Translate this page into:
Scanning electron microscopy and Fourier transmission analysis of polyhydroxyalkanoates isolated from bacteria species from abattoir in Ota, Nigeria
⁎Corresponding author. nwinyiobinna@gmail.com (Obinna C. Nwinyi) obinna.nwinyi@covenantuniversity.edu.ng (Obinna C. Nwinyi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study reports production of polyhydroxyalkanoates (PHA) from microbial species obtained from abattoir by altering different carbon source (acetate and molasses) in minimal salt medium. The isolates were characterized by understanding their physiological responses to biochemical tests, optical microscopy examination and comparison to standard reference organisms. They were presumably species of Lactobacillus, Bacillus, Corynebacterium, Arthrobacter and Rhodococcus. The PHA products from these isolates were confirmed to be of plastic origin using the Fourier transmission analysis and the microstructure analysis elucidated by the scanning electron microscopy coupled with electron dispersive spectra. The sourcing of this resource (PHA) from bacteria species domiciled in the high organic environment could provide amazing metabolic machinery that could create a cost effective means for PHA production.
Keywords
Abbatoir
Polyhydroxyalkanoates
Bacteria species
Molasses
Acetic acid
1 Introduction
In this day and age, the use of petroleum polymers, plastics and their derivatives has been intricately fused with man’s daily lives and activities (Khanna and Srivastava, 2005). Between the 1950 and 2008, the production capacities of plastics have geometrically increased with a yearly growth of 9% (Chanprateep, 2010; Muhammadi et al., 2015). Most of these plastics are used for short term purposes were they occupy land fill sites. In Nigeria and other developing world, plastics have been found to accumulate within the environment at an alarming rate due to its recalcitrance to degradation (Muhammadi et al., 2015). Over time, the increasing impact of these plastics is a huge concern to the management and disposal of solid waste in the developing countries. Some of the measures adopted for the management of the waste include open incineration, which gives rise to noxious gases such as hydrogen cyanide formed from acrylonitrile – based plastics. This can negatively affect the health of humans. Also, when recycling and sorting measures are adopted, it can be time consuming (Khanna and Srivastava, 2005). Alternately, an environment friendly polymer that can easily be degraded and mineralized is the best option.
Polyhydroxyalkanoates (PHAs) are naturally produced polymer or macromolecule polyesters produced by many species of microorganisms. PHAs accumulate as part of the carbon/energy, reducing-power storage granules within bacteria under nutrient limiting conditions where there is excess carbon source. PHA’s are a better alternative to the conventional plastics because of its fast rate of decomposition and mineralization. In terms of structure, PHAs have similar mechanical properties to those of polypropylene. Polyhydroxybutyrate are extremely crystalline, stereospecific with unequal carbon atoms that exhibit D (−) configuration (Doi and Abe, 1990; Steinbüchel, 1991). PHA’s consists of various hydroxyalkanoic acids with polyhydroxybutyrate PHB and hydroxyvalerate being the most common. PHA can undergo degradation in aquatic and terrestrial environment. Microorganisms release extracellular PHA depolymerases that breakdown PHA to form oligomers and monomers that are consequently assimilated into the cells (Khanna and Srivastava, 2005). Several bacteria, comprising gram-negative and gram-positive species, can store various PHAs (Steinbüchel, 1991, 1992; Lenz et al., 1994; Haas et al., 2008; Loureiro et al., 2015). This include but not limited to species of Alcaligenes latus, Azotobacter vinelandii, Pseudomonas oleovorans, recombinant Alcaligenes eutrophus and recombinant Escherichia coli have all been reported to produce PHA industrially (Grothe et al., 1999; Grothe and Chisti, 2000). High cost of production of PHA is an impediment to its extensive use, however the environmental challenges from the use of petroleum plastics makes its production worthwhile. Efforts to cut the cost of production and commercialization of PHA have been dedicated to isolation of new bacteria species and resourceful fermentation/recovery processes (Grothe et al., 1999). The choice of a suitable substrate for use by the microorganisms is necessary for the optimization of PHA production. This subsequently influences the PHA content, composition and the polymer properties. For a cost–effective approach towards increasing productivity and high yield, several researchers have used acetate as a resource during PHA production (Liu et al., 1997; Satoh et al., 1992; Hollender et al., 2002; Filipe et al., 2001a,b). In addition, the discovery of new strains of microorganisms with novel biocatalysts from pristine environments could benefit the PHA bioprocess technology (Pandian et al., 2010). This study therefore is aimed at creating alternatives to petroleum based plastics which will still maintain some of the salient properties of the conventional plastics. In this report, bacteria species from abattoir were isolated and showed capacity to produce PHA from varying concentrations of the carbon sources (molasses/acetic acid). This is the first report about production of PHA from microbial diversity obtained from an abattoir waste in Nigeria.
2 Material and methods
2.1 Chemicals and media
The chemicals, reagents and media used in this study were of analytical grade unless stated otherwise.
2.2 Sample collection
The waste water (slurry) of the animal waste was obtained from the drainage at the abattoir while the dried soil samples were from the surroundings of the dumpsite of the abattoir. The samples were placed into separate sterile plastic container with lid. The samples were immediately transported to the laboratory and stored in the refrigerator at 4 °C until use.
2.3 Media preparation
The minimal salt medium (MS) as described by (Nwinyi et al., 2013, 2016) was used for the enrichment. Appropriate dilutions for the working standards solution were prepared from the stock solutions (10X (0.4 M phosphate buffer, pH 7.3 and 10X –MS stock solution). The 10X –MS stock solution were made of (g/L) 5 (NH4)2SO4, 1 MgSO4.7H20, 0.76 Ca (NO3)2.4H2O, while the 0.4 M phosphate buffer, pH 7.3, contained (g/L) 34.84 K2HPO4, 27.22 KH2PO4. The working solution was prepared by adding of 100 mL of 0.4 M phosphate buffer, 100 ml 10X –MS stock solution into 800 mL of deionized water and supplemented with 1 mL of D lovely mineral and vitamins.
2.4 Enrichment of and isolation of bacteria species with potential to produce PHA
Wastewater slurry (5 ml) and soil sample (5 g) were mixed with minimal salt medium differently in two conical flasks (250 ml). The media were amended with few drops of molasses and acetic acid (0.3% v/v) added. The molasses was added as supplement to enhance the recovery of debilitated cells while the acetic acid served as the main carbon source. The flasks were plugged with sterile cotton and incubated on a shaker (Model H2Q-X 300) (Nwinyi et al., 2014). Transfers from this enrichment were made on sterile nutrient agar and sprayed with aerosols of acetic acid. Successive transfers from the enrichment were made on sterile nutrient agar using 3% inoculum to obtain pure bacteria cultures.
2.5 Characterization of PHA producing bacterial species
Axenic cultures from the wastewater slurry (5 ml) designated A and soil sample (5 g) designated B were characterized based on cultural and morphological differences (Gram stain, colony motility and morphology and comparison with standard type organisms). Thereafter, three axenic cultures from wastewater slurry A1–A3 was selected while three bacteria species B1–B3 were selected from the soil samples. These were further characterized using some biochemical tests which include nitrate reduction tests, gelatin hydrolysis, methyl red, indole, spore test, starch hydrolysis, coagulase, citrate, sugar utilization, catalase, voges proskauer and oxidase tests. Comparisons were made using standard reference/type organisms (Cowan and Steel, 1994; Olutiola et al., 1990).
2.6 Enrichment for polyhydroxyalkanoate (PHA)
The growth of the isolates A1–A3, B1–B3 from the minimal salt medium enrichment were supplemented with different proportions (ml) of molasses (M) and acetic acid (A) and incubated for 48 h, at different time regime (30 °C and 35 °C respectively). For this, five different media formulations were used in this study 6M, 6M: 1A, 6M: 2A, 6M: 3A and 6A. The growths of the isolates in the supplemented media were monitored by measuring the optical density daily at absorbance of 600 nm. On comparison with the control (minimal salt medium without any organism), it was observed that there was two orders of magnitude of increase from the control.
2.7 Extraction of polyhydroxyalkanoate from the bacteria isolates
The cultures were centrifuged at 10,000 rpm for 5 min at 4 °C at the end of 48 h incubation period. The pellets were purified according to (Santhanam and Sasidharan, 2010). For this, polyhydroxyalkanoate (PHA) was extracted using chloroform. For the complete digestion of other cell components except for PHA, cell pellets were re- suspended in sodium hypochlorite solution and incubated at 37 °C for 90 min. This mixture was separated by centrifugation at 10,000 rpm for 5 min. The PHA granules sediments and the supernatant were disposed-off. The obtained (PHA granules) were washed twice with sterile deionized distilled water. Lastly, the PHA granules were washed twice with acetone and diethyl ether (1:1). The resulting polymer (PHA granules) was dissolved in boiling chloroform placed in the fume chamber and air dried to obtain PHA powder and the estimate wet cell weight (WCW in g/ml) determined (Aravind et al., 2013; Du et al., 2001).
3 Results
3.1 Morphological, cultural and biochemical characterization of the bacteria species
Several bacteria species were isolated but was scaled down to five bacterial species tentatively named A1A, A2A, B1B, B2B and B3B. The isolates were all rod shaped with A1A, A2A and B3B occurring white in appearance. Isolates B1B and B2B were cream in appearance. All the isolates were non-motile, and reacted positive to the grams reaction showing effuse and filiform growth pattern on the nutrient agar. Generally all the isolates depicted moderate to abundant growth on the agar with no distinct pigmentation. For the carbohydrate utilization/hydrolytic (digestive) tests, the isolates reacted negative to the voges proskauer test. For the starch hydrolysis isolates B2B, B3B were positive and A1A, A2A were positive to urease activity. All the organisms were able to grow at 35 °C, 5% NaCl and pH 6.0. For the fermentation (sugar) tests, most of the isolates were able to produce acid only mainly while a few produced acid and gas in their responses. The numerical taxonomy (Morphological, cultural and biochemical) characterization results were compared with the standard reference/type organisms, the isolates A1A and A2A are members of the Lactobacillus and Bacillus species. Organisms B1B, B2B and B3B were members of Corynebacteria, Arthrobacter and Rhodococcus species respectively (See Table 1). +: Positive Reaction; −: Negative Reaction, A/G: Acid/gas production, A/-: Acid production only, ‡: no reaction.
