Translate this page into:
Corrosion inhibition of mild steel in 1 M HCl by sweet melon peel extract
⁎Corresponding author at: Dept. of General Studies, Jubail University College, Royal Commission-Jubail, P.O. Box-10074, Jubail Industrial City 31961, Saudi Arabia. saeedm@ucj.edu.sa (Mohammed Tariq Saeed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Corrosion inhibition of mild steel by sweet melon (Cucumis melo L) peel (SM) extract in 1 M HCl solution was evaluated by weight loss and potentiodynamic polarization methods. Various SM extracts concentrations such as 0.05, 0.1, 0.2, 0.3, 0.4, and 0.5 g/l were added and corrosion rate (CR) of mild steel and inhibition efficiency (IE) were determined at various temperatures from 295 to 333 K. The appreciable decrease in CR with increase in SM extract concentration was observed at each temperature. However, the typically accelerated CR at each SM extract with the rise in temperature corresponded to the increased kinetic activities at the metal/electrolyte interface. By the addition of 0.5 g/l SM extract, ∼5 times lower CR of mild steel at high temperature (333 K) than in blank acidic solution confirmed its strong inhibitive efficacy. The relatively large variation in the anodic Tafel slope and progressive decrease in CR with an increase in the SM extract concentration validated the restricted dissolution of mild steel. The barrier characteristics of the SM extract layer and its chemical interaction with the surface was evaluated from the low activation energy (Ea) values that fluctuated from ∼20 to 23 kJ/mole. The increase in kad increased from 0.602 to 1.053 (g/l)−1 and decrease in ΔG°ad (−3.74 to −4.91 kJ/mole) with an increase in temperature from 295 to 333 K assured the spontaneous interaction of SM extract molecules with the steel surface.
Keywords
Eco-friendly corrosion inhibitor
Sweet melon peels (Cucumis melo L.)
Mild steel
Weight loss and Potentiodynamic study
1 Introduction
Corrosion of metallic equipment has always remained a major challenge in any process industry. The corrosive attack of the environment to the metallic components may impact the functionality of the overall process. The sudden failure of the components due to corrosion result in the direct and indirect economic loss and could affect the product quality and delays in plant shutdown due to the necessary repairs. Various industries and operations, i.e., acid pickling, oil industry, oil-well, and heat exchangers cleaning processes utilize highly acidic media particularly HCl solutions. The acidic conditions could deleteriously affect the surface and may decrease the service life of the equipment (Khaled, 2003; Verma et al., 2015). It is, therefore, the precise control and monitoring of the corrosion processes are always required. For closed systems, the corrosion control by the chemical inhibitor is considered as the essential procedure. The inhibiting species in the aggressive media if added in small amount could significantly improve the corrosion resistance of the metallic materials and is considered as an effective way to avoid internal corrosion (Benabbouha et al., 2018; Umoren et al., 2018).
Generally, the corrosion inhibitors are expensive synthetic chemicals and may contaminate the product quality. Most effective and competitive organic inhibitors are those compounds having hetro-atoms such as nitrogen, oxygen, sulfur, and phosphorus, which permit adsorption on a metallic surface (Tariq Saeed, 2004; Rahman et al., 2005). Owing to the toxic effects of such corrosion inhibitors, the research on eco-friendly, green corrosion inhibitors is always encouraging (Al-Sehaibani, 2000; El-Etre and Ali, 2017). Green corrosion inhibitors may decompose and have the least toxic effects contrary to chromium or other heavy metals containing compounds (Khadom et al., 2018; Eddy and Odoemelam, 2009). The corrosion inhibition by the use of fruit peel extracts could be very beneficial due to their wide availability, economical, eco-friendliness, and compatibility with the environment (Verma et al., 2018). For instance, garlic (Raja and Sethuraman, 2008; Barreto et al., 2017), ginger (Narenkumar et al., 2017; Parthipan et al., 2018); azadirachta indica (Oguzie, 2008); strychnos nux-vomica (Bothi Raja and Sethuraman, 2009), aloe vera extract (Eddy et al., 2010; Abiola and James, 2010), and aquilaria crassna leaves extract (Helen et al., 2014) have been rigorously investigated in the last two decades. Similarly, the use of leaves and seeds of various plants and fruits as corrosion inhibitors has also been reported by many researchers (El-Etre, 2003; Noor, 2009; Noor and Al-Moubaraki, 2008; Caroline et al., 2015).
Sweet melon is known as cantaloupe and (الاشمام) in Arabic belongs to the family Cucurbitaceous. It is a good source of niacin, folate, and potassium (Kazuz and Elhadi, 2011). The fruit contains different vitamins including vitamin B6, pro-vitamin A and vitamin C in addition to riboflavin, thiamine and folic acid. Emran et al. (2014) reported the use of eco-friendly cantaloupe extract as a corrosion inhibitor for the protection of aluminum in acidic and alkaline solutions. The inhibitive effect of cantaloupe seed extract could also efficiently improve the corrosion resistance of cast iron in 1 M HCl solution (Emran et al., 2015). In this study, we utilize the Saudi origin sweet melon peel extract (SM extract) as an inhibitor and corrosion tendency of mild steel in 1 M HCl solution is monitored. Various concentration of SM extract (from 0.05 to 0.5 g/l) at different temperatures (from 298 to 333 K) was added in 1 M HCl solution and corrosion tendency of mild steel was evaluated via weight loss and potentiodynamic polarization methods.
