Translate this page into:
A facile ionic liquid-accelerated, four-component cascade reaction protocol for the regioselective synthesis of biologically interesting ferrocene engrafted spiropyrrolidine hybrid heterocycles
⁎Corresponding author. anatarajan@ksu.edu.sa (Natarajan Arumugam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Spiropyrrolidine engrafted ferrocene heterocycles were synthesized in excellent yields in a sustainable fashion employing an ionic liquid, 1-butyl-3-methylimidazoliumbromide accelerated one-pot multicomponent cycloaddition strategy. The in situ 1,3-dipole component derived from indenoquinoxalinone and L-phenylalanine that reacts with various substituted ferrocenyl chalcone in [bmim]Br affording spirocycloadduct.
Keywords
Multicomponent cycloaddition strategy
Ferrocene grafted spiroheterocyles
Indenoquinoxaline
Ionic liquids
1 Introduction
The construction of structurally diverse and pharmaceutically important heterocyclic hybrids from commercially available simple starting materials while combining propitious economic and environmental aspects constitutes a pronounced challenge in drug discovery especially in the area of combinatorial and diverse oriented synthesis (Cerulli et al., 2012; Sore et al., 2010). Among them, multi-component reactions (MCRs) were the preferred synthetic methods that creates several bonds in a single operation and affords notable benefits such as atom economy, tractability, simple operation, facile mechanization, less number of workup steps and easy extraction and purification methods, makes the conversions more environmental friendly (Ganem, 2009; Domling, 2006; Li and Chen, 2006) that enabled to obtain structurally interesting spirohybrid heterocycles without isolating intermediates (Sumesh et al., 2018).
Spiropyrrolidine heterocyclic motifs have attracted the attention of synthetic and medicinal chemists due to their stimulating biological properties (Trost and Brennan, 2009; Zhou et al., 2010) and these structural motifs are embodied in several natural alkaloids. For instance, (i) spirotryprostatine A and B, (Cui et al., 1996) were active against mammalian cell cycle at the G2/M phase (ii) horsfiline has utilized as a native medicine (Jossang et al., 1991) (iii) Mitraphylline was found to be inhibitor of human brain cancer cell lines, neuroblastoma SKN-BE(2) and malignant glioma GAMG (Prado et al., 2007). Apart from natural origin, many other synthetic spiropyrrolidine analogs have been reported to display anticancer (Yu et al., 2015), antimicrobial (Bhaskar et al., 2012), antimycobacterial (Rajesh et al., 2011), anti-inflammatory, analgesic (Rajanarendar et al., 2013), local anesthetics (Kornet and Thio, 1976) and AChE inhibition activities (Arumugam et al., 2019). Ferrocene is another important organometallic scaffold owing to their synthetic versatility, thermal and photochemical stability and potential biological properties. Ferrocene grafted organic molecules displays anti-cancer and antimalarial properties (Biot et al., 1997; Domarie et al., 1998; Motohashi et al., 1990; Allardyce et al., 2005). Many more ferrocene-based heterocycles possess antimicrobial properties (Fang et al., 2003a; Fang et al., 2003b; Jin et al., 2005).
Based on the above biological precedents, we reasoned the combination of spiropyrrolidine and ferrocene motifs in a single molecule would be of paramount interest in drug discovery. As a extension of our previous research interest in multicomponent cycloaddition strategy, we describe in this article the synthesis of these spirocycloadducts by employing a four-component sequence possessing 1,3-dipolar cycloaddition as crucial step (Gothelf and Jorgensen, 1998; Harju and Yli-Kauhaluoma, 2005). It is pertinent to note that the [3 + 2] cycloaddition involving a combination of ninhydrin and o-phenylenediamine along with L-phenylalanine to create 1,3-dipole comprising indenoquinoxaline unit, respectively, are relatively less explored in literature.
2 Materials and methods
2.1 Synthesis of spiropyrrolidine tethered ferrocene heterocyclic hybrids, 6a-h
An equimolar mixture of 1,2-phenylenediamine 1 (1 mmol), ninhydrin 2 (1 mmol), L-phenylalanine 4 and ferrocenyl chalcone 5 (1 mmol) were stirred in [bmim]Br for 1 h at 100 °C. After completion of the reaction (TLC), EtOAC (10 ml) and water (7 ml) was added. The ethyl acetate layer was separated and dried with sodium sulphate and then evaporated under reduced pressure to afford pure compound in excellent yields.