Isolates
Tests
A1A
A2A
B1B
B2B
B3B
Gram stain
+
+
+
+
+
Shape
Rods
Rods
Rods
Rods
Rods
Appearance (color)
White
White
Cream
Cream
White
Form
Effuse
Effuse
Filiform
Filiform
Effuse
Pigmentation
No pigmentation
No pigmentation
No pigmentation
No pigmentation
No pigmentation
Optical features
Transparent
Translucent
Translucent
Opaque
Transparent
Abundance of growth
Large
Large
Moderate
Large
Moderate
Voges Proskauer
-
−
−
−
−
Methyl red
−
−
+
+
+
Oxidase
−
−
−
−
−
Catalase
−
−
+
−
+
Citrate
+
−
+
+
+
Indole
−
−
−
−
−
Urease
+
+
−
−
−
Motility
−
−
−
−
−
Coagulase
+
+
+
+
+
Starch hydrolysis
−
−
−
+
+
Sucrose
A/-
A/-
‡
A/-
A/G
Fructose
A/-
A/-
A/-
A/-
A/G
Glucose
A/-
A/G
A/-
A/-
A/G
Galactose
‡
‡
A/-
‡
A/G
Lactose
A/-
A/-
A/-
A/-
A/-
Maltose
A/-
A/-
A/-
A/-
A/-
Endospore stain
−
+
+
+
+
Growth at 35 °C
+
+
+
+
+
Growth at pH 6.0
+
+
+
+
+
Growth in 5% of NaCl
+
+
+
+
+
Most probable organism
Lactobacillus
Bacillus
Corynebacterium
Arthrobacter
Rhodococcus
3.2 Extraction/recovery of polyhydroxyalkanoate from the bacteria isolates
The results for the pellet dry weight are shown in (Figs. 1–4). Based on the recovery rate, very low yield was obtained for Bacillus species. Thus further analysis on the pellets from other bacteria species: Rhodococcus, Corynebacterium, Lactobacillus and Arthrobacter species that had good recovery was carried out.
Data represent the mean of values of dry weight samples of PHA from Lactobacillus sp. at 30 °C and 35 °C respectively. The error bars were due to the differential recovery rate of PHA at different temperatures (α=0.05). Values are mean of the replicates.
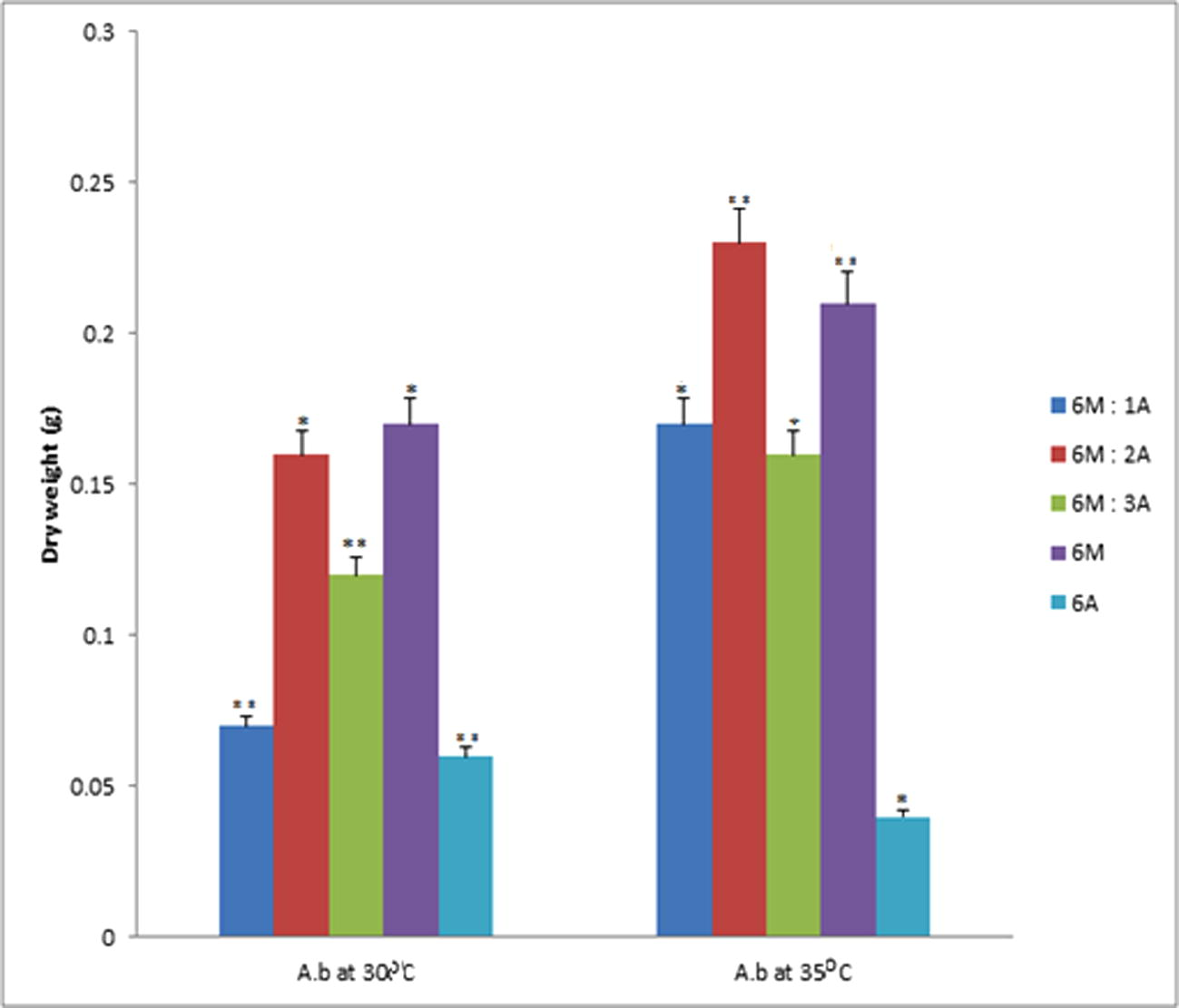
Data represent the mean of values of dry weight samples of PHA from Arthrobacter sp. at 30 °C and 35 °C respectively. The error bars were due to the differential recovery rate of PHA at different temperatures (α = 0.05). Values are mean of the replicates.
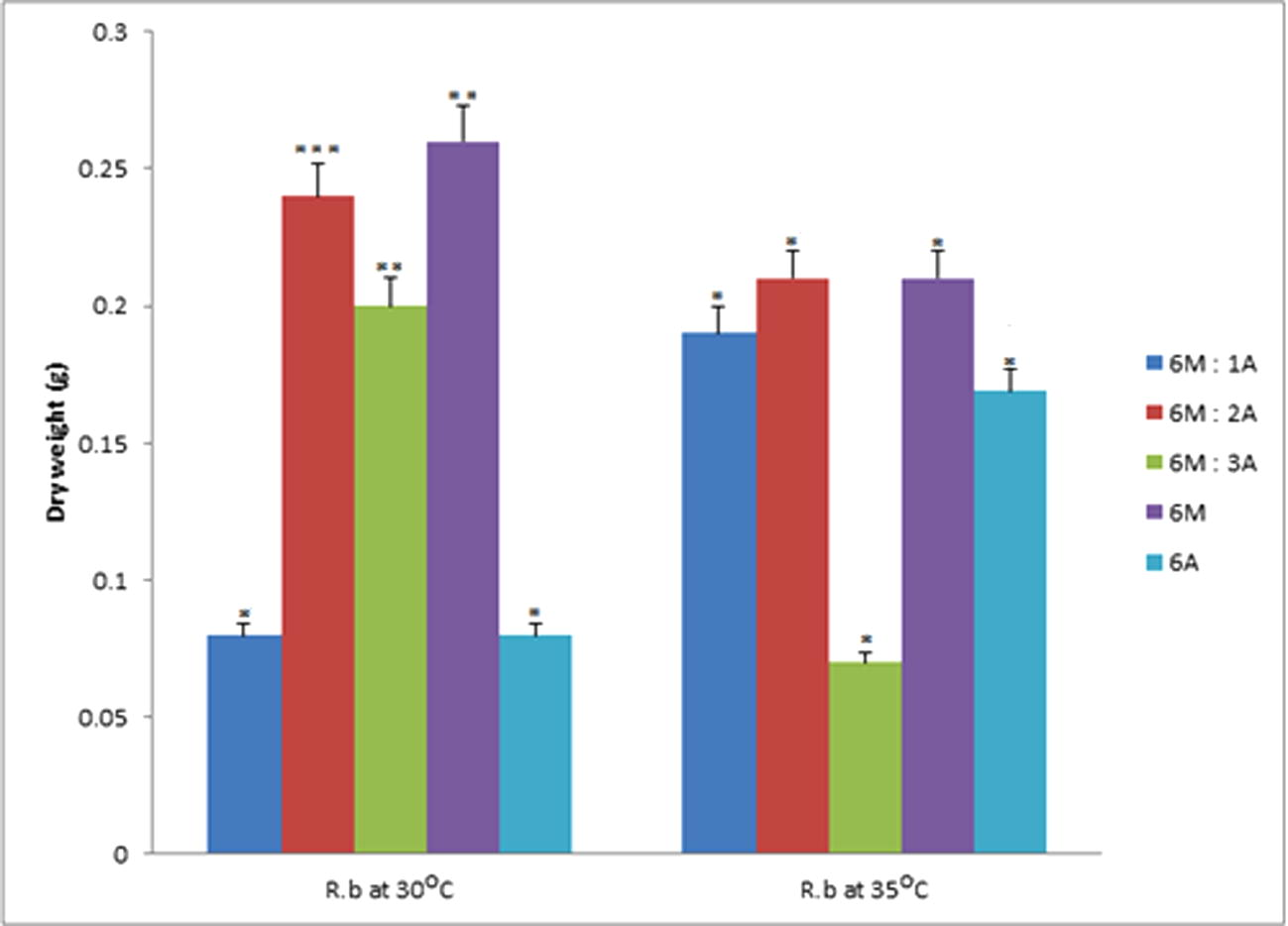
Data represent the mean of values of the dry weight samples of PHA from Rhodococcus sp. at 30 °C and 35 °C respectively. The error bars were due to the differential recovery rate of PHA at different temperatures (α = 0.05). Values are mean of the replicates.
