Translate this page into:
Antilipase activity guided fractionation of Vinca major
⁎Corresponding author. kanwarss2000@yahoo.com (Singh Shamsher Kanwar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Methanolic extract of flowers of Vinca major (VFE) reduced the activity of porcine pancreatic lipase (PPL) which was reported in our previous study. To identify the antilipase molecule(s) in the VFE, the antilipase activity-guided fractionation for the purification of lipase inhibitory molecule(s) by Silica Gel and Sephadex G-50 column chromatography was attempted, and the final anti-lipase fraction was examined by ESI-MS and FT-IR spectroscopy. Two molecules namely, Majoridine and Akuammine were elucidated to be present in the flowers of V. major which possessed high PPL inhibition activity. Further, both molecules were looked in silico for their interactions with PPL and other important proteins of lipid metabolism using molecular docking studies. Majoridine and Akuammine demonstrated significant binding interactions with active site residues of PPL.
Keywords
Vinca major
Fractionation
ESI-MS
FT-IR
Majoridine
Akuammine
Docking
1 Introduction
Obesity or adiposity, the ‘New World Syndrome’ has now been declared as a serious health concern in both developed and developing nations. It is one of the metabolic disorders which increases with age and reduce a person’s life anticipation by 5–15 years (Montesi et al., 2016). It is linked with serious clinical outcomes, viz. stroke, sleep apnea, osteoarthritis, cardiovascular problems and type-2 diabetes (Cliff et al., 2016). It is also tied up with some forms of cancer, periodontal diseases and pneumonia (Cabia et al., 2016). There were over 600 million adults worldwide as clinically obese and 1.9 billion as overweight up to 2014 (World Health Organization, 2016). In the European Union, 60% of adults and 20% of school-age children have been tagged as overweight or obese (Baker and Bate, 2016). In England, 24% of adult population is obese and further 36% is overweight (Public Health England, 2016). In USA, 13 million children (aged 2–19) and 79 million adults (aged 20 and older) have been declared obese by American Heart Association Statistics Committee (Skinner et al., 2016). In Australia, 80% adults have been predicted to be obese up to 2025 (Australian National Preventive Health Agency, 2014). Obesity figures have been demonstrated in Indian population too (Ranjani et al., 2016).
High energy diets and reduced physical movement are the major causes of development of this disorder. Obesity can be diagnosed by body mass index (BMI), weight distribution, waist circumference and associated comorbidities (Singh et al., 2013). A BMI of 30 or more indicates the person as obese or a person with waist circumference 40 (for man) and 35 (for woman) inches considered as obese. Medications for obesity and associated comorbidities are available in the market but they have intolerable side effects. Currently approved medicines (Orlistat, Phentermine and Sibutramine) lose only 5–10% weight (at 1 year use) which is very far from the cure of this epidemic. Additionally, patient regains the weight over a period of time. Therefore, prevention of this epidemic has become a major focus of research after cancer epidemic in the developed nations at present (Barnes, 2015; Pandita et al., 2016). Diminution of nutrient digestion and absorption through lowering the activity of enzymes involved in food digestion is the trending strategy for the same (Martin et al., 2015). Major components which contribute to obesity/adiposity are dietary fats, and the major responsible enzyme for the digestion of these fats is pancreatic lipase (PL). Consequently, inhibition or reduction of this enzyme will lead to the reduced digestion of dietary fats and concomitant absorption. Current clinically recommended pancreatic lipase inhibitor ‘Orlistat’ reduces the body fat (up to 30% during 1 year of use) with side effects i.e. fatty/oily spotting, gastric irradiations, stomach pain, increased bowel movements, reduced vitamin absorption, drowsiness, short of breath etc. Therefore, a safe pancreatic lipase inhibitor is still highly sought therapeutic molecule in the drug/pharmaceutical market, and plant derived natural products are suited option for the same. Several plants have been reported in literature for their lipase inhibitory effects, viz. Platycodon grandiflorus (Zhao et al., 2005), Cyclocarya paliurus (Kurihara et al., 2003), Dioscorea nipponica (Kwon et al., 2003), Scabiosa tschiliensis (Zheng and Koike, 2004), Panax japonicas (Han et al., 2005), Aesculus turbinate (Kimura et al., 2006), Acanthopanax sessiliflorus (Yoshizumi et al., 2008), Glycyrrhiza auralensis (Won et al., 2007), Peganum harmala, Inonotus hispidus (Benarous et al., 2015), Calatropis procera (Patil et al., 2015), Malus domestica, Vitaceae vitis (Danis et al., 2015) etc.
Our laboratory is engaged in discovering the lipase inhibitory natural products from the Western Himalayan flora (Shimla, India). Interestingly, the methanolic extract of flowers of Vinca major (VFE) has the potential to reduce the activity of porcine pancreatic lipase (Singh et al., 2014). Vinca major (the big leaf periwinkle) is an evergreen shrub and belongs to the Apocynaceae family which is very well known for its alkaloids. The plants of Vinca genus have been reported to possess medicinal properties against hypertension, leukemia and cancer. Vinca major is native to Mediterranean Europe, Asia Minor and Northern Africa. This plant bears flowers from mid January to mid March in Shimla, India. It spreads vegetatively by arching stolons which roots at tips. It has creepy non-flowering stem and erect flowering stem up to 2 ft. Leaves are glossy, 2–3 in number (4–7 cm long), caudate at base with ciliated hairy margins. All these characteristics are based on the observation recorded for Vinca major plants existing in Shimla and its surroundings. In the continuing work we evaluated the PPL inhibition kinetics of VFE and fractionated it on gel filtration matrices (Silica Gel and Sephadex-G-50) for the identification of lipase inhibitory molecule(s).
2 Materials and methods
2.1 Plant material and chemicals
Aerial parts of Vinca major were collected from forest areas of district Shimla (India), viz. Summer Hill, Potter Hill, Saangti, Chailii, Andari and Taradevi located at 31.61°N 77.10°E. Taxonomic identity of Vinca major was confirmed by Herbarium Staff, Systematic Botany Discipline, Forest Research Institute, Dehradun- 248 006, India. Porcine pancreatic lipase (PPL) and Steapsin (SRL, Mumbai, India); Lipolase100L (Novozymes, Bangalore, India); O’Stat (Orlistat; ARISTO Pharmaceuticals, Mumbai, India) and p-Nitrophenyl palmitate (p-NPP; Lancaster Synthesis, England) were procured from various commercial suppliers. All other chemicals were of analytical grade and were used as received.
