Translate this page into:
Molecular docking study and anticonvulsant activity of synthesized 4-((4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)urea/thiourea derivatives
⁎Corresponding author. aloksinghthakur19852@gmail.com (Alok Singh Thakur)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A novel series of 1-(4-substitutedphenyl)-3-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfon-yl)urea/thiourea (3a–l) derivatives were synthesized and evaluated for anticonvulsant activity. The sulfonylurea containing derivative 3a (unsubstituted), 3b (hydroxyl) and 3c (chloro) were found safer and active for protection against maximal electro shock (MES) and pentylenetetrazole (PTZ) induced convulsion. Out of these 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-(4-hydroxyphenyl)urea (3c) was found to be the most active molecule.
Abstract
A new series of 1-(4-substitutedphenyl)-3-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfon-yl)urea/thiourea (3a–l) derivatives were synthesized and screened for anticonvulsant activity by maximal electro shock (MES) and pentylenetetrazole (PTZ) induced convulsant model. All the derivatives were evaluated for neurotoxicity measures by rotarod method. Structures of synthesized compounds were established by spectroscopic techniques and elemental analysis. Pharmacological results were justified by the data obtained from the molecular docking studies along with the drug likeness and drug scores. From the experimental results, compounds 3a, 3b and 3c were found to be significantly effective. Out of these the chloro substituted derivative 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-(4-hydroxyphen-yl)urea (3c) was the most active convulsion protective compound. From the evaluation results it was concluded that the sulfonylurea series is better and safer than the sulfonylthiourea for anticonvulsant activity.
Keywords
Anticonvulsant
Sulfonylurea
Molecular docking
Neurotoxicity
Rotarod
Drug likeness
1 Introduction
Convulsion is neurological disorder that leads the recurrent neuronal firing from the cortical neurons (Henry, 2012). Due to overproduction of nerve impulses there is transitory loss of consciousness and other symptoms related to brain dysfunction. This bursting activity occurs from the prolonged depolarization of neuronal membrane due to opening of voltage gated Na+ channels which initiate the action potentials repeatedly (Köhling, 2002). Voltage gated sodium channel is the target for various cyclic anticonvulsant drugs i.e. phenytoin, carbamazepine, lamotrigine (Lipkind and Fozzard, 2010) and phenobarbitals (Rajak et al., 2010). This idea use to explore to develop some other tricyclic compounds along with some other basic structural characteristics like an aryl hydrophobic binding site (A), hydrogen bonding domain (HBD), hydrophobic-hydrophilic site controlling the pharmacokinetic properties (C) and an electron donor group (D) for efficient anticonvulsant activity (Rajak et al., 2010). In the earlier study sulfonylurea pharmacophoric model reported significant anticonvulsant activity (Thakur et al., 2015). Besides the anticonvulsant activity sulfonylurea reported for hypoglycemic (Zhang et al., 2009), antitumor (Samanta et al., 2004), cytotoxic (Zhang et al., 2010), antimalarial (León et al., 2007) and antifungal (Chen et al., 2015) activities in which the respective protein molecules were target for binding to exert the activity.
Here in the present work some new thiazine containing sulfonylurea and sulfonylthiourea derivatives have been prepared along with the mentioned structural requirements for efficient anticonvulsive effect.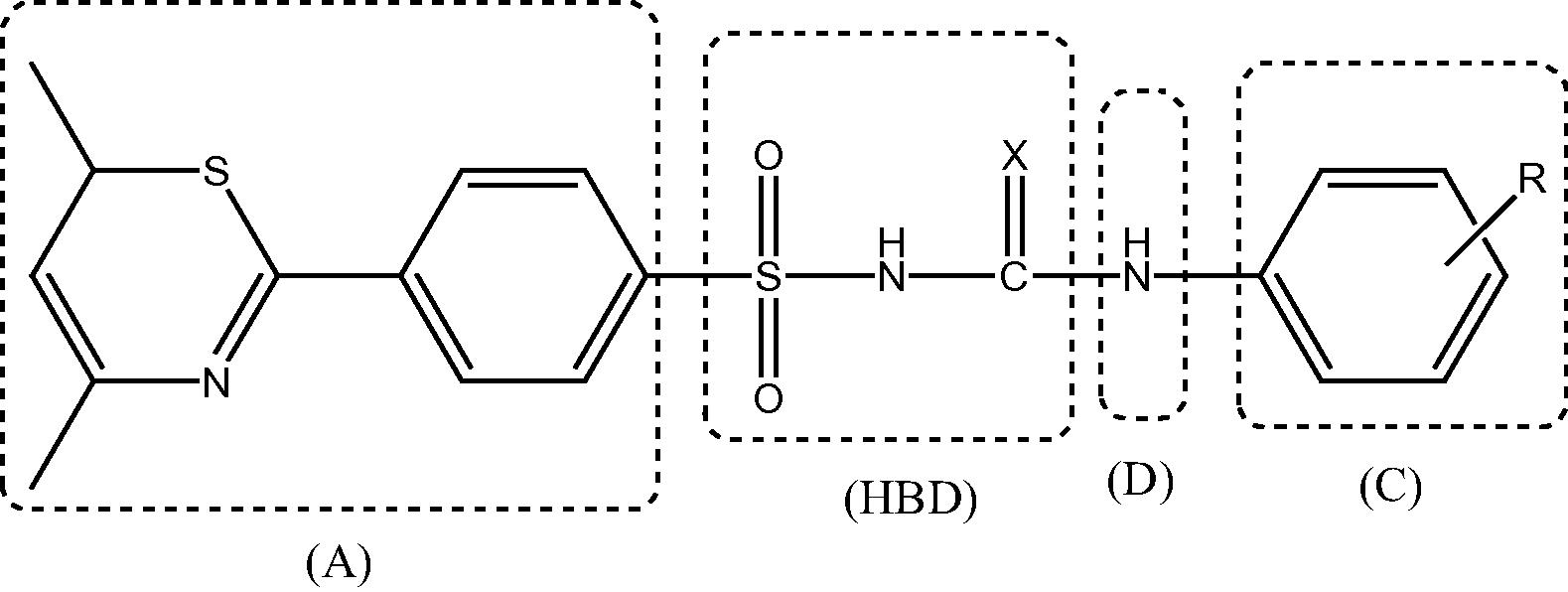
Sulfonylurea share structural features which closely resemble established molecule already in use as anticonvulsant viz. phenytoin, carbamazepine, Phenobarbitals, etc. These also contain sulfonamide moiety which show anticonvulsive properties as acetazolamide and topiramate (Masereel et al., 2002).