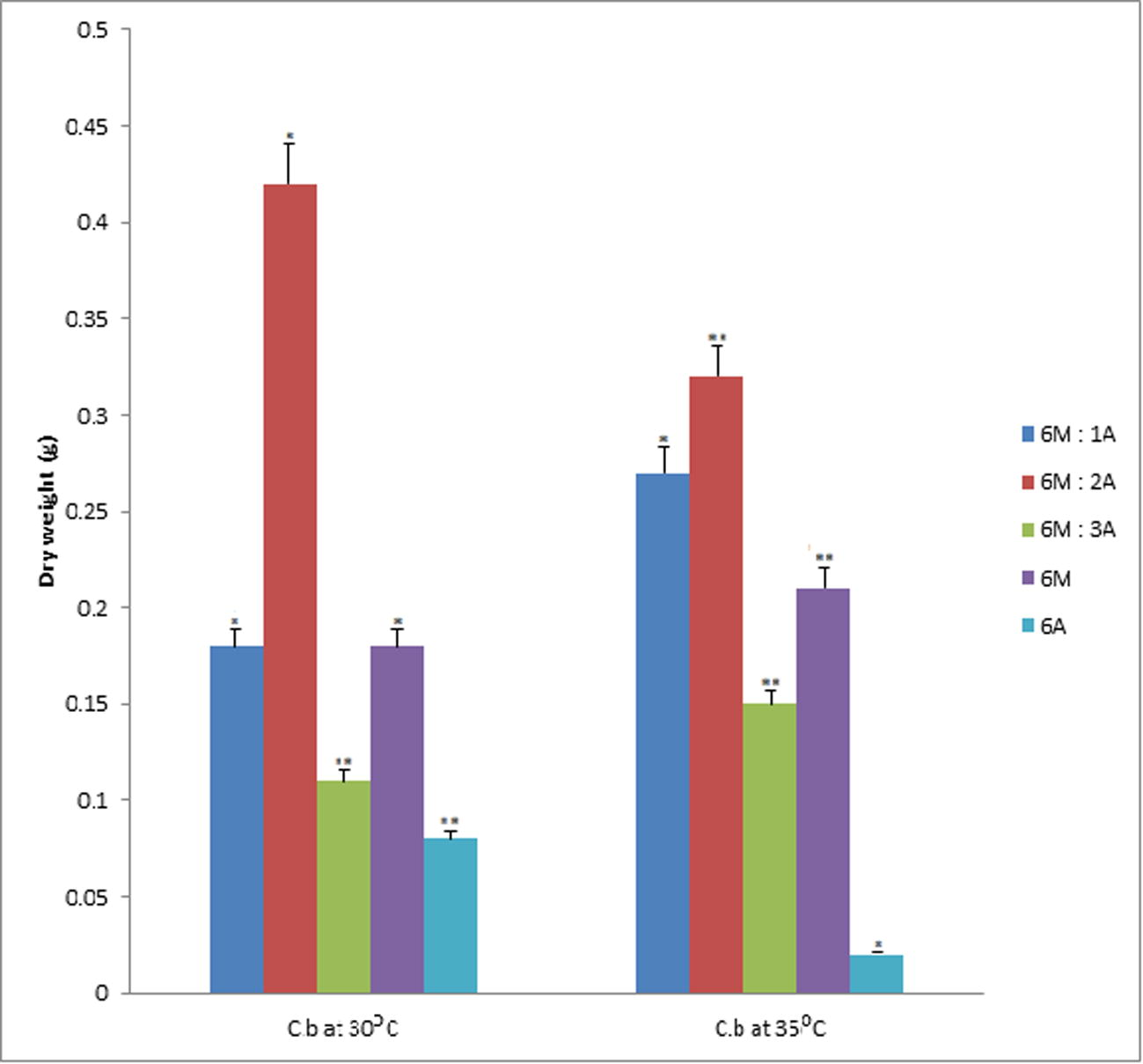
Data represent the mean of values of dry weight samples of PHA from Corynebacterium sp. at 30 °C and 35 °C respectively. The error bars were due to the differential recovery rate of PHA at different temperatures (α = 0.05). Values are mean of the replicates.
3.3 Statistical analysis
Graphs were plotted and computation for mean and standard error were carried out using Microsoft Excel (2010) and mathportal.org.
Considering the mean dry weight recovery of PHA obtained for Lactobacillus species at 30 °C, significant dry weight (P = 0.05) was obtained for the 6M: 2A, 6M: 3A and 6A enrichments (Fig. 1). The 6M: 1A and 6M enrichments did not record significant difference for the mean dry weight recovered. At 35 °C, the dry weight recovery of PHA for Lactobacillus species was not significantly different for the 6M: 1A, 6M: 3A and 6A enrichments. However, the 6M:2A and 6M enrichments recorded considerable significant values from the other three enrichments.
In Fig. 2, Arthrobacter species at 30 °C exhibited significant difference (P = 0.05) in the mean dry weight of PHA recovered from the enrichments 6M: 1A, 6M: 3A and 6A while the 6M:2A and 6M enrichments did not record any significant difference in the mean dry weight of PHA recovered from the enrichments. Similar pattern of recovery was also experienced at 35 °C with 6M: 1A, 6M: 3A and 6A enrichment showing significant difference (P = 0.05). The enrichments 6M:2A and 6M showed no different in the mean dry weight of PHA recovered.
Evaluating the mean dry weight of PHA recovered in Fig. 3, at 30 °C for Rhodococcus species there was significant difference (P = 0.05) in the enrichments for 6M: 2A, 6M:3A and 6M. The other two enrichments showed no significant difference in the mean dry weight of PHA recovered. Considering, the mean dry weight PHA recovered for Rhodococcus species at 35 °C, there was significant difference in all the enrichments (P = 0.05).
In Fig. 4, estimating the dry weight of PHA recovered from Corynebacterium species at 30 °C, there was no significance difference for enrichments 6M: 2A and 6M. However the enrichments 6M:1A, 6M:3A; 6A, recorded appreciable significant difference. At 35 °C, there was no significant difference in the PHA recovered for the enrichments 6M:1A and 6A conversely the 6M:2A, 6M:3A and 6M had significant difference in the dry weight of PHA recovered (p = 0.05).
