Translate this page into:
Active paper packaging material based on antimicrobial conjugated nano-polymer/amino acid as edible coating
⁎Corresponding author at: Cellulose & Paper Dept., National Research Centre, El-Buhouth St., Dokki, 12622, Egypt. sido_sci@yahoo.com (Mohamed Hasanin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Conjugated amino acid with nano-biodegradable polymer was applied as ecofriendly and edible coating. Polystyrene sulfate (PSS) was highly investigated with dynamic light scattering (DLS) to measure the particle size and over charge (zeta potential measurements) of nano-polymer system as well as the homogeny of particle distribution in presence and absence of Lysine mixture (AA). In addition, topographical structure of the coating was studied for PSS, AA and conjugated one as well as Fourier transform infrared (FTIR) and Differential Scanning Calorimetry (DSC). Antimicrobial test cleared that the prepared conjugate described as broad-spectrum antibacterial agent as well as antifungal with different concentrations. The hydrophilic active ingredient is absorbed into fiber, distributed homogenously and kept out the fiber loaded with antimicrobial properties over all areas. Tensile strength of the paper sheets coated with uni-layer 200 µ thickness presented good improving in physical and mechanical properties of coated paper sheets as well as gives more fiber condensation according to Scanning Electron Microscope (SEM) topography.
Keywords
Active packaging
Conjugated polymer/amino acid
Antimicrobial activity
And coating paper
1 Introduction
Packaging is the processing in which some technologies are used for enclosing or maintain the original product state luster till arrives to consumer (Choi and Burgess, 2007; Youssef and El-Sayed, 2018). Recyclable packaging material is new challenge where the reprocessing of materials (pre- and post-user) offer new products (Twede et al., 2014). Some materials such as steel, aluminum, papers, plastics, etc. are the largest primary components of a package, but the huge amounts of this components cannot be recycled because they are difficult to separate and do contaminate (Othman, 2014). Packages can sometimes be designed to separate components to better facilitate recycling. Additionally some of packaging materials are harmful to use as food contact otherwise it may be help the food to rot (Ibrahim et al., 2017, 2016). Free additives paper is the safest recycling packaging materials, which have not any hazard additives e.g. heavy metal, non-degradable petroleum polymers, and toxic materials as well as can be used as food contact. Nevertheless, the free additive papers have high safety profile as food contact but have many disadvantages in packaging such as, not resistant to microorganisms attacking and poisons as well as low mechanical and physical properties (Abou-Zeid et al., 2018; Youssef et al., 2019). Coating of paper with bio-base biological active coating may be reducing many disadvantages of free additive papers, since the traditional coating materials are mostly containing hazard and toxic materials (Abou-Zeid et al., 2018; Youssef et al., 2018; Youssef, Kamel, and El-Samahy, 2013). The convention antimicrobial coating in most preservation process is heavy metal ions and the drawback of this process is release of metal ions and contaminate throughout the food (Barhoum et al., 2014). In addition, to some paper surface coating is normally accompanied by some disadvantages such as, high porous structure, poor microbial resistance and low mechanical properties. These drawbacks negatively affect the quality of the paper used in food preservation mostly for food contact applications (El-Saied et al, 2003). Dual role coating materials e.g. bio-based composite has recently been employed for food packaging because it has various favorable properties such as biodegradability, renewability, recyclability, and mechanical flexibility as well as not cytotoxic and safe as food contact materials (Abou-Zeid et al., 2018; Wacoo et al, 2014). Various strategies have recently begun to synthesis non-toxic with anti-microbial properties coating the paper sheet to enhance its properties for food preservation avoid the heavy metal ions hazardous (Gomez-Carretero et al, 2017; Khalid et al., 2016; Levdik et al, 1967). Biomaterials, such as amino acids have unique features emerged as sustainable. Biocompatible and biocompatible are the main characters which draw attention to these materials to use as alternatives to plain petroleum-based polymers in biomaterials (Ghorab et al., 2004; Rehim et al., 2015). Amino acids are excellent example to these compounds (El-Henawy et al., 2018; Wu, 2009). Lysine is one of the essential amino acid which has not any toxically affect as well as having high biological important and also uses as nutrient supplant with great safety profile (Abelson, 1999). On the other hand, the most promising and interesting biodegradable as well as biocompatible polymers is sodium polystyrene sulfate which is sodium salt of polystyrene sulfate (PSS) derived from polystyrene by addition of sulfonate functional groups(Girard et al., 2013). PSS used as drug without any additive as well as approved from food and drug administration (FDA) as nutrient and drug (Sterns et al., 2010). PSS widely used as ion-exchange resins to remove ions such as potassium, calcium, and sodium from solutions in technical or medical applications (De Dardel and Arden, 2008). Bulky group growth inhibition technique is the new promising field in anti-biological activity applications (Basta et al., 2016; Nelson and O'Connor, 1964b; Van de Loosdrech et al, 1994) without any toxical effect. Using of excellent edible and highly safety profile materials as biobased-bioactive paper coating is added important value in active packaging and edible paper coating. Conjugation between PSS and amino acid is discussed in the present work as new trend and the most important challenge for using biodegradable, biocompatible, food contact and not cytotoxic materials as coating paper sheets with improving the mechanical and physical properties to use as food contact and preserved coated paper.