In silico (docking) study is the best way to develop mechanism base molecules, which could identify more precisely the bonding requirements in structure with targeted protein molecule. Here for anticonvulsant study open voltage gated sodium channel is chosen as the target protein for docking study to find its binding affinity to the test molecule by comparing with standard drug molecule (Iman et al., 2015).
2 Material and methods
The chemicals used for the experimental work were procured from Qualigens, Fine Chemicals Mumbai and CDH (P) Ltd. New Delhi. The ranges of melting point of synthesized compounds were measured by open capillary method and are uncorrected. The progress and completion of reactions were determined by thin layer chromatography using Silica gel G (E. Merck) using acetonitrile and methanol (60:40) as mobile phase. Detection of elements in synthesized compounds was carried out on Carlo Erba EA 1108 elemental analyser. KBr pellets of test compounds were used for taking IR spectra on FTIR spectrophotometer (JASCO), 1H NMR spectra were taken in DMSO (TMS as internal standard) on a Bruker Advance (400 MHz) NMR spectrophotometer using and mass spectra was taken on SHIMADZU-2010 AT.
2.1 In silico studies
The docking study is performed to elucidate mode of binding of compound with the target site. The open pore Na+ channel (retrieve from protein data bank, http://RCSB.org) was used as a target protein model based on homology of K+ channel structure for docking studies of synthesized compounds (Iman et al., 2015). Chem Draw ultra 10.0 has been used for drawing the structure of synthesized compound and converted to .pdb format from .mol file for docking. To investigate the mode of binding and related interactions of the synthesized compounds with target, Argus Lab 4.0 docking software was used. The protein–ligand interaction study was carried out by pymol 1.3 software. The drug likeness and drug score were calculated by Osiris online software.
2.2 Synthesis
2.2.1 General procedure for the synthesis of 4,6-dimethyl-2-phenyl-6H-1,3-thiazine (1)
Compound 1 was synthesized according to the reported procedures (Thakur et al., 2015).
2.2.2 General procedure for synthesis of 4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)benzene-1-sulfonyl chloride (2)
The chlorosulfonation of synthesized compound (1) was carried out using chlorosulfonic acid solution. Two separate equimolar (0.06 mol) solutions of compound 1 and chlorosulfonic acid were prepared in 1,4-dioxane (60 ml) with stirring at room temperature. Both the solutions were mixed together to produce a homogenous exothermic mixture by stirring at room temperature for half an hour. The resulting solution was added to the crushed ice for precipitation of compound 2. The precipitated solid was filtered and recrystallized from ethanol. Yield 74% and m.p. 240–244 °C.
IR Vmax (KBr, in cm−1): 1509–52 (Ar-C⚌C—), 1193 (C—N), 2360 (C—S—C), 1380 (C—CH3), 1359 and 1177 (C—SO2), 3058 (Ar—C—H); 1H NMR (300 MHz, DMSO-d6, δ): 5.60 (1H, d, C–H of thiazine), 3.46 (1H, m, CH-S of thiazine), 1.53 (3H, d, CH3 of thiazine) 1.72 (3H, s, CH3 of thiazine), 7.94–8.14 (4H, m, Ar-SO2); FAB-MS (m/z): 301 [M+].
2.2.3 General procedure for synthesis of 1-(4-substitutedphenyl)-3-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)urea/thiourea (3a–l) (Samanta et al., 2004)
Substituted phenylurea/phenylthiourea (Robert and Smith, 1948) (0.15 mol) was dissolved in alkaline sodium hydroxide solution (2 N) in a flask. To the resultant solution, chlorosulfonyl derivative (compound 2) was added slowly with constant stirring and maintained at 70 °C in water bath. In order to keep the reaction mixture alkaline further sodium hydroxide solution was added and allowed to cool to the room temperature. The solution was filtered and acidified with hydrochloric acid for complete precipitation.
2.2.3.1 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-phenylurea (3a)
Yield 79% (yellowish brown fine crystals), m.p. 222–224 °C, IR Vmax (KBr, in cm−1): 1286 (C—N), 2360 (C—S—C), 1326 and 1134 (SO2N), 1672 (—C⚌O), 3064 (Ar—C—H), 3336 (—NH—); 1H NMR (300 MHz, DMSO-d6, δ): 5.68 (1H, d, C–H of thiazine), 3.31 (1H, m, CH-S of thiazine), 1.55 (3H, d, CH3 of thiazine) 1.73 (3H, s, CH3 of thiazine), 7.84–8.02 (4H, m, Ar-SO2), 7.01–7.77 (5H, m, Ar), 6.02 (2H, s, —NH— of ureido); FAB-MS (m/z): 477 [M+]; Anal. Calcd for C19H19N3O3S2: C, 56.84; H, 4.77; N, 10.47; O, 11.95; S, 15.97 Found: C, 56.78; H, 4.95; N, 10.58; O, 11.86, S, 15.91.