3.4 Characterization of PHA using the Fourien Transform–Infrared (FT-IR) spectroscopy and Scanning electron microscope coupled with Energy dispersive spectroscopy
The surface functional groups and the structures of dried powder of PHA obtained from the different bacteria species was determined using the Fourien Transform–Infrared (FT-IR) spectroscopy (Shimadzu, Japan). The samples of PHA from the different bacteria species were mounted onto FT-IR; the various absorption bands depicting the presence of some functional groups were noted. See Table 2 and supplementary materials Figs. 5a–d.
ν (cm-1):
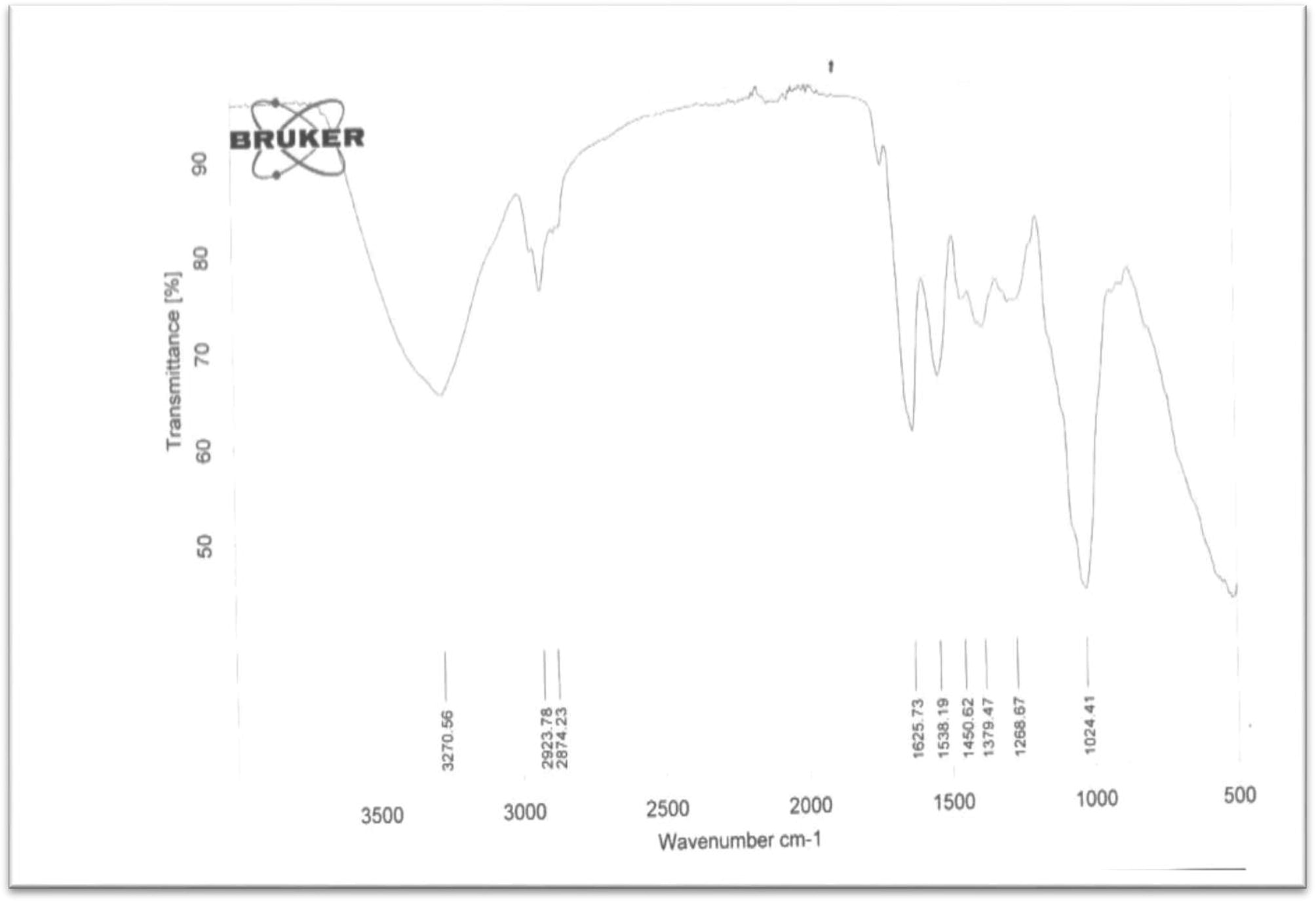
Sample: A (Rhodococcus @ 35 °C)3280 (O—H, broad), 2957 (C—CH3 aliphatic)
2924 (C—CH3 aliphatic), 2855(C—CH2 aliphatic,
1728(C⚌O of acid), 1629(C⚌C), 1024 cm−1(CH⚌CH2)
Sample: B
(Corynebacterium@ 30 °C)3271 (O—H, broad), 2924 (C—H aliphatic)
2874 (C—H aliphatic), 1626 (C⚌C),
1024 (CH⚌CH2)
Sample: C
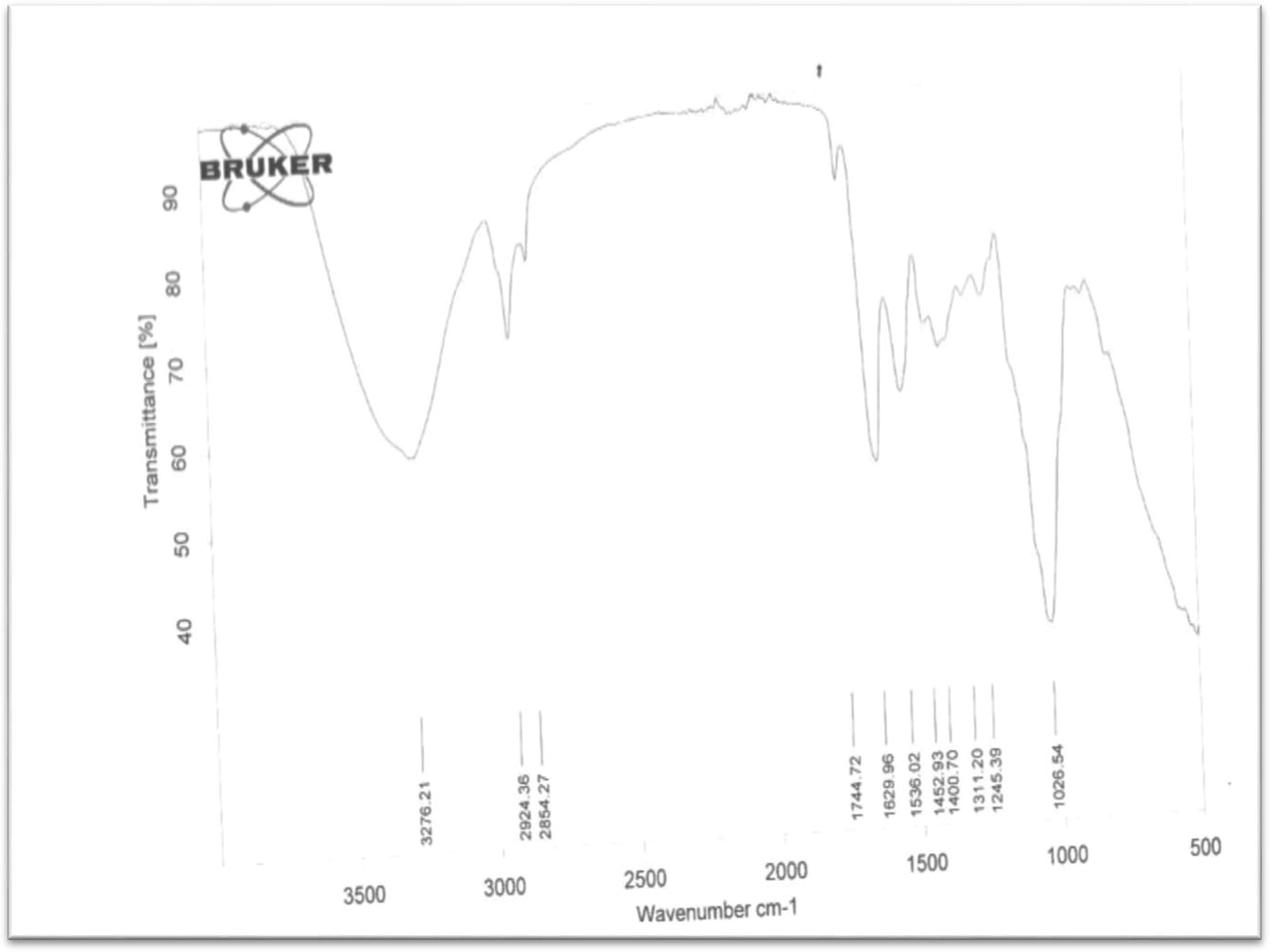
(Lactobacillus@ 35 °C)3276 (O—H, broad), 2924 (C—H aliphatic),
2854 (C—H aliphatic), 1745(C⚌O of ester), 1311 (C—O)
1630 (C⚌C), 1027 (CH⚌CH2)
Sample: D
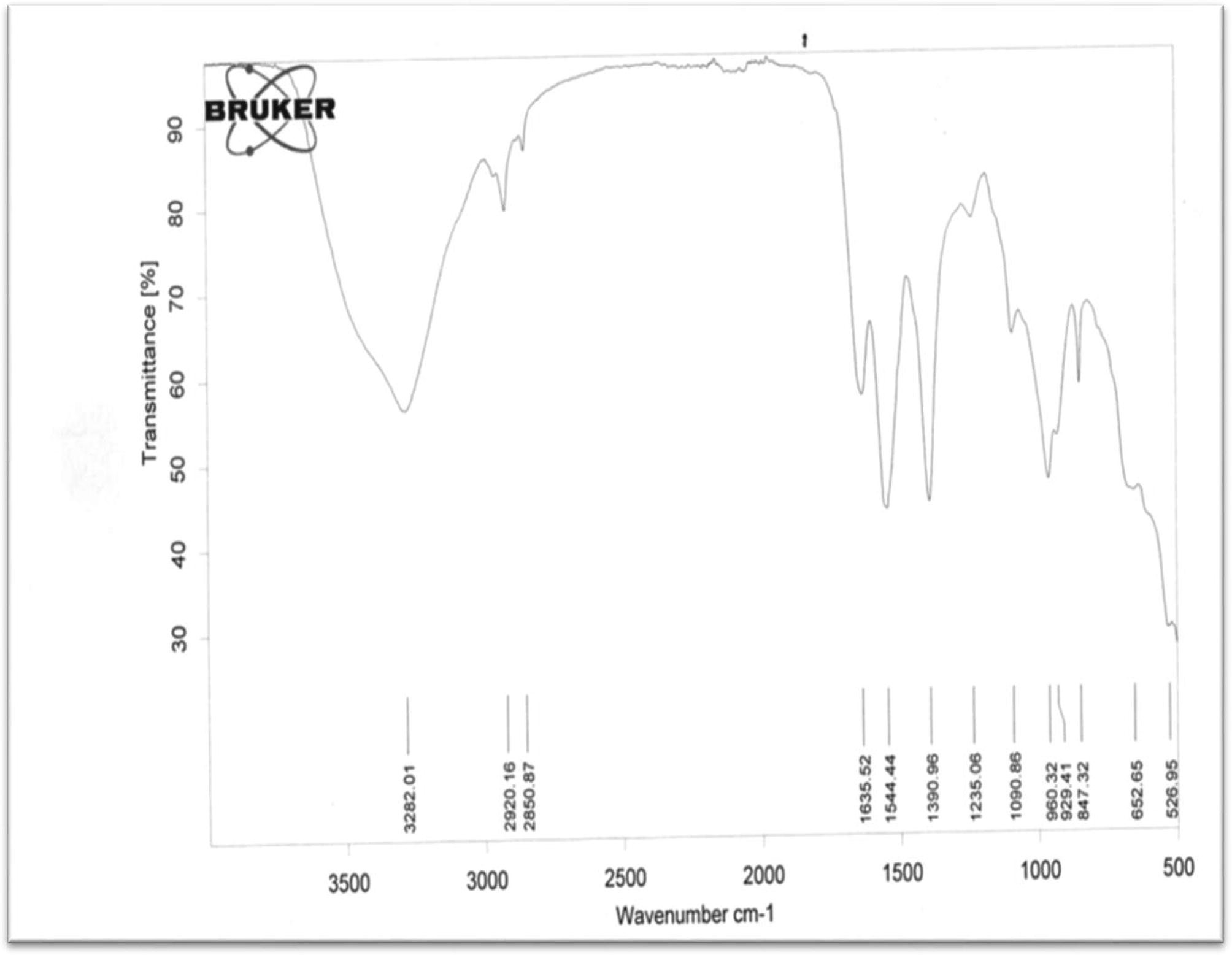
(Arthrobacter@ 35 °C)3282 (O—H, broad), 2920 (C—CH3 aliphatic),
2851 (C—CH2 aliphatic), 1636 (Non-conjugated), 1391 (CH3) aliphatic, 1091(CH⚌CH2), 960(⚌C—H, out of plane bending), 653 (cis-R-CH⚌CHR)
From the FT-IR spectrum of PHA obtained from Rhodococcus sp. (sample A), the highest noticeable absorption was found to be a broad band at 3280 cm−1. This band depicted the presence of —OH functionality. The stretching vibrational frequency at 2957 cm−1 confirmed the presence of C—CH3 bond of aliphatic group while the two bonds at 2924 cm−1 and 2855 cm−1 showed the presence of C—CH3 and C—CH2 bonds of aliphatic group respectively which may be from Sp3 hybridized carbon atom of CH3 and CH2. The C⚌O which is carbonyl of acid (—COOH) appeared at 1728 cm−1. This further confirmed that the broad band at 3280 cm−1 might be the OH of acid (COOH). The absorption bands at 1629 cm−1 depicted the presence of C⚌C of alkene.