2 Materials
Paper sheets are delivered from Quena Co. for paper production- Upper Egypt. Sodium salt of poly styrene sulfate (PSS) was purchased from AldrichCo., lysine mixture (AA) (monohydrochloride and base) purchased from BDH, Biochemical, England. All medium and its components were purchased from modern Lab Co, India.
2.1 Preparation of PSS lysine conjugate (Conj.)
PSS-AA Conjugate was prepared, using PSS and AA with different ratios (1: 1.25, 1.5, 1.75, 2.0, 2.5, and 3.0) PSS and Lysine respectively. Fusion method was carried out according to Basta et al., (Basta et al., 2016) by mixing PSS with different ratios AA, for 1.5 h at about 250 °C, then the product was subjected to microwave for about 30 s/gmixture. The resulted conjugates were washed several times by Millipore water, and air dried. Antimicrobial test was carried out as described below to select the promising coater as lasted in (Table 1).
Minimal inhibition concentration (MIC) calculation was carried out via plat diffusion method, Gram-positive and Gram- negative bacteria as well as uni-cellular fungi are presented. Five microorganisms are tested against conjugate Bacillus subtilis (NCID-3610) and Staphylococcus aureus (NCTC-7447) as Gram positive and Escherichia coli (NCTC-10416) and Pseudomonas aeruginosa (NCID-9016) as Gram-negative bacteria as well as Candida albicans (NCCLS 11) were used as unicellular fungi. One colony of each microbial strain was suspended in a physiological saline solution (NaCl 0.9% in distilled water at pH 6.5). The initial tested concentration of the PSS-AA was 100ug/ml. The MICs were calculated as stated previously (Basta et al., 2016; Remmal et al, 1993). Different concentrations (100, 50, 25, 12.5, 6.25, 3.125, 1.5625, 0.78125, 0.390625, 0.195313, 0.097656, and 0 µg/ml as positive control) of the tested conjugate were prepared through serial dilution method. Mueller Hinton Agar medium was inoculated by the above-mentioned bacterial strains and fungi are incubated individually at 37 °C for 24 h. After all incubation periods have been elapsed clear zones were measured in mm. The above concentrations were used to determine the MIC value under the same incubation conditions.
2.2 Characterization of coating
Fourier transform infrared (FTIR): The structure change between PSS, AA and its conjugated polymer was studied by attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy (Spectrum Two IR Spectrometer – PerkinElmer, Inc., Shelton, USA). All spectra were obtained by 32 scans and 4 cm−1 resolution in wave numbers ranging from 4000 to 450 cm−1. IR measurements which are including crystallinity index (Cr.I.) (Nelson and O'Connor, 1964a,b; Razva et al., 2014) and main hydrogen bond strength (MHBS) (Levdik et al., 1967) were calculated.
Differential Scanning Calorimetry (DSC): The DSC instrument (SETRAM DSC evo-131, France) with scan rate 5 °C/min was used to study the thermal behavior of PSS, AA and its conjugate compound/s.
Surface modification study (SEM): The morphology and topography of the prepared samples were analyzed by scanning electron microscopy (SEM, Quanta FEG 250, FEI). The SEM sample, a thin layer of Au was coated onto the sample by sputtering coating unit.
2.2.1 Particle size distribution and zeta potential measurements
Tإhe particle size distribution and zeta potential of the prepared samples were measured, using Nicomp™ 380 ZLS size analyzer, USA. Leaser light scattering was used at 170° in case of particle size detection where zeta potential was measured at 18°.
2.3 Paper coating
Coating formulation: PPS and AA was dissolved in aqueous medium (5 g in 100 ml) as separated solutions. Conjugated coating was prepared with solid content 5% (wt/wt) by mixing with water together under ultrasonic homogenizer Qsonics, USA (4 min/ 70% amplitude).
Coating process: Automatic film applicator (SMF-VII, 310) with different road coaters (150 and 200 µm) was used. Paper sheet was fixed over vacuum plate and applied constant speed in forward and backward movements. According to solid content calculation, coating thickness was varied as 150 µ and 200 µ coating thickness. The paper sheets were coated with one, two and three coating layers. Coated paper sheets were dried in thermal drying in air circulation oven at 80 °C for complete drying to avoid paper deformation.
