Translate this page into:
Adult emergence inhibition and adulticidal activity of leaf crude extracts against Japanese encephalitis vector, Culex tritaeniorhynchus
*Corresponding author. Tel.: +91 94423 10155; +91 04172 269009; fax: +91 04172 269487 abdulrahuman6@hotmail.com (A. Abdul Rahuman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 6 September 2010
Abstract
Mosquitoes have developed resistance to various synthetic insecticides, making its control increasingly difficult. Insecticides of botanical origin may serve as suitable alternative biocontrol techniques in the future. The aim of this study was to evaluate the adult emergence inhibition (EI) and adulticidal activity of the leaf hexane, chloroform, ethyl acetate, acetone, and methanol extracts of Aegle marmelos (Linn.) Correa ex Roxb, Andrographis lineata Wallich ex Nees., Andrographis paniculata (Burm.f.) Wall. ex Nees., Cocculus hirsutus L. Diels, Eclipta prostrata L. and Tagetes erecta L. were tested against japanese encephalitis vector, Culex tritaeniorhynchus Giles (Diptera: Culicidae). All plant extracts showed moderate EI and adulticidal activity effects after 24 h of exposure at 1,000 ppm; however, the highest EI activity was found in leaf methanol extracts of A. marmelos, A. paniculata, T. erecta, and chloroform extract of E. prostrata (EI50 = 141.94, 214.17, 166.43, and 184.58 ppm; EI90 = 590.26, 882.34, 532.00 and 571.81 ppm); and the effective adulticidal activity was observed in acetone extract of A.marmelos, hexane of A.lineata, ethlyl acetate of A.paniculata, methanol extracts of C.hirsutus, E.prostrata, and T.erecta (LD50 = 139.05, 251.24, 205.06, 222.10, 166.73, and 232.74 ppm; LD90 = 426.19, 837.09, 813.59, 794.42, 579.43 and 807.41 ppm), respectively against C. tritaeniorhynchus. These results suggest that the leaf methanol extract of C. hirsutus, E. prostrata, and T. erecta have the potential to be used as an ideal eco-friendly approach for the control of the C. tritaeniorhynchus. Therefore, this study provides first report on the mosquito EI and adulticidal activity of plant extracts against a vector.
Keywords
Plant extracts
Culex tritaeniorhynchus
Adult emergence inhibition
Adulticidal activity
Control
1 Introduction
Japanese encephalitis (JE) outbreaks occur frequently in 14 Asian countries with about 3,060 million people at the risk of infection (Sabesan, 2003). JE is a major public health concern due to its high epidemic potential, high case fatality, and neuropsychiatric sequel among survivors. The annual incidence and mortality estimates for JE are 30,000–50,000 and 10,000, respectively, (Solomon, 2004). Approximately 2 billion people live in countries where JE presents a significant risk to humans and animals, particularly in China and India, with at least 700 million potentially susceptible children (Gould et al., 2008) and it is highly endemic in few districts of Tamil Nadu, Southern India (Ravi et al., 1989; Reuben and Gajanana, 1997). C. tritaeniorhynchus is a primary vector of Japanese encephalitis virus, with a distribution throughout Southeast and South Asia. The larval habitat is mainly rice fields and shallow marshes. Mosquito control represents an important strategy for prevention of disease transmission and epidemic outbreaks. However, a high level of insecticide resistance has developed through chemical control of the vector and pests threatening the control strategies. To overcome these problems, it is necessary to search for alternative methods of vector control. The failure of chemical insecticides to control the insect and growing public concern for safe food and a healthy environment have catalyzed the search for more environmentally benign control methods for the management of the vectors. Biopesticides provide an alternative to synthetic pesticides because of their generally low environmental pollution, low toxicity to humans and other advantages (Liu et al., 2000).