2.2 Measurement of PPL inhibitory activity
Various amounts of plant extract or solvent fractions were pre-incubated with 20 μl of PPL (36–44 U/ml) at 37 °C for 20 min in 1X phosphate buffered saline (PBS; pH 7.2) with reaction volume 2.96 ml. Then 40 μl of p-NPP (10 mM in iso-propanol) was added as substrate in a final volume of 3.0 ml. This reaction mixture was incubated at 37 °C for 5 min and reaction was terminated by incubating at −20 °C for 10 min. Amount of p-nitrophenol released was measured at 405 nm wavelength in a UV–Visible spectrophotometer (3000+, Lab India, Mumbai). Assays were performed in duplicate and mean values were presented in each experiment. Initial and residual lipase activities of PPL preparation were recorded and percent PPL inhibition (if any) was calculated.
2.3 Determination of kinetic behaviour of lipase inhibition by VFE
PPL was assayed with increasing concentrations (2.5, 5, 10 and 20 mM) of p-NPP in the absence and presence of three concentrations (0.20, 0.25 and 0.30 mg/ml) of VFE. Inhibition mode for PPL activity was determined by the Vmax and Km values obtained from the Michaelis–Menten kinetics of VFE at the mentioned concentrations.
2.4 Antilipase activity guided fractionation of VFE
Shade dried flowers (5 g) of Vinca major were grounded, suspended in 100 ml petroleum ether and extracted overnight. Next day, petroleum ether layer was collected and evaporated at 37 °C to yield the ether fraction (31 mg; 0.62% percent extractive). Remaining residue was then extracted overnight with 100 ml benzene and fraction was collected by evaporation (34 mg; 0.68% percent extractive). Likewise, chloroform, acetone, methanol and water extracts were also obtained. The process resulted in completely dried 59 mg chloroform extract (1.18% percent extractive), 42 mg acetone extract (0.84% percent extractive), 88 mg methanol extract (1.76% percent extractive) and 107 mg aqueous extract (2.14%) from the V. major flowers. Each of the solvent extract was tested for PPL inhibition using same procedure as described above. In brief, 200 μl of each of the solvent extracts (1 mg/ml stock in 0.05 M PBS, pH 7.2) was pre-incubated with 20 μl PPL (36–44 U/ml) at 37 °C for 20 min in 1X PBS (pH 7.2). Reaction was then started by adding p-NPP (40 μl; 10 mM) in a final reaction volume of 3.0 ml. After 5 min incubation at 37 °C, A405 values were recorded, residual PPL enzyme activities calculated and percent PPL inhibition were calculated. Fractionation of the active methanolic extract was performed by Silica gel and Sephadex G-50 column chromatography. In brief, 4 g of silica gel (particle size 0.040–0.063 mm) was suspended in 20 ml n-hexanol and packed (wet packing) in a sintered glass column (15.4 cm length × 1.5 cm internal diameter). 2 ml of methanolic extract (stock 1 mg/ml) was loaded atop the column. Column was then subjected to elution using selected alcohols of increasing polarity with order being, n-octanol < n-hexanol < n-pentanol < n-butanol < n-propanol < ethanol < methanol. The aqueous mobile phases (1: 1 ratio; solvent: water) were used with octanol, hexanol and pentanol fractionation which didn’t affect the chromatography. Ten eluted fractions (each of 0.6 ml) with each alcohol were collected and stored at −20 °C for PPL inhibitory assays. Each of these alcohol fractions (200 μl) were pre-incubated with PPL for 20 min followed by addition of substrate p-NPP, and residual activities were recorded. The solvents alone were not observed to affect the activity of commercial PPL. Among the silica fractions, n-butanol fraction (fraction ID B8) showed maximum PPL inhibition (41%), and this fraction was also examined by spotting it on a preparative TLC plate coated with silica gel 60 F254 (Aluchro Sep Silica Gel 60, 50 mm × 100 mm × 0.2 mm). In brief, B8 fraction was spotted on the baseline drawn about 1 cm from the bottom of the TLC sheet by using 0.5 μl micropipette tip. Spots were dried and TLC plates were developed in a chromatographic tank saturated with vapors of the mobile phase (1:1 n-hexanol: n-methanol) at room temperature (average 28 °C). Compounds present in B8 fraction were detected by spraying 0.25% vanillin-sulfuric acid solution. The vanillin-sulfuric acid reagent detects natural compounds such as higher alcohols, phenols, steroids, flavonoids and essential oils. The colouring reagent was prepared by dissolving 0.25 g of vanillin crystals in sulfuric acid followed by addition of ethanol. Developed TLC plates were sprayed with this solution and heated at 65 °C in oven for 10 min. A smear was obtained in B8 fraction. The B8 fraction was further subjected to Sephadex G-50 column chromatography. In brief, 2.5 g of Sephadex G-50 was suspended in 25 ml of water, boiled in microwave oven, and left to stand for 2 h prior to use. The slurry was packed in a sintered glass column (length 16.8 cm × 1.5 cm internal diameter; Vt 29.7 cm3), allowed to settle and equilibrated with sec-Butyl alcohol. The 2 ml of above butanolic fraction was loaded onto the column and eluted with sec-Butyl alcohol < iso-Butyl alcohol < n-Butyl alcohol in the polarity gradient order. Eight fractions with each of solvent were collected and tested for PPL inhibition. Among these, n-Butyl alcohol fraction ‘B821’ was found to be most active with 38.1% PPL inhibition and this fraction was further analyzed by ESI-MS and FT-IR spectroscopy to identify the antilipase molecule(s).
2.5 ESI-MS and FT-IR spectroscopy of B821 fraction
The electrospray ionization time of flight mass spectra (ESITOFMS) was recorded on a Waters Micromass Q-TOF spectrometer by Sophisticated Analysis Instrumentation Facility, Panjab University, Chandigarh, India. The IR spectrum (KBr cell) was recorded on a Perkin Elmer FT-IR 400. The parameters used in ESI-MS have been provided as Supplementary material table 1.
