Translate this page into:
Biological effects of Naja haje crude venom on the hepatic and renal tissues of mice
*Corresponding authors. Address: Department of Biochemistry and Molecular Biology, Asturias Institute of Biotechnology, Asturias Institute of Biotechnology, University of Oviedo, Oviedo, Spain. Tel.: +34 611302236 (A.E. Abdel Moneim). Address: Department of Zoology and Entomology, Faculty of Science, Helwan University, Cairo, Egypt. Tel.: +20 225590000x1825; fax: +20 225552468 (M.S.M. Diab) abdelahmed.uo@uniovi.es (Ahmed E. Abdel Moneim), marwa.db@gmail.com (Marwa S.M. Diab)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 6 February 2014
Peer review under responsibility of King Saud University.

Abstract
Snake venoms are known to cause different metabolic disorders, altering cellular and enzymatic activities in animals and releasing pharmacological substances. In this study, the lethality as well as biochemical and histopathological effect of Egyptian cobra (Naja haje; N. haje) crude venom at a sublethal dose have been investigated on liver and kidney of male mice. Venom injected intramuscularly in mice with 1/2 LD50 (approximately 0.0115 μg/g body weight of mice) and the animals were sacrificed 6 days post injection. Results indicated that the injection of crude venom of the N. haje induced a significant disturbance in liver and kidney functions. In addition, results revealed that N. haje venom has a potent oxidative activity by increasing the level of reactive oxygen species with concomitant significant increase in hydrogen peroxide, lipid peroxidation, carbonyl protein and nitric oxide levels in hepatic and renal tissues. This activity was extended to decrease non-enzymatic and enzymatic antioxidant defense components such as glutathione, superoxide dismutase and catalase. Additionally, the biochemical alternations induced in hepatic and renal tissues were associated with significant alternations in the histological architecture of liver and kidney of injected mice. From this study, we can conclude that such injury could be considered among the factors that lead to death caused by N. haje venom.
Keywords
Naja haje venom
Liver
Kidney
Oxidative stress
Mice
1 Introduction
The study of structure and function of snake venom toxins is carrying out with medical application purposes. Snake venom is a complex mixture of many substances, such as toxins, enzymes, growth factors, activators and inhibitors with a wide spectrum of biological activities (Rahmy and Hemmaid, 2000; Al-Sadoon et al., 2013; Cherifi and Laraba-Djebari, 2013). They are also known to cause different metabolic disorders by altering the cellular inclusions and enzymatic activities of different organs (Aiesenberg, 1981). Cobra snakes are widely distributed in Africa and the Middle East. In Egypt, family Elapidae includes several toxic species of snakes, among which is the Egyptian cobra, Naja haje. In Egypt, N. haje distributes in Nile Valley and Delta, Faiyum and Western Mediterranean Coastal Desert. Envenomation causes local pain and swelling, and may be associated with blistering at the bite site. Neurotoxic and systemic symptoms develop within few hours, and deaths have occurred within 6–16 h after large snakes’ bites, despite the use of antivenom and mechanical ventilation. Cobra envenoming is known to induce multiple-organ failure, leading to death in case of severe envenoming (Cher et al., 2005).
Liver is considered as one of the targets for cobra venom factor (Fu et al., 1997). Hepatic injury due to cobra envenoming was reported by Rahmy and Hemmaid (2000) and Adzu et al. (2005). Doley and Mukherjee (2003) reported that the non-toxic phospholipase A2 isolated from the venom is responsible for inducing liver tissue damaging activity. In addition to hepatotoxicity, nephropathy induced by cobra was mentioned. In a previous study, a sub-lethal dose of the Egyptian cobra venom was found to induce a deleterious action on the histological and histochemical patterns of animal renal tissues. The histological alterations recorded in the tubular epithelial lining cells of most of the cortical renal tubules in all the envenomed mice with cobra venoms were previously reported by Rahmy and Hemmaid (2001) and Sitprija (2006). On reviewing the literatures, it was found that few studies were performed on the biochemical effects of the venom of N. haje snake, which is one of the most diverse and widespread genus of cobras.
Thus, the present study aimed to detect the hepatotoxic and nephrotoxic effects of a single dose of the venom of such snake after 6 days of envenomation from the biochemical point of view in relation to induce histopathological damages post cobra envenomation.