2.2 Spiropyrrolidine tethered ferrocene heterocycles, 6b
Mp: 265 °C; Light brown solid; 1H NMR: δ/ppm 2.92–2.96 (dd, J = 13.5, 8.0 Hz, 1H), 3.34–3.38 (dd, J = 13.5, 2.5 Hz, 1H), 3.91 (d, J = 9.5 Hz, 1H), 4.09–4.22 (m, 7H), 4.34–4.47 (m, 1H), 4.47–4.59 (m, 2H), 5.06 (d, J = 9.5 Hz, 1H), 6.89 (d, J = 8.5 Hz, 2H), 7.00 (d, J = 8.5 Hz, 2H), 7.16–7.35 (m, 7H), 7.60–7.84 (m, 4H), 8.09 (d, J = 7.5 Hz, 1H), 8.19 (d, J = 6.5 Hz, 1H): 13C NMR: δ/ppm 40.6, 45.6, 62.5, 65.6, 66.2, 67.4, 67.8, 68.7, 69.2, 69.9, 70.3, 71.7, 89.4, 118.5, 121.6, 126.5, 126.9, 128.1, 128.6, 128.9, 129.1, 129.3, 129.5, 129.8, 129.9, 130.0, 131.4, 131.8, 131.9, 135.8, 136.4, 138.8, 141.9, 142.7, 147.6, 147.7, 153.3, 166.1, 197.5; LC/MS(ESI): m/z = 732 (M+).
3 Results and discussion
The pre-requisite starting substrates, ferrocenyl chalcone 5a-h were prepared according to reported method (Mukhtari et al., 2014). The cycloaddition reaction of ferrocenyl chalcone with azomethine ylide derived from indenoquinoxalinone 3 and L-phenylalanine 4 under heating at 100 °C in 1-butyl-3-methylimidazoliumbromide ([bmim]Br) afforded new class of ferrocene engrafted spiropyrrolidine heterocycles in good to excellent yields (83–91%) (Scheme 1). Initially, the reaction was carried out with different solvents including methanol, ethanol, acetonitrile, 1,4-dioxane, mixture of solvents, methanol: acetonitrile (1:1, V/V) and methanol:1,4-dioxane (1:1, V/V) that afforded the cycloadduct in moderate yields as shown in Table 1. In order to enhance the yield, we attempted the reaction under the green synthetic protocol. The reaction was investigated with [bmim]Br as it possesses distinctive properties such as low vapor pressure, non-flammability, act as acidic or basic catalyst, recyclability and has more thermal stability. Thus, in a typical reaction, an equimolar amount of o-phenylenediamine 1, ninhydrin 2, L-phenylalanine 4 and ferrocenyl chalcone 5b were heated in [bmim]Br (Michael Rajesh et al., 2012) for 1 h which afforded 6b in excellent yields (Table 1, entry 7). With this optimized reaction conditions in hand and the structure established for 6b, the plausibility of all these ferrocenyl chalcones 5 towards multicomponent reaction with 1,3-dipole was explored.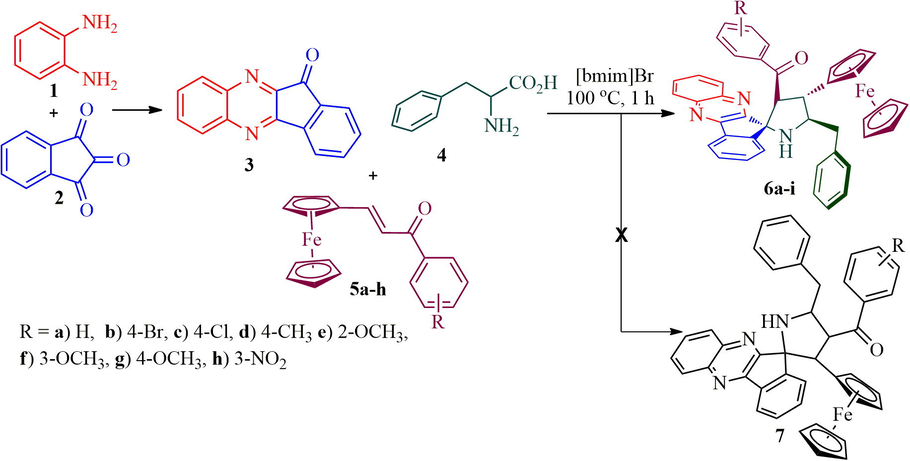
Synthesis of spiropyrrolidino-indenoquioxaline engrafted ferrocene heterocyclic hybrids, 6a-h.
