Translate this page into:
Antibacterial and immunity enhancement properties of anaesthetic doses of thyme (Thymus vulgaris) oil and three other anaesthetics in Sparidentax hasta and Acanthopagrus latus
*Corresponding author. Tel.: +965 22274959; fax: +965 25711293 azadis@hotmail.com (I.S. Azad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Available online 3 January 2014

Abstract
An effective alternative was discovered in the form of thyme oil for use as a fish anaesthetic (patent pending approval). The thyme oil along with a common aquaculture-grade commercial anaesthetic (AQUI-S), clove oil and quinaldine were investigated for their antimicrobial properties and its effect on the immune parameters of two important maricultured fish species, bluefin bream (Sparidentax hasta) and yellowfin bream (Acanthopagrus latus). In vivo studies indicated that both the fish species had highly reduced bacterial load after the treatments and the in vitro antibacterial activity of the of the thyme oil was superior to that of the other treatments. The effects of anaesthetic dose of thyme oil, clove oil, quinaldine and AQUI-S were evaluated and compared. The reduction in the total viable vibrio counts in the anesthetized fish indicated that the vibrio were sensitive to the thyme oil. Also thyme oil produced higher non-specific immune enhancements.
Keywords
Anaesthetics
Essential oils
Thyme oil
Antibacterial activity
Immunity
1 Introduction
Aquaculture operations under intensive production systems require frequent handling of the fish of different sizes, especially during transportation, for stocking the fingerlings into production ponds and during fish breeding operations. Apart from this the fish are handled for regular observations and for the treatment, if any. Though clove oil has been used in many aquaculture operations world over, its use has not been recommended. The US FDA has determined that eugenol is not Generally Recognised as Safe (GRAS) as a fish anaesthetic. Aquaculture, in the present day context, involves handling of cultured fish at different stages in the production network. Reduction of struggle during handling and trauma is the main reason for the use of anaesthetics in aquaculture. The chemicals used as anaesthetics in fish have generally been developed for purposes other than their use as anaesthetics. As a result, the potential side effects of these chemicals on users have not been investigated thoroughly. For example, Quinaldine (2-methylquinoline) is one of the most widely used aesthetics that is being used by marine biologists and by the aquaculture group of researchers at the Mariculture and Fisheries Department of Kuwait Institute for Scientific Research. However, there are some questions about its safety because of reported associations between quinaldine and thyroid abnormalities in humans and mice (Munday and Wilson, 1997). Another important anaesthetic used in fisheries is 3-aminobenzoic acid ethyl ester methanesulfonate (MS-222). There are limitations in using MS-222 in the field as the US Food and Drug Administration (US FDA) requires that fish exposed to MS-222 must have a minimum of 21 day withdrawal period before they can be consumed by humans (Bernstein et al., 1997; Waterstrat, 1999). Though the anaesthetic of plant origin, the clove oil, has been used for this purpose, the US FDA has determined that eugenol is not Generally Recognised as Safe (GRAS).
The choice of anaesthetics for field studies generally depends on several considerations (1) availability, (2) cost-effectiveness, (3) ease of use, (4) nature of the study, (5) allow for the immediate release of the fish into the food chain, (6) allow a swift induction of and recovery from anaesthesia, (7) not excessively disturb the physiological balance of the fish, which would reduce its chances of survival upon release and (8) safety for the user. The use of thyme oil in human medicine has been approved by the FDA.
Thyme (Thymus vulgaris) oil is being used traditionally to treat indigestion, to promote menstruation, and for the control of fever. The essential oil of thyme is made up of 20–55% thymol, a powerful antiseptic for both internal and external use. Thyme oil is employed as local anaesthetic in human medicine, and before modern antibiotics were developed, it was used to medicate bandages. Oil of thyme is the important commercial product obtained by distillation of fresh leaves and flowering tops of T. vulgaris. It is extensively used in processed food. The Greeks used thyme as an antiseptic. Thyme can be used in food systems to prevent the growth of food-borne bacteria and extend the shelf life of processed foods (Baydar et al., 2004).
While working on the anti parasitic effects of some essential oils in fish (Al-Yaqout and Azad, 2010), it was noticed that the thyme oil possessed an excellent additional property of an anaesthetic. Looking at the nature of the anaesthetic and its properties, we attempted to explore the qualities of antibacterial and immune enhancement properties of thyme oil as an anaesthetic and compare with that of three other commonly used anaesthetics. This property has a direct bearing on higher survival of the fish upon release into its natural environment or aquaculture rearing facility. Thus, the investigations were conducted to evaluate the antibacterial and immune enhancement properties of thyme oil as an anaesthetic and thereby suggest measures to reduce subsequent infections after handling.