2.2.3.2 1-(4-chlorophenyl)-3-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)urea (3b)
Yield 78% (faint brown powder), m.p. 248–250 °C, IR Vmax (KBr, in cm−1): 1258 (C—N), 2360 (C—S—C), 1334 and 1128 (SO2N), 1697 (—C⚌O), 3029 (Ar—C—H), 3234 (—NH—), 3411 (—OH); 1H NMR (300 MHz, DMSO-d6, δ): 5.69 (1H, d, C–H of thiazine), 5.02 (1H, m, Ar-OH) 3.32 (1H, m, CH-S of thiazine), 1.50 (3H, d, CH3 of thiazine) 1.69 (3H, s, CH3 of thiazine), 7.91–8.24 (4H, m, Ar-SO2), 7.26–7.61 (4H, m, Ar), 6.12 (2H, s, —NH— of ureido); FAB-MS (m/z): 435 [M+]; Anal. Calcd for C19H18ClN3O3S2: C, 52.35; H, 4.16; Cl, 8.13; N, 9.64; O, 11.01; S, 14.71 Found: C, 52.45; H, 4.16; Cl, 8.08; N, 9.70; O, 11.08; S, 14.67.
2.2.3.3 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-(4-hydroxyphenyl)urea (3c)
Yield 72% (creamy brown fine crystals), m.p. 244–247 °C, IR Vmax (KBr, in cm−1): 1274 (C—N), 2365 (C—S—C), 1348 and 1131 (SO2N), 1680 (—C⚌O), 3033 (Ar—C—H), 3449 (—NH—); 1H NMR (300 MHz, DMSO-d6, δ): 5.72 (1H, d, C–H of thiazine), 3.29 (1H, m, CH-S of thiazine), 1.55 (3H, d, CH3 of thiazine) 1.72 (3H, s, CH3 of thiazine), 7.88–7.93 (4H, m, Ar-SO2), 6.77–7.51 (4H, m, Ar), 6.08 (2H, s, —NH— of ureido); FAB-MS (m/z): 417 [M+]; Anal. Calcd for C19H19N3O4S2: C, 54.66; H, 4.59; N, 10.06; O, 15.33; S, 15.36 Found: C, 54.74; H, 4.47; N, 10.16; O, 15.28; S, 15.35.
2.2.3.4 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-(4-methoxyphenyl)urea (3e)
Yield 71% (reddish brown long crystals), m.p. 240–243 °C, IR Vmax (KBr, in cm−1): 1287 (C—N), 2389 (C—S—C), 1355 and 1148 (SO2N), 1719 (—C⚌O), 3031 (Ar—C—H), 3387 (—NH—), 1064 and 1240 (Ar-OCH3); 1H NMR (300 MHz, DMSO-d6, δ): 5.68 (1H, d, C–H of thiazine), 3.31 (1H, m, CH-S of thiazine), 1.57 (3H, d, CH3 of thiazine) 1.69 (3H, s, CH3 of thiazine), 7.90–8.12 (4H, m, Ar-SO2), 6.82–7.57 (4H, m, Ar), 6.10 (2H, s, —NH— of ureido), 3.54 (3H, s, OCH3); FAB-MS (m/z): 431 [M+]; Anal. Calcd for C20H21N3O4S2: C, 55.67; H, 4.91; N, 9.74; O, 14.83; S, 14.86 Found: C, 55.70; H, 4.88; N, 9.70; O, 14.89; S, 14.80.
2.2.3.5 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-p-tolylurea (3f)
Yield 75% (off brown fine powder), m.p. 230–233 °C, IR Vmax (KBr, in cm−1): 1264 (C—N), 2377 (C—S—C), 1336 and 1145 (SO2N), 1697 (—C⚌O), 3029 (Ar—C—H), 3378 (—NH—); 1H NMR (300 MHz, DMSO-d6, δ): 5.74 (1H, d, C–H of thiazine), 3.41 (1H, m, CH-S of thiazine), 1.54 (3H, d, CH3 of thiazine) 1.79 (3H, s, CH3 of thiazine), 7.92–8.08 (4H, m, Ar-SO2), 7.10–7.51 (4H, m, Ar), 6.07 (2H, s, —NH— of ureido), 2.82 (3H, s, CH3); FAB-MS (m/z): 431 [M+]; Anal. Calcd for C20H21N3O3S2: C, 57.81; H, 5.09; N, 10.11; O, 11.55; S, 15.43 Found: C, 57.75; H, 5.12; N, 10.15; O, 11.51; S, 15.38.
2.2.3.6 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-phenylthiourea (3g)
Yield 76% (dark yellow needled crystals), m.p. 235–238 °C, IR Vmax (KBr, in cm−1): 1254 (C—N), 2374 (C—S—C), 1365 and 1157 (SO2N), 3025 (Ar—C—H), 3321 (—NH—); 1H NMR (300 MHz, DMSO-d6, δ): 5.74 (1H, d, C–H of thiazine), 3.29 (1H, m, CH-S of thiazine), 1.51 (3H, d, CH3 of thiazine) 1.70 (3H, s, CH3 of thiazine), 7.90–8.07 (4H, m, Ar-SO2), 6.33–7.10 (5H, m, Ar), 2.07 (1H, s, SO2 —NH— of ureido), 4.20 (1H, s, CS-NH-Ar of ureido); FAB-MS (m/z): 417 [M+]; Anal. Calcd for C19H19N3O2S3: C, 54.65; H, 4.59; N, 10.06; O, 7.66; S, 23.04; Found: C, 54.54; H, 4.55; N, 10.10; O, 7.60; S, 23.06.
2.2.3.7 1-(4-chlorophenyl)-3-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)thiourea (3h)
Yield 81% (light yellow fine powder), m.p. 254–255 °C, IR Vmax (KBr, in cm−1): 1268 (C—N), 2329 (C—S—C), 1374 and 1135 (SO2N), 3041 (Ar—C—H), 3407 (—NH—); 1H NMR (300 MHz, DMSO-d6, δ): 5.63 (1H, d, C–H of thiazine), 3.34 (1H, m, CH-S of thiazine), 1.54 (3H, d, CH3 of thiazine) 1.74 (3H, s, CH3 of thiazine), 7.92–8.14 (4H, m, Ar-SO2), 6.41–7.09 (4H, m, Ar), 2.12 (1H, s, SO2 —NH— of ureido), 4.31 (1H, s, CS-NH-Ar of ureido); FAB-MS (m/z): 451 [M+]; Anal. Calcd for C19H18ClN3O2S3: C, 50.49; H, 4.01; Cl, 7.84; N, 9.30; O, 7.08; S, 21.28 Found: C, 50.41; H, 4.11; Cl, 7.78; N, 9.21; O, 7.12; S, 21.24.