2.4 Coating sheets testing
The mechanical properties, including tensile strength, Young’s modulus (using universal Instron testing machine), Roughness, and burst index as well as physical properties (water absorption and air permeability) were determined. Surface morphology of coated paper sheets were investigated with SEM as mentioned above. Morphological study of coating paper sheets was carried out as mentioned previously in SEM topography.
3 Results and discussion
3.1 Preparation of conjugate
Optimizing the PSS and AA suitable fusion ratiowas judged with the response of tested microorganisms detected as listed in (Table 1). The conjugate was getting his anti-microbial activity from bulky group growth inhibition mode of action (Hyldgaard et al., 2014). The bulky group growth inhibition is new promising field in antibiological activity applications (Hyldgaard et al., 2014; Salama, 2017). This application depends on the formation of conjugate from two substances, one of important biological role (AA), while the other one (PSS) not only has bulky characters but also biocompatible. Each conjugate procure component has not any antimicrobial activity, Exp. 1, 2, and 3 were shows undetected microbial sensitivity. This could have happened as a result of high concentration of AA which consumed by microorganism as nutrient in Free state. Exp. 4 show the suitable ratio in which the conjugation giving antimicrobial activity with lowest amount of amino acid. It is form interested economical point of view. Moreover, the prepared conjugate has new physical, chemical, and biological characters which emphasized conjugate is prepared.
3.2 Coating characterization
3.2.1 Antimicrobial and MIC calculation
The antimicrobial activity of this prepared conjugate was tested against microbial strains with different concentrations. MIC was calculated with measuring the surrounded clear inhibition zone diameter which refers to conjugate activity against tested organism (s), as shows in (Table 2 and Fig. 1). The obtained data shows that novel conjugate has strongest inhibitory effect on broad spectrum bacteria (Gram positive and negative). Where the antimicrobial activity against gram positive with MIC 25 µg/ml for Bacillus subtilis (NCID-3610) and 12.5 µg/ml for Staphylococcus aureus (NCTC-7447). These values are highly prospective values according to (Basta et al, 2018). While, for the case of gram negative bacteria the MIC value for Escherichia coli (NCTC-10416) was 50 µg/ml, as well as for Pseudomonas aeruginosa (NCID-9016) was 12.5 µg/ml and this was in agreement with (El-Saied et al., 2003; Rowlinson et al., 2003). Moreover, this conjugate showed significant activity against unicellular fungi Candida albicans (NCCLS 11), where its MIC was 3.125 µg/ml. The antimicrobial activity of the investigated conjugate ascribed to the presence of bulkier PSS molecules, which interprets the metabolic pathway and makes cells lost their ability to progress over stopping the protein building up using the bulky group (PSS) to block the active side of amino acid binding site (Hyldgaard et al., 2014; Menninger, 1995). Thereby, resulting significant activity is appeared. This explanation is in agreement with that reported for the role of bulky group and the exact relationship between bulk structure of agent and its inhibition activity as well as the conjugate can be described as broad-spectrum antimicrobial agent.
MIC μg/ml
inhibition zone/mm
B. subtilis NCID-3610
S. aureus NCTC-7447
P. aeruginosa NCID-9016
E. coli NCTC-10416
C. albicans
NCCLS 11
100
12 ± 0.9
19 ± 1.66
19 ± 1.1
17 ± 0.8
31 ± 2.3
50
9 ± 1.0
11 ± 1.1
11 ± 0.7
10 ± 0.6
22 ± 1.4
25
5 ± 0.4
7 ± 0.92
5 ± 0.39
0
17 ± 0.8
12.5
0
4 ± 0.55
3 ± 0.3
0
9 ± 1.11
6.25
0
0
0
0
6 ± 0.99
3.125
0
0
0
0
4 ± 0.7
1.5625
0
0
0
0
0
0.78125
0
0
0
0
0
0.390625
0
0
0
0
0
0.195313
0
0
0
0
0
0.097656
0
0
0
0
0
0
0
0
0
0
0
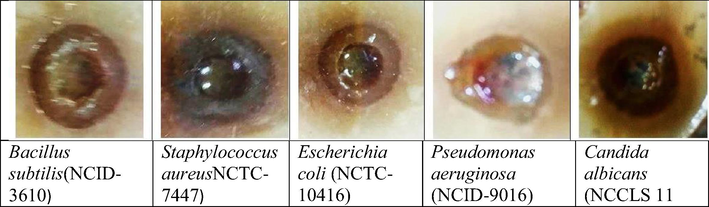
MIC antimicrobial test for conjugate (well diffusion method).