2 Materials and methods
2.1 Fish and anaesthesia optimisation
About 300 fish each of yellowfin bream (Acanthopagrus latus; 10.9–13.5 g) and bluefin bream (Sparidentax hasta; 9.9–11.7 g) were used in this study. All the fish were stocked in one ton tanks for two weeks for acclimatisation. Temperature, salinity and dissolved oxygen were recorded daily during the experimental period. The anaesthetic doses tested using clove oil, thyme oil, AQUI-S (a commercial product, AQUI-S, New Zealand Ltd.) and quinaldine were 20, 40 and 60 ppm exposed up to 10 min, respectively. These dose levels were selected based on our previous experience. Clove oil, thyme oil and quinaldine were procured from Sigma Aldrich, USA and the AQUI-S was purchased from AQUI-S New Zealand Ltd. Lower Hutt, New Zealand. All the anaesthetics prepared as aqueous suspension in absolute alcohol at 1:4 (anaesthetic: alcohol) ratio for the essential oils were not soluble in water. The control group was immersed in an aqueous suspension of PBS-alcohol mix (1:4). Twenty fish in each dose of an anaesthetic were treated to record sleep time, recovery time and mortalities for arriving at minimal anaesthetic dose. All the anaesthetics were tested at an exposure time of 10 min. A treatment with 20 ppm of different anaesthetics was carried out until the induction of deep sleep in 10 fish in each treatment. Blood from these fish was used in the assay of immunity parameters.
2.2 Antibacterial properties of anaesthesia
2.2.1 In situ bactericidal assay
After the fish anaesthetised by the optimised doses for each anaesthetic, surface swabs were taken from an approximate skin area of 4 cm2 from the fish. The swab was vortexed in sterile PBS for the enumeration of viable bacterial counts. The inactivation percentage was obtained by comparing the bacterial counts on the control group of fish with that of the anaesthetic treated fish. The surface swab suspension was serially diluted and plated onto brain heart infusion (BHI with 15% NaCl) agar and thiosulphate citrate bile salt sucrose (TCBS) agar (Conda, Spain). Bactericidal activity of anaesthetic doses was calculated using the control bacterial counts.
2.2.2 In vitro bacterial killing ability
Four species of standard (ATCC) bacterial isolates (Vibrio alginolyticus – 19108; Vibrio harveyi – 35084; Vibrio parahaemolyticus – 27519 and Streptococcus agalactiae – 27956) were procured. A 107 CFU/ml bacterial suspension of respective bacteria was obtained after 24-h growth (at 35 °C) in brain heart infusion broth. The bacterial suspensions were subjected to different concentrations of the anaesthetics (20, 40 and 60 ppm) in 1.5 ml microfuge tubes with a fixed exposure time of 10 min at room temperature (24 °C). The serial dilutions were enumerated using the spread plate method of plate count. The inactivation percentage was calculated using bacterial counts in the control (1:4 suspension of PBS:alcohol) for comparison.
2.3 Immune assays
After all the anaesthetic treatments with 20 ppm for 10 min, the fish serum was collected by bleeding via caudal vein. Serum was separated by centrifugation (2000g for 5 min). Five fish from each treatment were used for the serum collection. The serum was used in further assays. Another set of anti-coagulant (EDTA) coated vials were used to collect un-coagulated blood (0.2 ml fish−1 of) for phagocytic assay.
2.3.1 Lysozyme assay
Serum of the fish was collected for the serum lysozyme assay. Lysozyme (from hen’s egg white, Sigma) was used in a 96-well turbidometric method with concentrations ranging from 0–20 μg ml−1 (in 0.1 M phosphate citrate buffer, pH 5.8) as the standard. This along with the undiluted serum sample (25 μl) was placed in wells (96-well plate) in triplicate. Each well was loaded with 175 μl of Micrococcus lysodeikticus suspension (75 mg ml−1). After rapid mixing, the change in turbidity was measured every 30 s for 5 min at 450 nm, at approximately 20 °C, using a microplate reader. The equivalent unit of activity of the sample as compared to the standard was determined, the activity of the control group was compared with the treated groups and expressed as relative lysozyme activity (RLA) percent.
2.3.2 Phagocytic activity of blood leucocytes
The un-coagulated blood was used for assessing the phagocytic activity of blood leucocytes following Puangkaew et al. (2004) with some modifications. Flat bottom microtiter plates were coated with 50 μl of the blood containing 1% l-lysine, allowed to stand for 30 min at room temperature to facilitate the adhesion of blood leucocytes and then washed gently with sterile PBS. The leucocytes were reacted with 50 μl fluorescent latex beads (2 μm; Sigma) in tissue culture medium (L-15, Sigma) to reach a concentration of 107 beads ml−1. The plates were incubated at 22–23 °C for 1 h, rinsed with sterile PBS. The plate wells were carefully washed with L-15 medium and read under a fluorimeter (Microtek FL-800). Relative phagocytic activity (RPA) of the blood leucocytes of the sample was expressed in percentage relative to the control.