The band at 1024 cm−1 is a bending vibration frequency peculiar to the CH⚌CH2. These functionalities confirm the formation of plastic which might likely be of the polymer nature with COOH side chain.
From the FT-IR spectrum of PHA obtained from Corynebacterium sample B, the highest and lowest absorption bands that could be accounted for were found at 3271 cm−1 and 1024 cm−1 respectively, and they were found to represent the presence of OH and CH⚌CH2 functionalities respectively. The noticeable absorption bands at 2924 cm−1 and 2874 cm−1 showed the presence of Sp3-hydridized carbon of C—CH3 and C—CH2 respectively. Since there was no absorption at around 3030 cm−1 this showed the absence of aromaticity. Hence the absorption band found at 1626 cm−1was as a result of C⚌C of alkene. In a similar manner to A above the bending vibration frequency at 1024 cm−1 represent the presence of CH⚌CH2 groups from polyene.
According to FT-IR spectrum of PHA obtained from Lactobacillus sp. sample C, the band at 3276 cm−1 showed the presence of OH group. The lower value of frequency and the broad nature showed that the OH is hydrogen bonded and free. The presence of C—CH3 and C—CH2 was confirmed by the ν at 2924 cm−1 and 2854 cm−1 respectively. The band at 1745 cm−1 is as a result of the presence of C⚌O carbonyl (C⚌O) of ester which is further confirmed by the presence of C—O of alkoxy group at 1311 cm−1. The C⚌C of the alkene was found at 1626 cm−1 for sample C. It is also worthy to note that CH⚌CH2 occurred in this sample at 1027 cm−1. This PHA therefore, has high level of unsaturation obtainable from polyene but is devoid of benzene ring because of lack of band at around 3030 cm−1 which is a characteristic absorption band for aromatic C—H. This plastic might be polyester or a polyene with ester side chain because the ester and alkene functionalities were duly represented in this PHA.
From the result of PHA obtained from Arthrobacter Sample D, the highest and lowest assignable bands were found at 3282 cm−1 and 653 cm−1. While the band at 328 cm−1 stands for the presence of hydrogen bonded OH—, the band at 653 cm−1 represents cis RCH⚌CHR bending vibration. This showed that this polyene plastic has one or more of its alkene arranged in a cis isomeric form around that Sp2-hybridized carbon of alkene. In addition, the aliphatic linked CH3 and CH2 were also present in this plastic type at the frequency of 2920 cm−1 and 2851 cm−1 respectively. The band at 1636 cm−1 which is a bit highest than normal C⚌C (at 1600–1620 cm−1) showed that there is presence of at least one non-conjugated alkene in the plastic bond type in sample D. The absorption band at 1391 cm−1 further confirmed via bending vibrational mode, the presence of CH3 at the terminal end of the chain or as a substituent along the chain. The C⚌C—H was an out of plane bending at 960 cm−1 while the cis-RC⚌CHR appeared in the PHA at 653 cm−1 (Figs. 5a–d).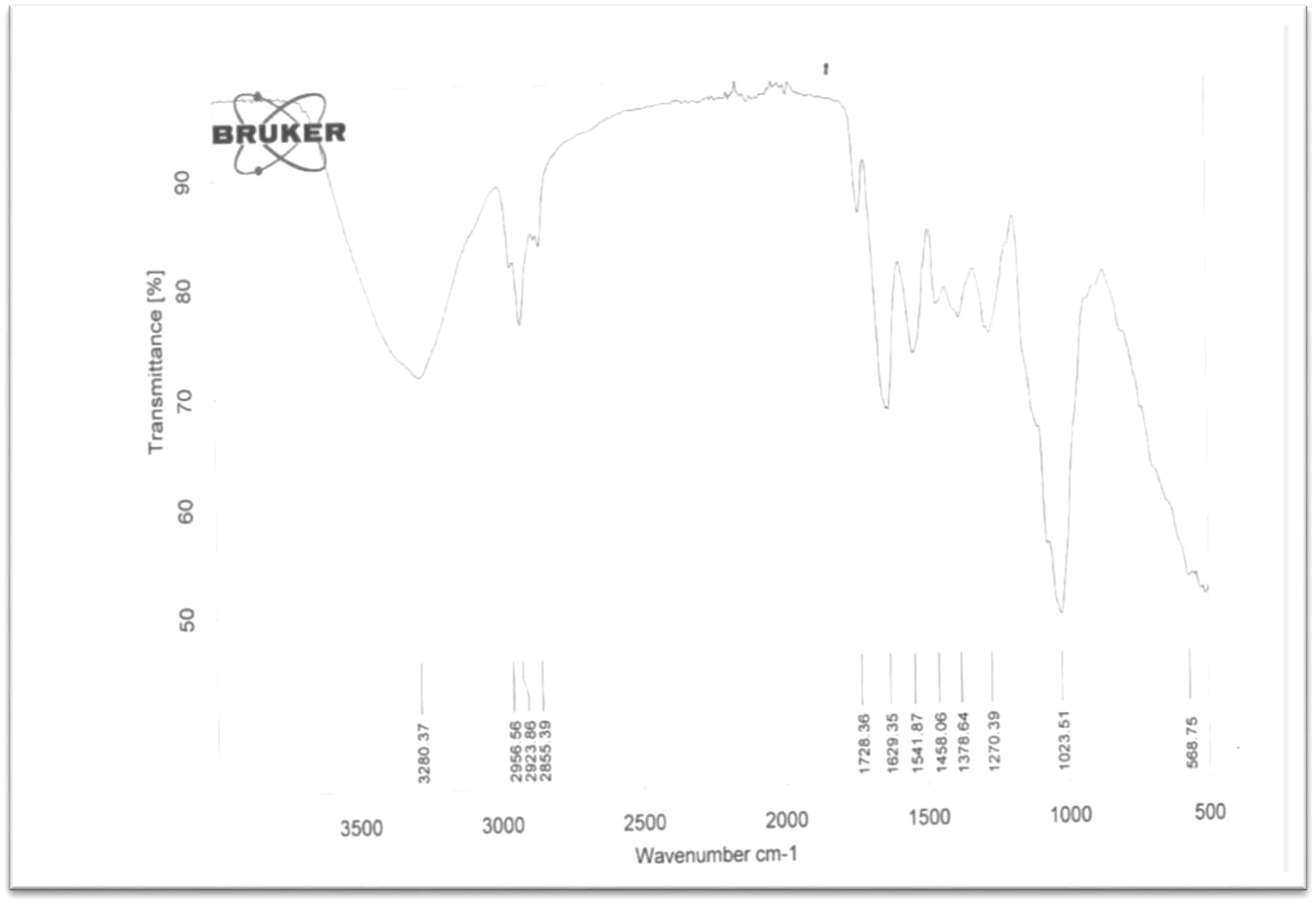
FT-IR analysis of PHA granules obtained from Rhodococcus species.
FT-IR analysis of PHA granules obtained from Corynebacterium species.
FT-IR analysis of PHA granules obtained from Lactobacillus species.
FT-IR analysis of PHA granules obtained from Arthrobacter species.
3.5 Scanning electron microscope coupled with Energy dispersive spectroscopy
The PHA samples were mounted onto the sample holder that was covered with carbon tape. The sample holders were placed into the TESCAN VEGA 3 LM SEM coupled with a selected EDS microanalysis (Oxford system). Voltage of 20 kv was supplied and used for analyzing the PHA materials. For quality control and assurance, about 85% of the study area of PHA was scanned leaving the edges that may have extraneous particles.
The microstructure, surface morphology and chemical composition of the samples were studied using Scanning electron microscope coupled with Energy dispersive spectroscopy. See Figs. 6a–d, Fig. 6a shows the microstructure of PHA produced from Rhodococcus species. The microstructure gives a fairly porous material with fine grains that are interconnected and a strong tendency to form multigrain agglomerates. The Morphology shows grains that are pseudo-spherical in shape with fairly uniform distribution.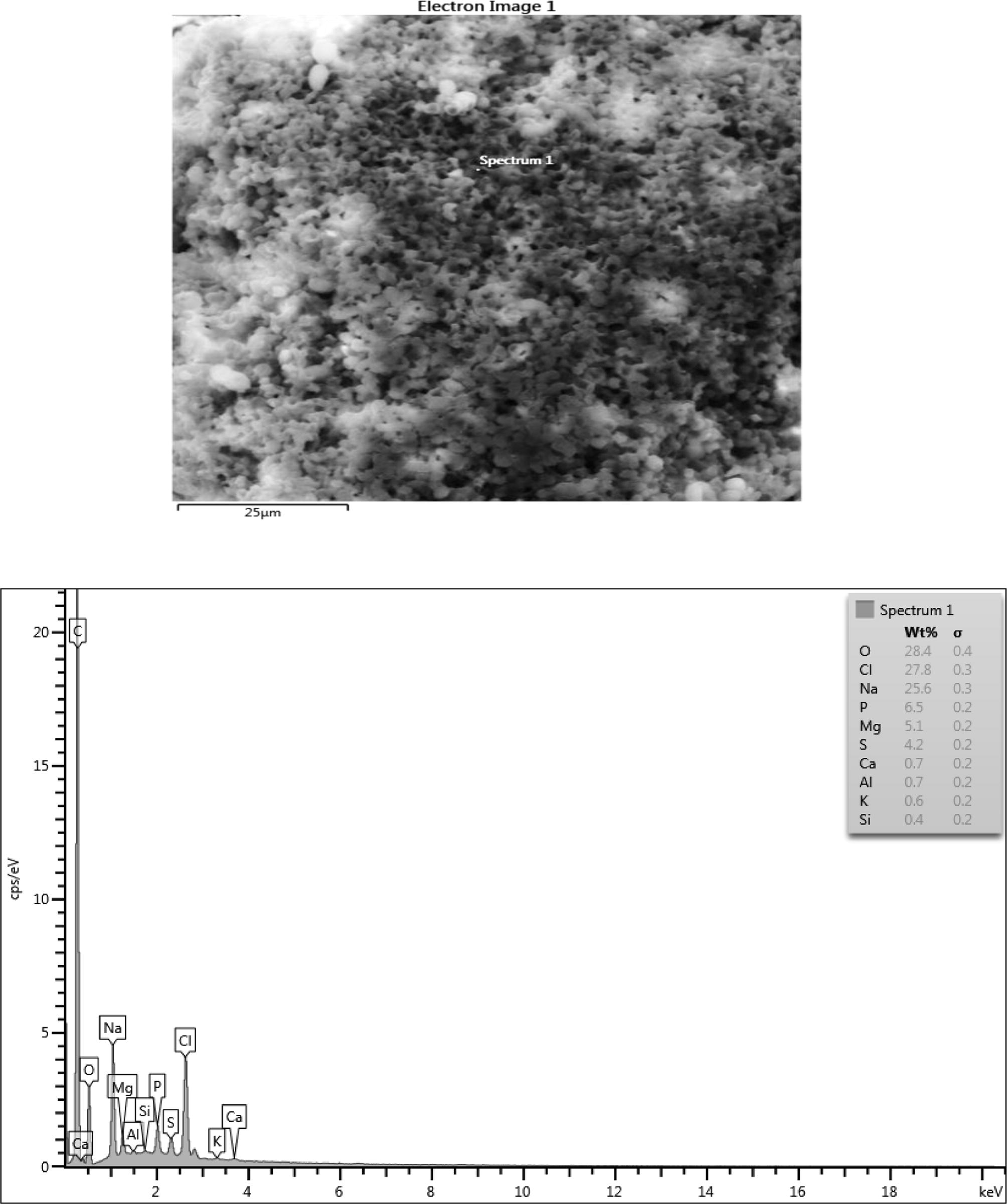
Microstructure, surface morphology and chemical composition of PHA from Rhodococcus species.