3.2.2 FTIR
The FT-IR spectra were illustrated in (Fig. 2), spectrum of PS-SO3H powders were taken over the range of wavenumbers from 450 to 4000 cm−1, but for the analysis of sulfonic groups, spectrum was shown over the range from 800 and 1400 cm−1. Sulfonic group vibration bands are reported at approximately 1040 and 1180 cm−1 (Martins et al, 2003). The characteristic infrared absorbance for PS-SO3H have suggested the bonding of the sulfonic groups to the aromatic ring of PS (out of plane deformation bands assigned to substituted aromatic ring γ (Car-H) at wavenumbers approximately from 830 to 850 cm−1). The absorption at 1040 cm−1resulted from the symmetric stretching vibration of SO3H groups and the absorption at 1127 cm−1results from a sulfonate anion attached to a phenyl ring. Our results are in agreement with the literature ones (Pantelić et al., 2009). We verified that the νas(S—O) vibration at 1180 cm−1 appears as a very broad band at approximately 1100 cm−1–1350 cm−1. AA shows the traditional amino acid IR spectra, however shows bands at 3316, 2944, 2104, 1576, and 1516 cm−1 which refer to (O and N)—H, CH2, C—H symmetrical deformation, and C⚌O respectively as well as fingerprint bands (Kitadai et al., 2009; Mostafa et al., 2017a,b). Consequence of after conjugation process, some function groups and new bands are appeared, as well as some are vanished. Conjugate spectra show bands positions as well as band sharpness were different than two started materials. Conjugate showed some changes in bands in the rang 4000–3000 cm−1 which regarded to OH starching vibration, NH vibration in AA and C—H in PSS, this is in agreement with IR calculations (MHBS and Cr.I.) in (Table 3) which are increased in conjugate in result of OH starching vibration and NH vibrations, this is emphasized that the increased of MHBS value of conjugate which increased than AA and decreased than PSS. Cr.I. value of conjugate was duplicated, this results is in agreement with SEM image. Blue shift is appeared in conjugate band at 3457 cm−1 which observed in PSS and AA at 3307 and 3358 cm−1 respectively. This may lead to liberate the hydrogen bonds during synthesis process. The bands which are characterized to sulfonate group in PSS show some changes in follow bands; 1192, 1124, 1034, and 1009 which have become sharper and recorded insanity higher than PSS spectra, this may be related to formation of crystal as mentioned. The forgoing data illustrated that the reaction between PSS and AA was going out via —SO3Na in PSS and —COOH group in AA and produces NaOH. Other evident is the pH of solution of conjugated material before washing was more than 8 whilst the pH of AA and PSS before reaction was less than 6.5 (Dhawan, 2002).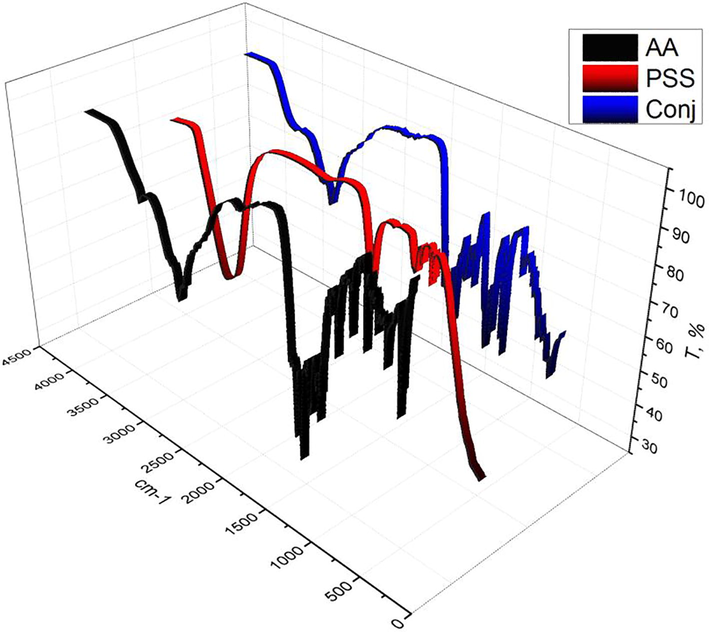
FTIR spectra for Amino acid, PSS, and conjugated compound.