2.3.3 Thiobarbituric acid reactive substances (TRARS) assay
Thiobarbituric acid reactive substances (TRARS) were used for screening and monitoring lipid peroxidation, a major indicator of oxidative stress (Yagi, 1998; Armstrong and Browne, 1994). Malondialdehyde (MDA) forms a 1:2 adduct thiobarbituric acid and the product can be measured by fluorometry or spectrophotometry. Biological specimens contain a mixture of thiobarbituric acid reactive substances (TBARS), including lipid hydroperoxidase and aldehydes, which increase as a result of oxidative stress. TBARS return to normal levels over time, depending upon the presence of anti-oxidants. In practice, TBARS are expressed in terms of malondialdehyde (MDA) equivalents. In this assay, an MDA standard is used to construct a standard curve against which unknown samples were plotted. A commercial kit was used to measure the product formation fluorimetrically. The relative activity (RA) was estimated as in the case of phagocytic activity. Three fish per treatment were used to collect the blood and separate the serum as explained above.
2.4 Statistical analysis
All results were subjected to Analysis of variance (ANOVA) for confirming the effects of thyme oil on different bacteria and immune responsiveness.
3 Results and discussion
Thyme oil among all anaesthetics in bluefin bream (S. hasta) and yellowfin bream (A. latus) showed a distinct antibacterial activity. Thyme oil exhibited the best effect in reducing the surface bacterial load in fish compared to the control groups. Data of the in vivo studies on the reduction of total bacterial load and the total Vibrio load on anaesthetic treated S. hasta and A. latus is depicted in Figs. 1(a and b) and 2(a and b), respectively. The in vitro studies indicated over 90% reduction of all the bacterial species exposed to all concentrations of the essential oils (Fig. 3) treated fish with thyme oil producing almost complete elimination of Vibrio from the surface swabs. Similar results on the antibacterial qualities of thyme oil have been also reported previous studies. Al-Yaqout and Azad, 2010 reported a high antibacterial activity of thyme oil against many marine Vibrio spp. and a Gram positive fish pathogen (S. agalactiae). Complete bactericidal activity was achieved with thyme oil in a majority of bacterial species tested and with all concentrations of the essential oils. V. alginolyticus, V. anguillarum, V. parahaemolyticus and V. vulnificus were completely killed at all levels tested with thyme oil (Al-Yaqout and Azad, 2010; Al-Yaqout et al., 2010). Most of the antimicrobial activities in essential oils from spices and culinary herbs appear to be associated with phenolic compounds (Davidson and Naidu, 2000). These observations suggest superior bactericidal and antiparasitic activity of thyme oil thus providing a very effective alternative anaesthetic that can reduce the bacterial load on fish in addition to serving as an anaesthetic.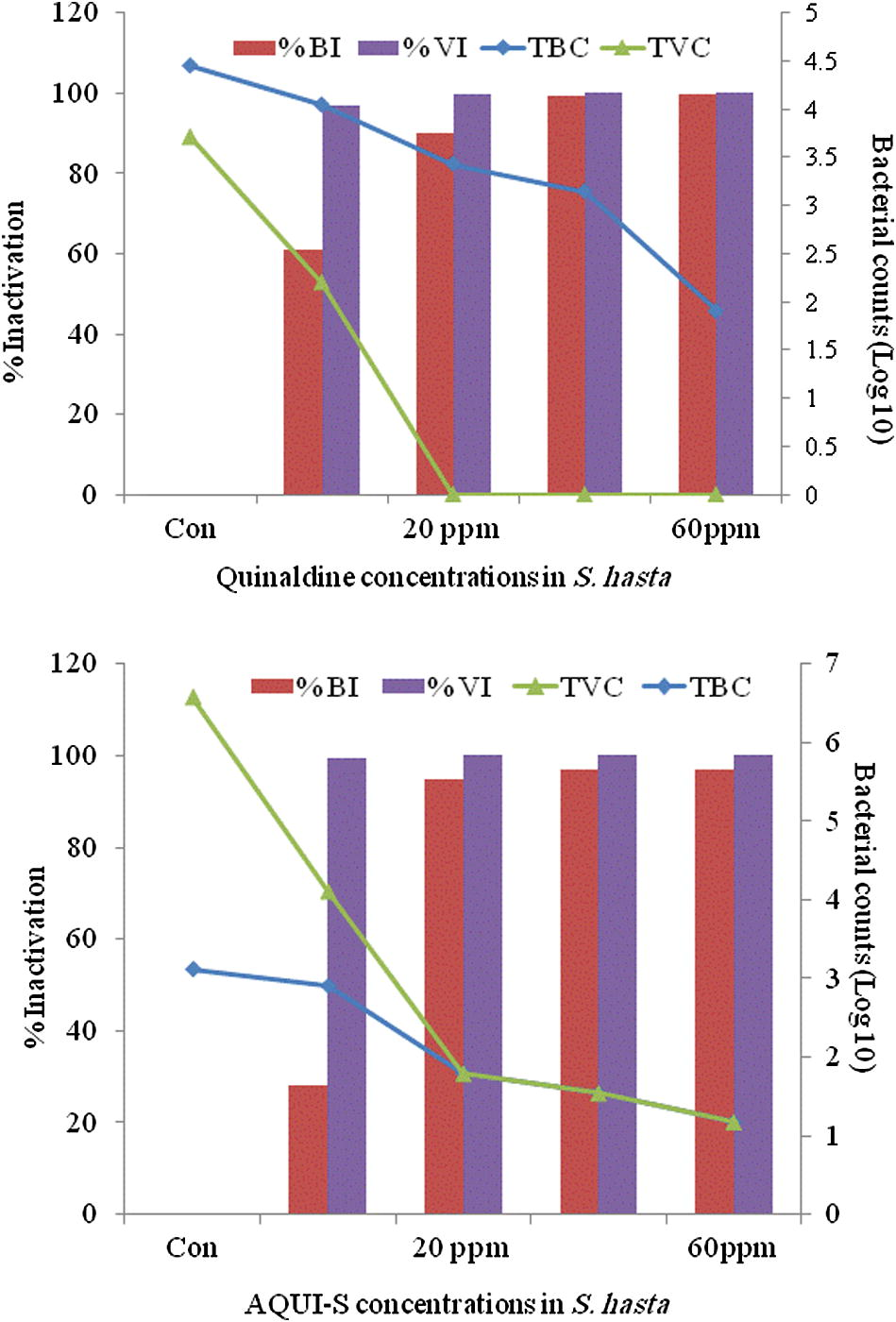
Surface swab from S. hasta for total bacterial and vibrio (log10) counts (TBC and TVC) and their percent inactivation after treatment with quinaldine (top) and AQUI-S (bottom).