2.2.3.8 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-(4-hydroxyphenyl)thiourea (3i)
Yield 74% (light brown crystals), m.p. 238–241 °C, IR Vmax (KBr, in cm−1): 1252 (C—N), 2354 (C—S—C), 1351 and 1143 (SO2N), 3032 (Ar—C—H), 3301 (—NH—), 3402 (—OH); 1H NMR (300 MHz, DMSO-d6, δ): 5.78 (1H, d, C–H of thiazine), 3.29(1H, m, CH-S of thiazine), 1.49 (3H, d, CH3 of thiazine) 1.71 (3H, s, CH3 of thiazine), 7.82–8.07 (4H, m, Ar-SO2), 6.11–6.89 (4H, m, Ar), 2.08 (1H, s, SO2 —NH— of ureido), 4.10 (1H, s, CS-NH-Ar of ureido), 5.13 (1H, s, Ar-OH); FAB-MS (m/z): 433 [M+]; Anal. Calcd for C19H19N3O3S3: C, 52.63; H, 4.42; N, 9.69; O, 11.07; S, 22.19 Found: C, 52.74; H, 4.35; N, 9.75; O, 11.12; S, 22.21.
2.2.3.9 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-(4-methoxyphenyl)thiourea (3k)
Yield 80% (dark yellow fine crystals), m.p. 238–239 °C, IR Vmax (KBr, in cm−1): 1271 (C—N), 2354 (C—S—C), 1348 and 1141 (SO2N), 3020 (Ar—C—H), 3324 (—NH—), 1048 and 1207 (Ar-OCH3); 1H NMR (300 MHz, DMSO-d6, δ): 5.72 (1H, d, C–H of thiazine), 3.33 (1H, m, CH-S of thiazine), 1.56 (3H, d, CH3 of thiazine) 1.74 (3H, s, CH3 of thiazine), 7.82–8.08 (4H, m, Ar-SO2), 6.29–6.61 (4H, m, Ar), 2.20 (1H, s, SO2 —NH— of ureido), 4.07 (1H, s, CS-NH-Ar of ureido), 3.92 (3H, s, Ar-OCH3); FAB-MS (m/z): 447 [M+]; Anal. Calcd for C20H21N3O3S3: C, 53.67; H, 4.73; N, 9.39; O, 10.72; S, 21.49 Found: C, 53.62; H, 4.79; N, 9.42; O, 10.68; S, 21.54.
2.2.3.10 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-p-tolylthiourea (3l)
Yield 77% (yellow fine crystals), m.p. 247–248 °C, IR Vmax (KBr, in cm−1): 1259 (C—N), 2344 (C—S—C), 1353 and 1139 (SO2N), 3034 (Ar—C—H), 3359 (—NH—); 1H NMR (300 MHz, DMSO-d6, δ): 5.68 (1H, d, C–H of thiazine), 3.37 (1H, m, CH-S of thiazine), 1.51 (3H, d, CH3 of thiazine) 1.71 (3H, s, CH3 of thiazine), 7.92–8.18 (4H, m, Ar-SO2), 6.30–6.85 (4H, m, Ar), 2.05 (1H, s, SO2 —NH— of ureido), 4.11 (1H, s, CS-NH-Ar of ureido), 2.43 (3H, s, Ar-CH3); FAB-MS (m/z): 431 [M+]; Anal. Calcd for C20H21N3O2S3: C, 55.66; H, 4.90; N, 9.74; O, 7.41; S, 22.29 Found: C, 55.67; H, 4.84; N, 9.68; O, 7.37; S, 22.35.
2.3 Anticonvulsant activity
Anticonvulsant screening was carried out on wistar rats (100–150 g) of either sex. The animals were grouped in six (n = 6) and housed under standard conditions and allowed to free access to food and water. First group of animals was used as negative control after administration of blank 1% tween20/water mixture. For positive control the second group of animals was administered phenytoin sodium (25 mg/kg, i.p.). The suspension of test compounds was prepared in 1% tween20/water mixture. Different doses containing 30, 100 and 300 mg/kg were administered intraperitoneally (i.p.) at the initial screening. All the experimental procedures followed as per the guidelines of CPCSEA (Reg no. 1171/c/08/CPCSEA). The review and approval was obtained from ethical committee of institution (IAEC).
2.3.1 Maximal electroshock seizure (MES) model
For the evaluation of anticonvulsant activity, observations carried after 0.5 h and 4 h of dose administration. MES was induced by 150 mA alternate current of 60 Hz for 0.2 s by means of ear clip electrodes. Prior placing the electrodes, a drop of 0.9% saline solution was poured into each ear. As a result of maximal seizure, initially tonic flexion and then tonic extension of hind limb occured. Abolition of hind limb extension shows the protection from seizure attack (Lotarski et al., 2014).
2.3.2 Pentylenetetrazole (PTZ) induced seizure test
The 0.9% sodium chloride solution was used to dissolve Pentylenetetrazole and administered subcutaneously at a dose of 85 mg/kg body weight. Severity of convulsion was noted in the control group. PTZ induces clonic seizures lasting for 3-5 s of forelimbs and/or hindlimbs. Animals were administered different doses (30, 100 and 300 mg/kg, i.p.) of test compounds and evaluated after 0.5, 4 h followed by PTZ administration. Animals were kept separately to reduce stress and observed for a period of 30 min for abolition of seizure threshold.
2.3.3 Neurotoxicity screening
Minimal motor impairment in rats was measured using standardized rotarod test. The rats were trained to balance on knurled plastic rod (1 inch diameter, 25 cm height), rotating at 6 rpm. The rats retained on the rotating rod for 1 min were selected for the study. Trained animals were treated with test compounds at doses of 100 and 300 mg/kg i.p. The neurotoxicity was defined as inability of rats to stay on rod for at least 1 min in each of four trials.