Sample
crystal index
MHBS
PSS
0.91
5.44
Lysine
0.79
3.06
Conjugate
1.67
4.49
3.2.3 DSC
(Fig. 3) showed the three DSC curves for started materials and conjugate. PSS curve shows after adsorbed water region only one peak with onset 136.25 °C and offset 180.7 °C with as well as peak temperature (TP) 158.5 °C. Similarly, in AA the DSC curve has one hump peak with TP105.76 °C. Additionally, conjugate curves show completely difference from both started materials. Clear splitting of started materials main peak to two peaks was appearing in conjugate DSC curve this accentual the produce new conjugate (Hatakeyama and Hatakeyama, 1990). Moreover, no significant variation in onset and outset values as shown in (Table 4). However, heat capacity is recorded with high value 117.46 J/g in conjugate one, comparison with started materials PSS and AA are 14.99 and 4.909 J/g respectively. This emphasized that the reaction between PSS and AA give highly thermal stable conjugate with high activation energy value as stated before (Garg et al., 2005).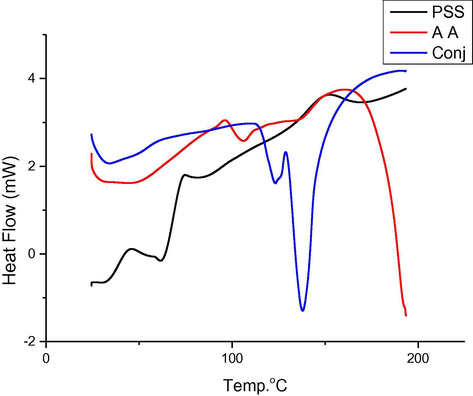
DSC curves for started materials and conjugate.
Code
To (°C)
Tf(°C)
TP (°C)
ΔT (°C)
Heat capacity (J/g)
Lysine
98.11
112.19
105.76
14.08
4.909
PSS
136.25
180.74
158.5
44.49
14.99
Conjugate
128.98
145.98
137.77
17.0
117.46
3.2.4 DLS and zeta potential
DLS results were plotted in (Fig. 4), polydispersity index (PDI) and mean particle size were lasted in (Table 5). The PDI is a measure of dispersion homogeneity. PDI value is ranged from 0 to 1.0 where from 0 to 0.3 is refer to the highest homogeneity distribution state (Cheng et al., 2018). The narrow uniform Gaussian distribution can be pointed to low PDI especially for PSS, and AA. as shown in (Fig. 3). Additionally, conjugated coating still presents few particle size variations with little higher PDI in related to AA and PSS as lasted in (Table 5). The mean diameters were measured in aqueous solutions with relative concentration as 439, 340 and 527 nm for PSS, AA and conjugated coating particles, respectively. Additionally, zeta potential was calculated from the three main measured factors (cell current, phase shift and mobility) that depending on the charge over particles. Zeta measurements of PSS, AA and conjugated coating were tabulated in (Table 5) and all related factors. Sulfonated group play a vital role in the net charge of PSS to be −0.68 mV where AA presented average zeta potential −12.48 mV. Combined system was detected at −7.38 mV which pointed to the connection side from COO— that reduced the value from −12 to −7.38 mV. FTIR supported this assumption by little decrease in C⚌O bond in conjugated chart than AA peak.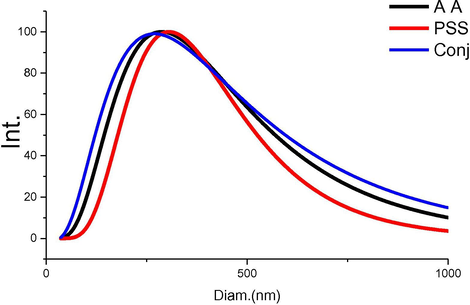
DLS for Amino acid, PSS, and conjugated compound.
zeta potential measurements
Particle size measurements
Cell current, mA
Av. Phase shift, rad/sec
Av. Mobility, M.U.
Av. Zeta potential, mV
PDI
Average particle size/nm
Lysine
8.95
−2.39
−0.05
−0.68
0.21
439
PSS
9.67
−23.11
−0.87
−12.48
0.34
340
Conjugate
17.13
−22.67
−0.52
−7.38
0.46
527
3.2.5 Microstructure investigation
PSS polymer showed nano-polymer in scale around 25 nm however after conjugation interaction between protein and polymer give uniform aggregated particles with as shown in (Fig. 5). Conjugated coating paper uniform particle in micron rang size with core/shell form as shown in (Fig. 5-E and F). Core size was recorded 689 nm while shell diameter was 3675 nm which confirmed in result to drying sample and aggregates the particles as core/shell form and distribute in solutions as colloids. These results have nice agreements with particle size distribution over DLS for AA and conjugate. However the results of PSS which show in (Fig. 5-A and B) in opposed to the DLS data this may be takes place as result of highly surface area either aggregate phenomena of PSS in water (Sen et al., 2007) as well as water hydrophilic properties of PSS which performed to swelling and recorded particle size more than SEM topography.