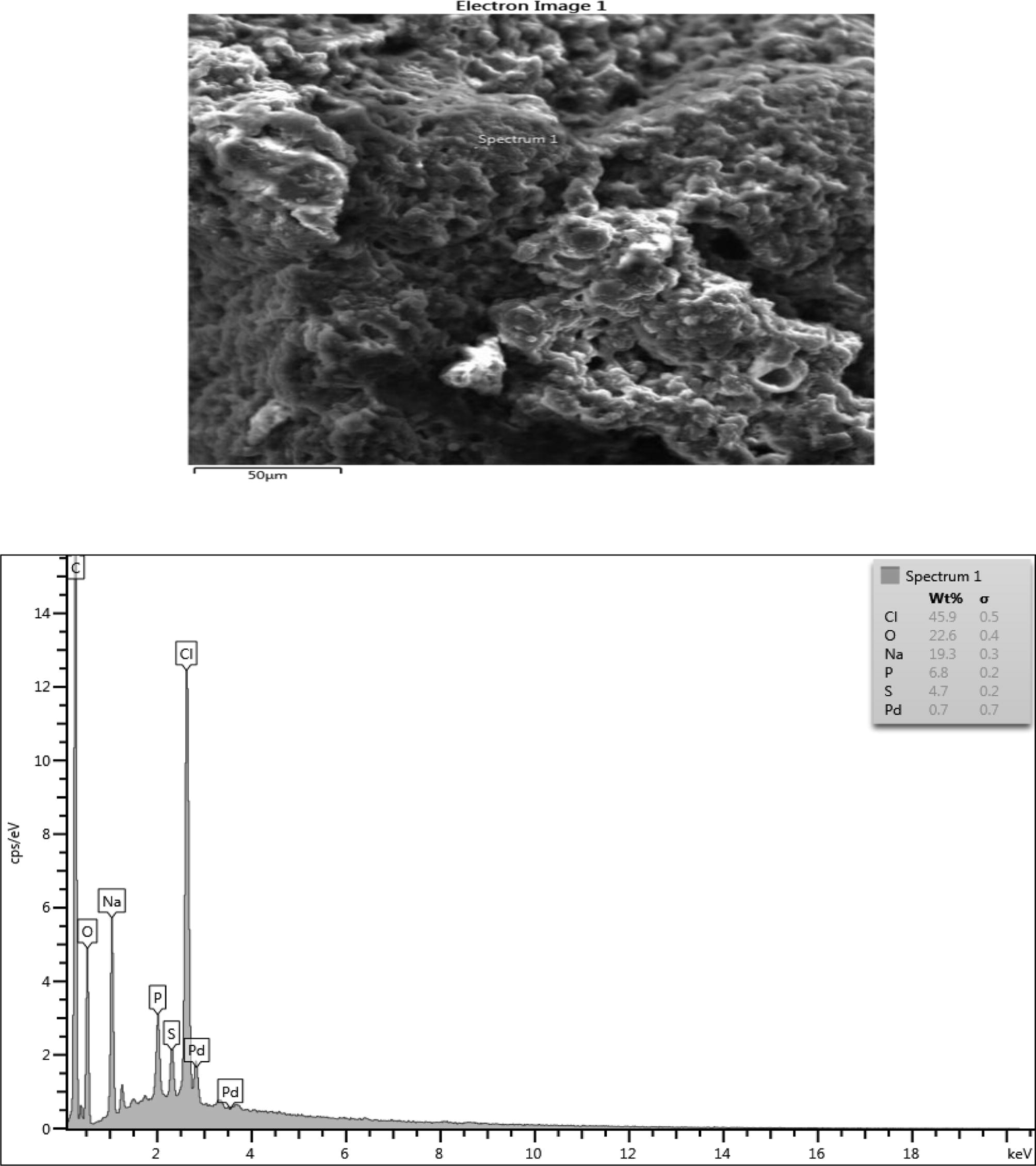
Microstructure, surface morphology and chemical composition of PHA from Corynebacterium species.
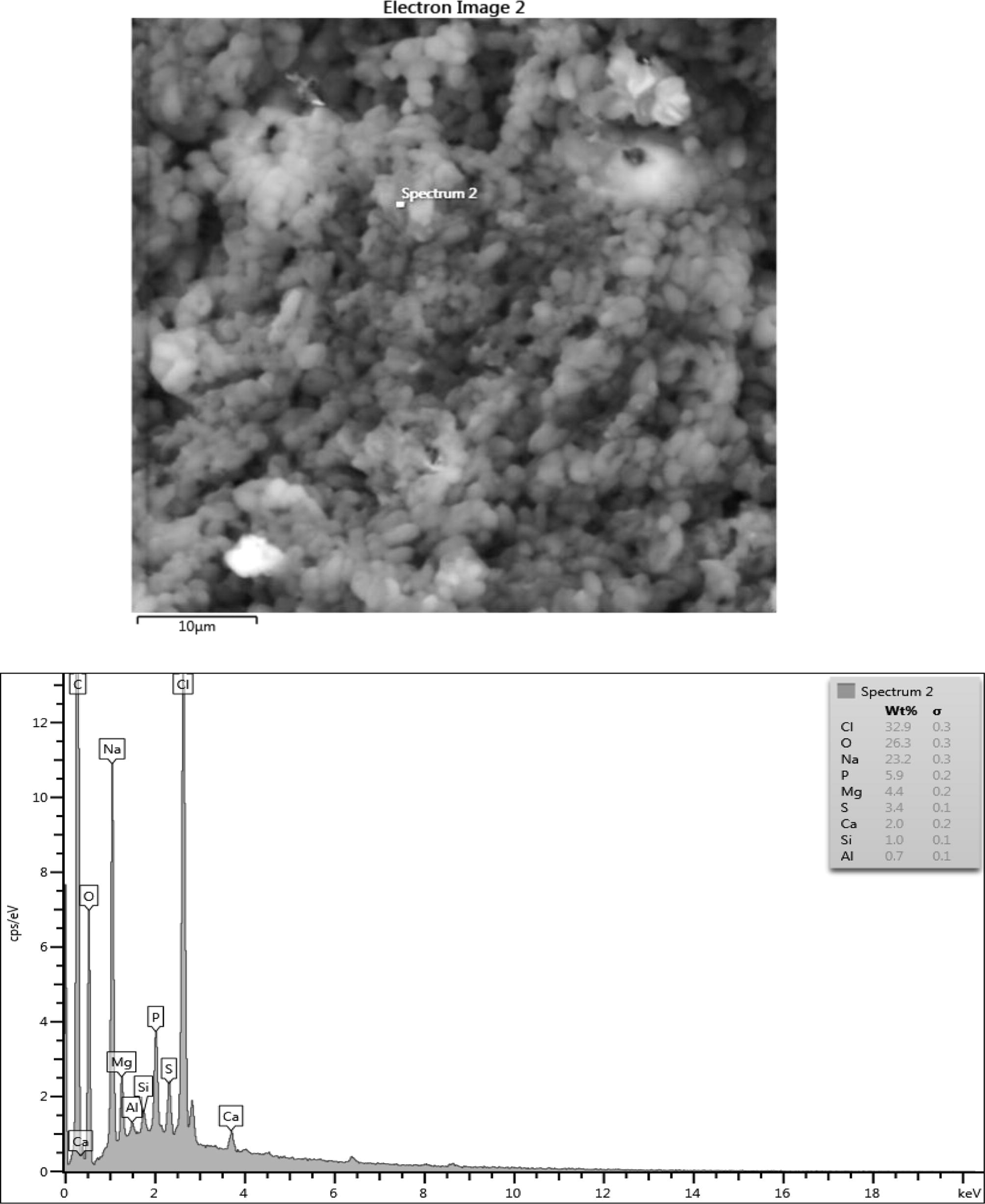
Microstructure, surface morphology and chemical composition of PHA from Lactobacillus species.