The present study was carried out on A. marmelos, an Indian plant with religious and medicinal importance and the stem bark ethyl acetate extracts exhibited moderate insecticidal activity against Phaedon cochleariae and Musca domestica (Samarasekera et al., 2004). Zaridah et al. (2001) have reported that the aqueous extract from the dried leaves of A. paniculata showed the strongest activity against adult worms of Brugia malayi. Elango et al. (2010) reported that the hexane extracts of A. marmelos and A. paniculata served as a potential repellent, ovicidal, and oviposition deterrent against C. tritaeniorhynchus. C. hirsutus is a widely growing plant found in the plains of India in dry localities and the methanol extract showed effective oviposition repellency against Anopheles subpictus (Elango et al., 2009a). E. prostrata, a member of the Asteraceae family and commonly known as False Daisy and the ethyl acetate extract of E. prostrata and leaf hexane extract of A. paniculata have the potential to be used against the fourth-instar larvae of A. subpictus and C. tritaeniorhynchus (Elango et al., 2009b). Macêdo et al. (1997) have reported that the ethanol extract of E. paniculata and Tagetes minuta were significantly active against the larvae of Aedes fluviatilis.
The callus tissue of T. erecta which showed the presence of insecticidal pyrethrin mixture was screened against Tribolium spp. Immediate ‘knock down’ effect was observed (Sarin, 2004), and the steam-distilled oils of T. patula, T. erecta and T. minuta were tested for larvicidal activity toward third instar A. aegypti and the results suggest a potential utilization of oil of T. minuta or its components for the control of A. aegypti and other species of mosquitoes (Green et al., 1991). The larvicidal potential of the essential oil of T. patula against A. aegypti, A. stephensi, and C. quinquefasciatus was evaluated (Dharmagadda et al., 2005); the compounds, 4 thiophenes, 5-(but-3-ene-1-ynyl)-2,2′-bithiophene,5-(but-3-ene-1-ynyl)-5′-methyl-2,2′-bithiophene,2,2′,5′,2″- terthiophene, and 5-methyl-2,2′,5′,2″-terthiophene isolated from the floral extract of T. minuta were largely responsible for the toxicity exhibited against the adults of A. aegypti and A. stephensi (Perich et al., 1995).
The bioactive constituents of these plants could be either a single substance or a mixture of substances. The separation of the mixture is neither practical nor advantageous in the insect economic control strategies. The aim of this study was to investigate the activity of different solvent extracts of six plant species from Tamil Nadu, South India. As far as our literature survey could ascertain, no information was available on the adult emergence inhibition and adulticidal activities of the experimental plant species given here. Therefore, the present study was an attempt to assess the adult emergence inhibition and adulticidal activity leaf extracts against C. tritaeniorhynchus. This is the first report of the solvent extracts of selected plants.
2 Materials and methods
2.1 Collection of plants
The leaf of A. marmelos (Linn.) Correa ex Roxb (Rutaceae), A. lineata Wallich ex Nees. (Acanthaceae), A. paniculata (Burm.f.) Wall. ex Nees. (Acanthaceae), C. hirsutus (L.) Diels (Menispermaceae), E. prostrata L. (Asteraceae) and T. erecta L. (Compositae) were collected from the Tamil Nadu Medical Plant Farms and Herbal Medicine Corporation Limited, medicinal plant farm, Arumbakkam (13° 13′ 4N, 79° 59′ 7E Altitude 118 feet), Chennai, Tamil Nadu and the taxonomic identification was made by Dr. B. Annadurai, Department of Plant Biology and Biotechnology, C. Abdul Hakeem College, Melvisharam, India. The voucher specimen was numbered and kept in our research laboratory for further reference.
2.2 Collection and rearing of mosquitoes
C. tritaeniorhynchus larvae were collected from a rice field and the stagnant water area of Melvisharam (12° 56′ 23″ N, 79° 14′ 23″ E) and identified in Zonal Entomological Research Centre, Vellore (12° 55′ 48″ N, 79° 7′ 48″ E), Tamil Nadu, to start the colony and the larvae were kept in plastic and enamel trays containing tap water. They were maintained, and all the experiments were carried out, at 27 ± 2 °C and 75–85% relative humidity under 14:10 light and dark cycles. Larvae were fed with a diet of brewer’s yeast, dog biscuits, and algae collected from ponds in a ratio of 3:1:1, respectively. Pupae were transferred from the trays to a cup containing tap water and were maintained in our insectary (45 cm× 45 cm× 40 cm) where adults emerged. Adults were maintained in glass cages and were continuously provided with 10% sucrose solution in a jar with a cotton wick. On day 5, the adults were given a blood meal from a pigeon placed in resting cages overnight for blood feeding by females. They were maintained and reared in the laboratory as per the method of Kamaraj et al. (2009).