Entry
Solvents
Time (h)
Yield (%)
1
MeOH
3
68
2
|EtOH
3
65
3
MeCN
3
62
4
1,4-Dioxane
3
60
5
MeOH:MeCN
3
66
6
MeOH:1,4-Dioxane
3
64
7
[bmim]Br
1
86
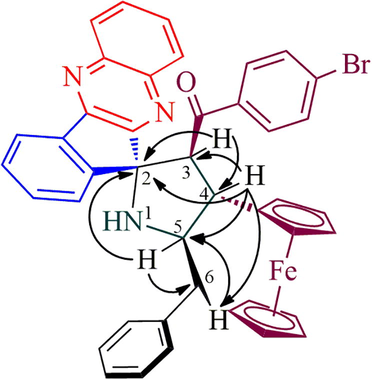
The structure of spiropyrrolidine tethered ferrocene hybrid heterocycles was confirmed through spectroscopic data (vide Supplementary data). The structural elucidation of 6b (Fig. 1) is discussed as follows. In its 1H NMR spectrum, the compound 6b showing a doublet at δ 5.06 (J = 9.5 Hz) due to H-3 of pyrrolidine ring hydrogen which showed H, H-COSY correlation with the triplet at δ 3.91 (J = 9.5 Hz), being assigned to H-4 pyrrolidine ring hydrogen. Further, H-3 shows HMBCs with spiro carbon C-2 and C-4 at δ 89.4 and δ 45.6, respectively. H-4 shows HMBCs (Fig. 2) with H-3, H-5 and H-6 at δ 66.2, 65.6 and δ 40.6, respectively. The multiplet at δ 4.34–4.38 is assigned to H-5 hydrogen which showed H, H-COSY correlation with two doublet of doublets at δ 3.38–3.47 and 2.92–2.96 assigned to 6-CH2 hydrogens. The correlation of H-4 hydrogen with C-6 confirms the obtained regioisomer. Further, the structure of the compound 6a (CCDC deposition number. 1989690) was determined by single crystal X-ray diffraction analysis (Fig. 3).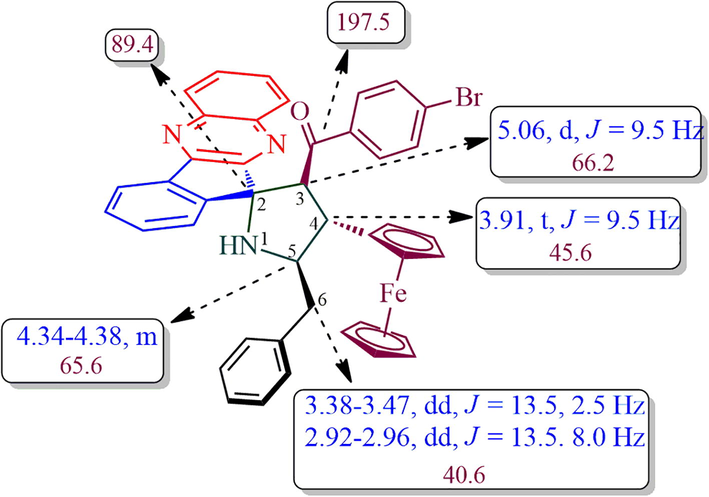
Selected chemical shift of 6b.
Selected HBMCs of 6b.
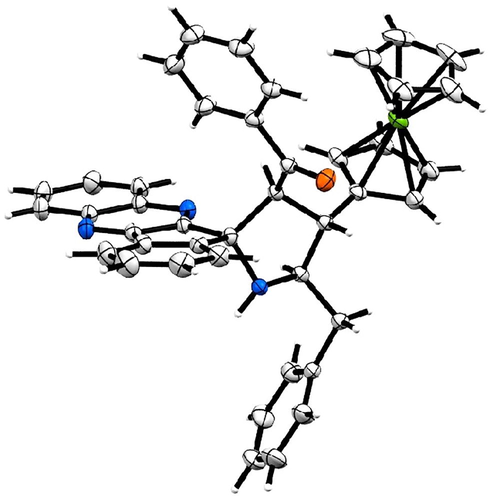
ORTEP diagram of 6a.