2 Materials and methods
2.1 N. haje venom preparation and lethality
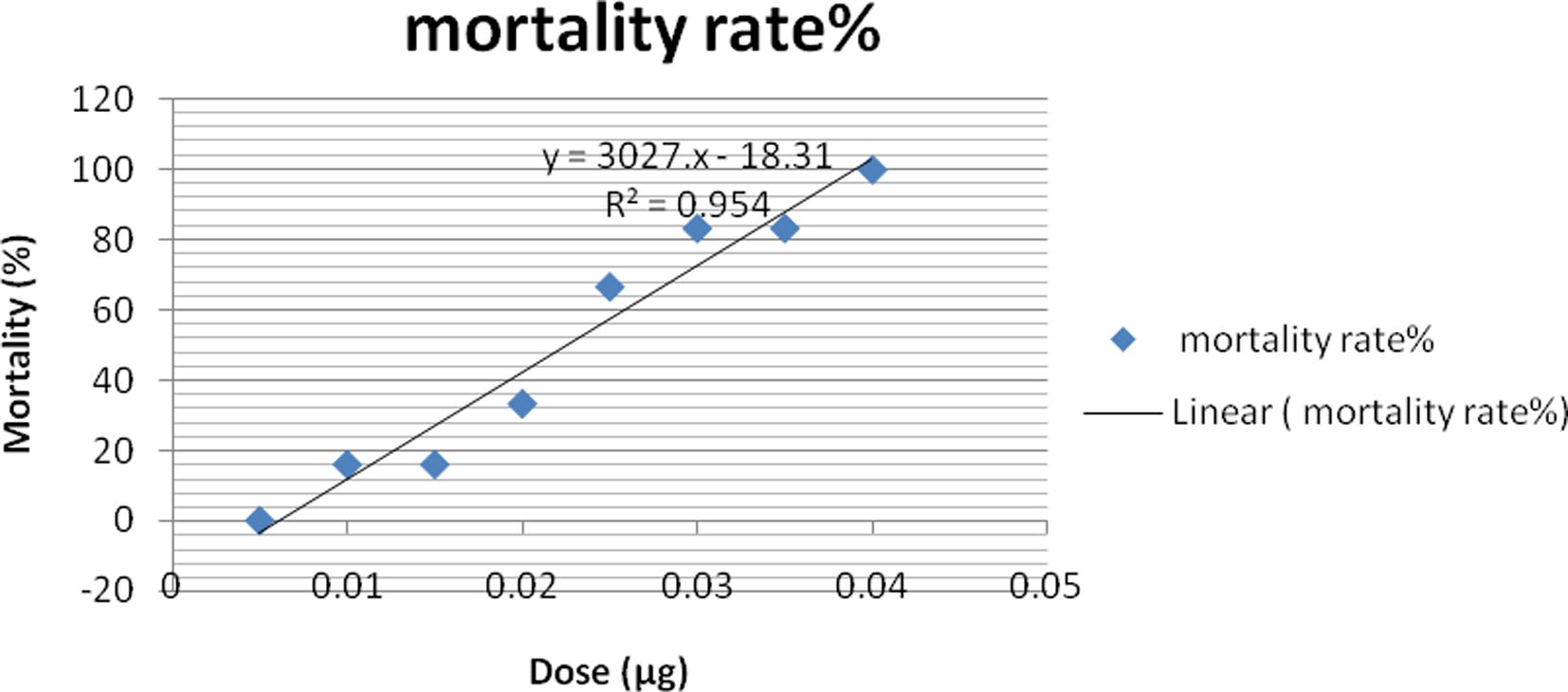
Crude venom was obtained from N. haje snakes collected from the West Delta of Egypt. In VACSERA laboratory; snake venom was milked, lyophilized, stored in a desiccator at 4 °C in the dark and reconstituted in saline solution prior to use. LD50 of crude venom was determined as described by Meier and Theakston (1986). Briefly, LD50 of the venom was determined by intravenous injection of different concentrations of venom in 0.2 ml of physiological saline into the tail vein of albino mice. Four mice were used per group for each dose. Venom dose of each group was increased by 1 μg venom protein until 50% mortality was observed within 24 h. The LD50 of venom was found to be 0.023 μg/g of mice body weight.
2.2 Experimental design
Thirty adult male Swiss Albino mice weighing 22 ± 5 g were used. Animals were selected from the Animal house facility of research institute of ophthalmology, Egypt. Animals were housed in standard condition and fed with normal diet and water ad libitum. The experiments were approved by the state authorities and it followed the Egyptian rules on animal protection.
Animals were divided into two groups of 15 mice per group. The first group was injected intramuscularly (i.m.) with 200 μL physiological saline (0.9% NaCl) solution and the second group was injected intramuscularly with 1/2 LD50 (0.0115 μg/g b.wt.) of N. haje crude venom in 200 μL saline solution at a single dose.
Animals of both groups were sacrificed at the 6th day post-crude venom injection. Blood samples were collected, centrifuged at 500g for 15 min at 4 °C to separate serum and stored at −40 °C until liver and kidney function analysis. Livers and kidneys were weighed and divided into two parts. The first part is for histological studies and the second part for biochemical determinations.
2.3 Histological studies
Pieces of liver and kidney were quickly removed, fixed in 10% neutral buffered formalin, dehydrated, embedded in wax and 4- to 5-μm paraffin sections were cut and stained with hematoxylin and eosin. Histological damage in liver and kidney was scored as follows: 0: absent; +: mild; ++; moderate; +++: severe (Jamshidzadeh et al., 2008). The liver activity index was estimated using a modified quantitative Ishak Scoring System (Ishak et al., 1995); scores of 1–3 were assigned to cases of minimal liver damage, scores of 4–8 to mild, scores of 9–12 to moderate and scores of 13–18 to severe cases.
2.4 Liver and kidney functions test
Colorimetric determination of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was estimated by measuring the amount of pyruvate and oxaloacetate respectively produced by forming 2,4-dinitrophenylhydrazine, according to the method of Reitman and Frankel (1957). The color of which was measured at 546 nm. l-γ–glutamyl transferase (γGT), uric acid (UA), blood urea nitrogen (BUN) and serum creatinine (Cr) were assayed in serum using kits provided from Biodiagnostic Co. (Giza, Egypt).
2.5 Biochemical studies
Pieces of liver and kidney were homogenized immediately to give 50% (w/v) homogenate in ice-cold medium containing 50 mM Tris–HCl, pH 7.4. The homogenates were cold centrifuged at 500g for 10 min. The supernatant (10%) was used for various biochemical determinations.
2.5.1 Quantification of reactive oxygen species (ROS)
A modified version of a previously described assay for the intracellular conversion of nitro blue tetrazolium (NBT) to formazan by superoxide anion was used to measure the generation of ROS (Vrablic et al., 1995) with slight modification. Briefly, 200 μL NBT (1.00 mg/ml) was added to the hepatic and renal homogenates, followed by additional incubation for 1 h at 37 °C. The solutions were then treated with 100 μL KOH (2 M). The absorbance at 570 nm was determined spectrophotometrically and expressed as μmol NBT reduced/g tissue.