2.4 Results and discussion
2.4.1 Chemistry
The route of synthesis given in scheme 1 was very handy for preparation of derivatives (3a–l) with high yield. 4,6-dimethyl-2-phenyl-6H-1,3-thiazine (1) was prepared according to the procedure reported in literature (Thakur et al., 2015). Addition of sulfonyl chloride group at the para position of aromatic ring in compound 1 was carried out by preparing two separate solution of chlorosulfonic acid and compound 1 in 1,4-dioxane. The solutions were mixed together at room temperature which produces 4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)benzene-1-sulfonyl chloride (2). In the final step of synthesis, the compound 2 was stirred with different alkaline solution of phenylurea/thiourea in 2 N sodium hydroxide solution at 70 °C to form a homogenous mix. After cooling, the mix was completely neutralized with concentrated HCl to get precipitate of 1-(4-substitutedphenyl)-3-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)urea/thiourea (3a–l) (Table 1).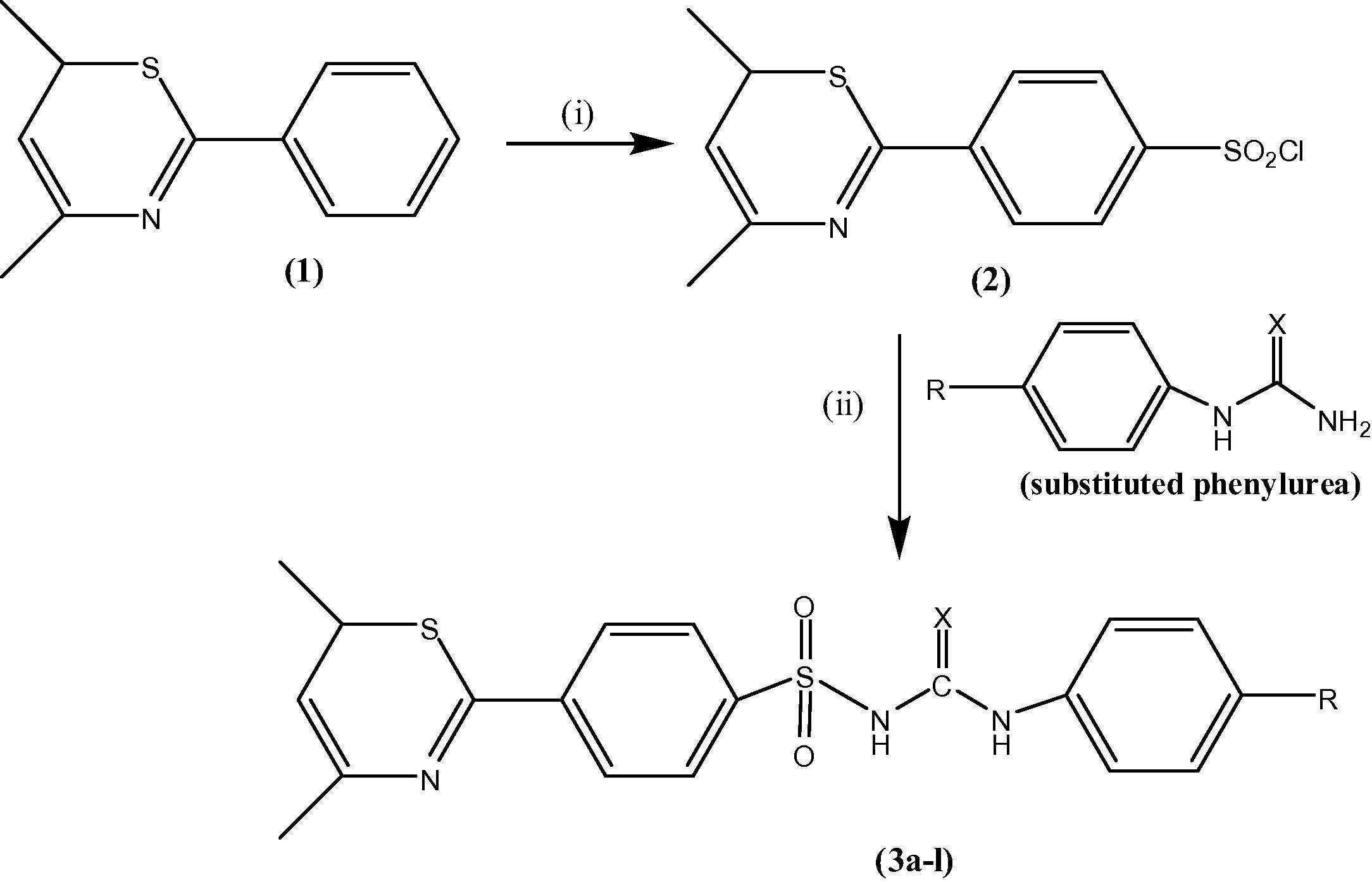
Reagents and reaction conditions: (i) Chlorosulfonic acid, 1,4 dioxane, stirring at r.t., (ii) NaOH solution (2 N), conc. HCl, stirring at 70 °C.
Compounds
X
R
C log Pa
3a
O
H
3.90
3b
O
Cl
4.91
3c
O
OH
3.22
3d
O
NO2
4.20
3e
O
OCH3
4.00
3f
O
CH3
4.40
3g
S
H
3.38
3h
S
Cl
4.14
3i
S
OH
3.03
3j
S
NO2
3.22
3k
S
OCH3
3.48
3l
S
CH3
3.88
The structure of different synthesized compounds was elucidated by IR, 1H NMR, mass spectra and elemental analysis. The absence of singlet peak around δ 6.0 ppm and δ 4.0 ppm in the NMR spectra of compound 2 shows the absence of —NH (of urea) moiety. Also absence of IR peak around 3400 cm−1 confirmed the nonexistence of urea moiety. There were two IR peaks around 1177 and 1359 cm−1, which assure the addition of –SO2Cl group in compound 2. Further the structure of target molecule (3a–l) was elucidated by the presence of characteristic singlet peak around δ 6.0 ppm for —NH of urea and δ 4.0 ppm, δ 2.0 ppm for thiourea along with the IR peak around 3400 cm−1. There was also a doublet peak at δ 5.2 ppm which characterizes the C–H of thiazine ring. Additional spectrum at δ 1.5 ppm and δ 1.7 ppm helps to elucidate the presence of methyl groups in thiazine ring. The multiplates around δ 7.8–8.1 ppm and δ 6.4–7.2 ppm conclude the presence of two aromatic rings in the structure. IR bands around 1680 for C⚌O and 3032 for Ar—C—H support the NMR data for the confirmation of the structure. Molecular weight and purity of compounds were supported by mass spectral data.