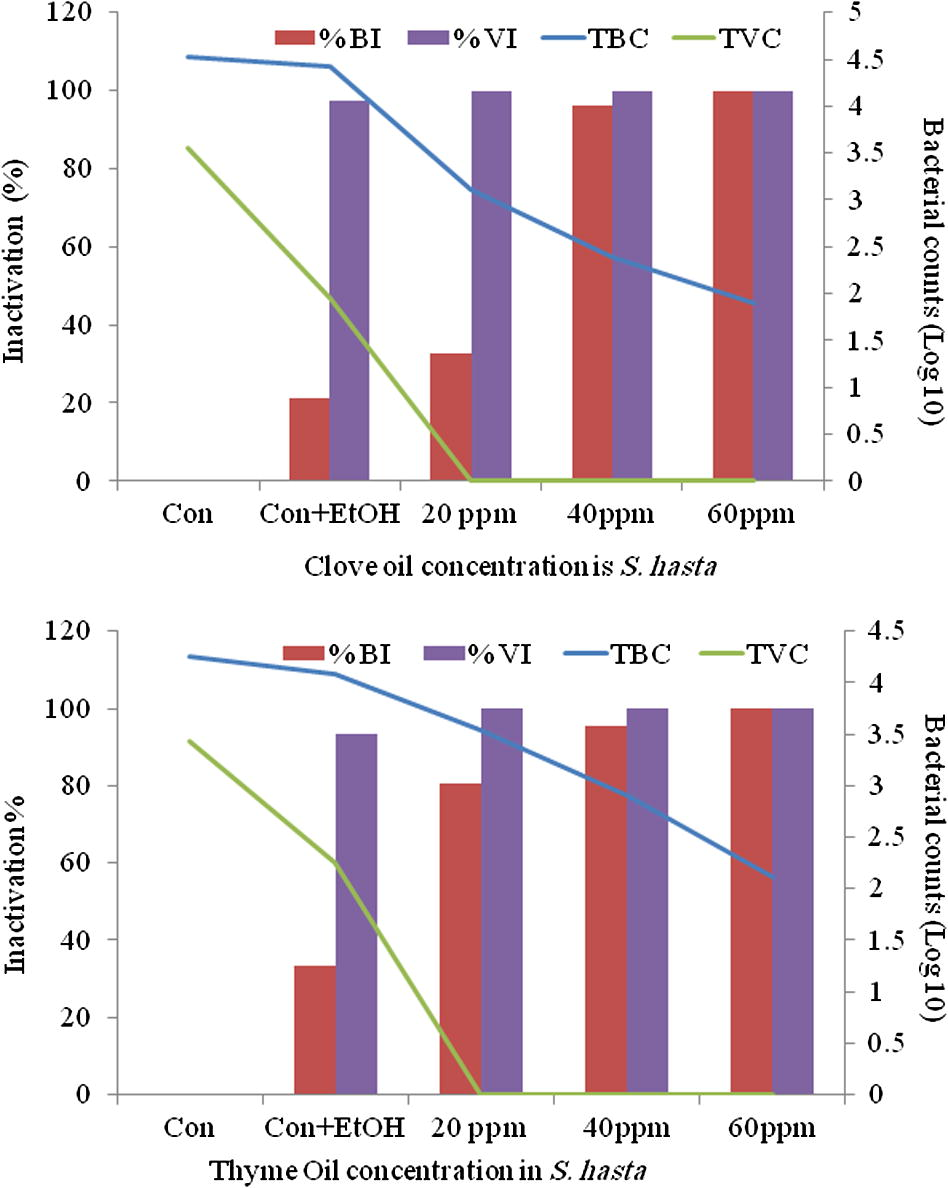
Surface swab from S. hasta for total bacterial and vibrio (log10) counts (TBC and TVC) and their percent inactivation after treatment with clove oil (top) and thyme oil (bottom).