2.5.2 Hydrogen peroxide assay
Hydrogen peroxide content (H2O2) in liver and kidney homogenates was determined according to the method of Fossati et al. (1980). Briefly, in the presence of peroxidase, H2O2 of hepatic and renal homogenates was reacted with 3,5-dichloro-2-hydroxybenzensulfonic acid and 4-aminophenazone to form a chromophore determined spectrophotometrically at 510 nm.
2.5.3 Determination of lipid peroxidation and nitrite/nitrate
Homogenates of liver and kidney were used to determine lipid peroxidation (LPO) by the reaction of thiobarbituric acid (TBA) according to the method of Ohkawa et al. (1979). Similarly, these homogenates were used to determine nitrite/nitrate (nitric oxide; NO) using the Griess reagent (Green et al., 1982).
2.5.4 Estimation of glutathione and anti-oxidant enzymes
The hepatic and renal glutathione (GSH) levels were determined according to Ellman (1959). In addition, activities of hepatic and renal antioxidant enzymes were determined. According to the method of Aebi (1984), catalase (CAT) reacts with a known quantity of H2O2. Although, superoxide dismutase (SOD) activity was assayed by the method of Nishikimi et al. (1972).
2.5.5 Determination of protein carbonyl content
Protein carbonyl content was determined as described by Levine et al. (1990), with slight modifications. Hepatic and renal homogenates were incubated with 0.5 ml of 10 mM dinitrophenylhydrazine in 2 M HCl (or 2 M HCl alone for the blanks), for 1 h at room temperature. The protein hydrazone derivatives were precipitated with 0.5 ml of 20% trichloroacetic acid and the precipitates were washed three times with 1 ml ethanol:ethylacetate (1:1). During each washing, the homogenized pellet was vortexed and left in the washing solution for 10 min at room temperature before centrifugation. The final pellet was resuspended in 6 M guanidine HCl, and incubated for 15 min at 37 °C. The carbonyl content was determined spectrophotometrically at 360 nm on the basis of molar absorbance coefficient of 22,000 M−1 cm−1.
2.6 Statistical analysis
The obtained data were presented as means ± standard error of the mean (SEM). Statistical analysis was performed using an unpaired Student’s t-test using a statistical package program (SPSS version 17.0).
3 Results
3.1 Lethality test result
Results indicated that the approximate lethal dose fifty (LD50) of the venom is equal to 0.023 μg/g mice b.wt. (Supplementary data; Fig. S1).
3.2 Histological findings
