenerating plantlets from embryogenic calli of Tripogon loliiformis is represented in Fig. 4.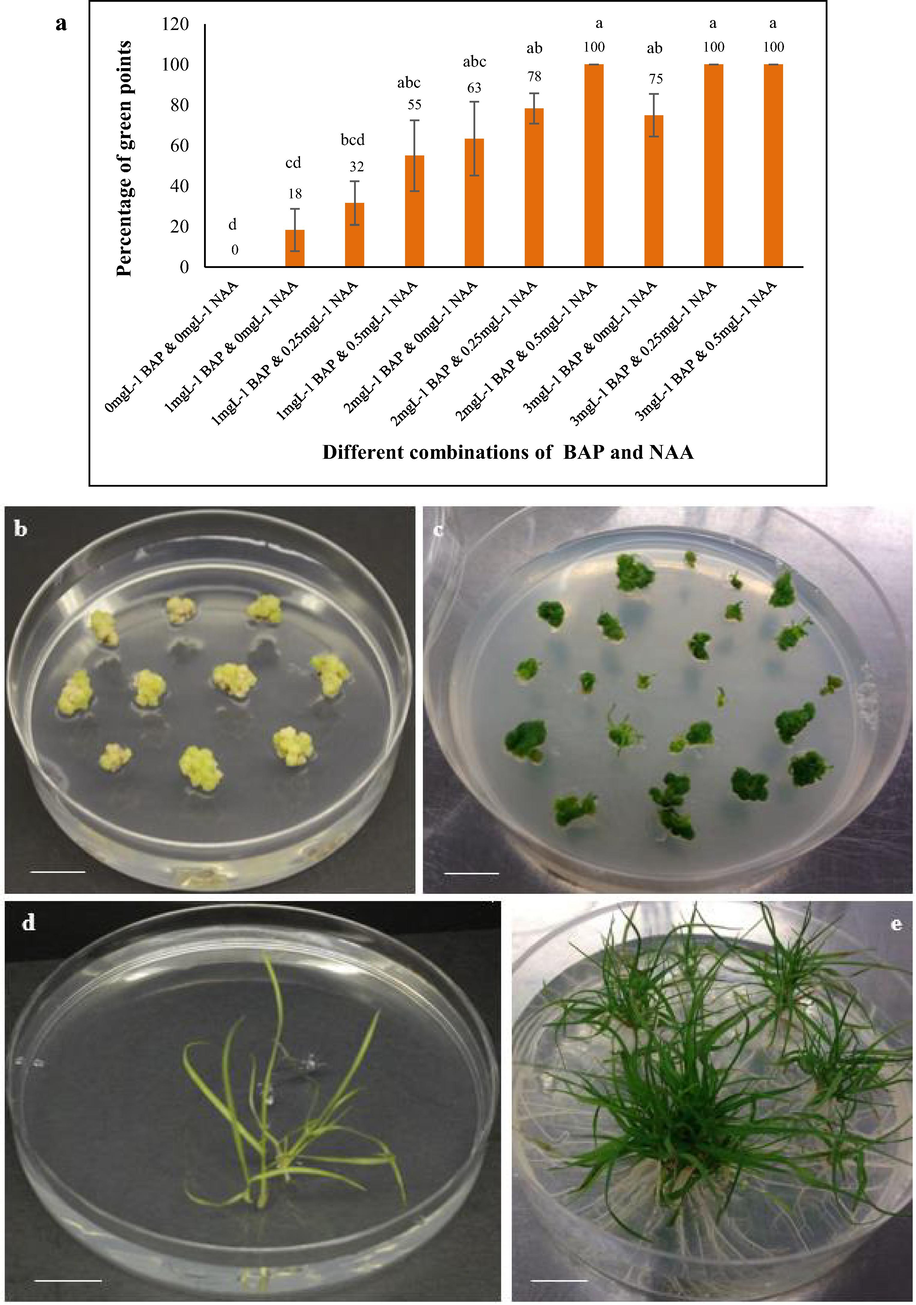
Regeneration of Tripogon loliiformis plantlets from calli. a) Mean percentage of green points coming on MS medium containing different concentrations of BAP and NAA. n = 6 (corresponding to 6 independent biological replications with 10 calli each). Error bars represent standard error, (b) emergence of green points on calli after 2 weeks and (c) shoot initiation from calli after 5 weeks on MS medium with 2.0mgL−1 BAP and 0.5mgL−1 NAA, (d) shoot regeneration after 8 weeks and (e) profuse rooting from shoots after 11 weeks in MS medium supplemented with 2.0mgL−1 BAP and 0.25mgL−1 NAA. Calli were exposed in 16 h light/8h dark. Scale bar = 14 mm.