2.6 In silico determination of binding affinities of Majoridine and Vincamajoridine towards lipases
After analyzing the molecular mass peaks of ESI-MS and functional group frequencies of FT-IR spectra, two molecules namely, Majoridine and Vincamajoridine (also known as Akuammine) were elucidated to be present being dominant in the final lipase inhibitory B821 fraction. Binding affinities of these molecules were further investigated in silico towards lipases involve in fat digestion. Additionally, previously reported lipase inhibitory and fat reducing drug molecules were also included in this in silico investigation. Docking experiments were performed using Autodock Vina. This study is the first report to show the affinity of discovered lipid inhibitory molecules towards critical proteins involved in lipid digestion metabolism thus provides a comprehensive platform for designing the lipase inhibitory drug molecules. Brief methodology of this in silico investigation is described in the following sections.
2.6.1 Softwares employed to check the affinity of ligands/ phytomolecules towards PPL and other lipases
Complete modules and setups of Molecular Graphics Laboratory Tools (MGL Tools-1.5.6), Autodock Vina and Python Molecular Viewer (PyMOL) were downloaded from www.scripps.edu. LigPlot+ (version v.1.4.5) setup was downloaded from www.ebi.ac.uk/thornton-srv/software/LigPlus/download.html. RasMol (version 2.7.5.2) was downloaded from www.openrasmol.org/OpenRasMol.html and EPS Viewer setup was downloaded from http://epsviewer.org.
2.6.2 Preparation of macromolecules
Crystal structures of porcine pancreatic lipase (pdb ID 1ETH), human pancreatic lipase (pdb ID 1LPB), human gastric lipase (pdb ID 1HLG) and fat mass obesity protein (pdb ID 3LFM) were retrieved from RCSB Protein Data Bank (www.rcsb.org/pdb) and saved in pdb file formats.
2.6.3 Preparation of ligands
Forty nine lipid inhibitory or fat loss molecules (including Majoridine and Akuammine) were used as ligands (Supplementary material table 2). SDF files of these ligands were retrieved from PubChem and converted to pdb file format using OpenBabelGUI.
2.6.4 Docking methodology
Molecular docking was performed using the docking software Autodock Vina. Proteins (1LPB, 1HLG, 1ETH and 3LFM) and ligands were prepared by Autodock Tools 1.5.6. Initially, the software was run and pdb file of the target protein was loaded. Bonds were built by distance. Any water molecule present was deleted. Polar hydrogens were added by selecting ‘no bond order’ method. Kollman charges were added. Molecule visualization was colored by selecting ‘Atom type’ in ‘All Geometries’. Grid map types were set ‘Directly’. Three-Dimensional affinity grids of size 40 × 40 × 40 Å with 0.375 Å spacing were centered on geometric center of the target protein. One configuration file ‘conf.txt’ containing information i.e., receptor = protein.pdbqt, ligand = ligand.pdbqt and points in x-y-z dimension from grid center was prepared for each of the proteins. Docking parameters for the genetic algorithm were used as default. These parameters were as follows: number of GA runs 10, population size 150, number of energy evaluations 2.5 million, number of generations 27,000, number of top individuals to automatically survive to next generation 1, mutation rate 0.02, crossover rate 0.8, GA crossover mode ‘two pt’, and random initial positions and conformations. The probability of performing local search on an individual in the population was set to 0.06. After performing above settings, ‘pdbqt’ file of the protein was saved by choosing target protein from ‘Macromolecule’ icon of the ‘Grid’. Each of the ligand was prepared by loading the ligand molecule in Autodock and active torsions & torsional degree of freedom (TORSDOF) were presented. PDBQT file of each ligand was saved by selecting ‘Ligand → Output → Save as PDBQT’.
2.6.5 Autodock Vina run
Vina was run to get various docked conformations which were subsequently analyzed for the binding affinity of each lipid inhibitory molecule towards the lipase and fat mass obesity protein. The docking space was kept according to the coordinates of active site amino acid residues of each of the protein. All the docking runs were performed in an Acer system of Intel®Core™ i3-2330M, 4GB RAM CPU. All the necessary files of ligands and proteins i.e. pdb, pdbqt and conf.txt were compiled and run under Windows 7 Ultimate operating system. Docking command was run in command prompt as follows:
For each docked ligand, best poses were generated and binding affinity and distance from best mode (RMSD) was presented.
3 Results and discussion
Methanolic extract of Vinca flowers (VFE) was found most potent (34%) fraction followed by the water fraction (19%). However, less than 20% inhibitions were also detected in ether and chloroform extracts (Table 1).
Solvent fraction
PPL Inhibition (%)
Petroleum ether
13.6
Benzene
7.2
Chloroform
10.6
Acetone
3.7
Methanol
34.1
Aqueous
19.7
Interestingly, the VFE (250 μg/ml) reduced the PPL activity in a reversible manner when incubated up to 40 min. The maximum PPL inhibition was detected (52%) at 20 min pre-incubation (Table 2), however degree of inhibition was observed to be declined after 20 min and was recovered at 40 min. It might be the case that active compounds in VFE preparation may dissociated from PPL and enzyme restored the activity. To address which type of reversible inhibition (competitive/uncompetitive or mixed) VFE exerted on PPL, lipase activities were recorded at different VFE concentrations, general reaction rate was plotted (Fig. 1) and kinetic parameters (i.e. Vmax, Km, Kcat) were calculated (Table 3). From the recorded results VFE demonstrated mixed type of inhibition on PPL as VFE preparation affects Km and Vmax in a random manner not in a same ratio as happen in uncompetitive inhibition.