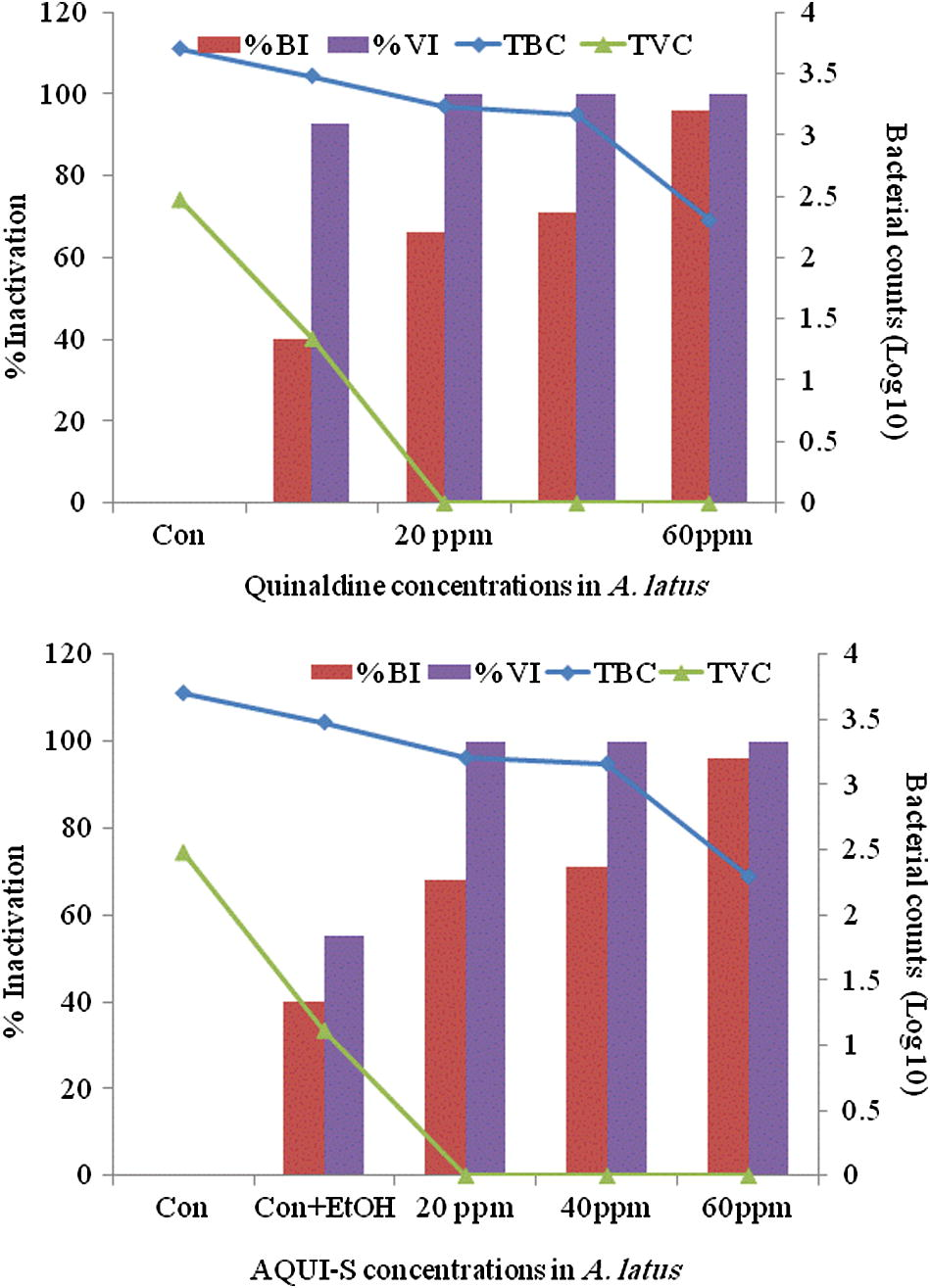
Surface swab from A. latus for total bacterial and vibrio (log10) counts (TBC and TVC) and their percent inactivation after treatment with quinaldine (top) and AQUI-S (bottom).