2.3 Preparation of plant extracts
The dried leaf (800 g) were powdered mechanically using commercial electrical stainless steel blender and extracted with hexane (1400 ml, Fine), chloroform (1400 ml, Fine),ethyl acetate (2200 ml, Qualigens), acetone (1200 ml, Qualigens), and methanol (2500 ml, Qualigens) in a soxhlet apparatus separately until exhaustion. The extract was concentrated under reduced pressure 22–26 mm Hg at 45 °C and the residue obtained was stored at 4 °C.
2.4 Adult emergence inhibition (EI) bioassay
The inhibition of adult emergence was evaluated by following the WHO standard procedure for testing insect growth regulators (WHO, 2005).Only 3rd instars larvae were used and followed the method of larvicidal activity. Because of the long duration of the test the larvae were fed with yeast at two-days intervals until the mortality counts were made. The yeast powder was prepared as stock suspension in water from which one or two drops were added per cup. One gram of crude extract was first dissolved in 100 mL of acetone (stock solution). From the stock solution, 15.625–1000 ppm were prepared with dechlorinated tap water. Polysorbate 80 (Qualigens) was used as an emulsifier at the concentration of 0.05% in the final test solution. All the treated and control cups containing the pupae were kept separately in the net cage to prevent the successfully emerged adults from escaping into the environment. Mortality of the pupae was recorded at 24 h intervals. Observation was continued in treated and control cups (acetone, polysorbate 80 and de-chlorinated tap water) until the complete emergence of the adults. At the end of observation period, the impact was expressed as EI% based on the number of pupae that did not develop successfully into viable adults. In recording EI% for each concentration, moribund and pupae, as well as adult mosquitoes not completely separated from the pupal case, were considered as dead. The experiments stop when all the pupae in the controls have died or emerged as adults. The experimental media, in which 100% (EI) pupae occurs alone, were selected and dose response bioassay test done (Elimam et al.,2009b).
2.5 Adulticidal bioassay
C. tritaeniorhynchus mosquitoes were selected for the testing of adulticidal activities. The bioassay was performed by WHO (1981) method. Appropriate concentrations of the leaf plant solvent extracts were dissolved in 2.5 ml of acetone and applied on Whatman no. 1 filter papers (size 12 × 15 cm2) as described by Dua et al. (2008). Control papers were treated with acetone under similar conditions. The plant extracts were evaluated at seven concentrations 15.625, 31.25, 62.5, 125, 250, 500 and 1000 ppm to produce a range of mortality from 10% to 100% along with the control. Twenty mosquitoes (2–5 days old glucose fed, blood starved) were collected and gently transferred into a plastic holding tube. The mosquitoes were allowed to acclimatize in the holding tube for 1 h and then exposed to test paper for 1 h. At the end of exposure period, the mosquitoes were transferred back to the holding tube and kept 24 h for the recovery period. A pad of cotton soaked with 10% glucose solution was placed on the mesh screen. Mortality of mosquitoes was determined at the end of 24 h recovery period. Percent mortality was corrected by using Abbott’s formula (1925).
2.6 Dose response bioassay
From the stock solution, different concentrations ranging from 15.62 to 1000 ppm were prepared. Based on the preliminary screening results, crude different solvent leaf extracts prepared from A. marmelos, A. lineata, A. paniculata C. hirsutus, E. prostrata, and T. erecta were subjected to dose response bioassay for percentage adult emergence inhibition and adulticidal activity were counted after 24 h of exposure, against the C. tritaeniorhynchus. The percentage adult mortality and EI% was reported from the average of five replicates.
2.7 Statistical analysis
Adult mortality counts were made after 24 h exposure. Bioassay test showing more than 10% control mortality was discarded and repeated. However, when control mortality ranged from 5% to 10%, the corrected mortality was calculated using Abbott’s formula (Abbott, 1925). LD50, LD90 and the 90% confidence intervals (CI) of the lethal dosage of 50% and 90% calculated by a computerized log-probit analysis (Harvard Programming; Hg1, 2) was used to measure differences between test samples.