2 Experimental
2.1 Specimen preparation
The mild steel coupons (2.0 cm × 2.5 cm × 0.1 cm) having composition, 0.16 wt% C, 0.033 wt% V, 0.017 wt% P, 0.181 wt% Mn, 0.035 wt% Cr, 0.017 wt% Al, 0.054 wt% Mo, and Fe (balance) were used in this study. The surface of the coupon was mechanically abraded with emery grit paper from 320 to 1200 grades sequentially to prepare the polished surface prior to each test. After polishing, the coupons were washed with double distilled water, degrease with acetone and finally dried in hot air stream before use.
2.2 SM extract preparation
The SM peels were air dried for 10 days under natural sunlight followed by drying in an oven at 50 °C for 2 h. The dried peels were crushed and ground to fine powder. About 5 g of powder was dissolved in 100 ml of 1 M HCl and kept for digestion overnight and stirred for 2 h to dissolve all the soluble species from the peel powder. The solution was filtered and designated as “SM extract”. The residue was refluxed in ethanol for at 2 h, filtered, and the SM extract solution was separated from the ethanol through fractional distillation. This stock (SM extract) solution was stored in the airtight glass bottle and kept in the refrigerator for further use. To study the corrosion efficiency of mild steel in the 1 M HCl, containing 0.05, 0.1, 0.2, 0.3, 0.4, and 0.5 g/l, SM extract were prepared from this stock solution.
2.3 Weight loss measurements
Mild steel coupons were first degreased with ethanol, air dried and etched in 5% HCl solution for 1 min. The weight loss was calculated by subtracting the weight of the sample before and after immersion in 100 ml of acidic solution without and in the presence of SM extract. Various concentrations of SM extract i.e., 0 (control), 0.05, 0.1, 0.2, 0.3, 0. 4 and 0.5 (g/l) in 1 M HCl solutions were prepared and used as electrolytes for weight loss measurement. The temperature of each electrolyte was maintained constant by using the thermostatically controlled water bath during experiments and the mild steel samples were exposed to the above-mentioned electrolytes for 6 h (control and in the presence of SM extract) at 295 K, 318 K, and 333 K. All experiments were performed in triplicate to achieve reproducibility and average weight loss values were determined. The CR was calculated from the weight loss data according to ASTM standards method (ASTM PA 2012) and by using Eq. (1) (Oparaodu and Okpokwasili, 2014).
The inhibition efficiency (IE) was calculated using Eq. (2) (Tian et al., 2018):
The surface coverage (Θ) was calculated using Eq. (3).
2.4 Electrochemical measurements
Potentiodynamic polarization study was conducted in a water jacketed three-electrode glass cell. The cell comprising of mild steel as the working electrode, a platinum electrode was used as auxiliary electrodes, and saturated calomel electrode (SCE) was used as the reference, in this study.
The working electrodes of 1.0 cm2 were ground with SiC paper up to 1200 grit size, rinsed with distilled water, and degreased with acetone before each test. The electrochemical experiments were conducted in 100 ml of the solution containing various concentrations of SM extract (from 0 to 0.5 g/l) at various temperatures i.e., 295, 318, and 333 K. The initial delay of 30 min was imposed to establish a steady-state condition in the solution and to achieve the constant open circuit potential (OCP) value before each experiment. All the electrochemical test were conducted by using a Band Corr-test Potentiostat (Model CS-315) connected via an electrometer. The potential range of
vs. OCP and a scan rate of 0.5 mV/s were selected. Extrapolation of the Tafel regions was carried out in the built in software application; the corrosion potential (Ecorr) and corrosion current density (icorr) values were determined. Tafel slopes ‘ba’ and ‘bc’ obtained from the slope of the linear Tafel portion of the anodic and cathodic curves, respectively (McCafferty, 2005). For the polarization study, the IE was calculated by using the Eq. (4) (Yang et al., 2017; Murulana et al., 2015).