Two different models viz. subcutaneous pentylenetetrazole (scPTZ) and maximal electroshock seizure (MES) were used for the estimation of anticonvulsant activity. Three dose levels 30, 100 and 300 mg/kg by intraperitoneal (i.p.) route were used for study. All the synthesized derivatives except 3d and 3k are active in both the anticonvulsant models (MES and scPTZ). Out of all these, the compounds 3a, 3b and 3c are found as the most active derivatives devoid of neurotoxicity at any dose. Compounds 3g and 3h are also active but show neurotoxicity at higher dose. In scPTZ model, the compounds were positively active within half an hour but not so after 4 h, which suggest that the drug is fast acting but having short duration in this type of seizure. (Table 2).
Compounds
Intraperitoneal injection in ratb
MES Screen
scPTZ Screen
Neurotoxicity Study
0.5 h
4.0 h
0.5 h
4.0 h
0.5 h
4.0 h
3a
100
300
100
300
–a
–
3b
100
100
100
100
–
–
3c
100
100
100
300
–
–
3d
NDc
–
ND
–
300
ND
3e
300
–
300
–
–
–
3f
100
300
300
–
–
–
3g
100
100
300
–
300
–
3h
100
100
100
300
300
–
3i
100
300
300
ND
–
–
3j
ND
–
ND
–
100
ND
3k
300
–
300
–
–
–
3l
–
300
ND
–
100
–
Phenytoin
30
100
30
100
–
300
Out of 3a, 3b and 3c, the compound 3b is the most active derivative containing electronegative chloride group showing anticonvulsant effect at 100 mg/kg body weight. Another electronegative derivative 3b containing hydroxyl group manifests the promising anticonvulsant effect at the same dose level except at 300 mg/kg body weight after 4 hrs in scPTZ model. The unsubstituted aromatic ring as in compound 3a is also a remarkable candidate for protection against MES induced convulsion after compounds 3b and 3c. The electron donating group (—CH3) (3g and 3l) is less active as compare to the presence of electronegative atom in the same position. On the other hand an attempt for bioisosteric replacement of oxygen (sulfonylurea) with sulfur atom (sulfonylthiourea) is not convincing to get an efficient molecule, as it has less anticonvulsant activity with minor neurotoxicity (3g–l).