The mechanism for the construction of ferrocene grafted spiroheterocycles 6a-h is described in Scheme 2. Initially, o-phenylenediamine 1 reacts with ninhydrin 2 affording indenoquinoxalinone 3 by the elimination of water molecules via cyclocondensation which further reacts with the α-amino acid, L-phenylalanine 4 to form spiro-oxazolidinone intermediate 9 via 8 followed by generation of 1,3-dipole 10. Consequently, the 1,3-dipole 10 attacks the C⚌C bond of the ferrocenyl chalcone 5 regioselectively furnishing the spirocycloadduct 6 forming four contiguous setereocenter in a one-pot conversion, out of which one is quaternary carbon. The ionic liquid, [bmim]Br played an essential role in the cycloaddition sequence as described in Scheme 2 which is supported by our earlier report (Arumugam et al., 2019).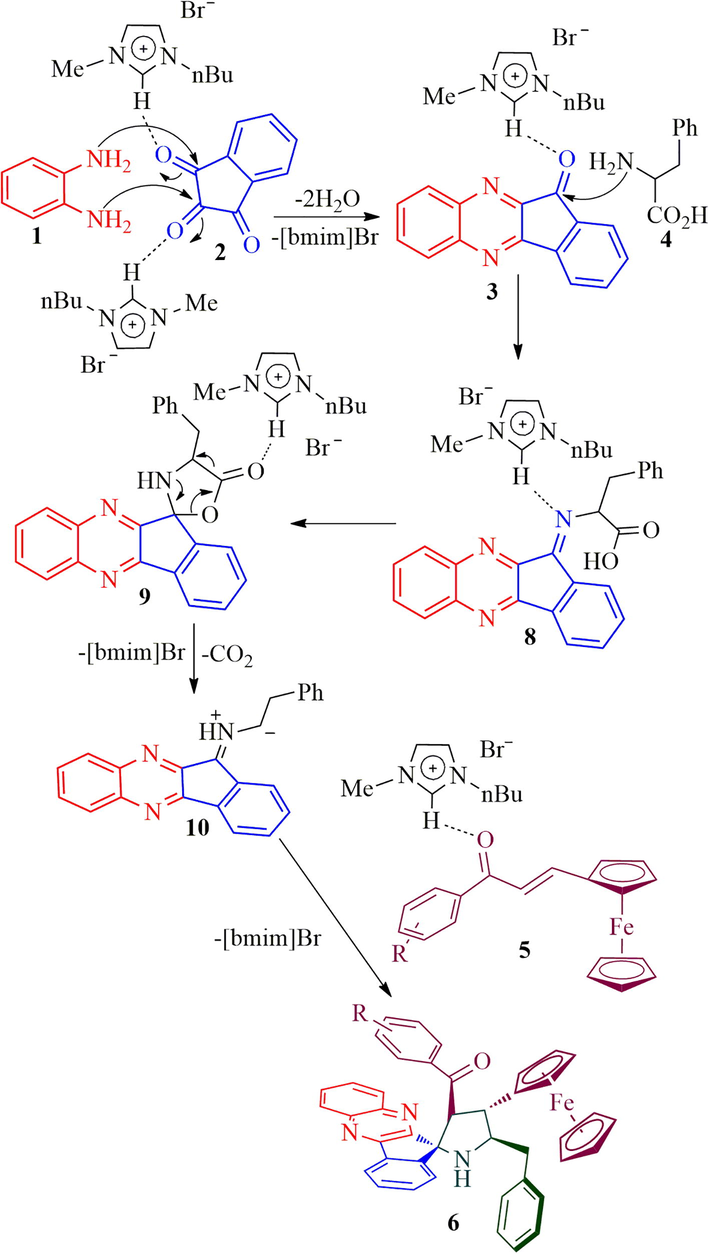
The mechanism for the formation of ferrocene grafted spiropyrrolidines, 6a-h.
4 Conclusion
A small library of hitherto unexplored heterocycles comprising spiropyrrolidine, indenoquinoxaline and ferrocene structural sub-units has been accomplished in good to excellent yields using [bmim]Br mediated multicomponent cycloaddition approach. A relatively less explored non-stabilized 1,3-dipole, derived from indenoquinoxalinone and L-phenylanine was employed. The structure of synthesized spiropyrrolidine hybrids were assigned through 1H, 13C, and 2D NMR spectroscopic analysis. The biological activity of synthesized compounds will be done in due course.
Acknowledgement
The project was supported by Researchers Supporting Project number (RSP-2019/143), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Development of organometallic (organo-transition metal) pharmaceuticals. J. Appl. Organomet. Chem.. 2005;19:1-10.
- [Google Scholar]
- Dispiropyrrolidinyl-piperidone embedded indeno[1,2-b]quinoxaline heterocyclic hybrids:Synthesis, cholinesterase inhibitory activity and their molecular docking simulation. Bioorg. Med. Chem.. 2019;27:2621-2628.