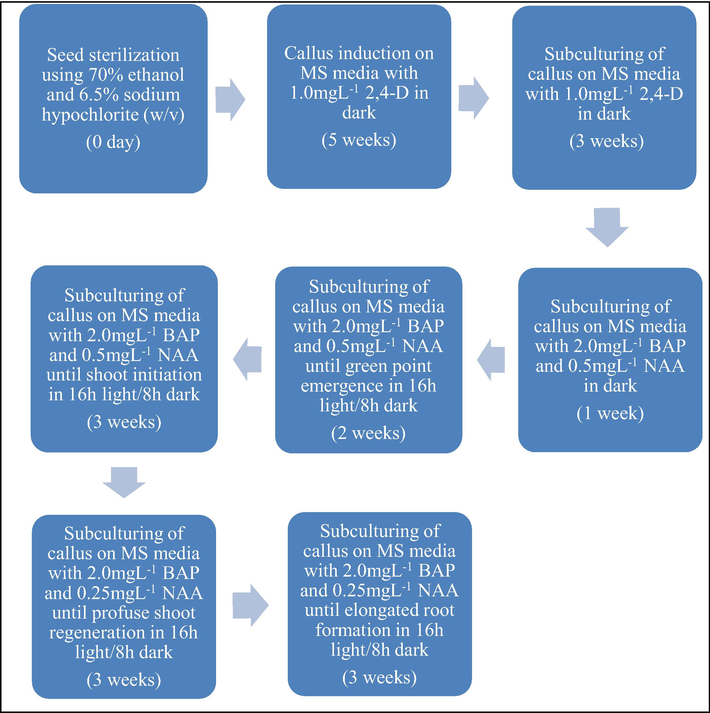
Tripogon loliiformis tissue culture procedure.
3.2 Transient expression UidA gene after Agrobacterium-mediated transformation of T. loliiformis
Tripogon loliiformis is closely related to rice (Oryza sativa) as both are monocotyledonous plants belonging to the grass family. We conducted experiment for Tripogon transformation following the high efficiency rice transformation protocol by Hoang (2014) where embryogenic calli raised from rice grains were used as the explants. Histochemical GUS staining for transient UidA gene expression of 5 weeks old calli after 3 days post transformation showed intense blue colouration in 59% of the transformed calli. However, the control embryonic calli without Agrobacterium transformation did not turn blue indicating that gene has been successfully delivered to the embryogenic transformed calli after Agrobacterium infection (Fig. 5).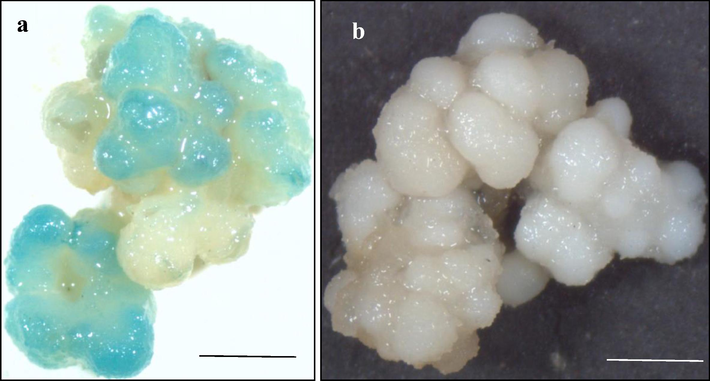
UidA gene expression assay after 3 days of transformation following rice transformation protocol (Hoang 2014). (a) Calli transformed with AGL1pCE104-UidA- EYFP, (b) non-transformed control calli, n = 3 (corresponding to 3 independent biological replications with 10 calli each) . Scale bar = 1 mm.