The alternation in the liver architecture of envenomated animals, compared with that in control animals is shown in Fig. 1 and Table 1. Fig. 1A displays a histological section of liver from a control mouse. The center-lobe vein has normal morphological characteristics while fig. 1B–D show a histological section of liver after 6 days injection of 0.0115 μg venom/g mice b.wt. Cellular alteration was verified on liver. There are leukocyte aggregations near blood vessels and evident vascular congestion. Histological investigation of hepatic tissue sections reveals that N. haje envenomation caused a severe inflammatory response of the liver, as indicated by inflammatory cellular infiltration as well as cytoplasmic vacuolation and degeneration of hepatocytes. In addition, the hepatic sinusoids were dilated and apparently contained more Kupffer cells. SV: represented N. haje crude venom. Score: 1–3, minimal; 4–8, mild; 9–12, moderate; 13–18, severe. 0: absent; +: mild; ++; moderate; +++: severe (Jamshidzadeh et al., 2008).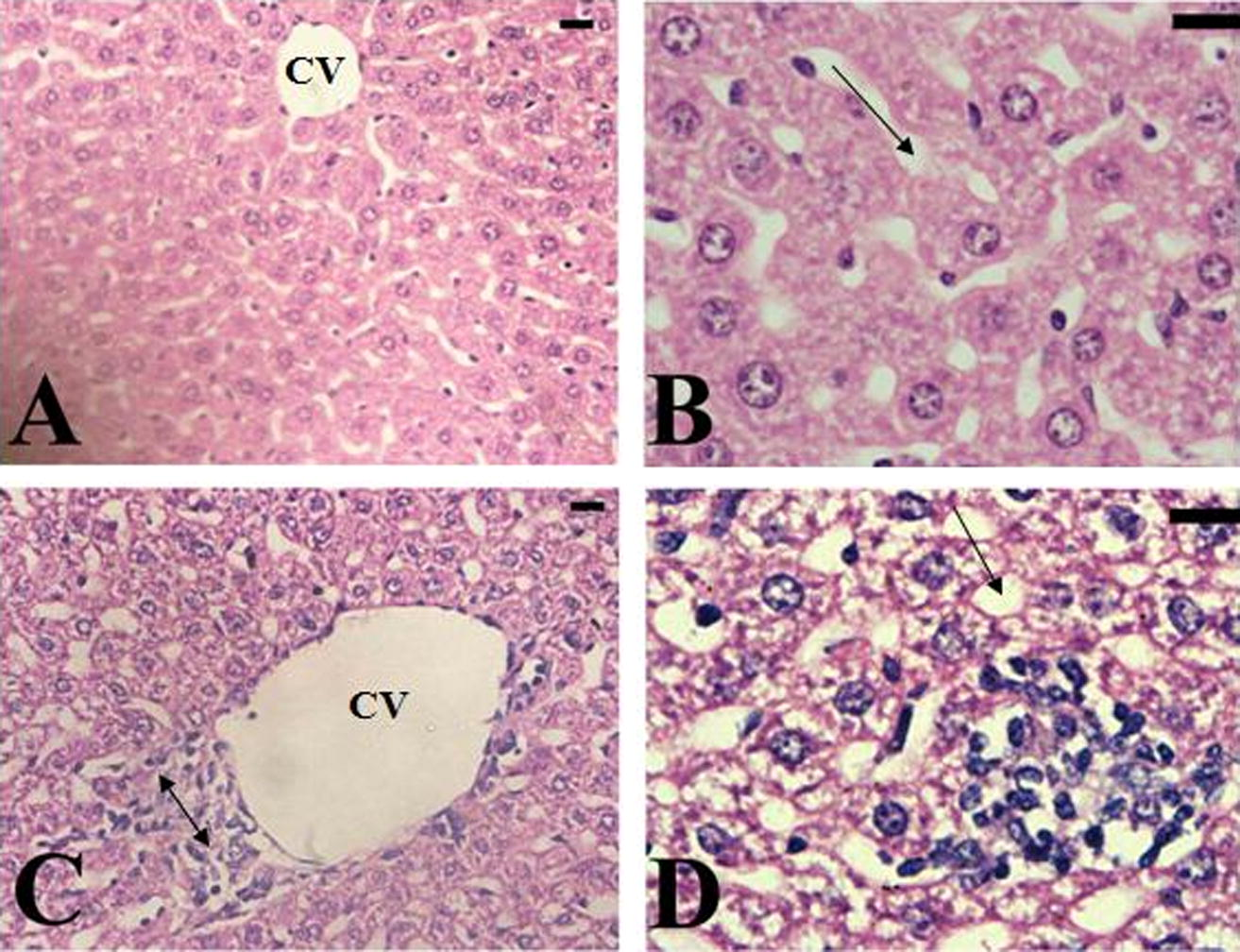
(A) Liver of mice in the control group, showing that the center-lobe vein (CV) has normal morphological characteristics. (B–D) Hepatic tissue of mice in the envenomated group; showing a severe inflammatory response in liver indicated by inflammatory cellular infiltration, cytoplasmic vacuolation (
 ), degeneration of hepatocytes (
), degeneration of hepatocytes ( ), the hepatic sinusoids were dilated and apparently contained more Kupffer cells. Sections were stained with hematoxylin and eosin, ×400.
), the hepatic sinusoids were dilated and apparently contained more Kupffer cells. Sections were stained with hematoxylin and eosin, ×400.
Group
Microscopic observation
Histological activity indexa
Necrosis or apoptosis
Hemorrhage
Disorganized sinusoids
Infiltration of lymphocytes
Hyperplasia of Kupffer cells
Hepatocytic swelling
Control
2
+
0
0
0
0
0
SV
13
++
+++
++
+++
++
++
Histological structure of the renal tissue of control mice shows normal architecture represented in Fig. 2A. Histological changes in the renal tissue treated with N. haje crude venom are represented in Table 2 and Fig. 2B–D. Treated kidney of mice appeared with moderate inflammatory cellular infiltration. Some kidney tubules were vacuolated. Most of the glomeruli appeared shrunken. SV: represented N. haje crude venom, 0: absent; +: mild; ++: moderate; +++: severe.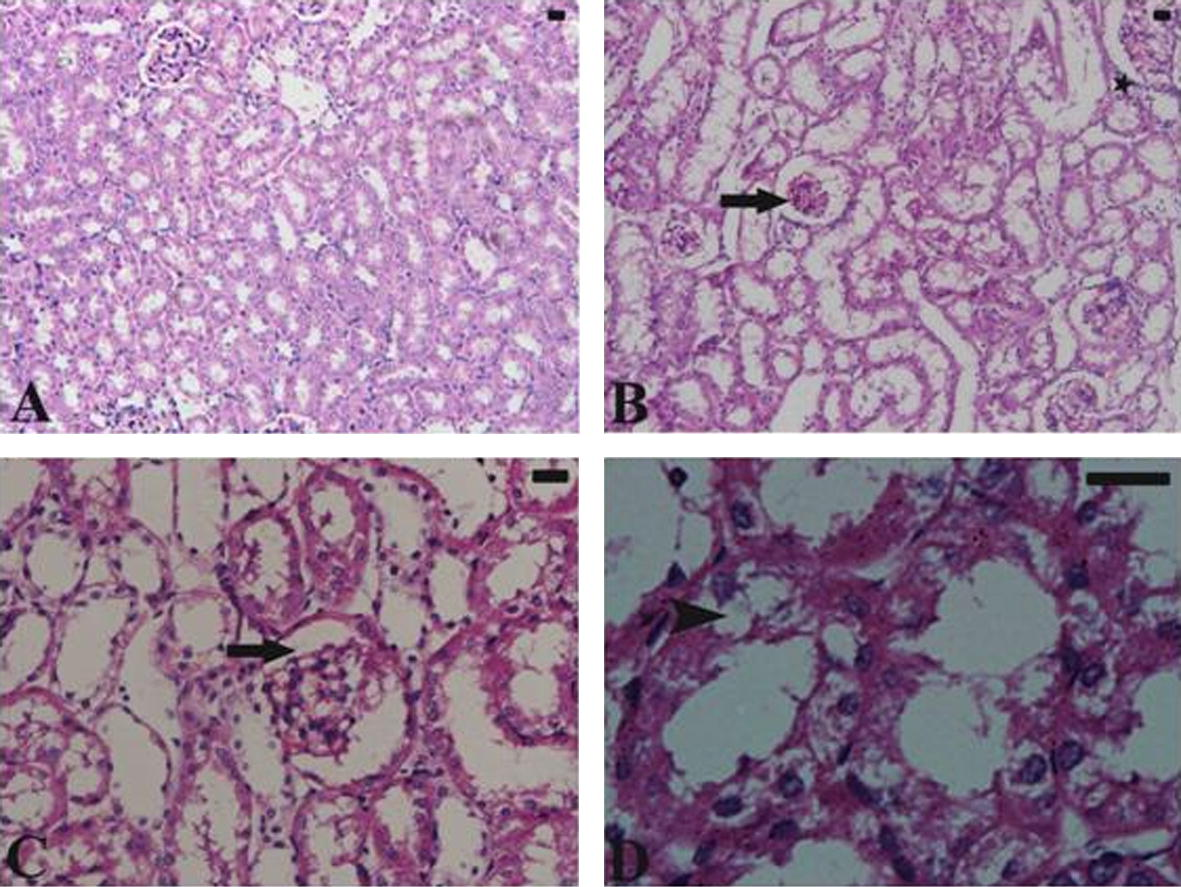
(A) Untreated control kidney shows normal architecture. (B–D) Histological changes in the renal tissue of mice treated with N. haje crude venom. Treated kidney of mice appeared with moderate inflammatory cellular infiltration (star). Some kidney tubules are vacuolated (arrow head). Most of the glomeruli appeared shrunken (arrow). Sections were stained with hematoxylin and eosin. Bar = 25 μm.