These results suggest that the synthesized derivatives are efficiently bound and inhibit voltage gated sodium channel to protect the spread of neuronal action potential by impairing the prolonged depolarization of neuronal membrane which helps to reduce the excitatory motor activity.
2.4.2 Docking study using open voltage gated sodium channel model
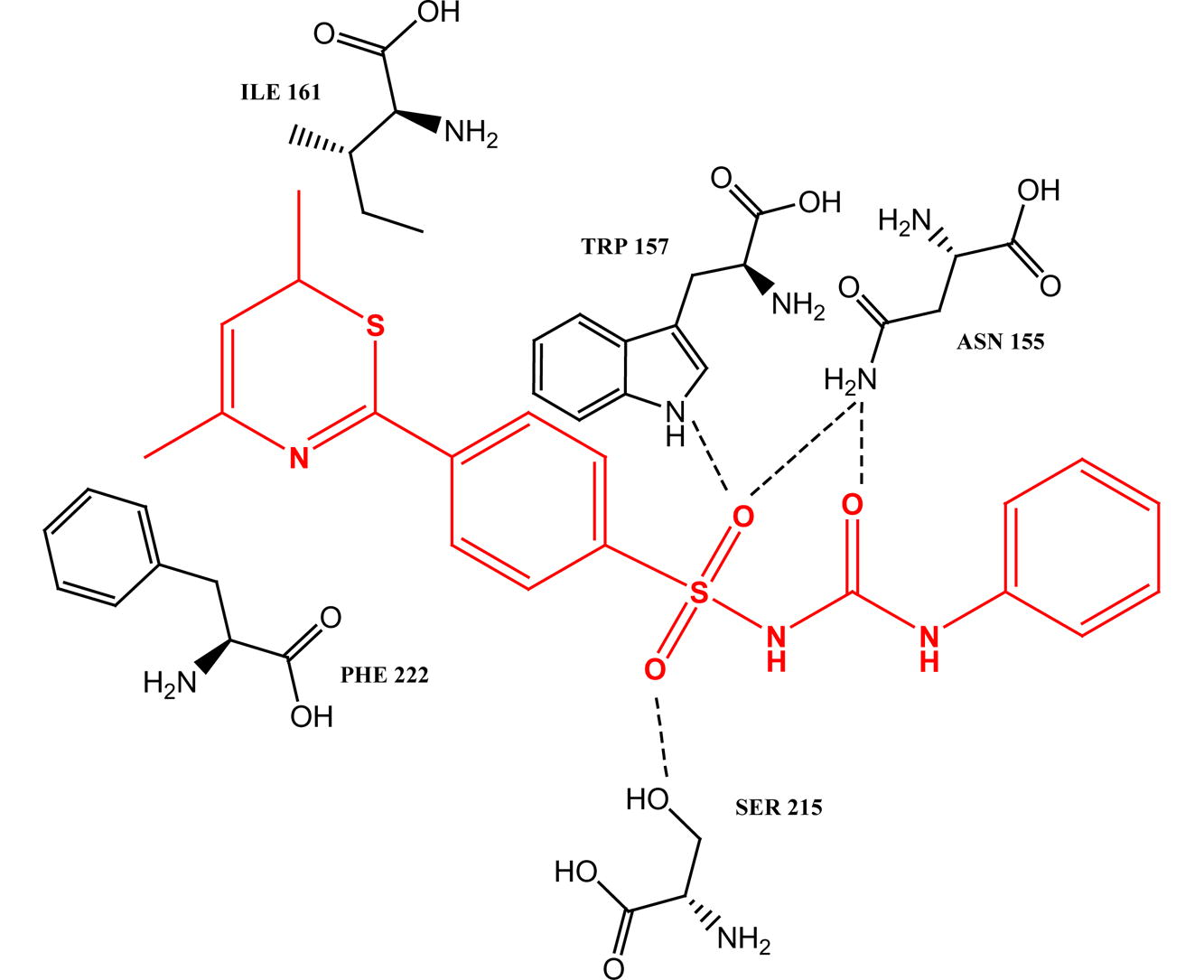
For comparing the effective binding of test compounds which have been docked to the active site, where the following amino acid residues were found in the protein i.e. 147 ARG, 151 ASN, 155 ASN, 161 ILE, 15 MET, 165 MET, 154 PRO, 162 PRO, 157 TRP, 218 LEU, 216 LYS, 222 PHE, 215 SER. All the designed derivatives were docked (Argus Lab) in the active site of open voltage gated sodium channel (Fig. 1).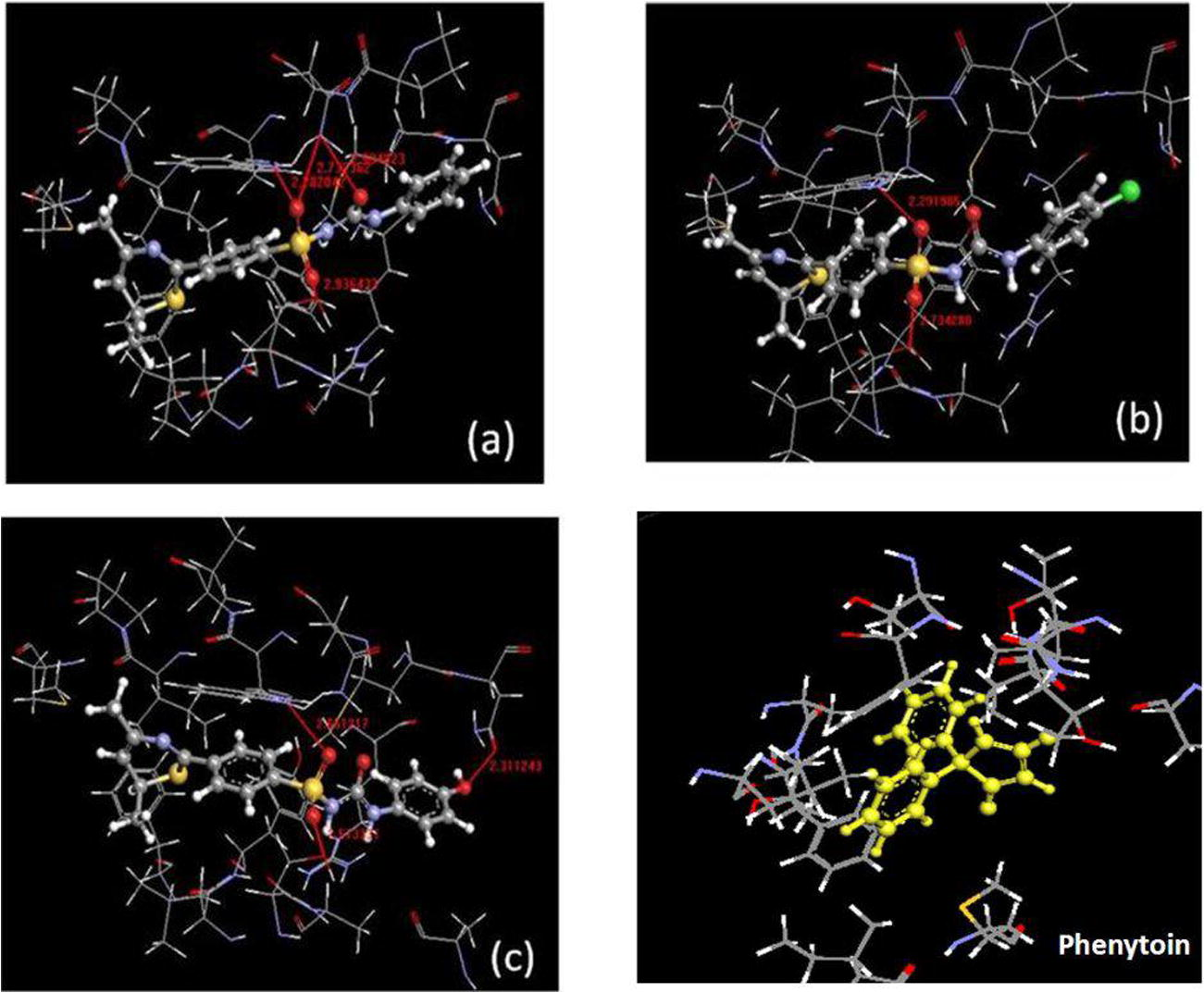
(a) Best possible pore of compound 3a (boll and stick structure) showing hydrogen bond (red color line) and bond distance, (b) best possible pore of compound 3b, (c) best possible pore of compound 3c, (d) Possible interaction of amino acids with phenytoin.
The phenytoin is the standard ligand bearing the less docking score (−12.54) than the most active test compound. The oxygen atom of sulfonyl (—SO2) and keto moiety of urea were taking part in the formation of hydrogen bond with channel protein. The amino acid residues 147 ARG, 154 PRO, 151 ASN and 150 MET formed a hydrophobic domain around the distal aromatic ring of test compounds. Out of all these the sulfonylurea derivatives, compounds 3a, 3b and 3c were showing the highest docking score without any toxic profile.