3 Results and discussion
3.1 Weight loss data
The CR of mild steel was determined by weight loss measurement in 1 M HCl at 295, 318, and 333 K in blank and in the presence of SM extract. The quantitative information is presented in Table 1. Form these results, the appreciable decrease in wright loss was observed with the increase in SM extract concentration at each temperature. For instance, in the blank acidic solution, the CR of mild steel was 1.974 mm/y (equivalent to 77.78 mpy), which decreased to 1.533 mm/y (60.4 mpy) with the addition of 0.05 g/l SM extract at 295 K. Also, approximately 5 times lower CR of mild steel in the presence of 0.5 g/l SM extract compared to the blank solution was evident from these experiments. The significant increase in CR of mild steel from 1.974 to 16.305 mm/y with an increase in temperature from 295 to 333 K, respectively was observed in blank 1 M HCl solution. However, in the presence of SM extract, an appreciable decrease in CR at high temperatures was evident. This indicated the strong inhibitive tendency of the SM extract and its stability at high temperature (up to 333 K). The inhibitive action of SM extract at a relatively high temperature than 295 K was an impressive feature of SM extract. Approximately 12 and 5 times lower corrosion rate at 318 and 333 K, respectively, in 1 M HCl solution containing 0.5 g/l SM extract was registered by the mild steel. Similarly, in the presence of 0.5 g/l SM extract, the appreciable improvement in the IE from 79.69% to 91.59% with an increase in temperature from 295 to 318 K represented the thermal stability and effectiveness of inhibitor at relatively high temperature. Further increase in temperature to 333 K resulted in the decrease of IE (81.26%), which was most likely associated with the degradation and incompatibility of SM extract species with the steel surface at high temperatures. This also indicated that the inhibitive action of SM extract was hampered by the increase of ionic mobility at the metal/electrolyte interface at high temperature. Generally, independent of the SM extract concentration the corrosion of mild was accelerated with an increase in solution temperature. However, at each temperature, the effectiveness of SM extract and its inhibitive action was predicted from the considerable decrease in CR and/or from the improvement in the IE (Table 1). From these results, it was deduced that SM extract species could adsorb on the surface of mild steel and provide a barrier to the aggressive environment to interact with the surface. The direct relation of IE with the SM extract concentration also suggested the effective surface protection by providing a physical barrier to steel dissolution in highly acidic and aggressive medium. To ensure the potency and efficacy of SM extract as an inhibitor in 1 m HCl solution, the Potentiodynamic polarization tests (Tafel scans) were also conducted. Based on the kinetic information, the surface coverage and thermodynamic parameters, the inhibitive action of SM extract is explained in the following section.
SM extract
Temperature of the electrolyte
295 K
318 K
333 K
Concentration (g/l)
Loss in wt. (mg)
CR (mm/y)
IE %
Loss in wt. (mg)
CR (mm/y)
IE %
Loss in wt. (mg)
CR (mm/y)
IE%
Blank
11.590
1.974
–
35.985
6.131
–
95.687
16.305
–
0.05
9.001
1.533
22.34
15.650
2.666
56.51
48.958
8.342
48.84
0.1
7.021
1.196
39.42
10.658
1.816
70.38
38.562
6.571
59.70
0.2
6.500
1.107
43.92
8.135
1.386
77.39
28.965
4.935
69.73
0.3
5.006
0.853
56.81
6.557
1.117
81.78
23.650
4.030
75.28
0.4
3.600
0.613
68.94
5.659
0.964
84.27
20.540
3.500
78.53
0.5
2.354
0.401
79.69
3.025
0.515
91.59
17.932
3.055
81.26
3.2 Electrochemical testing and thermodynamic evaluation of the SM extract inhibitive action
To elucidate the effect of temperature on the corrosion inhibition by SM extract, the CR of the mild steel was calculated from the Tafel scans. The extrapolation of the Tafel region (linear portion in the log(i) vs. potential trends) as shown in Fig. 1 was carried out to determine the corrosion current density (icorr) and the values obtained at 295, 318, and 333 K are presented in Tables 2a–c, respectively. With the addition of SM extract in 1 M HCl solution at 295 K, a noticeable effect on the anodic Tafel slope (βa) was observed. The increase in βa (>120 mV/dec for steel) with the addition of SM ≤ 0.1 g/l indicated its possible adsorption on the steel surface and controlled the anodic dissolution process. The dissolution tendency of steel in 1 M HCl solution was initially decreased rapidly from 3.11 to 1.67 mm/y (approx. 