Group
Microscopic observation
Tubular vacuolization
Hydropic degeneration change
Glomerular damage
Inflammatory cellular infiltration
Control
+
0
0
0
SV
+++
+++
++
++
3.3 Liver and kidney functions
A significant increase in serum AST activity was observed after 6 days of 0.0115 μg/g b.wt. venom injection, compared with the control group. On the other hand, a significant decrease in serum ALT levels and γGT was observed in envenomated mice (Table 3).The injection of N. haje crude venom to mice caused a significant decrease in the serum uric acid level (−28.76%) and significant increase in serum creatinine and urea, these increases were 34.06% and 11.25% at p < 0.05, respectively, after 6 days of injection when compared to the control group (Table 3). Values are means ± SEM (n = 6). SV: represented N. haje crude venom.
Parameter
Control
SV
ALT (U/L)
9.24 ± 0.110
7.06 ± 0.521*
AST (U/L)
4.63 ± 0.101
28.25 ± 0.259*
γ-GT (U/L)
26.47 ± 2.635
4.92 ± 2.158*
Uric acid (mg/dl)
8.52 ± 0.262
6.07 ± 0.509*
Urea (mg/dl)
46.86 ± 1.826
52.13 ± 0.581*
Creatinine (mg/%)
0.23 ± 0.019
0.31 ± 0.019*
3.4 Biochemical results
Results clearly demonstrated that a single injection of N. haje venom at a dose of 0.0115 μg/g b.wt. caused a significant elevation in hepatic and renal ROS levels (231.77% and 354.79% at p < 0.05, respectively) with a concomitant increase in LPO and NO in hepatic and renal homogenates compared to the control group (Table 4). GSH levels show a non significant change in hepatic homogenate (−7.00% at p > 0.05) and a significant decrease in renal tissue (−17.00% at p < 0.05) compared to the control group. While H2O2 level in hepatic tissue (−22.22% at p < 0.05) recorded a significant decrease and a non-significant change in H2O2 level in renal tissue compared to the control group (Table 4). In addition, data in Table 4 show a significant decrease in SOD and CAT activities in hepatic tissues (−21.26% and −20.97% at p < 0.05, respectively), while show a non-significant change in SOD and CAT activities in renal homogenate when compared to the control group. N. haje envenomation also had shown a significant increase in carbonyl protein content of hepatic tissues (69.11% at p < 0.05) and a non-significant change in carbonyl protein content of renal tissue compared to the control group (Fig. 3). Values are means ± SEM (n = 6). SV: represented N. haje crude venom.
Tissues and groups
Hepatic tissue
Renal tissue
Parameter
Control
SV
Control
SV
ROS (μmol NBT/g tissue)
720.62 ± 57.77
2390.77 ± 13.19*
282.46 ± 11.88
1284.62 ± 117.24*
H2O2 (mM/g tissue)
1.08 ± 0.394
0.84 ± 0.759*
1.09 ± 0.749
1.12 ± 0.609
LPO (nmol/g tissue)