We also followed Brachypodium distachyon transformation protocol developed by Alves et al. (2009) because Brachypodium is a monocot grass and embryogenic calli is the explant for transformation like Tripogon. This protocol with few modifications was experimented for transforming Tripogon calli by using two different Agrobacterium strains viz. AGL1 (pCE104-UidA-EYFP and pCAMBIA2301) and LBA4404 (pCE104-UidA-EYFP). 61% of calli expressed UidA gene for transformation performed with AGL1 (pCE104-UidA-EYFP), followed by 58% and 36% GUS expression for LBA4404 (pCE104-UidA-EYFP) and AGL1 (pCAMBIA2301), respectively in the transient expression essay of UidA gene at 3 days after transformation (Fig. 6).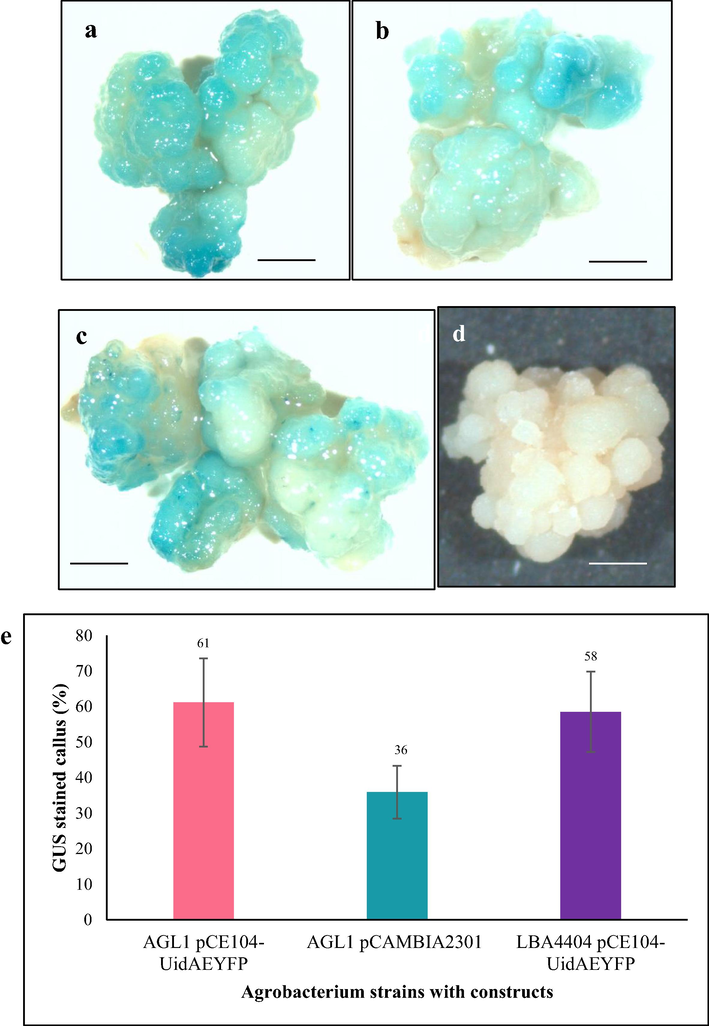
UidA gene expression in Tripogon calli 3 days post-transformation with Agrobacterium strains AGL1 pCE104-UidAEYFP, AGL1 pCAMBIA2301 and LBA4404 pCE104-UidA-EYFP following Brachypodium transformation protocol. (a) AGL1 pCE104-UidA-EYFP, (b) AGL1 pCAMBIA2301, (c) LBA4404 pCE104-UidA-EYFP, (d) control, (e) Percentages of GUS stained calli. n = 3 (corresponding to 3 independent biological replications with 10 calli each). Scale bar = 1 mm.