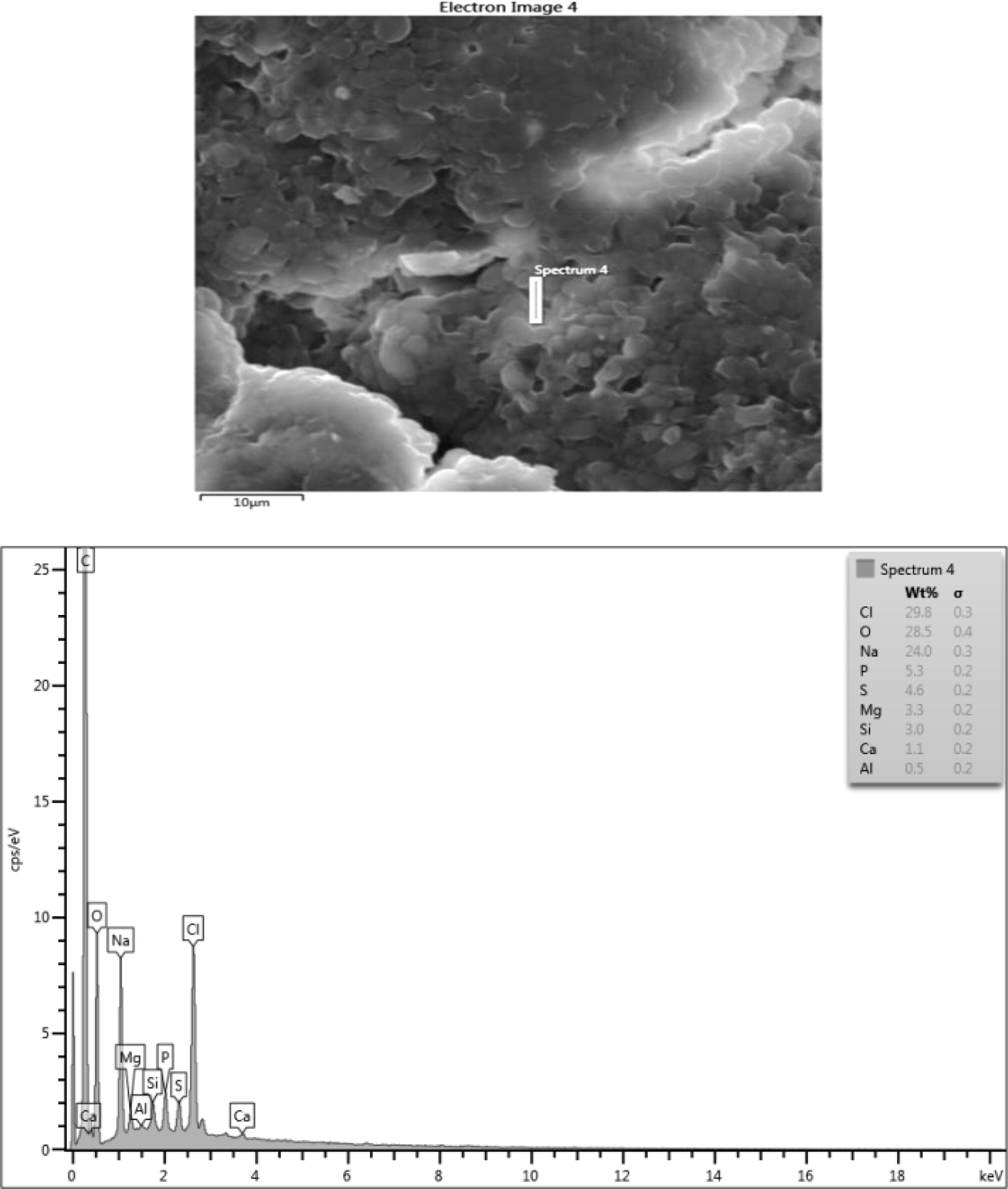
Microstructure, surface morphology and chemical composition of PHA from Arthrobacter species.
The chemical composition result consisted of (wt%) 28.4 Oxygen, 27.8 Chlorine, 25.6 Sodium, 6.5 Phosphorus, 5.1 Mg and 4.2 Sulphur in the sample which are consistent with their elemental signals. Ca, Al, K and Si peaks are not from the sample since their peaks are on the level of background noise or they might be artifacts. The presence of C peak which is so intense in the EDX spectrum of sample might be due to contributions from the carbon tape that was used to hold the sample. Fig. 6b shows a nearly uniform microstructure that showed some degrees of porosity and sphericity of the image. The elemental composition of the microlayer of PHA consisted of (wt%) 26.3 Oxygen, 32.9 Chlorine, 23.2 Sodium, 5.9 Phosphorus, 4.4 Magnesium and 3.4 Sulphur. The Ca Si and Al could be artifacts. The high presence of carbon is from the impurities from the carbon coated sample during the sample preparation. When carbon coats the surface of PHA it allows electrons to radiate through the PHA sample thus enabling the elucidation of the micrograph information of the PHA. In Fig. 6c, there exists a high degree of agglomeration with lesser degree of porosity when compared with the previous micrographs. The elements composition had (wt%) 45.9 Chlorine, 22.6 Oxygen, 19.3 Sodium, 6.8 Phosphorus and 4.7sulphur. The lead at the background is an artifact. Fig. 6d showed some degree of agglomeration with little or no porosity. The chemical composition of the microlayer showed (wt%) 29.8 chlorine, 28.5 oxygen, 24.0 sodium, 5.3 phosphorus, 4.6 sulphur, 3.3 Magnesium. Silicone, calcium and aluminum are perceived to be artifacts.
4 Discussion
The adoption of polyhydroxyalkanoates a biodegradable plastic for daily use will be of immense importance to the developing countries’ that have been negatively impacted by plastic wastes. Polyhydroxyalkanoates have continued to receive great attention because it can easily be synthesized by bacteria from renewable feed costs and malleable with other polymer (Braunegg et al., 2004). Based on the unique properties of PHA, it can successfully replace petroleum based polymers that pollute the environment. PHA can be applied to many areas because of its unique attributes. It can be used as packaging films in bags, paper coatings, and disposable items such as diapers, shampoo bottles, cups, razors and utensils (Loureiro et al., 2015). It has been applied during synthesis of compounds such as osteosynthetic materials for bone growth in the bone plates, surgical sutures, blood vessel replacement and drug release vectors (Khanna and Srivastava, 2005; Xiong et al., 2010). Under aerobic conditions, PHA undergoes complete mineralization into CO2 and water and under anaerobic condition methane is produced (Pandian et al., 2009). The abundance of biodiversity of polymer synthesizing microorganisms varies depending on the milieu. These include the soil, sea, compost, and other refuse dump sites. Thus it is important to explore from such environments for polymer-synthesizing microorganisms with the view of finding microbial systems that can ease the impeding cost of PHA production in terms of time and processing of PHA (Haas et al., 2008). Notable bacteria species isolated for PHA production include but not limited to species of Pseudomonas, Comamonas, Acidovorax faecalis, Aspergillus fumigatus, Variovorax paradoxus Alcaligenes faecalis, and Comamonas testosterone (Chanasit et al., 2016). In this study, it is noteworthy that five bacteria species isolated from the soil and slurry wastes: Bacillus, Lactobacillus, Corynebacteria, Arthrobacter and Rhodococcus showed capacity to produce PHA. The abatoir environment generates large amount of solid and liquid wastes typified by high biological oxygen demand (BOD/COD) and such could be a repository for amazing metabolic diversity.
Phenotypic characterization has been known to elucidate the metabolic activities of bacterial species in terms of their survival and development. This technique was deployed during the characterization of our bacteria species. From the characterization of these bacteria species, we showed more direct functional information by comparing the physiological traits of our bacteria species to those of standard type strains. Two of the bacteria species (Bacillus and Lactobacillus) belonged to the Phylum Firmicutes while Arthrobacter, Corynebacterium and Rhodococcus species belonged to the Actinobacteria. The phylum Actinobacteria are known to be widely distributed in soil where they play key role in degradation of organic matter and humus formation. They group are gram positive and produce mycelia that are non-septate and more slender. Because this group serves as repository for various metabolites, they have been known to be of biotechnological value. The phylum Firmicutes are gram positive, heterotrophic in terms of metabolism with preference to oxygen-rich environments. They possess cell walls that enable them exist in various habitats.
Bacteria have the potential to manufacture and store PHA as a carbon and energy source. This is usually under unfavorable state that limits essential nutrients such as nitrogen or phosphorus or where these elements are in limited concentrations but in the presence of excess carbon source (Byron, 1994). Our obtained species proved their capacity of producing PHA production following enrichment on carbon sources (molasses and acetate). The carbon sources were combined in different proportions and the recovery of the PHA determined (See Figs. 1–4). Several researchers have reported using acetate as sole substrate during enrichment of mixed cultures for PHA production (Satoh et al., 1998; SeraWm et al., 2004; Takabatake et al., 2000). In addition, other workers have reported of combining different substrates, acetate, propionate and lactate during PHA production (Dionisi et al., 2004). The combined substrates offered the chances of realizing different grades of PHA. These include polylactides, aliphatic polyesters, polysaccharides, and the copolymer (Chanasit et al., 2016). The blends of these grades have been used in several industries as raw materials to meet their definite needs (Ojumu et al., 2004). Thus in this study to the best of our knowledge, the combination of molasses and acetate is a new approach to production of polyhydroxyalkanoates (PHAs). Thus the alternative use of molasses may provide huge economic returns for the companies from which this resource could be obtained.
It is noteworthy that with proper combination of the different carbon sources, PHA could be recovered as shown in Figs. 1–4 for our bacteria species, Lactobacillus, Arthrobacter, Rhodococcus and Corynebacterium. In Fig. 1, the Lactobacillus species showed significant recovery of PHA from enrichments 6M: 2A, 6M: 3A and 6A at 30 °C, while at 35 °C a combination of 6M:2A and 6M showed significant dry weight PHA recovery when compared to other media. It could be inferred that there are three possible media combination that could offer significant yield of PHA for Lactobacillus at 30 °C, while at 35 °C a combination of 6M:2A and 6M served as the best regime for the recovery of PHA. This report further validates the temperature tolerance (35 °C) and heterotrophic metabolism expressed by our Lactobacillus species. Thus, with good optimization of the temperature (30–35 °C) efficient production of PHA could be harnessed from this bacteria species. In Fig. 2, PHA recovered for Arthrobacter species at 30 °C and 35 °C was significant in the following enrichments 6M:1A, 6M:3A and 6A. It was obvious from this study that the enrichment media 6M:1A, 6M:3A and 6A was best for the Arthrobacter species even at the different temperature regimes. The Arthrobacter species are chemoorganotrophic and exhibited oxidative metabolism thus utilizing effectively low cost sugar sources. There was significant difference in the PHA recovered from Rhodococcus species in the enrichment media 6M:2A, 6M:3A and 6M at 30 °C while all the five media showed significant difference in the recovered PHA at 35 °C (See Fig. 3). It is obvious from this study that Rhodococcus species optimum temperature for effective utilization of carbon sources for effective production of PHA was 35 °C. This result further corroborates to the ability of Rhodococcus species to utilize wide array of organic compounds effectively. Amongst the organisms that yielded the highest amount of PHA at the different temperatures was Corynebacterium. It yielded PHA at 0.396 ± 0.001 g at 30 °C and 0.317 ± 0.001 g at 35 °C for 6M:2A combination. Using 6M alone PHA recovered was 0.210 ± 0.001 g at 35 °C and 0.181 ± 0.001 g at 30 °C. Evaluating the PHA yield statistically, only the media 6M:1A, 6M:3A and 6A showed significant difference at 30 °C (See Fig. 4) while at 35 °C, the Corynebacterium the media 6M: 2A, 6M:3A and 6A were significant. In all, it was obvious that the low yield of PHA may be due to the harsh impact of chloroform during the recovery process.