- [Google Scholar]
- Synthesis of novel spirooxindole derivatives by one pot multicomponent reaction and their antimicrobial activity. Eur. J. Med. Chem.. 2012;51:79-91.
- [Google Scholar]
- Synthesis and antimalarial activity in vitro and in vivo of a new ferrocene-chloroquine analogue. J. Med. Chem.. 1997;40:3715-3718.
- [Google Scholar]
- Diversity oriented and chemoenzymatic synthesis of densely functionalized pyrrolidines through a highly diastereoselective Ugimulticomponent reaction. Org. Biomol. Chem.. 2012;10:1255-1274.
- [Google Scholar]
- Novel mammalian cell cycle inhibitors, spirotryprostatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron. 1996;52:12651-12666.
- [Google Scholar]
- In vitro antimalarial activity of a new organometallic analog, ferrocene-chloroquine. Antimicrob. Agents Chemother.. 1998;42:540-544.
- [Google Scholar]
- Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev.. 2006;106:17-89.
- [Google Scholar]
- Synthesis, structure and antibacterial activities of novel ferrocenyl-containing 1-phenyl-3-ferrocenyl-4- triazolyl-5-aryl-dihydropyrazole derivatives. J. Organomet. Chem.. 2003;674:1-9.
- [Google Scholar]
- Preparation, characterization and biological activities of novel ferrocenyl-substituted azaheterocycle compounds. Appl. Organomet. Chem.. 2003;17:145-153.
- [Google Scholar]
- Strategies for innovation in multicomponent reaction design. Acc. Chem. Res.. 2009;42:463-472.
- [Google Scholar]
- Recent advances in 1,3-dipolar cycloaddition reactions on solid supports. Mol. Divers.. 2005;9:187-207.
- [Google Scholar]
- Synthesis, structures and biological activity research of novel ferrocenyl-containing 1H–1,2,4-triazole derivatives. J. Organomet. Chem.. 2005;690:1226-1232.
- [Google Scholar]
- Horsfiline, an oxindole alkaloid from Horsfieldia superba. J. Org. Chem.. 1991;56:6527-6530.
- [Google Scholar]
- Oxindole-3-spiropyrrolidines and piperidines. Synthesis and local anesthetic activity. J. Med. Chem.. 1976;19:892-898.
- [Google Scholar]
- Synthesis and activity of potential antitumor ferrocenes. J. Organomet. Chem.. 1990;398:205-217.
- [Google Scholar]
- Multi-component, 1,3-dipolar cycloaddition reactions for the chemo-, regio and stereoselective synthesis of novel hybrid spiroheterocycles in ionic liquid. Tetrahedron Lett.. 2012;53:5367-5371.
- [Google Scholar]
- Synthesis, characterization, antiamoebic activity and toxicity of ferrocenyl chalcones. Asian. J. Chem.. 2014;26:8407-8412.
- [Google Scholar]
- Antiproliferative effects of mitraphylline, a pentacyclic oxindole alkaloid of Uncaria tomentosa on human glioma and neuroblastoma cell lines. Phytomedicine. 2007;14:280-287.
- [Google Scholar]
- A facile synthesis, anti-inflammatory and analgesic activity of isoxazolyl-2,3-dihydrospiro[benzo[f]isoindole-1,3′-indoline]-2′,4,9-triones. Bioorg. Med. Chem. Lett.. 2013;23:3954-3958.
- [Google Scholar]
- Antimycobacterial activity of spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio- and stereoselective 1,3-dipolar cycloaddition. Med. Chem. Commun.. 2011;2:626-630.
- [Google Scholar]
- Diversity-oriented synthesis of disubstituted alkenes using masked silanols. Org. Lett.. 2010;12:2806-2809.
- [Google Scholar]
- Synthesis of spiro-linked quinolinone-pyrrolidine/pyrrolo[1,2-c] thiazole-oxindole/acenaphthalene hybrids via multi-component [3 + 2] cycloaddition. Tetrahedron Lett.. 2018;59:4086-4089.
- [Google Scholar]
- Trost, B.M., Brennan, M.K., 2009. Asymmetric Syntheses of Oxindole and Indole Spirocyclic Alkaloid Natural Products Synthesis. 3003–3025.
- Spirooxindoles: promising scaffolds for anticancer agents. Eur. J. Med. Chem.. 2015;97:673-698.
- [Google Scholar]
- Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3 position. Adv. Synth. Catal.. 2010;352:1381-1407.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.04.007.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