50%) with 0.1 g/l SM extract addition which further reduced to 0.45 mm/y at 0.5 g/l SM extract concentration. However, the βc (cathodic Tafel slope) values of steel were found to be independent of the concentration of SM extract. In other words, in support to the weight loss measurement results, the variation in βa suggested the formation of an adsorbed barrier layer, which restricted the dissolution of steel in aggressive HCl solution. With an increase in temperature (from 318 to 333 K), the βa decreased significantly, but an overall decreasing trend of icorr was observed with increase in SM extract concentration as given in Tables 2b and 2c. This behavior illustrated that the SM extract could cover the surface by forming a protective layer on the steel surface and considerably improved the corrosion resistance as evident from the decrease in CR with an increase in SM extract concentration. In agreement with the weight loss measurements, the highest IE of 85.52, 90.12, and 87.24%, respectively were observed at 295, 318 and 333 K. To further elucidate the inhibitive action of SM extract and to diagnose the adsorption capability at various temperatures, the thermodynamic parameters were also determined as discussed below. The quantitative information about the thermodynamic parameters was obtained based on the electrochemical results as given in Tables 2a–c.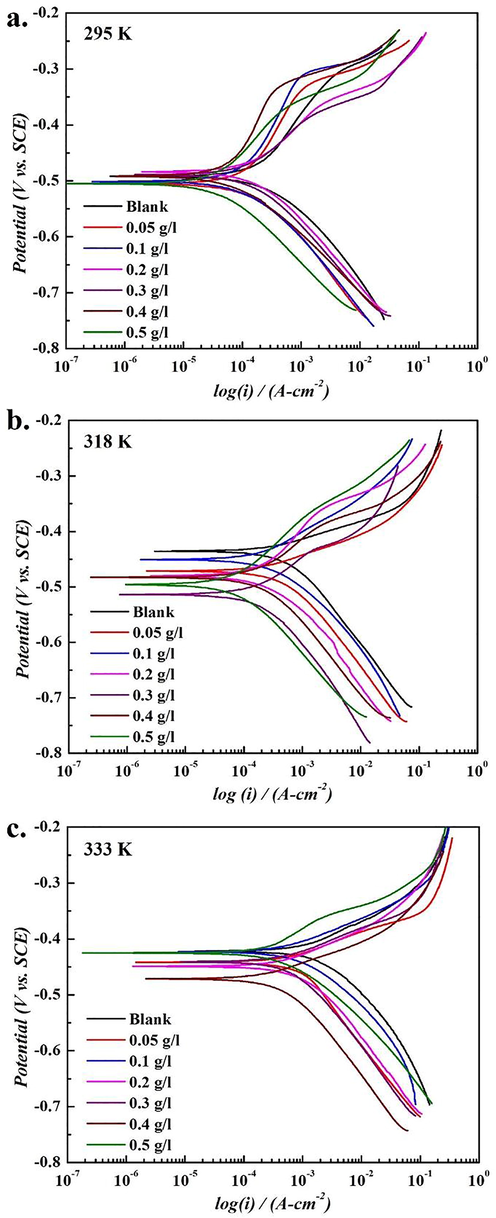
Tafel plots of SM extract at different temperatures (a) 295 K, (b) 318 K, and (c) 333 K).
Concentration (g/l)
ba (mV)
bc (mV)
icorr (mA/cm2)
Ecorr (V)
CR (mm/y)
IE %
Blank
174.81
113.16
0. 0265
−0.492
3.11
–
0.05
221.06
123.79
0. 0165
−0.505
2.33
25.33
0.1
239.05
118.96
0. 0142
−0.501
1.67
46.44
0.2
89.54
104.90
0. 0136
−0.484
1.59
48.87
0.3
78.76
99.49
0. 0100
−0.489
1.18
62.21
0.4
237.59
96.45
0. 0082
−0.492
0.96
69.18
0.5
112.32
99.65
0. 0038
−0.505
0.45
85.52
Concentration (g/l)
ba (mV)
bc (mV)
icorr (mA/cm2)
Ecorr (V)
CR (mm/y)
IE %
Blank
73.28
152.91
0. 0747
−0.436
8.76
–
0.05
71.95
140.49
0. 0574
−0.471
6.74
23.11
0.1
82.21
108.38
0. 0328
−0.451
3.85
56.09
0.2
117.56
116.34
0. 0248
−0.480
2.91
66.81
0.3
82.62
132.71
0. 0201
−0.514
2.36
73.05
0.4
67.49
106.35
0. 0135
−0.483
1.58
81.94
0.5
96.95
110.82
0. 00738
−0.496
0.87
90.12
Concentration (g/l)
ba (mV)
bc (mV)
icorr (mA/cm2)
Ecorr (V)
CR (mm/y)
IE %
Blank
90.181
133.1
0. 267
−0.42165
31.215
–
0.05
75.814
175.7
0. 135
−0.44158
15.792
49.41
0.1
76.897
114.4
0. 124
−0.42294
14.589
53.26
0.2
81.630
134.2
0. 111
−0.44899
13.006
58.33
0.3
76.169
152.5
0.0955
−0.44098
11.201
64.12
0.4
71.950
140.5
0.0574
−0.47144
6.7356
78.42
0.5
65.443
86.3
0.034
−0.42519
3.9839
87.24
It has been observed that in the blank and the presence of inhibitor, the CR increased with increase in temperature. It has been found that in 1 M HCl solution and at each concentration of SM, the dissolution rate of steel was approximately 10 times higher at 333 K than observed at 295 K. This behavior suggested that the increased dissolution of steel at high temperature was affiliated with the accelerated kinetics behavior of steel and deterioration in the barrier characteristics of the SM extract layer after interaction with ionic species. In other words, the decrease in the activation energy for the oxidation reactions (dissolution of steel) is expected. Similar to the weight loss measurements, the appreciable decrease in CR was noticed at each temperature. The effectiveness of the SM inhibitive action with an increase in SM extract concentration in 1 M HCl solution was predicted from the significant increase in the IE. The IE was increased from 25.3 to 85.5% when the SM extract concentration was increased from 0.05 to 0.5 g/l at 295 K. The high CR in the presence of SM extract and relatively lower IE at 333 K compared to 318 K revealed the deterioration in the inhibitive performance of SM extract at high temperature. The decrease in IE with an increase in temperature suggested the formation of an adsorbed layer on the surface, physical in nature (electrostatic) (Qiang et al., 2018). The influence of temperature and inhibitive action of SM extract on mild steel was estimated from the Arrhenius relation (5) which can be used to calculate the activation energy required for the progress of corrosion reaction (Loganayagi et al., 2014).