Pre-incubation
PPL inhibition (%)
5 min
−0.97
10 min
10.38
15 min
22.51
20 min
52.88
25 min
30.38
30 min
6.01
35 min
11.01
40 min
39.01
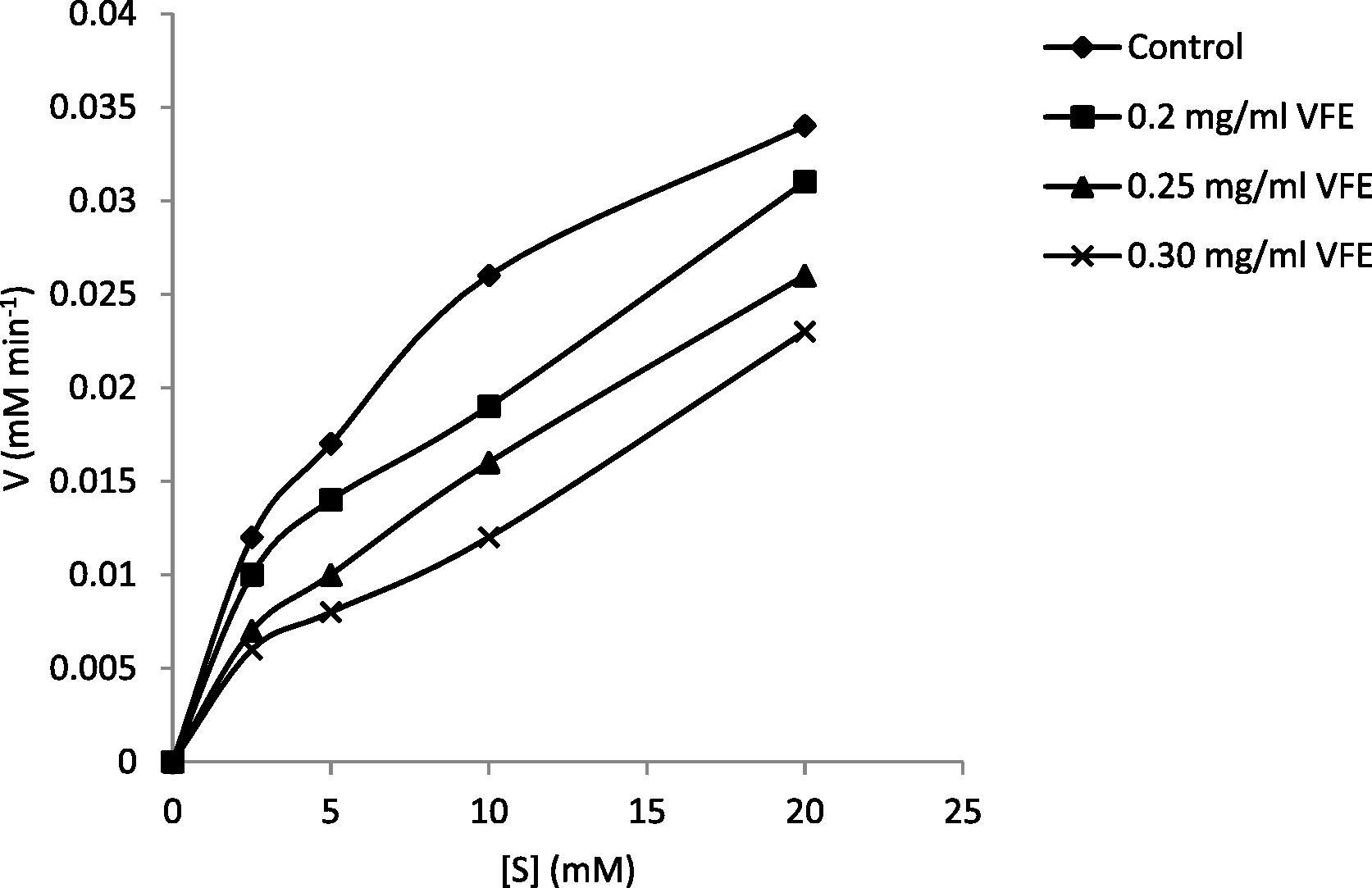
General reaction rate by PPL in the presence of VFE.
VFE mg/ml
Vmax (mM min−1)
Km (mM)
Kcat (S−1)
Kcat/Km (M−1 S−1)
0
0.043
6.65
997.17
1.49 × 105
0.20
0.033
5.81
765.27
1.31 × 105
0.25
0.029
7.41
672.51
0.9 × 105
0.30
0.023
6.82
533.37
0.78 × 105
The active methanolic extract was then fractionated on silica gel and Sephadex G-50 column and elution profile was constructed (Fig. 2). After Sephadex column fractionation, n-Butyl alcohol sub-fraction B821 was found most active with 38% PPL inhibition hence analyzed by ESI-MS and FT-IR spectroscopy. Two molecules namely, Majoridine (C23H28N2O3, calculated mass 380.480, observed mass 381.1) and Vincamajoridine (C22H26N2O4, calculated mass 382.452, observed mass 383.1) were elucidated to be present in dominance in B821 fraction based on the major peak appearance (Figs. 3 and 4). Further, the functional group frequencies of FT-IR spectrum also supported this observation