17.03 ± 0.780
18.20 ± 0.356
6.70 ± 0.339
12.562 ± 0.452*
NO (μmol/g tissue)
97.39 ± 6.468
114.16 ± 0.548*
43.64 ± 1.827
49.354 ± 2.541*
GSH (mg/g tissue)
20.34 ± 2.953
18.93 ± 2.973
20.59 ± 1.894
17.08 ± 2.810*
SOD (U/g tissue)
1000 ± 14.999
787.380 ± 11.710*
1978 ± 29.029
1879 ± 18.537
CAT (U/g tissue)
0.534 ± 0.013
0.422 ± 0.053*
1.842 ± 0.030
1.779 ± 0.149
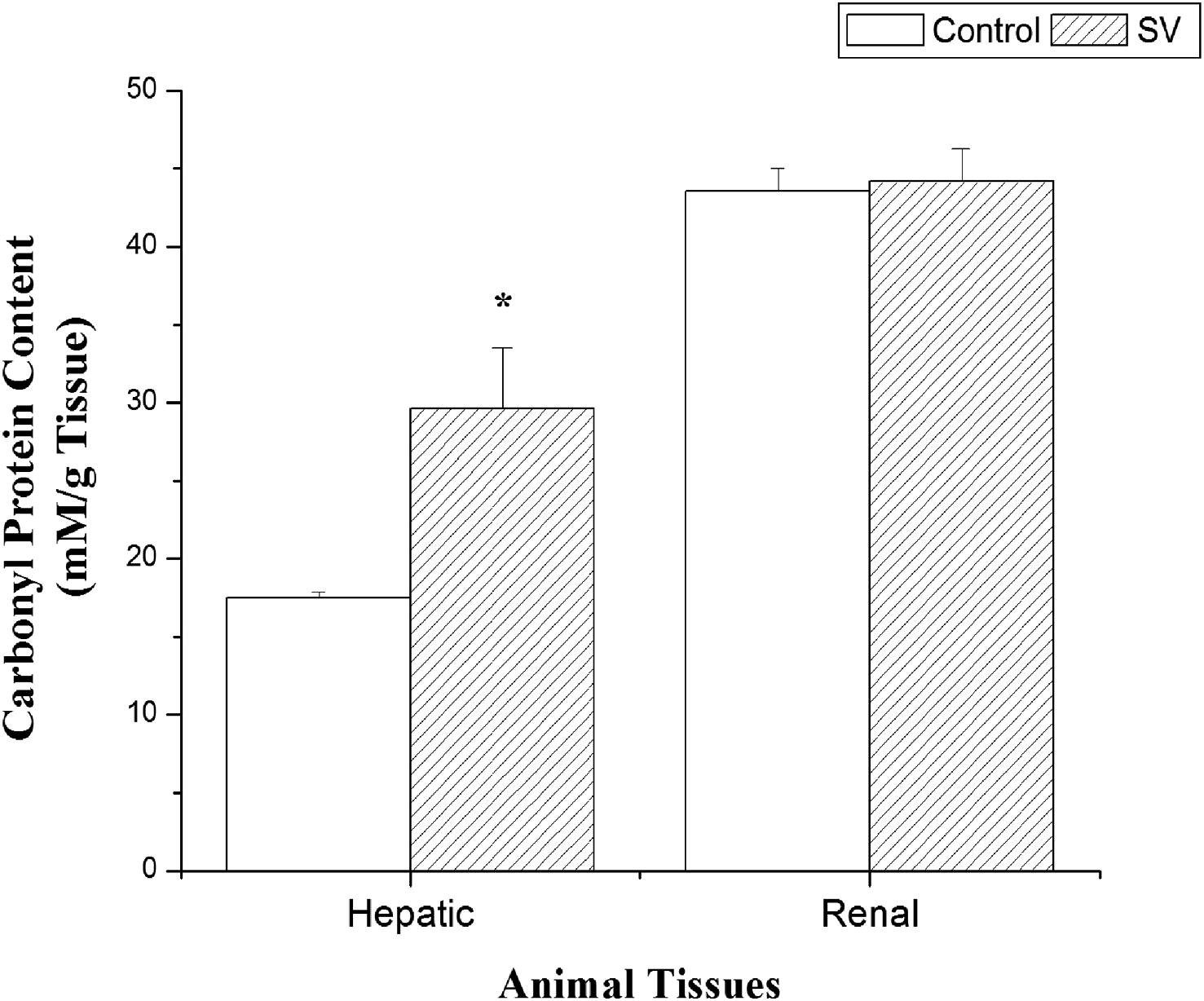
Effect of N. haje envenomation on renal and hepatic carbonyl protein content of mice. Values are means ± S.E (n = 6). SV: represented N. haje crude venom. ∗Significant change at p < 0.05 with respect to the control group.
4 Discussion
Venomous snakes possess extensively developed oral glands that secrete various enzymes and toxic proteins used for the immobilization and digestion of their prey and represent the most sophisticated integrated weapons systems in the natural world (Fry, 2005). Snake venoms are complex mixtures; mainly it has proteins, which have enzymatic activities. Protein and peptides make 90–95% of the dry weight of venom. In addition to that snake venoms contain inorganic cations such as sodium, calcium, potassium, magnesium and small amounts of zinc, nickel, cobalt, iron, manganese. Zinc is necessary for anti-cholinesterase activity; calcium is required for the activation of enzyme like phospholipase. Some snake venoms also contain carbohydrate, lipid, biogenic amines and free amino acids (de Vieira Santos et al., 2008). Snake venoms contain at least 25 enzymes, but no single venom contains all of them. Enzymes are protein in nature, but few depend on certain nonprotein prosthetic groups or cofactors.
Three authors have reported that sublethal doses of N. haje venom induced potent histopathological, histochemical, and pathophysiological alterations in the heart, liver, kidney, and brain of rats (El-Fiky, 1999; Imam and Rahmy, 2001).These pathological changes included severe degrees of cellular damage concomitant with marked signs of both myolytic and coagulative necrosis (Rahmy and Hemmaid, 2000).
AST and ALT enzymes are markers for cellular damage and ALT enzyme is essentially present in hepatocytes. These enzymes are of importance in assessing and monitoring liver inflammation and necrosis which result in the release of both enzymes in circulation due to increased permeability of the cell membrane or breakdown of the cells (Abdel Moneim et al., 2013). Animals inoculated with copra venom showed an increase in AST and ALT activities as demonstrated by James et al. (2013) for Naja nigricollis in rats. Moreover, liver damage was found after bitten by the family of Elapidae (Omran et al., 2004).