3 Results
The activity of crude plant extracts is often attributed to the complex mixture of active compounds. The preliminary screening is a good means of evaluation of the potential of plants adult emergence inhibition and adulticidal activity of crude plant extracts is often attributed to the different solvent extracts of six plants which are noted and presented in Table 1 and Fig 1. Among the crude extracts tested, the present results showed that emergence inhibition of leaf methanol extract of A. marmelos, A.paniculata, T. erecta and chloroform extract of E. prostrata (EI50 = 141.94, 214.17, 166.43, and 184.58 ppm; EI90 = 590.26, 882.34, 532.00 and 571.81 ppm), and the maximum adulticidal activity observed in acetone extract of A. marmelos, hexane extract of A. lineata, ethyl acetate extract of A. paniculata, methanol extract of C. hirsutus, E. prostrata, and T. erecta (LD50 = 139.05, 251.24, 205.06, 222.10, 166.73, and 232.74 ppm; LD90 = 426.19, 837.09, 813.59, 794.42, 579.43 and 807.41 ppm), respectively against C. tritaeniorhynchus. The chi-square values calculated during the analysis did not show any heterogeneity of the response. Table 2 and 3 and Fig. 2 and 3. EI = Emergence inhibition. UCL = upper confidence limit; LCL = lower confidence limit; χ2 = Chi-square; df = degree of freedom. UCL = Upper confidence limit; LCL = lower confidence limit; χ2 = Chi-square; df = degree of freedom. Mosquitoes was exposed for 1 h and mortality was recorded at 24 h recovery period.
Botanical name/Family (Herbarium numbers) Vernacular names