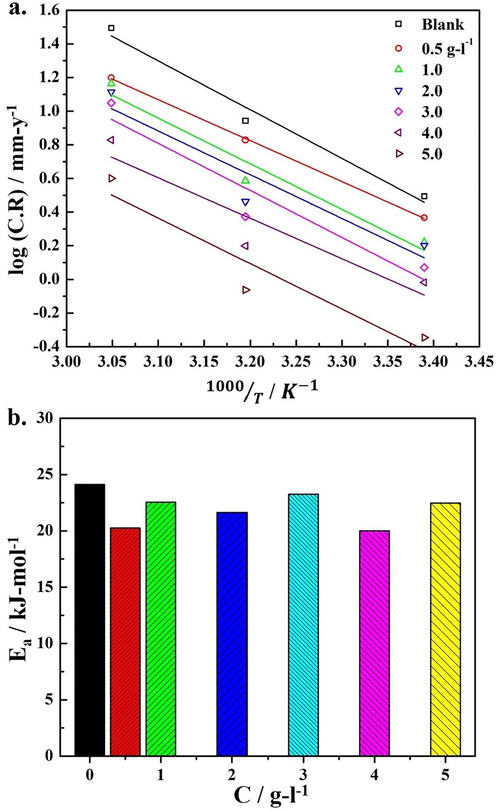
The kinetic model of steel corrosion in HCl solution containing various amount of SM (a) Arrhenius plots showing the linear regression of log (CR) vs. 1000/T at each concentration of SM extract (b) variation in the Ea at various SM extract concentrations.
Fig. 2b illustrates the variation of Ea in 1 M HCl solution at various concentrations of SM extract. The increase in IE with temperature and slightly lower Ea in the presence of SM extract (varied within 20–23 kJ/mole) compared to the Ea (24.1 kJ/mole) in the blank 1 M HCl solution corresponding to the chemisorption of SM extract species on the steel surface as suggested in other studies (Hosein Zadeh et al., 2013; Popova et al., 2003). The relatively low Ea in the presence of SM extract than in the un-inhibited solution indicated the formation of coordinated complex between Fe and SM extract molecules. This behavior assured the inhibition potency of SM extract by forming a dense blocking layer that restricted the dissolution of steel in HCl solution by covering the larger surface area possibly due to orientation of hydrocarbon chains towards solution, which may increase the hydrophobicity of the surface (Hamdy and El-Gendy, 2013; Szauer and Brandt, 1981).
The corrosion inhibition tendency of SM extract was attributed to specific adsorption (chemical adsorption) due to the formation of Fe atoms–SM extract molecule complex and re-orientation of the hydrocarbon chains towards solution which restricted approach of aggressive ionic species towards steel surface. The formation of the adsorbed layer at a constant temperature depends on the concentration of SM extract and was estimated from the surface coverage (θ) (by using Eq. (3).
The progressive adsorption of SM extract also depends on the availability of the occupied sites or fraction of exposed surface area. This is represented by the fractional occupancy or surface coverage, and from this parameter, the adsorption/desorption equilibrium constant (kad) was also calculated at each temperature by using Eq. (6). Where C is the SM extract concentration in g/l in this relation (Qiang et al., 2018).
The adsorption and desorption of SM species followed the Langmuir isotherm and the plot of C/θ vs. C at each temperature showed linear trends with a sufficient degree of certainty as shown in Fig. 3a. These isotherms were used to calculate the kad that was related with the Gibbs free energy (ΔGad) of adsorption-desorption of the SM extract according to Eq. (7), where 0.018 is the inverse of the molar concentration of water (55.5 mol/l) (Qiang et al., 2018; Hamdy and El-Gendy, 2013).
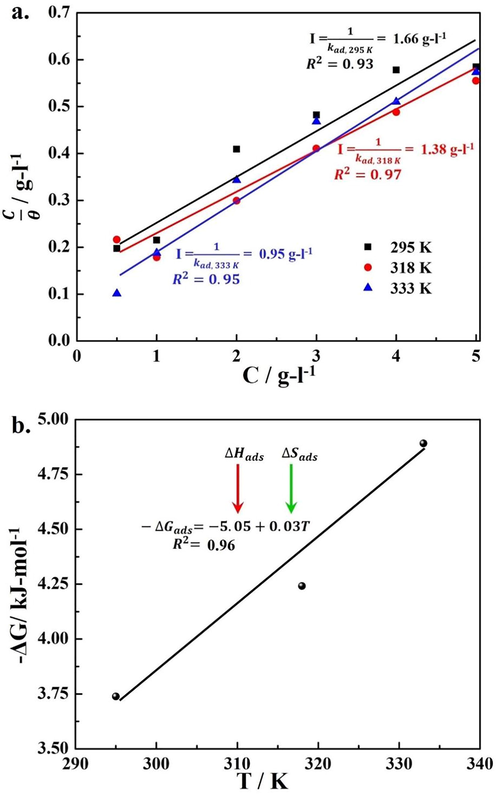
Thermodynamic evaluation of SM extract (a) Langmuir isotherms used to evaluate the kad (b) variation in the (ΔG°ad) as a function of temperature.