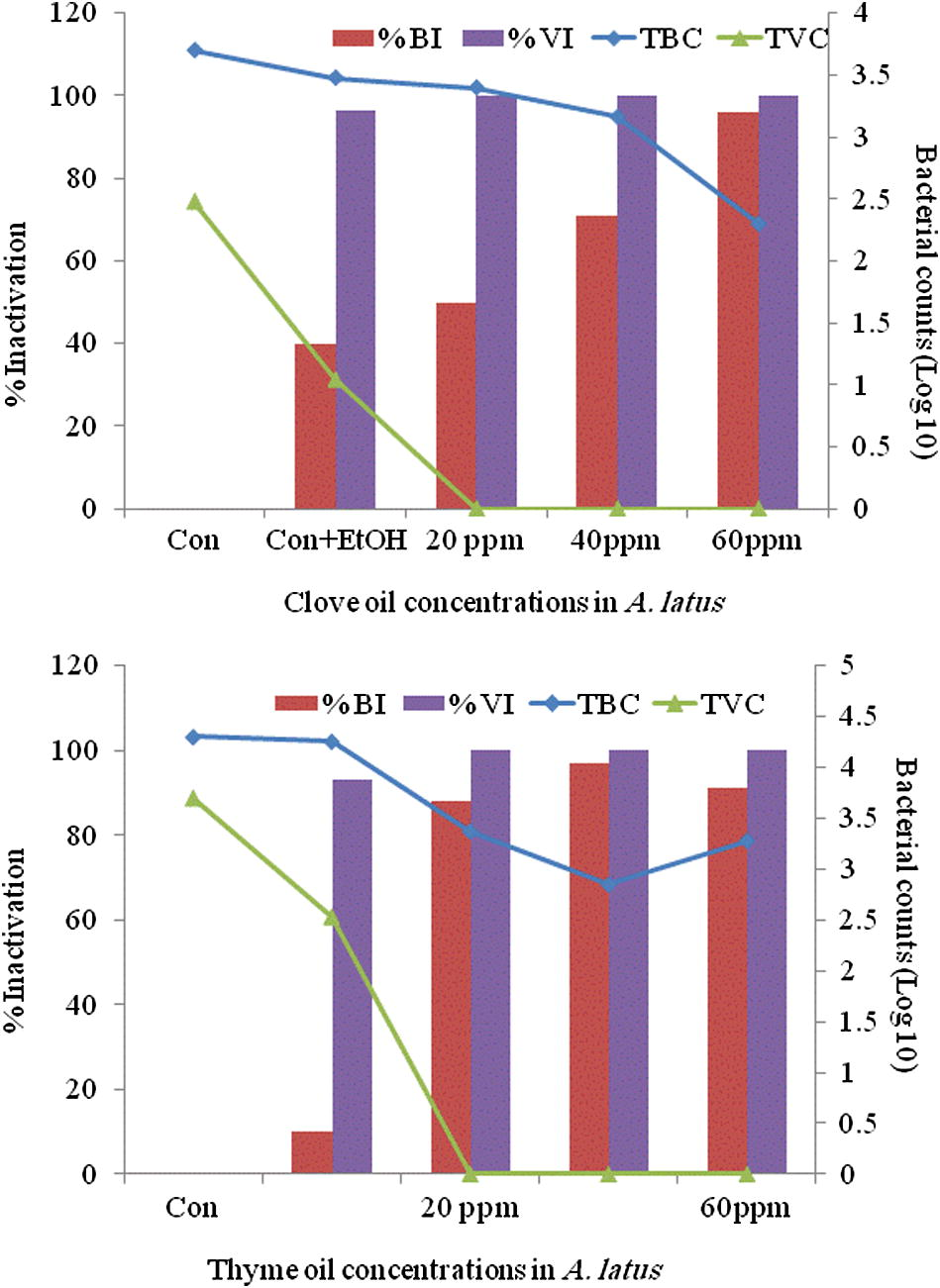
Surface swab from A. latus for total bacterial and vibrio (log10) counts (TBC and TVC) and their percent inactivation after treatment with clove oil (top) and thyme oil (bottom).
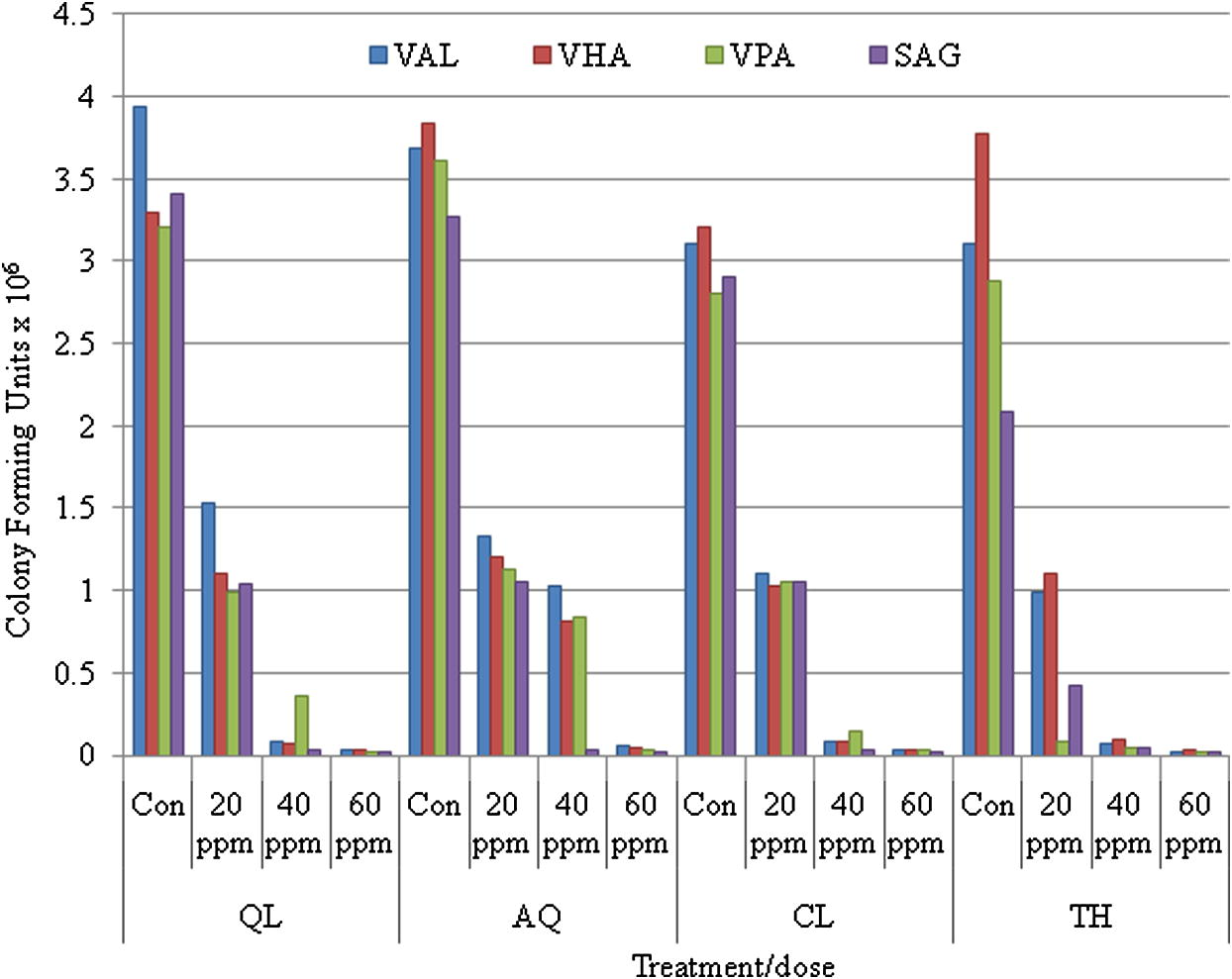
Antibacterial activity of different anaesthetics on standard (ATCC) bacterial isolates.
Effect of different anaesthetics on the non-specific immune parameters such as the serum lysozyme activity, TBARS activity and the phagocytic capabilities of serum and blood leucocytes are depicted in Table 1 and Fig. 3. In this study it was clearly demonstrated that these non-specific immune parameters get activated thus enhancing disease resistance properties of the fish following anaesthesia. The best effect was recorded in fish (irrespective of the species tested) anaesthetised with thyme oil followed by clove oil and AQUI-S. The quinaldine treatment had the least influence on the immune parameters. The relative percent lysozyme activities Fig. 4 in S. hasta treated with thyme oil and clove oil (165.58 ± 32.09 and 168.73, respectively) were significantly (p < 0.05) higher than that of the others. Effect of thyme oil on the immune parameters of fish has not been documented so far. Treament means f each row sharing a common super scripts are not sinifivanty (P = 0.05) different.
Parameter
Species
QN
AQ
CL
TH
RLA