Solvents
*%EI
%*mortality at 24 h
Aegle marmelos (Linn.) Correa ex Roxb/Rutaceae(LE/ZB/007–05) Vilvam
Hexane
72 ± 2.21
76 ± 2.11
Chloroform
80 ± 2.16
90 ± 1.60
Ethylacetate
84 ± 1.72
74 ± 1.58
Acetone
89 ± 1.68
100 ± 0.00
Methanol
100 ± 0.00
86 ± 1.76
Andrographis lineata Wallich ex Nees./Acanthaceae (ZD/AL/040–08) Siriyanangai
Hexane
72 ± 3.00
100 ± 0.00
Chloroform
66 ± 1.42
68 ± 1.86
Ethyl acetate
84 ± 1.38
66 ± 1.20
Acetone
76 ± 0.00
85 ± 1.11
Methanol
86 ± 1.65
85 ± 1.70
Andrographis paniculata (Burm.f.) Wall. ex Nees./Acanthaceae (ZD/AP/143–08) Periyanangai or Nilavembu
Hexane
65 ± 1.79
86 ± 1.62
Chloroform
69 ± 1.98
54 ± 1.66
Ethyl acetate
58 ± 1.84
100 ± 0.00
Acetone
72 ± 1.00
88 ± 1.20
Methanol
100 ± 0.00
90 ± 1.11
Cocculus hirsutus (L.) Diels/Menispermaceae (ZD/CH/142–08) Vellakattukkodi
Hexane
75 ± 2.44
65 ± 1.40
Chloroform
55 ± 1.41
78 ± 1.76
Ethyl acetate
81 ± 0.00
80 ± 1.32
Acetone
72 ± 1.82
76 ± 1.72
Methanol
86 ± 1.71
100 ± 0.00
Eclipta prostrata L./Asteraceae (ZD/EP/ 114–08) Manjal Karisallankannai
Hexane
54 ± 2.14
89 ± 1.98
Chloroform
100 ± 0.00
92 ± 1.68
Ethyl acetate
62 ± 1.22
68 ± 1.86
Acetone
84 ± 1.91
84 ± 1.76
Methanol
82 ± 2.07
100 ± 0.00
Tagetes erecta L./Compositae(ZD/TE/ 156–08) Tulukkaccevvanti
Hexane
86 ± 1.48
63 ± 1.79
Chloroform
80 ± 1.67
76 ± 1.75
Ethyl acetate
76 ± 1.72
92 ± 1.68
Acetone
70 ± 1.20
85 ± 1.62
Methanol
100 ± 0.00
100 ± 0.00
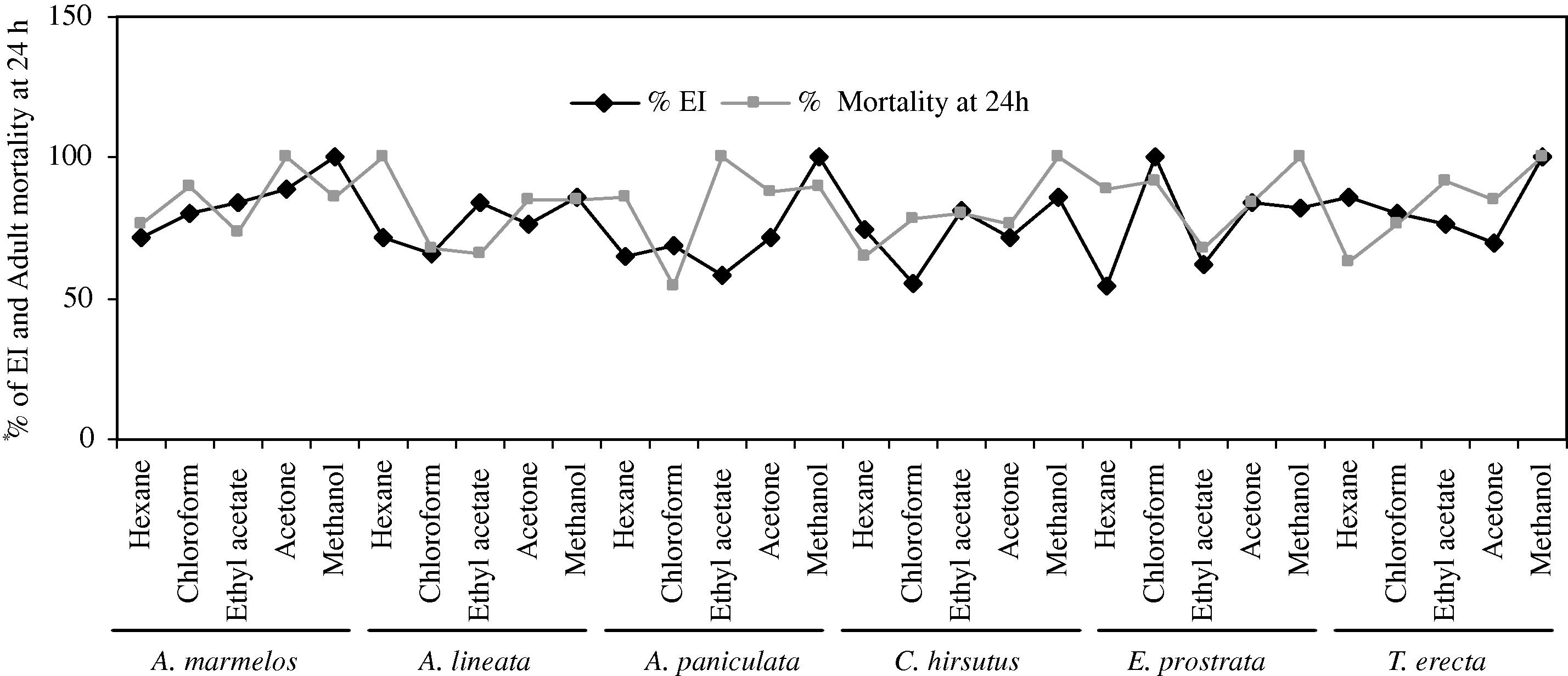
Adult emergence inhibition and adulticidal activity of different plant extracts against C. tritaeniorhynchus at 1000 ppm.