4 Discussion
Establishment of an efficient tissue culture protocol could be a criterion for an effective transformation system of a plant (Sant, 2012). Tripogon loliiformis is a inimitable resurrection plant that shares the identical family with important cereals including rice (Karbaschi et al., 2016; Williams et al., 2015). It has been reported that the well designated methods for cereal transformation generally involve Agrobacterium tumefaciens-mediated transformation of cultured tissue, immature and mature embryos followed by callus culture to regenerate plants (Shrawat and Lörz, 2006). Among the materials that may be used for initiating callus, mature grains are the best suited because they can be obtained in quantity and kept viable for an extended time in the laboratory (Hiei and Komari, 2008). Not only that, different concentrations of 2,4-D starting from 1mgL−1 upto 4mgL−1, are used for inducing callus in rice along with either MS or N6 media (Binte Mostafiz and Wagiran, 2018; Hiei et al., 1994; Verma et al., 2011). While each rice cultivar may have an optimum 2,4-D concentration for callus induction, one common thing between them is that calli are often induced from mature grains using different concentrations of 2,4-D in MS or N6 media. Similarly, in this study we found that embryogenic callus can be induced from mature Tripogon grains on MS or N6 media supplemented with 3% sucrose and different concentrations of 2,4-D ranging from 0.5mgL−1 to 3mgL−1, pH 5.8 and solidified with 2.5gL−1 gelzan (Fig. 1). However, 1mgL−1 2,4-D in MS media generated the best frequency of callus induction (73%) from mature Tripogon grains and provided the superlative quality of callus compared to other 2,4-D concentrations tested (Fig. 2). Therefore, it was absolutely selected as the most suitable concentration of 2,4-D for callus induction from Tripogon mature grains. Beside callus induction, shoot regeneration is additionally a necessary step within the protocol of any plant tissue culture. In many plants, shoots are often regenerated in high frequency from embryogenic callus employing a suitable combination of a cytokinin, most of the time BAP, and an auxin such as NAA. Poeaim et al. (2016) reported that a amalgamation of 2.0mgL−1 BAP and 0.5mgL−1 NAA in MS media produced highest plant regeneration frequency from embryogenic rice callus in Thai rice variety Nam Roo. A combination of 3mgL−1 BAP and 0.5mgL−1 NAA in 2N6 media has been practised to regenerate shoot from callus of Nipponbare rice cultivar (Hoang, 2014). During this study, we subcultured embryogenic Tripogon callus on MS media supplemented with 9 different combinations of BAP and NAA (Fig. 3a). We found that at 14 days post exposure to 16h light and 8h dark at 25 ± 2°C, 100% callus cultured on MS media supplemented with 3 different combinations of BAP and NAA including 2.0mgL−1 BAP and 0.5mgL−1 NAA (Fig. 3b and 3c), 3.0mgL−1 BAP and 0.25mgL−1 NAA, and 3.0mgL−1 BAP and 0.5mgL−1 NAA turned green, an honest signal for shoot regeneration. Callus cultured on MS supplemented with another 6 combinations of BAP and NAA also turned green but at lower frequency. However, only callus cultured on MS supplemented with 2.0mgL−1 BAP and 0.25mgL−1 NAA developed to shoots and roots with a frequency of roughly 60% in our experiments (Fig. 3d and 3e). Therefore, we developed a tissue culture system for Tripogon loliiformis using embryogenic callus as suitable material for transformation (Fig. 4).