Temperature (K)
Kad (g/l)–1
ΔG°ad (kJ/mole)
ΔH°ad (kJ/mole)
ΔS°ad (kJ/mole-K)
295
0.602
−3.74
−5.24
0.03
318
0.725
−4.24
333
1.075
−4.91
The kad increased from 0.602 to 1.075 (g/l)–1 with an increase in temperature from 295 to 333 K also indicated the increase in the energetics of the SM molecules to interact with the steel surface that facilitated the formation of coordinated complex with the Fe atoms (chemical interaction). In agreement with the increase in the kad value, the ΔG°ad decreased to more negative value (from −3.74 to −4.91 kJ/mole) with increase in temperature from 295 to 333 K. This behavior attributed to the spontaneous reaction of SM molecules with the Fe atoms and formation of barrier adsorbed layer on the surface of steel which led to decrease in CR in the aggressive acidic environment. Plotting the ΔG°ad vs. absolute temperature also presented a linear trend with R2 = 0.96 as shown in Fig. 3b. From the intercept and slope of this linear regression, the standard enthalpy (ΔH°ad) and entropy (ΔS°ad) values, respectively were derived as given in Table 3. The ΔH°ad and ΔS°ad were calculated to be −5.24 kJ/mole and 0.03 kJ/mole-K, respectively. These values suggested that during the interaction of SM extract molecules with the steel surface, the energy is released (exothermic reaction) as depicted by the negative sign of enthalpy of activation (Fiori-Bimbi et al., 2015; Lodhi et al., 2018). From these results, it is predicted that the dissolution of mild steel is controlled by the diffusion of ionic species through this barrier surface layer (Tian et al., 2018). In other words, with increase in temperature and concentration of SM extract in 1 M HCl, promoted the hydrophobization at the steel surface due to chemical interaction of SM molecules with the Fe atoms (on steel surface) and due to possible orientation of hydrocarbon chains towards solution as discussed in the literature (Szauer and Brandt, 1981). On the other hand, the +0.03 kJ/mol-K, ΔS°ad was associated with the increase in disorder at the steel/electrolyte interface by the SM extract species to circumvent the activation complex attributing to the breakdown of solvation sheath of SM molecules followed by formation of coordinating complex with the surface atoms. In simple words, the adsorption of SM extract molecules on the steel was thermodynamically favorable, and its addition in 1 M HCl solution could effectively slow down the kinetics of corrosion processes by developing a barrier layer on the steel surface. However, in contrast to the kinetic parameter (e.g., Ea), the thermodynamic calculation suggested the steel inhibition via physisorption process. From these results it is predicted that SM molecules interaction with the steel surface was very complex. In simple words, it is estimated that inhibitive action of SM molecules involved both chemisorption (on the local active sites) and physisorption processes (i.e., electrostatic interaction of SM molecules with the cathodic sites) on the steel surface.
The micrographs of steel samples before and after exposure to 1 M HCl containing 0.5 g/l SM extract are shown in Fig. 4. The un-exposed sample displayed the presence of unidirectional scratches attributing to the polished surface. However, the severely etched surface of steel (Fig. 4b) indicated the aggressiveness of 1 M HCl solution. This also presents the accelerated dissolution of mild steel in HCl solution as confirmed from the weight loss and electrochemical analyses, which exhibit relatively large CR of mild steel in the blank solution. However, Fig. 4c illustrates the surface of mild steel sample in 1 M HCl solution containing 0.5 g/l SM extract. The relatively smooth steel surface (compared to un-exposed steel sample) was observed due to the slight dissolution of the surface. Presence of localized corrosion product and bright surface in the presence of 0.5 g/l SM extract validated the results presented above, which also explained the decrease in CR and improvement in the IE.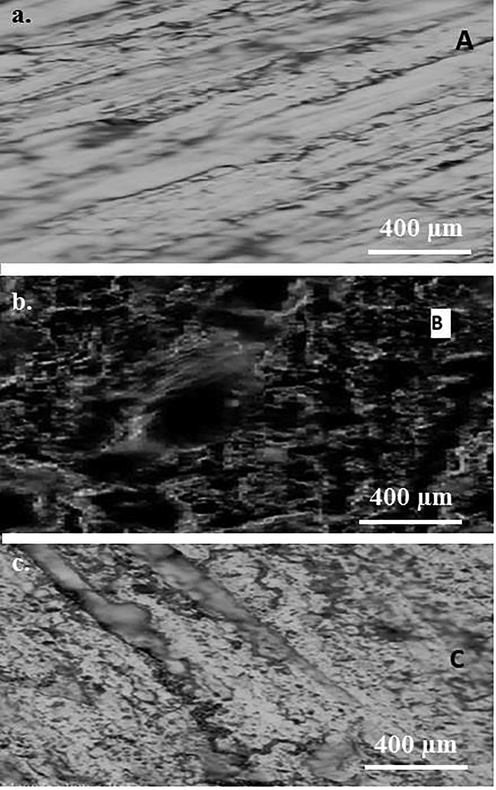
Carbon steel sample (a) Polished surface before exposure, (b) surface after 6 h exposure to 1 M HCl, (c) and surface morphology of steel sample exposed to 1 M HCl containing 0.5 g/l SM extract.