SEM for PSS at low magnification (A), high magnification (B), amino acid at low magnification (C), high magnification (D), and Conjugate at low magnification (E), High magnification (F).
3.3 Coated paper evaluation
3.3.1 Coating sheets properties
The mechanical properties, including tensile strength, Young’s modulus, roughness, and burst index were carried out for uncoated (blank) and coating papers sheets. The results were calculated and displayed in (Table 6). Tensile strength of the paper sheets coated with 1 layer 150 and 200 µm thickness was increased by about 2% and 17%, respectively in comparing to the blank paper sheets. Additionally, the Young’s modulus was also increased by about 19% and 22%, respectively. On the contrary were the both thickness (150 and 200 µm) of two and three layers sheets drawback are noticed in the tensile strength and Young‘s modulus. This is referring to the high swelling degree of paper fiber in two and three coating layers on papers sheets. This is due to water base coating conjugate which penetrates the fibers easily. Moreover, the hydrophilic coating gives the paper fiber highly microbial resistance. Where the active ingredient is adsorbed into fiber and distributed homogenously and kept out the fiber loaded with antimicrobial properties over all fibers areas. In one-layer paper sheets, paper fibers shape converted from flat form to round form, which help in compact the fibers together and became closed than in blank sheets. Antithesis in the two and three layers coated paper sheets, increasing in coating layers give significant draw back variations because paper fibers have over swell with coating matrix and become fragile.
Tensile strength (MPa)
Young’s modulus (MPa)
Roughness
Burst index (KPa.m2/g)
Water absorption (g/m2)
Air permeability (ml/min)
Reference Paper
23.15 ± 1.92
2.05 ± 0.77
10
312
21
94 ± 2.44
PSS
25.17 ± 1.7
2.78 ± 0.92
41
308
36
87 ± 2.21
1 Layer 150 µ
23.44 ± 1.81
2.49 ± 0.44
31
Less than 200
40
53.5 ± 2.51
1 Layer 200 µ
27.12 ± 2.19
2.43 ± 0.49
412
Less than 200
55
27.5 ± 2.12
2 Layer 150 µ
20.85 ± 0.97
1.81 ± 0.19
51
Less than 200
45
32.5 ± 077
2 Layer 200 µ
12.81 ± 2.11
1.61 ± 0.14
412
Less than 200
60
30.5 ± 2.71
3 Layer 150 µ
9.3 ± 2.13
1.35 ± 0.21
108
Less than 200
50
46 ± 2.43
3 Layer 200 µ
10.38 ± 1.21
1.31 ± 0.23
434
Less than 200
59
60.5 ± 2.12
On the other hand increasing the coating thicknesses gives significant variation in roughness values in comparison with reference sheets value (Abou-Zeid et al., 2018; Barhoum et al., 2014). One layer paper sheets roughness of 200 µm coating thickness increased with 13 times than 150 µm thickness. Additionally, double layers paper sheets with coating thickness 200 µm show increase in roughness value with 8 times than 150 µm thickness ones. Triple layers paper sheets showed less improvement with 4 times only. Burst index shows monotonous values, this may be takes place according to the swelling of fibers in all layers types lead to close all pours. However water absorption gives high values for coated paper sheets than blank one, the reason of this phenomena is similar to what happened in mechanical properties layers behaviors (Khwaldia Bet al., 2014). In addition, coating thickness and water absorption are directly proportion together. Air permeability in one layer paper sheets with coating thickness 200 µm was the lowest value, this is an advantage can synergized the antimicrobial properties of paper coated sheets (Basta et al., 2015; El-Gendy et al., 2017) as well as reinforce preservation characteristics behavior.
3.3.2 Coating sheets morphology
SEM image (Fig. 6) displays surfaces morphological structure of blank paper sheet (Fig. 6-A and B) and papers coated with one layer with 200 µm thickness (Fig. 6-C and D) and three-layer 150 µ thickness (Fig. 6-E and F) conjugate. Blank shows a typical morphology of the normal papers, which is a flat fiber network with free pores (Abou-Zeid et al., 2018). The fibers of coated papers were presented with thick diameter, that is can be attributed to highly soluble of conjugate which obtained from PSS (Essafi et al., 2009).
SEM for blank paper sheet at low magnification (A), high magnification (B), 1layer with 150 µ thickness at low magnification (C), high magnification (D), and 3 layers with 200 µ thickness. at low magnification (E), high magnification (F).
Conjugate can be penetrating paper fibers and filling space of paper filament after drying. This is elucidating the roughness values as well as air permeability results. SEM images were emphasized the mechanical and physical measurements. In addition, this phenomenon enhanced the antimicrobial efficiency not only as a coating but also at active filler between cellulose fibers.