Liver injury is among the common and most serious symptoms of cobra snake envenoming (Adzu et al., 2005). In the present study, i.m. injection of 1/2 LD50 of N. haje venom induced histopathological alterations. The changes included variable degrees of cellular swelling, cytoplasmic changes, cellular necrosis and cellular damage accompanied with loss of the common architecture of the hepatic parenchyma at different stages of envenoming. The recorded cytoplasmic granulation of the hepatocytes was also observed by Rahmy and Hemmaid (2000). Cytoplasmic granulation and vacuolization suggested hydropic degeneration, which were accompanied by swelling of the hepatocytes after injection of 1/2 LD50. Cellular swelling might be due to the action of venom phospholipase, which causes disturbance of the cell membrane permeability with consequent influx of Na+ and water (Segelke et al., 1998). Chethankumar and Srinivas (2008) concluded that the exposure of cellular membranes to Naja naja venom phospholipase significantly decreased the Na+/K+ ATPase activities, thereby altering the ionic gradients, disorganizing the membrane lipid bilayer and eventually leads to cell death. According to Mukerjee and Maity (1998), the progression of hepatic cellular swelling together with the effect of the venom phospholipase on the membranous phospholipids during envenoming might be among the factors responsible for the rupture of the hepatic cell membranes and the occurrence of the recorded cellular damage of envenoming as seen in the present study.
Hypertrophied von-Kupffer cells were reported by Rahmy and Hemmaid (2000) in hepatic parenchyma of mice envenomed with N. haje snake venom. The activation of these cells indicated the phagocytic action of the hepatic tissues as a response to cell injury and as a defense against envenoming (Rahmy et al., 2005).
The tubular lesions observed in the renal tissue from envenomated animals were accompanied by invasion of inflammatory cells to the intertubular tissues in a trial to minimize the injury. Some of these external stressors apparently caused the tubular lesions. Renal tubules appeared with cytoplasmic vacuolation which is mainly a consequence of considerable disturbances in lipid inclusions and fat metabolism occurring under pathological cases (Ebaid et al., 2007).
In the present work, the increment in serum urea and creatinine levels and the reduction in uric acid of envenomated mice compared to the control group indicated the impairment of renal function. Renal damages with different types of lesions (glomerular, tubular, interstitial or vascular) and the increase in the urea serum level were also recorded by Schneemann et al. (2004). However, the precise mechanisms whereby the venoms cause alterations of these parameters are not fully known. It might be assumed that the changes in levels of these serum constituents could be due to disturbances in renal function as well as hemorrhages in some internal organs. In fact, increased vascular permeability and hemorrhages in vital organs due to the toxic action of various snake venoms were described by Meier and Stocker (1991) and Marsh et al. (1997).
The results of this study also demonstrated that envenomation by N. haje venom caused a significant increase in free radicals and other reactive oxygen species levels with a concomitant increase in LPO in hepatic and renal homogenates. ROS is known to play an important role in tissue damage in a variety of pathological processes (Kumar et al., 2006). Over-production of ROS leads to oxidative damage to macromolecules, resulting in lipid peroxidation and breakage of DNA (Chaudhuri et al., 2008). The lethal effects of snake venom were largely attributed to its active ingredient of phospholipase A2 (PLA2). Phospholipid hydrolysis by PLA2 enzyme releases arachidonic acid whose metabolism results in the formation of potentially toxic ROS and lipid peroxides (Adibhatla et al., 2003; Abdel-Rahman et al., 2013).
To understand this type of envenomation and improve the therapeutic strategy, besides clinical and epidemiological studies, it is necessary to understand the role of inflammatory mediators such as NO, cytokines and the complement system. NO also participates in the pathogenesis of snake envenomation by two different mechanisms; it may lead to tissue damage due to its ability to generate peroxynitrite and hydroxyl radicals after interaction with superoxide ions and it may provoke hypotension by its vasodilator action (Radi et al., 1991; Hogg et al., 1992). The precise function of NO in snake envenomation has yet to be investigated, but it is probably important in systemic effects caused by snake envenomation. Moreover, the mechanism by which snake venoms induce the production of cytokines and NO is unknown (Petricevich et al., 2000).
SOD as antioxidant enzyme takes part in maintaining GSH homeostasis in tissues (Abdel-Moneim et al., 2010). Also, the decrease in CAT and SOD activities was found to correlate with elevated LPO and NO levels and depressed GSH level in envenomated mice, showing the oxidative activity of snake venom.
5 Conclusion
On the basis of the results obtained from our study, it can be stated that the damage in hepatic and renal tissues caused by snake envenomation is related to its oxidative stress. Also, such injury could be considered among the factors that lead to death caused by N. haje snake. Therefore, further detailed studies on time and dose dependent manner and isolated purified venom toxins are essentially required in order to clarify the mechanism of action of this venom.
Conflict of interest
The authors have no relationships with any person that may be considered conflict of interest.
References
- The redox status in rats treated with flaxseed oil and lead-induced hepatotoxicity. Biol. Trace Elem. Res.. 2010;143(1):457-467.
- [Google Scholar]
- Pomegranate peel attenuates aluminum-induced hepatorenal toxicity. Toxicol. Mech. Methods. 2013;23(8):624-633.
- [Google Scholar]
- Conus vexillum venom induces oxidative stress in Ehrlich’s ascites carcinoma cells: an insight into the mechanism of induction. J. Venom. Anim. Toxins Incl. Trop. Dis.. 2013;19(1):10.
- [Google Scholar]
- Phospholipase A2, hydroxyl radicals, and lipid peroxidation in transient cerebral ischemia. Antioxid. Redox Signal.. 2003;5:647-654.
- [Google Scholar]
- Effect of Annona senegalensis root bark extracts on Naja nigricollis nigricollis venom in rats. J. Ethnopharmacol.. 2005;96(3):507-513.
- [Google Scholar]
- Aiesenberg, A.C., 1981. The Glycolysis and Respiration of Tumors. Acad. Press 21, pp. 314–317.
- Al-Sadoon, M.K., Abdel Moneim, A.E., Diab, M.M., Bauomy, A.A., 2013. Hepatic and renal tissue damages induced by Cerastes cerastes gasperetti crude venom. Life Sci. J. 10, 191–197.