and thus clearly demonstrated the stretches of major functional groups (phenolics –OH, tertiary amine C–N, ester & ether C–O stretchings) of these molecules which have been elaborated in spectrum legend (Supplementary material Fig. 1). No reference molecule of known molecular weight was fractionated through Sephadex so it was impossible to correlate the exact elution volume of active compounds with their molecular weight. However, the total column volume (of Sephadex column) was 29.67 ml (πr2h) and the active molecules were observed to elute after 9.6 ml (Ve) based on observed PPL inhibition.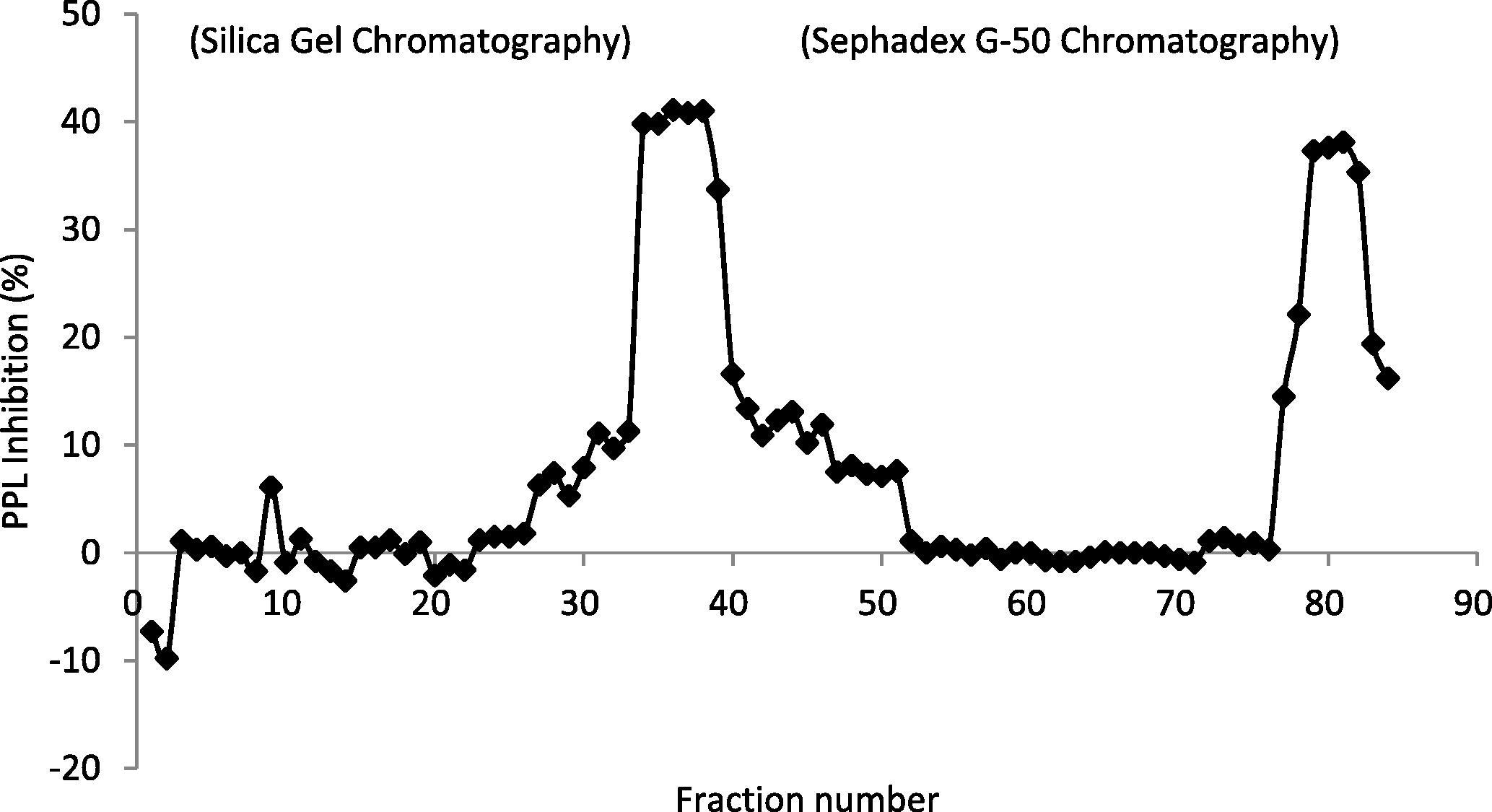
PPL inhibition profile of collected fractions from Silica Gel (fractions 1–60) and Sephadex G-50 column (fractions 61–85).
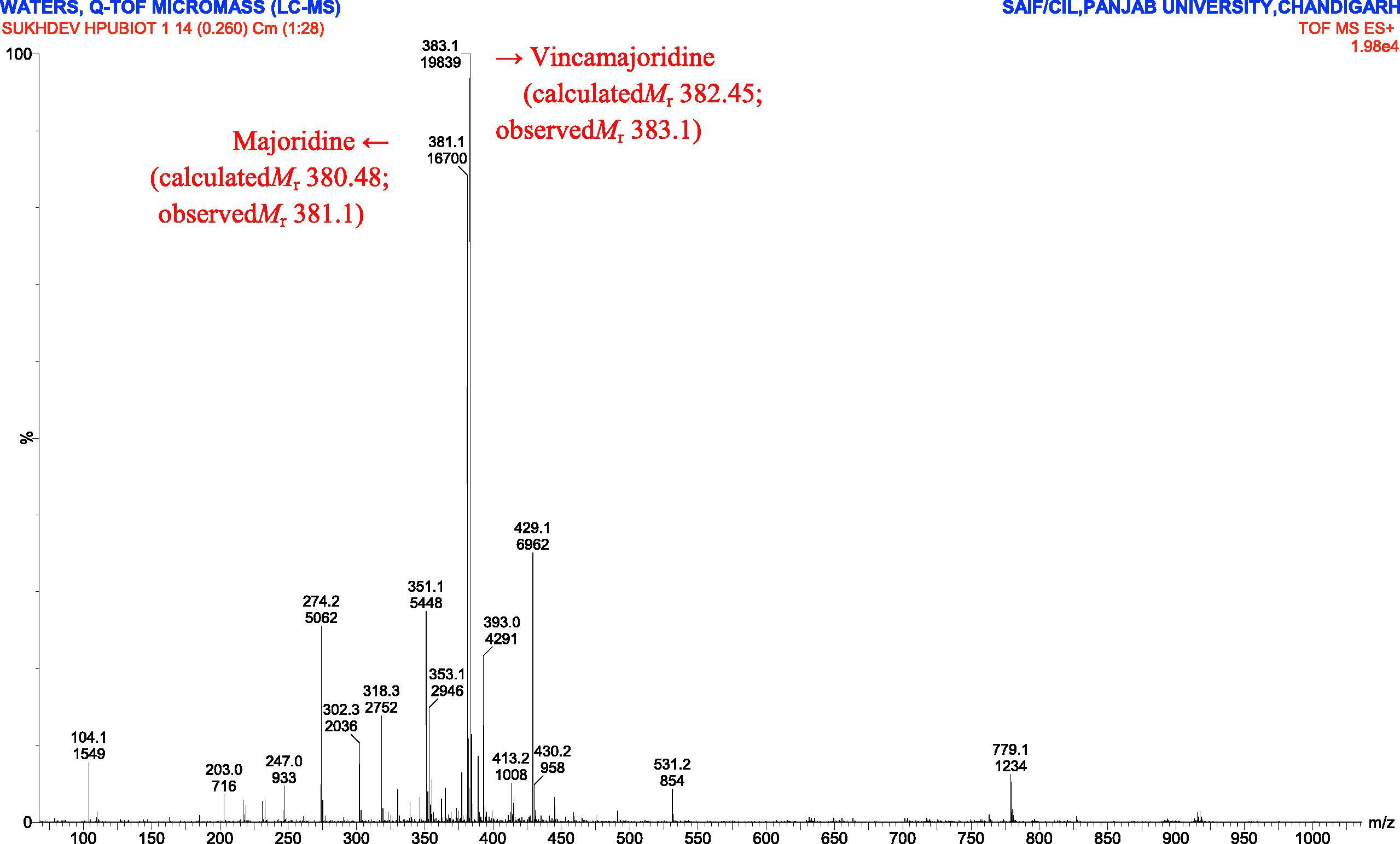
ESI-MS of lipase inhibitory VFE fraction B821. Two major peaks (383.1 and 381.1) were detected. These molecular peaks were elucidated to contain Vincamajoridine (also known as Akuammine) and Majoridine, respectively on the basis of molecular mass(es). The peaks of other molecules were also recorded.