Compound 3a has highest docking score (−13.18) among the three, but the drug likeness (4.61) and drug score (0.64) were lower than the other two (3b and 3c). Compound 3a formed four hydrogen bonds, one with —NH group of indole ring in 157 TRP (2.28 Å), two with oxygen (–SO2 and >C⚌O) of —NH2 group of 155 ASN (2.73 Å and 2.99 Å) and the last one with 215 SER (2.93 Å) amino acid residues on the active site. Compound 3b having docking score −13.05 was also a suitable candidate for synthesis as it is having the highest drug likeness (6.92) with drug score (0.48) while the most favorable compound is 3c with good docking score (−13.10) as well as drug likeness (6.37) and highest drug score (0.66). Compound 3c is a hydroxyl derivative which helps to form an additional hydrogen bond with the oxygen atom of 151 ASN even in the hydrophobic domain. Out of the all sulfonylthiourea derivatives (3g–l), compound 3h was shown a considerable docking score (−13.08) with low drug likeness (4.80) and drug score (0.30), but due to toxic profile (minor mutagenic) cannot be suited for synthesis. The nitro derivatives 3d and 3k showed negative drug likeness and very low drug score with poor docking score made these unsuitable for synthesis (Table 3).
Compounds
Docking (binding)score
Drug likeness
Drug-score
3a
−13.18
4.61
0.64
3b
−13.05
6.92
0.48
3c
−13.10
6.37
0.66
3d
−11.79
−5.38
0.30
3e
−12.73
4.84
0.60
3f
−12.22
4.49
0.58
3g
−12.37
2.71
0.47
3h
−13.08
4.80
0.30
3i
−12.30
4.20
0.50
3j
−13.19
−6.97
0.22
3k
−12.50
2.97
0.36
3l
−13.14
2.77
0.42
2.5 Conclusion
In the present work, different analogs of sulfonylurea and sulfonylthiourea were prepared. To evaluate the approach for structural development, docking study was performed. It contributes very much to carry out further experimental work. Different spectroscopic analysis helped to confirm the structure of synthesized derivatives. The chloro and hydroxyl substituted 1-(4-substitutedphenyl)-3-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)urea analogs 3c and 3b respectively were found to be very potent anticonvulsant compound, whereas the compound 1-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)-3-phenylurea (3a) also showed a good analog for the treatment of convulsion. All these biological results were well substantiating the outcome of in silico studies. Few analogs of 1-(4-substitutedphenyl)-3-(4-(4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)thiourea series i.e. compounds 3h and 3i also given a considerable result regarding the anticonvulsant activity but can’t be acceptable for further study due to their minor neurotoxicity. The nitro derivatives (3d and 3k) have not been synthesized as they were showing negative drug likeness scores. This research reveals a new eligible candidate for development of anticonvulsant agents. However, more precise mechanism is still remain to prove which inspire to explore this pharmacophore for further study.
Conflict of interest
Author’s had no conflict of interest.
Acknowledgement
We want to convey our thanks to IISER, Bhopal for 1H NMR, and Mass spectral analysis, School of Pharmaceutical Sciencs, SOA, University, Bhubaneswar for providing the facilities to carry out FTIR spectral and pharmacological studies and also grateful to the management of FPSc, SSTC, Bhilai for facilitating our research work.
References
- Synthesis and evaluation of novel N-(4′-arylpyrimidin-2′-yl) sulfonylurea derivatives as potential antifungal agents. Chem. Res. Chin. Univ.. 2015;31:218-223.
- [Google Scholar]
- Henry, T.R., 2012. Seizures and Epilepsy: pathophysiology and principles of Diagnosis. Hospital Physician Epilepsy Board Review Manual, (Part 1) p. 1.
- Molecular docking analysis and molecular dynamics simulation study of ameltolide analogous as a sodium channel blocker. Turk. J. Chem.. 2015;39:306-316.
- [Google Scholar]
- Synthesis and evaluation of sulfonylurea derivatives as novel antimalarials. Eur. J. Med. Chem.. 2007;42:735-742.
- [Google Scholar]
- Molecular model of anticonvulsant drug binding to the voltage-gated sodium channel inner pore. Mol. Pharmacol.. 2010;78:631-638.
- [Google Scholar]
- Anticonvulsant activity of pregabalin in the maximal electroshock-induced seizure assay in a2d1(R217A) and a2d2R279A) mouse mutants. Epileps. Res.. 2014;108:833-842.
- [Google Scholar]
- Carbonic anhydrase inhibitors: anticonvulsant sulfonamides incorporating valproyl and other lipophilic moieties. J. Med. Chem.. 2002;45:312-320.
- [Google Scholar]
- Synthesis and anticonvulsant evaluation of some novel 2,5-disubstituted 1,3,4-thiadiazoles: pharmacophore model studies. Acta. Pol. Pharm.. 2010;65:503-510.
- [Google Scholar]
- 5-N-Substituted-2-(substituted benzenesulphonyl) glutamines as antitumor agents. Part II: synthesis, biological activity and QSAR study. Bioorg. Med. Chem.. 2004;12:1413-1423.
- [Google Scholar]
- Synthesis and oral hypoglycemic effect of novel thiazine containing trisubstituted benzenesulfonylurea derivatives. Saudi Pharm. J.. 2015;23:475-482.
- [Google Scholar]
- Synthesis and biological evaluation of sulfonylurea and thiourea derivatives substituted with benzenesulfonamide groups as potential hypoglycemic agents. Bioorg. Med. Chem. Lett.. 2009;19:1740-1744.
- [Google Scholar]
- Design, synthesis and cytotoxic activity of novel sulfonylurea derivatives of podophyllotoxin. Bioorg. Med. Chem.. 2010;22:204-210.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jksus.2016.12.006.
Appendix A
Supplementary data