4 Conclusions
The present study evaluates the potential of Saudi origin SM peel extract as a corrosion inhibitor for the protection of mild steel in 1 M HCl solution. The CR and IE were determined via weight loss measurements and potentiodynamic polarization methods. The CR of mild steel and IE in 1 M HCl solution was directly related with the increase in temperature from 295 to 333 K even in the presence of SM extract. The IE increased from 22.34% to 79.69% when the SM extract concentration increased from 0.05 to 0.5 g/l in HCl solution at 295 K. Similarly, at each temperature the decrease in CR and increase in IE indicated the strong inhibitive action of SM extract molecules in the highly acidic environment. The IE at the highest SM extract concentration (0.5 g/l) was increased from 79.69% to 91.59% with an increase in temperature from 295 to 318 K, respectively. However, further increase in temperature to 333 K, the IE decreased to 81.26% which indicated the formation of a strong barrier layer on the steel surface, which stability is deteriorated at a relatively high temperature. Based on the Tafel polarization results, the low Ea (20–23 kJ/mole) and thermodynamic assessment of the adsorption phenomenon revealed the strong interaction of SM extract molecules with the mild steel. The increase in kad from 0.602 to 1.075 (g/l)–1 and decrease in ΔG°ad from −3.74 to −4.91 kJ/mole with the rise in temperature from 295 to 333 K highlighted the preferential adsorption of SM extract on the steel surface. Based on the kinetic parameters, the formation of chemisorbed surface layer restricted the direct approach of aggressive ionic species towards the steel surface and inhibited the dissolution of active sites. The preferential physisorption of SM molecules on the local cathodic sites was predicted from the thermodynamic ΔH°ad and ΔS°ad parameters, which were −5.24 kJ/mole and 0.03 kJ/mole-K, respectively. The barrier characteristics of this complex adsorbed surface layer and positive effects of SM extract (0.5 g/l) were validated from the relatively smooth and shiny surface compared to steel surface exposed to blank 1 M HCl solution.
Acknowledgment
The authors are grateful to the Jubail University College for providing financial support to conduct this study.
References
- The effects of Aloe vera extract on corrosion and kinetics of corrosion process of zinc in HCl solution. Corrosion Sci.. 2010;52(2):661-664.
- [Google Scholar]
- Evaluation of extracts of henna leaves as environmentally friendly corrosion inhibitors for metals. Mater. Wissenschaft und Werkstofftechnik. 2000;31(12):1060-1063.
- [Google Scholar]
- Evaluation of the anticorrosion performance of peel garlic extract as corrosion inhibitor for ASTM 1020 carbon steel in acidic solution. Matéria (Rio de Janeiro). 2017;22(3)
- [Google Scholar]
- Red algae halopitys incurvus extract as a green corrosion inhibitor of carbon steel in hydrochloric acid. J. Bio- Tribo-Corrosion. 2018;4(3):39.
- [Google Scholar]
- Strychnos nux-vomica an eco-friendly corrosion inhibitor for mild steel in 1 M sulfuric acid medium. Mater. Corrosion. 2009;60(1):22-28.
- [Google Scholar]
- Inhibitive performance of bitter leaf root extract on mild steel corrosion in sulphuric acid solution. Am. J. Mater. Eng. Technol.. 2015;3(2):35-45.
- [Google Scholar]
- Inhibition of corrosion of mild steel in acidic medium using ethanol extract of Aloe vera. Pigment & Resin Technol.. 2009;38(2):111-115.
- [Google Scholar]
- Ethanol extract of Ocimum gratissimum as a green corrosion inhibitor for the corrosion of mild steel in H2SO4. Green Chem. Lett. Rev.. 2010;3(3):165-172.
- [Google Scholar]
- Inhibition of aluminum corrosion using Opuntia extract. Corrosion Sci.. 2003;45(11):2485-2495.
- [Google Scholar]
- A novel green inhibitor for C-steel corrosion in 2.0mol·L−1 hydrochloric acid solution. Chinese J. Chem. Eng.. 2017;25(3):373-380.
- [Google Scholar]
- Cantaloupe extracts as eco friendly corrosion inhibitors for aluminum in acidic and alkaline solutions. J. Mater. Environ. Sci.. 2014;5(6):1940-1950.
- [Google Scholar]
- Corrosion and corrosion inhibition of cast Iron in hydrochloric acid (HCl) solution by cantaloupe (Cucumis melo) as green inhibitor. Afr. J. Pure Appl. Chem.. 2015;9(3):39-49.
- [Google Scholar]
- Corrosion inhibition of mild steel in HCL solution by pectin. Corrosion Sci.. 2015;92:192-199.
- [Google Scholar]
- Thermodynamic, adsorption and electrochemical studies for corrosion inhibition of carbon steel by henna extract in acid medium. Egypt. J. Petrol.. 2013;22(1):17-25.