4 Conclusion
Active coating of papers sheets with prepared conjugate is promising technique for producing safety antimicrobial and biocompatible packaging materials suitable to be used as food contact. The treated papers sheets are characterized by improved physical and mechanical properties as well as microbial resistance and antimicrobial behavior.
Acknowledgment
The authors express their sincere thanks to National Research Centre Of Egypt for providing the necessary research facilities. The authors would like to acknowledge the facilities available at Central Laboratory Network (CLN), National Research Centre, Dokki, Giza, Egypt.
References
- Surfactant-assisted poly (lactic acid)/cellulose nanocrystal bionanocomposite for potential application in paper coating. J. Renewable Mater. 2018
- [Google Scholar]
- Effect of cationic and anionic surfactants on the application of calcium carbonate nanoparticles in paper coating. ACS Appl. Mater. Interfaces. 2014;6(4):2734-2744.
- [Google Scholar]
- Enhancing the performance of carboxymethyl cellulose by chitosan in producing barrier coated paper sheets. Nord. Pulp Pap. Res. J.. 2015;30(4):617-625.
- [Google Scholar]
- Properties of modified carboxymethyl cellulose and its use as bioactive compound. Carbohydr. Polym.. 2016;153:641-651.
- [Google Scholar]
- Green carboxymethyl cellulose-silver complex versus cellulose origins in biological activity applications. Int. J. Biol. Macromol.. 2018;107:1364-1372.
- [Google Scholar]
- Surfactant-free hybrid latexes from enzymatically hydrolyzed starch and poly(butyl acrylate-methyl methacrylate) for paper coating. Prog. Org. Coat.. 2018;118:40-47.
- [Google Scholar]
- Practical mathematical model to predict the performance of insulating packages. Packag. Technol. Sci.. 2007;20(6):369-380.
- [Google Scholar]
- De Dardel, F., Arden, T.V., 2008. Ion exchangers. In: Ullmann's encyclopedia of industrial chemistry.
- Design and construction of novel molecular conjugates for signal amplification (II): use of multivalent polystyrene microparticles and lysine peptide chains to generate immunoglobulin–horseradish peroxidase conjugates. Peptides. 2002;23(12):2099-2110.
- [Google Scholar]
- TEMPO-oxidized cellulose nanofibers/polylactic acid/TiO2 as antibacterial bionanocomposite for active packaging. Egypt. J. Chem.. 2017;60(6):1007-1014.
- [Google Scholar]
- Discovery potent of bagasse (CMC-L-Phe) as bioactive material based on DFT calculations. Eurasian J. Anal. Chem.. 2018;13(5)
- [Google Scholar]
- Cellulose membranes for reverse osmosis Part I. RO cellulose acetate membranes including a composite with polypropylene. Desalination. 2003;159(2):171-181.
- [Google Scholar]
- Synthesis of antimicrobial cellulosic derivative and its catalytic activity. J. King Saud Univ. – Sci. 2018
- [Google Scholar]
- Hydrophobic polyelectrolytes in better polar solvent. Structure and chain conformation as seen by SAXS and SANS. Macromolecules. 2009;42(24):9568-9580.
- [Google Scholar]
- Development and characterization of bioadhesive vaginal films of sodium polystyrene sulfonate (PSS), a novel contraceptive antimicrobial agent. Pharm. Res.. 2005;22(4):584-595.
- [Google Scholar]
- Antimicrobial activity of amino acid, imidazole, and sulfonamide derivatives of pyrazolo[3,4-d]pyrimidine. Heteroat. Chem.. 2004;15(1):57-62.
- [Google Scholar]
- Development of a polystyrene sulfonate/silver nanocomposite with self-healing properties for biomaterial applications. C. R. Chim.. 2013;16(6):550-556.
- [Google Scholar]
- Electroenhanced antimicrobial coating based on conjugated polymers with covalently coupled silver nanoparticles prevents staphylococcus aureus biofilm formation. Adv. Healthcare Mater.. 2017;6(20)
- [Google Scholar]
- Thermal and nuclear magnetic relaxation studies of water–sodium polystyrene sulfonate systems. Polym. Adv. Technol.. 1990;1(5–6):305-310.
- [Google Scholar]
- The antimicrobial mechanism of action of epsilon-poly-l-lysine. Appl. Environ. Microbiol.. 2014;80(24):7758-7770.
- [Google Scholar]
- Water soluble cationic xylan-alginate polymer as packaging retardant-release peptide drug delivery. Int. J. PharmTech Res.. 2016;9(5):119-128.