S. hasta
83.70c
130.98b
168.73a
165.58a
±18.23
±21.53
±46.08
±32.09
A. latus
159.267c
176.27b
182.97b
195.87a
±6.161
±4.61
±1.34
±5.33
RPA
S. hasta
105.17c
121.06b
125.35ab
127.82a
±1.14
±12.51
±7.10
±2.92
A. latus
108.07c
119.61b
124.16b
132.92a
±3.76
±2.94
±0.52
±3.90
RTBARSA
S. hasta
79.27c
45.38b
40.59b
30.63a
±2.15
±1.88
±2.14
±1.95
A. latus
89.13c
56.95b
52.61b
26.09a
±3.41
±2.65
±3.11
±3.07
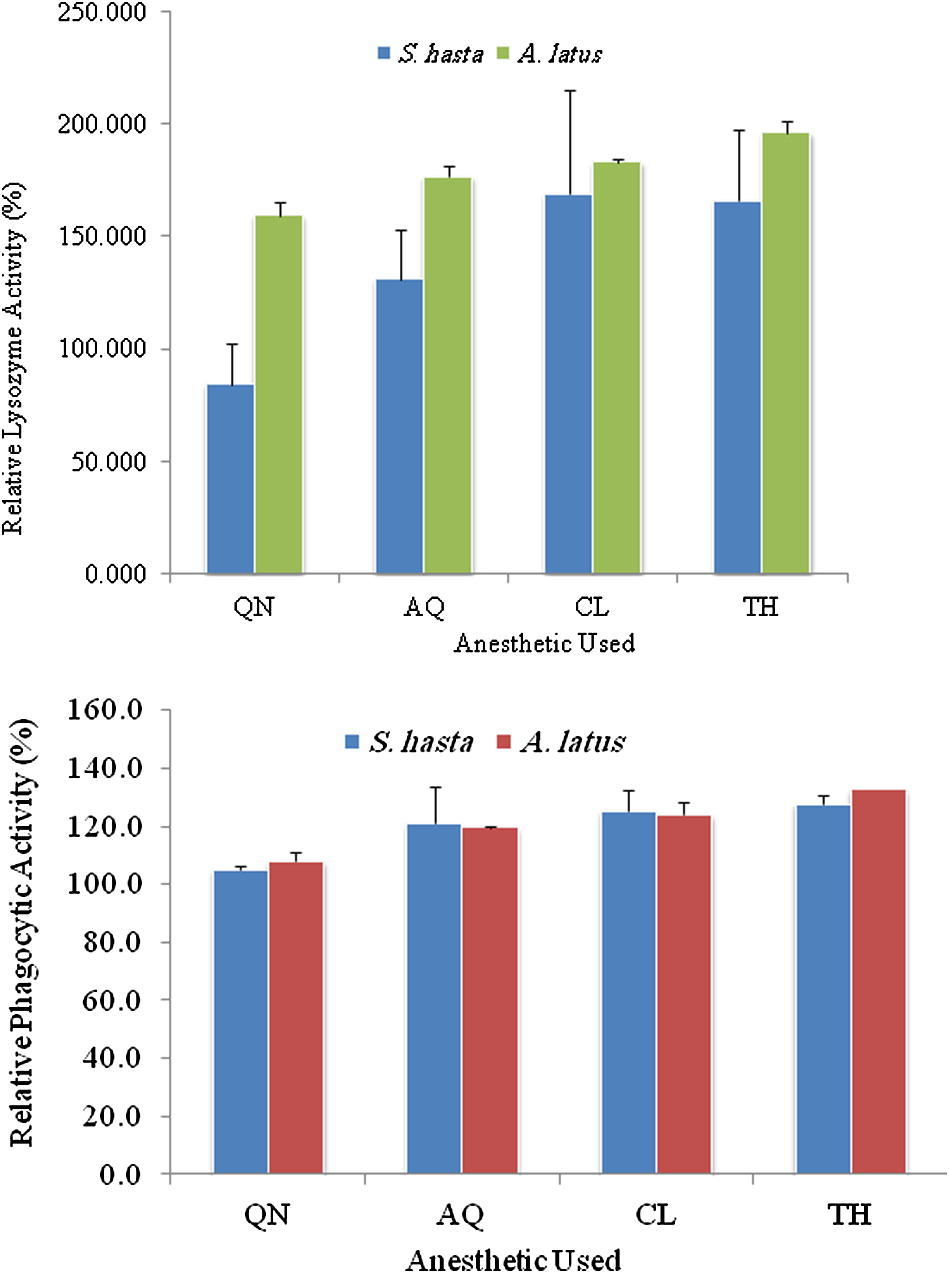
Relative lysozyme activity (top) and relative phagocytic activity (bottom) in S. hasta and A. latus after treatment with different anaesthetics.
The study while establishing the excellent anaesthetic qualities of thyme oil in aquacultured marine fish, it provided important information to supplement the recommendation for the use of thyme oil as an anaesthetic with an additional benefit of disinfection and enhancement in immunity. However, further work about the antibacterial and immunity enhancement properties of thyme oil need to be investigated for a developing complete application guide lines for the use of thyme oil in aquaculture.
References
- Antiparasitic effects of some essential oils on the scuticociliate, Uronema Sp. Res. J. Biotechnol.. 2010;5(4):20-25.
- [Google Scholar]
- Al-Yaqout, A., Azad, I.S., Al-Roumi, M., Al-Abdul Elah, K., Al-Kamdari, M., Yaseen, S., 2010. Comparative analysis of thyme oil, clove oil, quinaldine and AQUI-S as anesthetics for the cultured marine fish. FM068K. Report No. KISR 10220, Kuwait.