Following the success of establishment of the tissue culture system for Tripogon loliiformis, we investigated a transformation system for Tripogon loliiformis based on rice transformation protocol that was well established in QUT lab (Hoang, 2014). The GUS staining assay showed UidA gene transient expression in 58.81% of transformed calli (Fig. 5). We also employed Brachypodium distachyon transformation protocol (Alves et al., 2009) for Tripogon transformation because like Tripogon, Brachypodium is a monocot grass and its embryogenic callus was used for Agrobacterium–mediated transformation. As shown in result section, Tripogon embryogenic callus showed transient expression of GUS 3 days post transformation with different Agrobacterium strains with constructs. 61% of calli expressed UidA gene after transformation performed with AGL1 (pCE104-UidA-EYFP), followed by 58% and 36% for LBA4404 (pCE104-UidA-EYFP) and AGL1 (pCAMBIA2301), respectively (Fig. 6). These events ensured the successful delivery of UidA gene from Agrobacterium strains to Tripogon calli.
5 Conclusion
We successfully established the regeneration system for Tripogon loliiformis, the first time described tissue culture set up for a monocot resurrection plant. In addition to this, the transient expression of UidA gene in the Agrobacterium tumefaciens- transformed callus reveals that stable transformation system can be optimized in future with more attempts.
Acknowledgements
We are grateful to the Australian Government Department of Education for funding the study through ‘Endeavour Postgraduate (Master) Scholarship Program round 2017’ and Queensland University of Technology (QUT) for providing tuition fee waiver for 1 year of this study through ‘QUT HDR Scholarship’. We also would like to thank Centre for Agriculture and Bioeconomy (CAB) and Central Analytical Research Facility (CARF) of QUT for the research facilities.
Author contribution
SRC contributed in the planning and implementation of experiments and data collection. SH and NA contributed in writing of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability. 2020;12:1736.
- [Google Scholar]
- Effect of some osmoregulators on photosynthesis, lipid peroxidation, antioxidative capacity, and productivity of barley (Hordeum vulgare L.) under water deficit stress. Environ. Sci. Pollut. Res.. 2018;25:30199-30211.
- [Google Scholar]
- Desiccation tolerance in Pleurostima purpurea (Velloziaceae) Plant. Growth Regulation. 2010;62:193-202.
- [Google Scholar]
- A protocol for Agrobacterium-mediated transformation of Brachypodium distachyon community standard line Bd21. Nature Protocols. 2009;4:638-649.
- [Google Scholar]
- Saving for a rainy day: Control of energy needs in resurrection plants. Plant. Sci.. 2018;271:62-66.
- [Google Scholar]
- Roots of the resurrection plant Tripogon loliiformis survive desiccation without the activation of autophagy pathways by mainataining energy reserves. Front. Plant Sci.. 2019;10:459.
- [Google Scholar]
- Comparative metabolic profiling of Haberlea rhodopensis, Thellungiella halophyla, and Arabidopsis thaliana exposed to low temperature. Front. Plant Sci.. 2013;4:499.
- [Google Scholar]
- Efficient callus induction and regeneration in selected indica rice. Agronomy. 2018;8:77.
- [Google Scholar]
- Desiccation tolerance in resurrection plants: new insights from transcriptome, proteome and metabolome analysis. Front. Plant Sci.. 2013;4:482.
- [Google Scholar]
- Photosynthesis in desiccation tolerant plants: energy metabolism and antioxidative stress defense. Plant Sci.. 2012;182:29-41.
- [Google Scholar]
- Leaf and inflorescence structure and phylogenetics of Tripogon and affiliated genera (Poaceae: Chloridoideae). Queensland University of Technology; 2015.
- Friedrich, T. (2015). A new paradigm for feeding the world in 2050: The sustainable intensification of crop production. Resource Magazine22, 18-18.
- Desiccation tolerant vascular plants of Southern Africa. Oecologia. 1977;31:95-109.
- [Google Scholar]
- The occurrence of resurrection plants in the Australian flora. Australian J. Botany. 1978;26:485-492.