In furtherance, the recovery method used in this study was adopted from (Hesselmann et al., 1999; Khanna and Srivastava, 2005; Santhanam and Sasidharan, 2010). Thus, there is need for the development of efficient bioprocess technology since the ease of recovery of PHA is a very important factor in the economics of PHA production. For the purposes of potential applications of the obtained PHA, the microstructure, surface morphology and chemical composition based on Fourien Transform–Infrared (FT-IR) spectroscopy and Scanning electron microscopy was done. The FT-IR spectroscopic analysis showed a peak at 1728 cm−1 confirming polyester or a polyene with ester side chain because the ester and alkene functionalities were duly represented in this PHA (See supplementary material Figs. 5a–d. This data agrees to previous reports of (Hong et al., 1999).
Parameters such as temperature, moisture level, pH, nutrient supply, crystallinity, polymer aggregates, additives and composition of the surface area of PHA have been reported to influence the extent PHA biodegradation in a given environment (Khanna and Srivastava, 2005). Considering the application of our PHA in replacing the petroleum type that has been a huge concern in the developing countries’ a good understanding of the influences of some of these parameters will provide a guideline on the proposed use of our obtained PHA. The microstructure, surface morphology and chemical composition of the samples obtained using Scanning electron microscope coupled with Energy dispersive spectroscopy (See Figs. 6a–d), showed microstructures that are fairly porous interconnected and with strong tendency to form multigrain agglomerates. This is an attribute that are common to most plastic material. This further confirms our report on deriving PHA from our isolates.
Thus the obtained yield based on the combination (molasses and acetate) at different proportions/ temperature may provide a better approach to the production of PHA.
5 Conclusion
The innovative ways of production of PHAs can lead to cost reduction thus stirring wide use of PHA’s in daily life. The sourcing of microbial diversity from abatoir has been shown to provide microbial diversity that could have direct impact on the economics and final price of PHA production. In addition the use of cheap raw materials such as molasses in combination with acetic acid certainly will reduce the cost of the biopolymers. Based on the findings presented in this scientific report, it is clear that Nigeria and other developing countries may utilize PHA as a resource in the management of plastic waste challenges.
Competing interests
Authors declare that no competing interests exist.
Acknowledgements
Authors are grateful to Dr. O.O Ajani for assisting with the interpretation of the data from FTIR and other reviewers whose inputs improved the quality of the manuscript.
References
- Production of Polydroxyalkanote (PHA) using hydrolyzed grass and Syzygium cumini seed as low cost substrates. J. Microbiol., Biotech. Food Sci.. 2013;2(3):970-982.
- [Google Scholar]
- Polyhydroxyalkanoates. In: Mobley D.P., ed. Plastics from Microbes: Microbial Synthesis of Polymers and Polymer Precursors. Munich: Hanser; 1994. p. :5-33.
- [Google Scholar]
- Sustainable polymer production. Polymer-plastics Technol. Eng.. 2004;43(6):1779-1793.
- [Google Scholar]
- Efficient production of polyhydroxyalkanotes (PHAs) from Pseudomonas mendocina PSU using a biodiesel liquid waste (BLW) as the sole carbon sources. Biosci. Biotechnol. Biochem.. 2016;80(7):1440-1450.
- [Google Scholar]
- Current trends in biodegradable polyhydroxyalkanoates. J. Biosci. Bioeng.. 2010;110(6):621-631.
- [Google Scholar]
- Manual for the Identification of Medical Bacteria. Cambridge: Cambridge University Press; 1994.
- Biosynthesis and characterization of a new bacterial copolyester of 3-hydroxyalkanoates and 3-hydroxy-ω-chloroalkanoates. Macromolecules. 1990;23:3705-3707.
- [Google Scholar]
- Biodegradable polymers from organic acids by using activated sludge enriched by aerobic periodic feeding. Biotechnol. Bioeng.. 2004;85(6):569-579.
- [Google Scholar]
- Continuous production of poly-3- hydroxybutyrate by Ralstonia eutropha in a two stage culture system. J. Biotechnol.. 2001;88:59-65.
- [Google Scholar]
- A metabolic model for acetate uptake under anaerobic condition by glycogen accumulating organisms: stoichiometry, kinetics, and the effect of pH. Biotechnol. Bioeng.. 2001;76:17-31.
- [Google Scholar]
- Stoichiometry and kinetics of acetate uptake under anaerobic conditions by an enriched culture of phosphate accumulating organisms at different pHs. Biotechnol. Bioeng.. 2001;76:32-43.
- [Google Scholar]
- Fermentation optimization for the production of poly(h-hydroxybutyric acid) microbial thermoplastic. Enzyme Microb. Technol.. 1999;25:132-141.
- [Google Scholar]
- Poly(h-hydroxybutyric acid) thermoplastic production by Alcaligenes latus: behavior of fedbatch cultures. Bioprocess. Eng.. 2000;22:441-449.
- [Google Scholar]
- Production of poly -3-hydroxybutyrate from waste potato starch. Biosci. Biotechnol. Biochem.. 2008;72(1):253-256.
- [Google Scholar]
- Determination of polyhydroxyalkanoates in activated sludge by ion chromatographic and enzymatic methods. J. Microbiol. Methods. 1999;35:111-119.
- [Google Scholar]
- Effect of different carbon sources on the enhanced biological phosphorous removal in a sequencing batch reactor. Microbiol. Biotechnol.. 2002;18:355-360.
- [Google Scholar]
- A rapid method for detecting bacterial polyhydroxyalkanoates in intact cells by fourier transform-infrared spectroscopy. Appl. Microbiol. Biotechnol.. 1999;51:523-526.
- [Google Scholar]
- Recent advances in microbial polyhydroxyalkanoates. Process Biochem.. 2005;40:607-619.
- [Google Scholar]
- Bacteria synthesis of poly(-β-) hydroxyalkanoates with functionalized side chains. In: Doi Y., Fukuda K., eds. Biodegradable Plastics and Polymers. Amsterdam: Elsevier science; 1994. p. :109-119.
- [Google Scholar]
- Internal energy based competition between polyphosphate and glycogen accumulating bacteria in biological phosphorus removal reactors-effect of P/C feeding ratio. Water Res.. 1997;31:1430-1438.
- [Google Scholar]
- Thermal characterization of polyhydroxyalkanoates and poly(lactic acid) blends obtained by injection molding. Polymer-Plastics Technol. Eng.. 2015;54(4):350-356.
- [CrossRef] [Google Scholar]
- Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev.. 2015;8(3–4):56-77.
- [CrossRef] [Google Scholar]
- Degradation of polynuclear aromatic hydrocarbons by two strains of Pseudomonas. Braz. J. Microbiol.. 2016;47:551-562.
- [Google Scholar]
- Characterization of diesel degrading bacterial species from contaminated tropical ecosystem. Braz. Arch. Biol. Technol.. 2014;57(5):789-796.
- [Google Scholar]
- Aerobic degradation of naphthalene, fluoranthene, pyrene and chrysene using indigenous strains of bacteria isolated from an old polluted industrial site. Can. J. Pure Appl. Sci.. 2013;7(2):2303-2314.
- [Google Scholar]
- An introduction to general microbiology, a practical approach (2nd ed.). Oxford printing press; 1990. p. :12-50.
- Production of Polyhydroxyalkanoates, a bacterial biodegradable polymer. Afri. J. Biotech.. 2004;3(1):18-24.
- [Google Scholar]
- Recovery of biodegradable plastics from activated sludge process. Water Sci. Technol.. 2000;42(3–4):351-356.
- [Google Scholar]
- Synthesis of PHB nanoparticles from optimized medium utilizing diary industrial waste using Brevibacterium casei SRKP2: a green chemistry approach. Colloids Surf. B. 2009;74:266-273.
- [Google Scholar]
- Polyhydroxyalkanoic acids. In: Byrom D., ed. Biomaterials: Novel Materials from Biological Sources. New York: Stockton; 1991. p. :124-213.
- [Google Scholar]
- Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acid in bacteria. FEMS Microbiol. Rev.. 1992;103:217-230.
- [Google Scholar]
- Microbial Production of Polyhydroxyalkanoates (PHA) from Alcaligens spp. and Pseudomonas oleovorans using different carbon sources. Afr. J. Biotechnol.. 2010;9:3144-3150.
- [Google Scholar]
- Optimization of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol. Bioeng.. 2004;87(2):145-160.
- [Google Scholar]
- Activated sludge as a possible source of biodegradable plastic. Water Sci. Technol.. 1998;38(2):103-109.
- [Google Scholar]
- Uptake of organic substrate and accumulation of poly hydroxyalkanoates linked with glycolysis of intracellular carbohydrates under anaerobic conditions in biological excess phosphorus removal process. Water Sci. Technol.. 1992;1992(26):933-942.
- [Google Scholar]
- Application of polyhydroxyalkanoates nanoparticles as intracellular sustained drug-release vectors. J. Biomater. Sci.. 2010;21:127-140.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jksus.2017.08.003.
Appendix A
Supplementary data