Name of the plants
Solvents
EI50 ± SE (ppm)
UCL–LCL
EI90 ± SE (ppm)
UCL–LCL
Regression coefficient (slope)
χ2 (df = 4)
A. marmelos
Methanol
141.94 ± 9.64
160.84–123.04
590.26 ± 66.61
720.81–459.72
2.070
6.70
A. paniculata
Methanol
214.17 ± 14.04
241.69–186.65
882.34 ± 93.42
1005.6–639.22
2.305
18.96
E. prostrata
Chloroform
184.58 ± 10.79
205.73–163.42
571.81 ± 53.97
677.59–466.03
2.609
5.34
T. erecta
Methanol
166.43 ± 9.99
185.93–146.94
532.00 ± 51.38
632.69–431.30
2.539
4.76
Name of the plants
Solvents
LD50 ± SE (ppm)
UCL–LCL
LD90 SE (ppm)
UCL–LCL
Regression coefficient (slope)
χ2 (df = 4)
A. marmelos
Acetone
139.05 ± 8.21
155.15–122.95
426.19 ± 40.25
505.25–347.29
2.634
2.89
A. lineata
Hexane
251.24 ± 15.23
281.67–221.82
837.09 ± 89.39
1012.30–661.88
2.452
16.72
A. paniculata
Ethyl acetate
205.06 ± 13.72
231.95–178.17
813.59 ± 95.94
1001.62–625.54
2.141
17.71
C. hirsutus
Methanol
222.10 ± 14.18
249.91–194.31
794.42 ± 87.12
965.17–623.67
2.315
16.67
E. prostrata
Methanol
166.73 ± 10.35
187.03–146.43
579.43 ± 58.59
694.28–146.43
2.633
6.57
T. erecta
Methanol
232.74 ± 14.60
261.36–204.22
807.41 ± 87.58
979.07–635.76
2.372
15.02
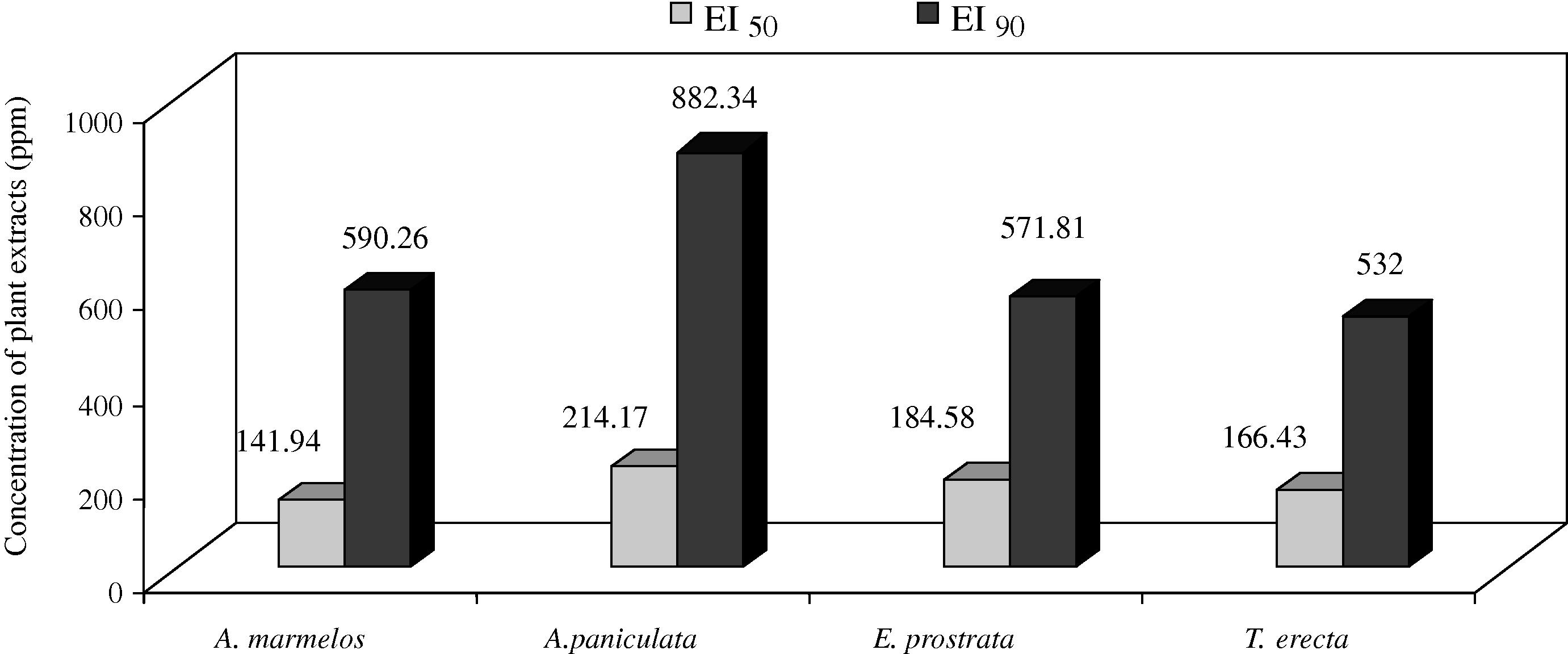
The adult emergence inhibition activity of leaves extract against C. tritaeniorhynchus expressed as EI50 and EI90.