- [Google Scholar]
- The evolution of desiccation tolerance in angiosperm plants: A rare yet common phenomenon. Funct. Plant Biol.. 2013;40:315-328.
- [Google Scholar]
- Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy. 2020;2020(10):630.
- [Google Scholar]
- Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nature Protocols. 2008;3:824-834.
- [Google Scholar]
- Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J.. 1994;6:271-282.
- [Google Scholar]
- Engineering salinity tolerance in rice by exogenous expression of cell death regulators. Queensland University of Technology; 2014.
- High efficiency transformation of Escherichia coli with plasmids. Gene. 1990;96:23-28.
- [Google Scholar]
- GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J.. 1987;6:3901-3907.
- [Google Scholar]
- Structural, physiological and molecular characterisation of the Australian native resurrection grass Tripogon loliiformis (F. Meull) In: CE Hubb during dehydration and rehydration. Queensland University of Technology; 2015.
- [Google Scholar]
- Tripogon loliiformis elicits a rapid physiological and structural response to dehydration for desiccation tolerance. Funct. Plant Biol.. 2016;43:643-655.
- [Google Scholar]
- An osmotin from the resurrection plant Tripogon loliiformis (TlOsm) confers tolerance to multiple abiotic stresses in transgenic rice. Physiologia Plantarum. 2018;162:13-34.
- [Google Scholar]
- McGuire, S. (2013). WHO, World Food Programme, and International Fund for Agricultural Development. 2012. The State of Food Insecurity in the World 2012. Economic growth is necessary but not sufficient to accelerate reduction of hunger and malnutrition. Rome, FAO. Oxford University Press.
- Towards a systems-based understanding of plant desiccation tolerance. Trends Plant Sci.. 2009;14:110-117.
- [Google Scholar]
- Genome-wide investigation of the role of microRNAs in desiccation tolerance in the resurrection grass Tripogon loliiformis. Plants. 2018;7:68.
- [Google Scholar]
- Optimization for callus induction and plant regeneration from mature seeds of Thai rice variety: Nam Roo (Oryza sativa L.) Bioeng. Biosci.. 2016;4:95-99.
- [Google Scholar]
- Development of a transformation system for sorghum (Sorghum bicolor L.). Queensland University of Technology; 2012. PhD
- Differences in rehydration of three desiccation-tolerant angiosperm species. Ann. Botany. 1996;78:703-710.
- [Google Scholar]
- Protection mechanisms against excess light in the resurrection plants Craterostigma wilmsii and Xerophyta viscosa. Plant Growth Regul.. 1998;24:203-210.
- [Google Scholar]
- Agrobacterium-mediated transformation of cereals: A promising approach crossing barriers. Plant Biotechnol. J.. 2006;4:575-603.
- [Google Scholar]
- An effective and reproducible transformation protocol for the model resurrection plant Craterostigma plantagineum Hochst. Plant Cell Reports. 2002;21:63-69.
- [Google Scholar]
- Agrobacterium-mediated genetic transformation of the desiccation tolerant resurrection plant Ramonda myconi (L.) Rchb. Plant Cell Reports. 2006;25:442.
- [Google Scholar]
- World Population Prospects: The 2017 Revision, Key Findings and Advance Tables. Department of Economic and Social Affairs, Population Division: United Nations; 2017.
- Verma, D., Joshi, R., Shukla, A., and Kumar, P. (2011). Protocol for in vitro somatic embryogenesis and regeneration of rice (Oryza sativa L.).
- Cell wall characteristics and structure of hydrated and dry leaves of the resurrection plant Craterostigma wilmsii, a microscopical study. J. Plant Physiol.. 1999;155:719-726.
- [Google Scholar]
- Trehalose accumulation triggers autophagy during plant desiccation. PLoS Genet.. 2015;11:e1005705
- [Google Scholar]
- Effect of drought on agronomic traits of rice and wheat: A meta-analysis. Int. J. Environ. Res. Public Health. 2018;15
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.10.010.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