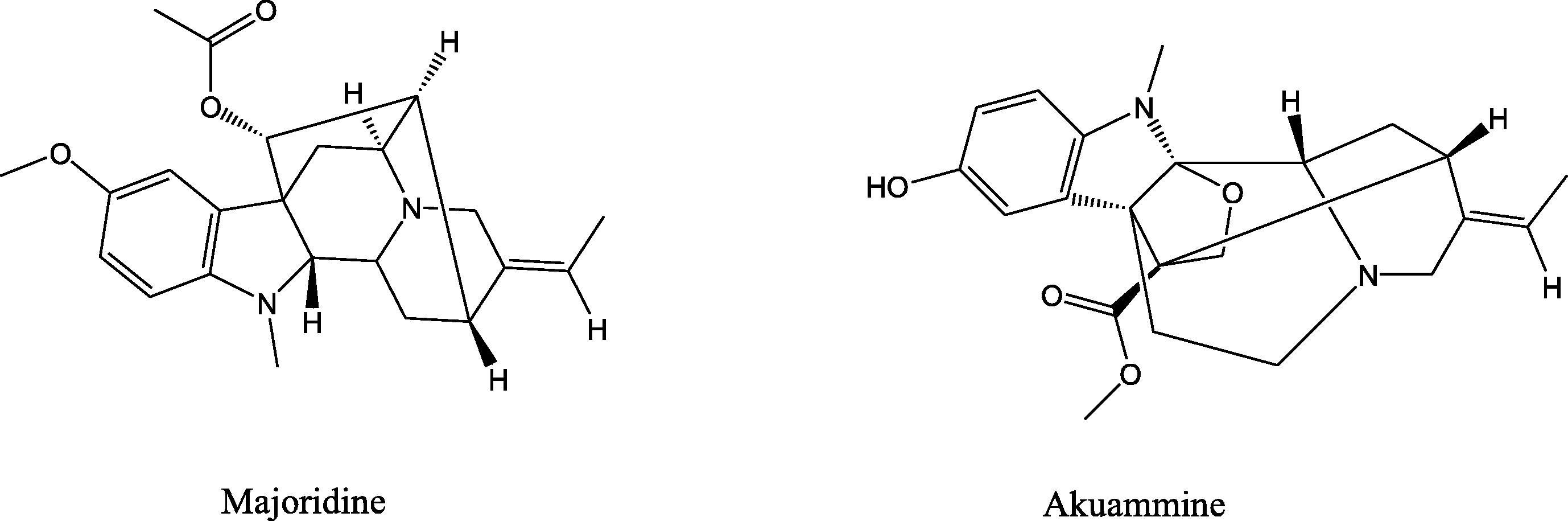
The chemical structures of Majoridine and Vincamajoridine (Akuammine).
Vinca major belongs to the Apocynaceae family which is well known for its alkaloids. To date, several alkaloids have been isolated from this plant (Table 4). Majoridine and Akuammine are indole alkaloids present in the aerial parts of Vinca major and are known for their astringent, antihaemorrhagic and hypotensive effects and used to treat menorrhagia and leucorrhoea (Wren, 1988). Akuammine is strong sympathomimetic and showed antimalarial, antipyretic, anti inflammatory and analgesic activities (Okada et al., 2012). The demonstrated biological activity of a plant or its tissue(s) may be due to the consequence of a specific bioactive molecule or it can be the result of synergistic interactions of bioactive molecules (Singh et al., 2013). So it was hypothesized that these molecules or their interactions with other molecules might account for antilipase activity of Vinca major.
Name
Molecular formula
Reserpinine
C22H26N2O4
Majoridine
C23H28N2O3
Vincamajoridine
C22H26N2O4
Vincamine
C21H26N2O3
Vincamajine
C22H26N2O3
Perivincine
C22H28N2O4
Pubescine
C20H26N2O4
Vinine
C19H26N2O4
Vincawajine
C24H28N2O5
Majorinine
C22H24N2O4
10-Methoxyvinorine
C22H24N2O3
Further, binding affinities of Majoridine and Akuammine towards lipases involved in lipid metabolism were evaluated by molecular docking. Pancreatic lipase is not the only lipase responsible for dietary fats, gastric lipase also aids in lipid digestion. Another important protein which is functionally involved in energy homeostasis and whole body metabolism is fat mass & obesity associated protein FTO (Han et al., 2010). FTO is a Fe2+/2-oxoglutarate-dependent oxidative DNA/RNA demethylase and belongs to AlkB family. Single nucleotide polymorphism studies have revealed the association of FTO gene with increased body mass and obesity risks. This protein was also included in the in silico investigation. Besides Majoridine and Akuammine, the previously reported lipase inhibitory and fat loss molecules were also examined. The docking analyses revealed binding energy ranging between −16.4 and −4.4 kcal/mol (Supplementary material table 3). Majoridine demonstrated inhibition constant (Ki) ∼ 1.015 mM with binding energies −9.8, −9.2, −10.3 and −9.3 kcal/mol towards 1ETH, 1LPB, 1HLG and 3LFM, respectively whereas Akuammine demonstrated inhibition constant (Ki) ∼ 1.014 mM with binding energies −9.0, 8.3, −7.6 and −7.1 kcal/mol. These features are comparable with the recommended lipase inhibitor drug Orlistat (1.010 mM, −6.8, −7.1, −6.7 and −6.2 kcal/mol).