- The analysis of free radicals, lipid peroxidases, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Free Radic. Diagn. Med.. 1994;366:43-58.
- [Google Scholar]
- Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control. 2004;15:169-172.
- [Google Scholar]
- Retinal toxicity associated with occupational exposure to the fish anaesthetic MS-222. Am. J. Ophthalmol.. 1997;124(6):843-844.
- [Google Scholar]
- Phyto-phenol. In: Naidu A.S., ed. Natural Food Antimicrobial Systems. Boca Raton, FL: CRC Press; 2000. p. :265-294.
- [Google Scholar]
- Comparative efficacy of clove oil and other chemicals in anesthetization of Pomacentrus amboinensis, a coral reef fish. J. Fish Biol.. 1997;51:931-938.
- [Google Scholar]
- Nonspecific immune response of rainbow trout (Oncorhynchus mykiss, Walbaum) in relation to different status of vitamin E and highly unsaturated fatty acids. Fish Shellfish Immunol.. 2004;16:25-39.
- [Google Scholar]
- Induction and recovery from anaesthesia in channel catfish Ictalurus punctatus fingerlings exposed to clove oil. J. World Aquaculture Soc.. 1999;30(2):250-255.
- [Google Scholar]
- Simple procedure for specific assay of lipid hydroperoxides in serum or plasma. Free Radic. Antioxid. Protoc.. 1998;108:101-106.
- [Google Scholar]