- [Google Scholar]
- Aquilaria crassna leaves extracts – a green corrosion inhibitor for mild steel in 1 M HCl medium. Int. J. Electrochem. Sci. 2014;9(2):830-846.
- [Google Scholar]
- Thermodynamic and adsorption behaviour of medicinal nitramine as a corrosion inhibitor for AISI steel alloy in HCl solution. J. Mater. Sci. Technol.. 2013;29(9):884-892.
- [Google Scholar]
- Postharvest Biology and Technology of Tropical and Subtropical Fruits. Woodhead Publishing; 2011.
- Xanthiumstrumarium leaves extracts as a friendly corrosion inhibitor of low carbon steel in hydrochloric acid: Kinetics and mathematical studies. South Afr. J. Chem. Eng.. 2018;25:13-21.
- [Google Scholar]
- The inhibition of benzimidazole derivatives on corrosion of iron in 1 M HCl solutions. Electrochimica Acta. 2003;48(17):2493-2503.
- [Google Scholar]
- Electrochemical characterization and thermodynamic tendency of β-Lactoglobulin adsorption on 3D printed stainless steel. J. Industr. Eng. Chem.. 2018;65:180-187.
- [Google Scholar]
- Opuntiol: an active principle of opuntia elatior as an eco-friendly inhibitor of corrosion of mild steel in acid medium. ACS Sustain. Chem. Eng.. 2014;2(4):606-613.
- [Google Scholar]
- Validation of corrosion rates measured by the Tafel extrapolation method. Corrosion Sci.. 2005;47(12):3202-3215.
- [Google Scholar]
- Experimental and theoretical studies on the corrosion inhibition of mild steel by some sulphonamides in aqueous HCl. RSC Adv.. 2015;5(36):28743-28761.
- [Google Scholar]
- Ginger extract as green biocide to control microbial corrosion of mild steel, 3. Biotech. 2017;7(2):133.
- [Google Scholar]
- Potential of aqueous extract of Hibiscus sabdariffa leaves for inhibiting the corrosion of aluminum in alkaline solutions. J. Appl. Electrochem.. 2009;39(9):1465-1475.
- [Google Scholar]
- Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4[4′(-X)-styryl pyridinium iodides/hydrochloric acid systems. Mater. Chem. Phys.. 2008;110(1):145-154.
- [Google Scholar]
- Evaluation of the inhibitive effect of some plant extracts on the acid corrosion of mild steel. Corrosion Sci.. 2008;50(11):2993-2998.
- [Google Scholar]
- Comparison of percentage weight loss and corrosion rate trends in different metal coupons from two soil environments. Int. J. Environ. Bioremed. Biodegr.. 2014;2(5):243-249.
- [Google Scholar]
- Allium sativum (garlic extract) as a green corrosion inhibitor with biocidal properties for the control of MIC in carbon steel and stainless steel in oilfield environments. Int. Biodeterioration Biodegr.. 2018;132:66-73.
- [Google Scholar]
- AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corrosion Sci.. 2003;45(1):33-58.
- [Google Scholar]
- Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corrosion Sci.. 2018;133:6-16.
- [Google Scholar]
- Cyclic nitrones as novel organic corrosion inhibitors for carbon steel in acidic media. Anti-Corrosion Methods Mater.. 2005;52(3):154-159.
- [Google Scholar]
- Natural products as corrosion inhibitor for metals in corrosive media – a review. Mater. Lett.. 2008;62(1):113-116.
- [Google Scholar]
- Adsorption of oleates of various amines on iron in acidic solution. Electrochimica Acta. 1981;26(9):1253-1256.
- [Google Scholar]
- Corrosion inhibition of carbon steel in sulfuric acid by bicyclic isoxazolidines. Anti-Corrosion Methods Mater.. 2004;51(6):389-398.
- [Google Scholar]
- Controlled delivery of multi-substituted triazole by metal-organic framework for efficient inhibition of mild steel corrosion in neutral chloride solution. Corrosion Sci.. 2018;131:1-16.
- [Google Scholar]
- Comparative studies on the corrosion inhibition efficacy of ethanolic extracts of date palm leaves and seeds on carbon steel corrosion in 15% HCl solution. J. Adhesion Sci. Technol.. 2018;32(17):1934-1951.
- [Google Scholar]
- 2-Amino-5-nitro-4,6-diarylcyclohex-1-ene-1,3,3-tricarbonitriles as new and effective corrosion inhibitors for mild steel in 1M HCl: Experimental and theoretical studies. J. Mole. Liquids. 2015;212:804-812.
- [Google Scholar]
- A green and sustainable approach for mild steel acidic corrosion inhibition using leaves extract: experimental and DFT studies. J. Bio- Tribo-Corrosion. 2018;4(3):33.
- [Google Scholar]
- Enhanced Corrosion Resistance of Carbon Steel in Hydrochloric Acid Solution by Eriobotrya Japonica Thunb. Leaf Extract: Electrochemical Study, Materials (Basel, Switzerland). 2017;10(8):956.
- [Google Scholar]