Hydrogen bonds and hydrophobic interactions contribute to the affinity, orientation and stabilization of a ligand in its receptor molecule. A favorable interaction is possible only through its perfect geometrical fit and low binding energy. Poor solubility and low oral bioavailability are major limitations of Orlistat. Through in silico molecular docking, the suggested limitations may be alleviated, and drugs can be designed with improved therapeutic efficacy. LigPlot+ is a favorable tool to address covalent and hydrophobic interactions in a protein-ligand complex. So Ligplots were generated for Majoridine and Akuammine complexes with above mentioned proteins and amino acid residues of catalytic space involved in the interactions were developed (Fig. 5). Ligplots revealed that Majoridine is interacting through hydrogen bonding with Ser153 residue of ETH which might be the interaction responsible for the reduction in the activity of this lipase (PPL). On other hand, the Akuammine has hydrophobic interactions with Ser153 & His264. These in silico outcomes are in accordance with in vitro outcomes which indicated that Majoridine and Akuammine have comparable binding sites for interaction with PPL. So it could be safely predicted that Vinca major VFE preparation exerted its lipase inhibitory effects by virtue of presence of Majoridine and Akuammine molecules. Additionally, Majoridine (molecular weight 380.48 Da, hydrogen bond donors 0, hydrogen bond acceptors 5 and log P 2.7) and Akuammine (molecular weight 382.45 Da, hydrogen bond donors 1, hydrogen bond acceptors 6 and log P 1.5) fulfill Lipinski’s druggability criteria and suitable candidates for clinical development.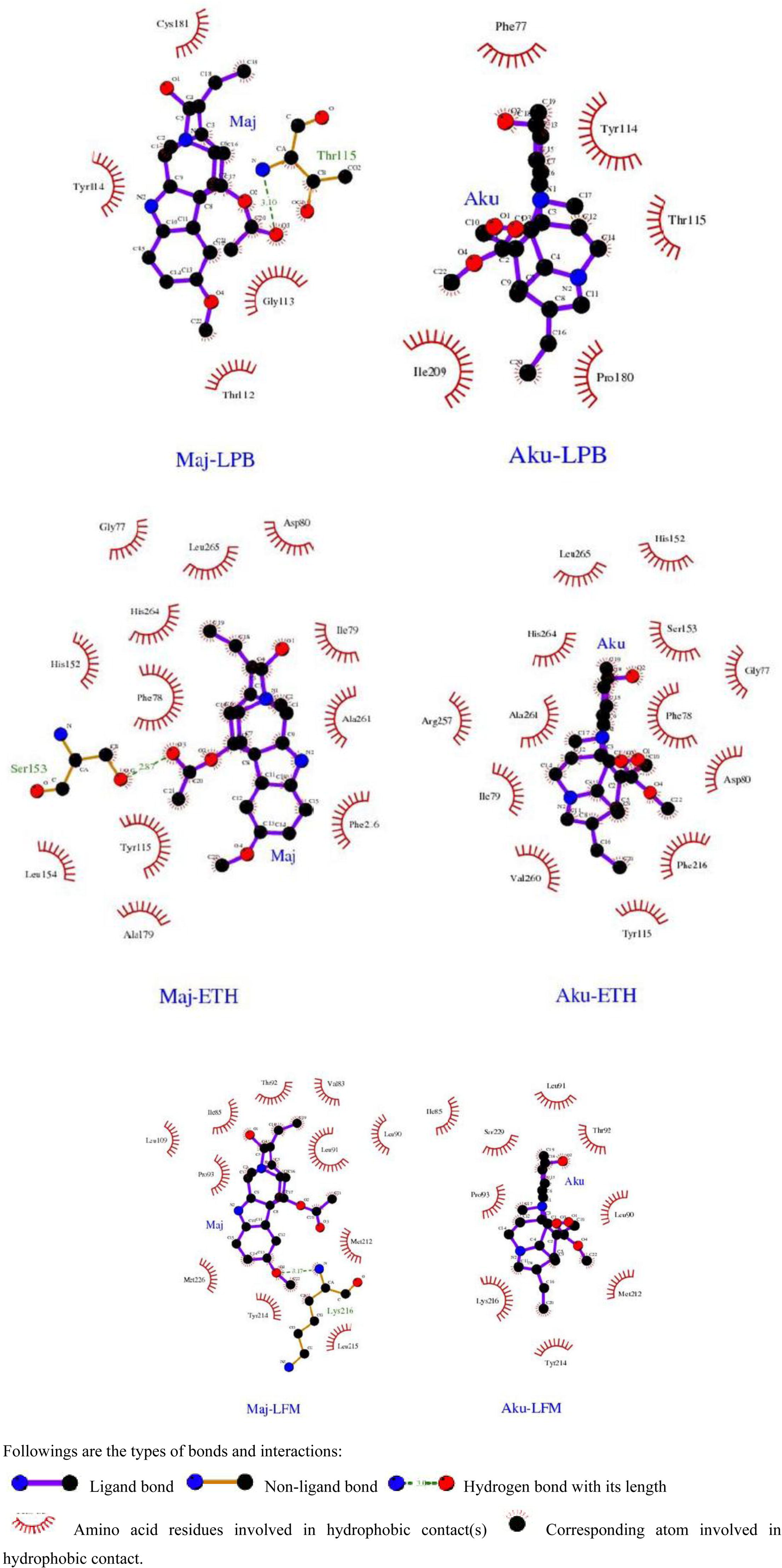
LigPlot diagrams of 1LPB, 3LFM and 1ETH catalytic domains with their respective inhibitors (majoridine and akuammine). The atoms of the protein and lipase inhibitors involved in the interaction are shown as colored spheres: carbon in black, oxygen in red and nitrogen in blue. Intermolecular hydrogen bonds are shown as green dashed lines with their respective bond distances in angstrom units. The lipase inhibitors are depicted with their chemical structure and labelled as Maj for Majoridine and Aku for Akuammine. These images were generated using LigPlot+ (version v.1.4.5).