- Erythrocytic antioxidant defense, lipid peroxides level and blood iron, zinc and copper concentrations in dogs naturally infected with Babesia gibsoni. Res. Vet. Sci.. 2008;85:120-124.
- [Google Scholar]
- Molecular basis of cardiotoxicity upon cobra envenomation. Cell. Mol. Life Sci.. 2005;62(1):105-118.
- [Google Scholar]
- Isolated biomolecules of pharmacological interest in hemostasis from Cerastes cerastes venom. J. Venom. Anim. Toxins Incl. Trop. Dis.. 2013;19:11.
- [Google Scholar]
- Gangliosides as potential inhibitors of Naja naja venom PLA2 (NV-PLA2) induced human erythrocyte membrane damage. Afr. J. Biochem. Res.. 2008;2(1):8-14.
- [Google Scholar]
- Antitumoural effect of an l-amino acid oxidase isolated from Bothrops jararaca snake venom. Basic Clin. Pharmacol. Toxicol.. 2008;102:533-542.
- [Google Scholar]
- Purification and characterization of an anticoagulant phospholipase A(2) from Indian monocle cobra (Naja kaouthia) venom. Toxicon. 2003;41:81-91.
- [Google Scholar]
- Piroxicam induced hepatic and renal histopathological changes in mice. Lib. J. Med.. 2007;2:82-89.
- [Google Scholar]
- Hyperglycemic effect of a neurotoxic fraction (F3) from Naja haje venom: role of hypothalamo-pituitary adrenal axis (HPA) J. Nat. Toxins. 1999;8:203-212.
- [Google Scholar]
- Use of 3,5-dichloro-2 hydroxybenzenesulfonic acid/4-aminophenazone chromogenic systems in direct enzymic assay of uric acid in serum and urine. Clin. Chem.. 1980;26:227-231.
- [Google Scholar]
- From genome to “venom”: molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins. Genome Res.. 2005;15:403-420.
- [Google Scholar]
- Multiple scattering parameterization in thermal infrared radiative transfer. J. Atmos. Sci.. 1997;54:2799-2812.
- [Google Scholar]
- Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem.. 1982;126(1):131-138.
- [Google Scholar]
- Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem. J.. 1992;281:419-424.
- [Google Scholar]
- Reactive astrocytic response and increased proliferatic cell nuclear antigen expression in cerebral cortex of envenomated rats. J. Toxicol. Toxin Rev.. 2001;20:245-259.
- [Google Scholar]
- Histological grading and staging of chronic hepatitis. J. Hepatol.. 1995;22:696-699.
- [Google Scholar]
- In vivo neutralization of Naja nigricollis venom by Uvaria chamae. Am. J. Biochem. Biotechnol.. 2013;9:224-234.
- [Google Scholar]
- Effects of tomato extract on oxidative stress induced toxicity in different organs of rats. Food Chem. Toxicol.. 2008;46:3612-3615.
- [Google Scholar]
- A comparative study on oxidative stress in dogs infected with Ehrlichia canis with or without concurrent infection with Babesia gibsoni. Vet. Res. Commun.. 2006;30:917-920.
- [Google Scholar]
- Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol.. 1990;186:464-478.
- [Google Scholar]
- The Gaboon viper, and Bitis gabonica: hemorrhagic, metabolic, cardiovascular and clinical effects of the venom. Life Sci.. 1997;61(8):763-769.
- [Google Scholar]
- Approximate LD50 determinations of snake venoms using eight to ten experimental animals. Toxicon. 1986;24(4):395-401.
- [Google Scholar]
- The composition of Naja naja venom samples from three districts of West Bengal, India. Comp. Biochem. Physiol.. 1998;119:621-627.
- [Google Scholar]
- The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun.. 1972;46:849-854.
- [Google Scholar]
- Essay for lipid 385 peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Biochemical and morphological analysis of cell death induced by Egyptian cobra (Naja haje) venom on cultured cells. J. Venom. Anim. Toxins Incl. Trop. Dis.. 2004;10(3):219-241.
- [Google Scholar]
- Increments in serum cytokine and nitric oxide levels in mice injected with Bothrops asper and Bothrops jararaca snake venom. Toxicon. 2000;38:1253-1266.
- [Google Scholar]
- Peroxynitriteoxidation of sulphydryls: the cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem.. 1991;266:4244-4250.
- [Google Scholar]
- Immunohistochemical detection of hepatic injury after cobra snake – envenoming. Egypt. J. Nat. Toxins. 2005;2:119-134.
- [Google Scholar]
- Histological and histochemical alterations in the liver following intramuscular injection with a sublethal dose of the Egyptian cobra venom. J. Nat. Toxins. 2000;9:21-32.
- [Google Scholar]
- Prophylactic action of garlic on the histological and histochemical patterns of hepatic and gastric tissues in rats injected with a snake venom. J. Nat. Toxins. 2001;10:137-165.
- [Google Scholar]
- Colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol.. 1957;28:56-63.
- [Google Scholar]
- Life-threatening envenoming by the Saharan horned viper (Cerastes cerastes) causing micro-angiopathic haemolysis, coagulopathy and acute renal failure. Clin. Cases Rev.. 2004;97(11):717-727.
- [Google Scholar]
- Structure of two novel crystal forms of Naja naja phospholipase A2 lacking Ca2+ reveals trimeric packing. J. Mol. Biol.. 1998;279(1):223-232.
- [Google Scholar]
- Clinical toxicology of snakebite in Africa and the Middle East/Arabian peninsula. In: Meier J., White J., eds. Handbook of Clinical Toxicology of Animal Venoms and Poisons. Florida: CRC Press; 1995. p. :433-492.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jksus.2014.01.003.
Appendix A
Supplementary data
Supplementary Figure.