- [Google Scholar]
- Synthesis of super-hydrophobic polymer nanocomposites as a smart self-cleaning coating films. Polym. Compos.. 2017;38:E147-E156.
- [Google Scholar]
- Antibacterial behavior of laser-ablated copper nanoparticles. Acta Metall. Sin. (English Letters). 2016;29(8):748-754.
- [Google Scholar]
- Chitosan–caseinate bilayer coatings for paper packaging materials. Carbohydr. Polym.. 2014;99:508-516.
- [Google Scholar]
- ATR-IR spectroscopic study of L-lysine adsorption on amorphous silica. J. Colloid Interface Sci.. 2009;329(1):31-37.
- [Google Scholar]
- Study of pulp structure by infrared spectroscopy. Tr. Vses Nauch. Issled. Irst. Tsellyul Bum. Prom. 1967;52:109-111.
- [Google Scholar]
- Synthesis in pilot plant scale and physical properties of sulfonated polystyrene. J. Braz. Chem. Soc.. 2003;14(5):797-802.
- [Google Scholar]
- Mechanism of inhibition of protein synthesis by macrolide and lincosamide antibiotics. J. Basic Clin. Physiol. Pharmacol.. 1995;6(3–4):229-250.
- [Google Scholar]
- Synthesis of cadmium oxide nanoparticles by pulsed laser ablation in liquid environment. Optik-Int. J. Light Electron Opt.. 2017;144:679-684.
- [Google Scholar]
- Au@ CdO core/shell nanoparticles synthesized by pulsed laser ablation in Au precursor solution. Appl. Phys. A. 2017;123(12):774.
- [Google Scholar]
- Relation of certain infrared bands to cellulose crystallinity and crystal latticed type. Part I. Spectra of lattice types I, II, III and of amorphous cellulose. J. Appl. Polym. Sci.. 1964;8(3):1311-1324.
- [Google Scholar]
- Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J. Appl. Polym. Sci.. 1964;8(3):1325-1341.
- [Google Scholar]
- Bio-nanocomposite materials for food packaging applications: types of biopolymer and nano-sized filler. Agric. Agric. Sci. Procedia. 2014;2:296-303.
- [Google Scholar]
- Characterization of Partially Sulfonated Polystyrene-block-poly (ethylene-ran-butylene)-block-polystyrene Thin Films for Spectroelectrochemical Sensing. Anal. Chem.. 2009;81(16):6756-6764.
- [Google Scholar]
- Calculation of quarzite crystallinity index by infrared absorption spectrum. In: IOP Conference Series: Earth and Environmental Science. IOP Publishing; 2014. p. 012006
- [Google Scholar]
- Polystyrene/hydrophobic TiO 2 nanobelts as a novel packaging material. Polym. Bull.. 2015;72(9):2353-2362.
- [Google Scholar]
- Improved method for the determination of antimicrobial activity of essential oils in agar medium. J. Essent. Oil Res.. 1993;5(2):179-184.
- [Google Scholar]
- A novel mechanism of cyclooxygenase-2 inhibition involving interactions with Ser-530 and Tyr-385. J. Biol. Chem.. 2003;278(46):45763-45769.
- [Google Scholar]
- Dicarboxylic cellulose decorated with silver nanoparticles as sustainable antibacterial nanocomposite material. Environ. Nanotechnol. Monit. Manage.. 2017;8:228-232.
- [Google Scholar]
- Effect of structure on solution and interfacial properties of sodium polystyrene sulfonate (NaPSS) Polym. Int.. 2007;56(2):167-174.
- [Google Scholar]
- Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J. Am. Soc. Nephrol.. 2010;21(5):733-735.
- [Google Scholar]
- Cartons, Crates and Corrugated Board: Handbook of Paper and Wood Packaging Technology. DEStech Publications Inc.; 2014.
- A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods. 1994;174(1–2):311-320.
- [Google Scholar]
- Methods for detection of aflatoxins in agricultural food crops. J. Appl. Chem.. 2014;2014:1-15.
- [Google Scholar]
- Bionanocomposites materials for food packaging applications: concepts and future outlook. Carbohydr. Polym. 2018
- [Google Scholar]
- Morphological and antibacterial properties of modified paper by PS nanocomposites for packaging applications. Carbohydr. Polym.. 2013;98(1):1166-1172.
- [Google Scholar]
- Novel bionanocomposite materials used for packaging skimmed milk acid coagulated cheese (Karish) Int. J. Biol. Macromol.. 2018;115:1002-1011.
- [Google Scholar]
- Development of bionanocomposite materials and its use in coating of Ras cheese. Food Chem.. 2019;270:467-475.
- [Google Scholar]