4 Conclusion and future perspectives
Majoridine and Akuammine are hypothesized to be the responsible molecules for the antilipase activity of Vinca major. These molecules (especially Majoridine) have vital interactions with active site amino acid residues of PPL. Both molecules are druggable candidate as per Lipinski’s norms so these must be further examined for weight loss effects in vivo using an appropriate animal model. Further investigations are still needed to develop safer lipase inhibitory molecules for the prevention and treatment of adiposity related diseases and disorders.
Conflict of interest
Authors declare no conflict of interest regarding publication of this paper.
Acknowledgements
Sophisticated Analysis Instrumentation Facility of Panjab University, Chandigarh (India) is gratefully acknowledged. This work was funded by Department of Biotechnology, Ministry of Science and Technology, Govt. of India (Grant no. DBT-JRF/F-19/487) under a DBT-SRF Fellowship awarded to Mr. Sukhdev Singh. The authors are thankful to DBT-Sub-Distributed Information Centre, Himachal Pradesh University, Shimla for providing necessary laboratory facilities.
References
- Australian National Preventive Health Agency, 2014. Evidence brief obesity: prevalence trends in Australia, pp. 1–32.
- Baker, C., Bate, A., 2016. Obesity statistics. House of Commons Library 3336, 1–22.
- Overweight versus obese: different risk and different management. Tex. Heart Inst. J.. 2015;42:237-238.
- [Google Scholar]
- Harmalin and hispidin from Peganum harmala and Inonotus hispidus with binding affinity to Candida rugosa lipase: in silico and in vitro studies. Bioorg. Chem.. 2015;62:1-7.
- [Google Scholar]
- A role for novel adipose tissue secreted factors in obesity related carcinogenesis. Obes. Rev.. 2016;17:361-376.
- [Google Scholar]
- Objectively measured sedentary behavior and health and development in children and adolescents: systematic review and meta-analysis. Obes. Rev.. 2016;17:330-344.
- [Google Scholar]
- Inhibition of pancreatic lipase by culinary plant extracts. Int. J. Plant Biol. Res.. 2015;3:1038.
- [Google Scholar]
- Anti-obesity effects of chikusetsusaponins isolated from Panax japonicus rhizomes. BMC Complement. Altern. Med.. 2005;5:9-18.
- [Google Scholar]
- Crystal structure of the FTO protein reveals basis for its substrate specificity. Nat. Lett. 2010
- [CrossRef] [Google Scholar]
- Identification of novel saponins from edible seeds of Japanese horse chestnut (Aesculus turbinate Blume) after treatment with wooden ashes and their nutraceutical activity. J. Pharm. Biomed. Anal.. 2006;41:1657-1665.
- [Google Scholar]
- Hypolipidemic effect of Cyclocarya paliurus (Batal) Iljinskaja in lipid-loaded mice. Biol. Pharm. Bull.. 2003;26:383-385.
- [Google Scholar]
- Anti-obesity effect of Dioscorea nipponica Makino with lipase-inhibitory activity in rodents. Biosci. Biotechnol. Biochem.. 2003;67:1451-1456.
- [Google Scholar]
- New targets to treat obesity and the metabolic syndrome. Eur. J. Pharmacol.. 2015;763:64-74.
- [Google Scholar]
- Long term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab. Syndr. Obes.: Targets Ther.. 2016;9:37-46.
- [Google Scholar]
- Developmental potential for endomorphin opioidmimetic drugs. Int. J. Med. Chem.. 2012;715123:1-10.
- [Google Scholar]
- Childhood obesity: prevention is better than cure. Diabetes Metab. Syndr. Obes.: Targets Ther.. 2016;9:83-89.
- [Google Scholar]
- In vitro lipase inhibitory effect and kinetic properties of di-terpenoid fraction from Calatropis procera (Aiton) Biocatal. Agric. Biotechnol.. 2015;4:57-585.
- [Google Scholar]
- Public Health England (2016). UK and Ireland prevalence and trends [http://www.noo.org.uk/NOO_about_obesity/adult_obesity/UK_prevalence_and_trends]. Accessed on 6th November 2015.
- Epidemiology of childhood overweight & obesity in India: a systematic review. Indian J. Med. Res.. 2016;143:160-174.
- [Google Scholar]
- Antilipase, antiproliferatic and antiradical activities of methanolic extracts of Vinca major. J. Pharmacognosy Phytochem.. 2014;3:53-64.
- [Google Scholar]
- Therapeutic effect of herbal medicines on obesity: herbal pancreatic lipase inhibitors. Wudpecker J. Med. Plants. 2013;2:53-65.
- [Google Scholar]
- Prevalence of obesity and severe obesity in US children, 1999–2014. Obesity. 2016;24:1116-1123.
- [Google Scholar]
- Licochalcone A: a lipase inhibitor from the roots of Glycyrrhiza uralensis. Food Res. Int.. 2007;40:1046-1050.
- [Google Scholar]
- Chiisanoside is not absorbed but inhibits oil absorption in the small intestine of rodents. Biosci. Biotechnol. Biochem.. 2008;72:1126-1129.
- [Google Scholar]
- World Health Organization (2016). Obesity and Overweight [http://www.who.int/mediacentre/factsheets/fs311/en/].Accessed on 6th November 2015.
- Potter’s New Cyclopaedia of Botanical Drugs and Preparations. Saffron Walden: CW Daniel Co.; 1988.
- Antiobese and hypolipidemic effects of Platycodin saponins in diet-induced obese rats: evidences for lipase inhibition and calorie intake restriction. Int. J. Obesity. 2005;29:983-990.
- [Google Scholar]
- New biologically active triterpenoid saponins from Scabiosa tschiliensis. J. Nat. Prod.. 2004;67:604-613.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jksus.2017.03.005.
Appendix A
Supplementary data