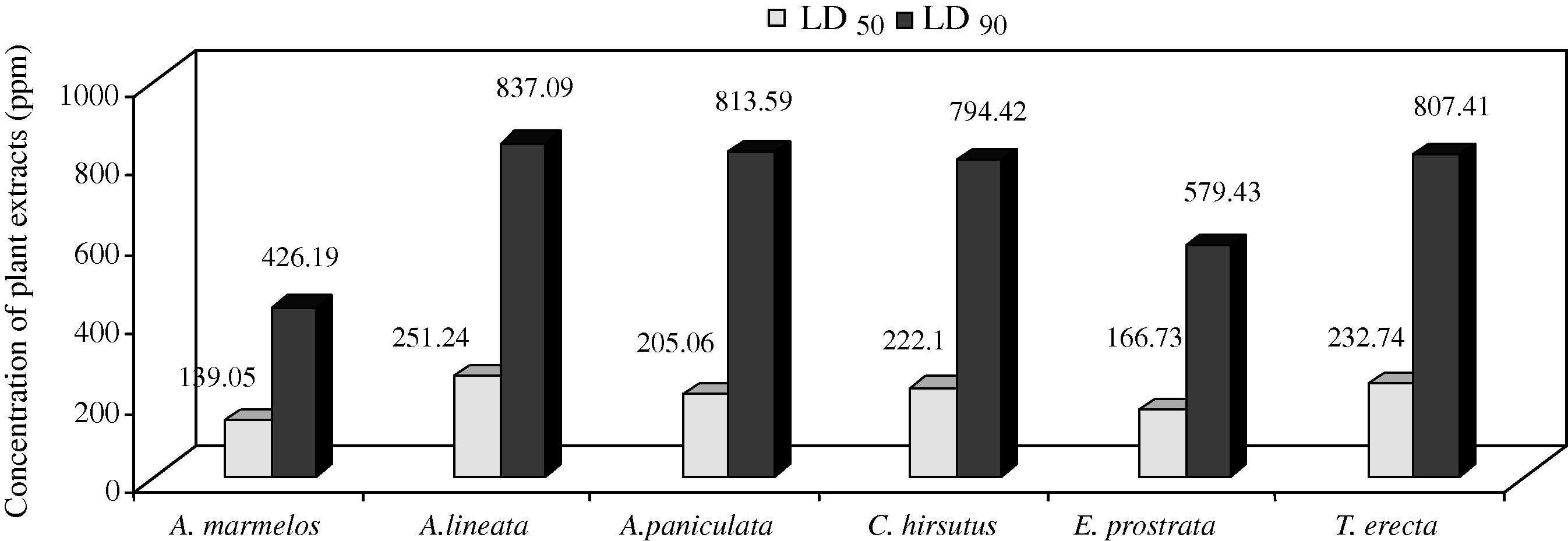
Adulticidal activity of different plant extracts against C. tritaeniorhynchus expressed as LD50 and LD90.
4 Discussion
In this study it was observed that, six plant leaves extract of different solvents have tested adult emergence inhibition and adulticidal activity against C. tritaeniorhynchus. The biological activity of the experimental plant extracts may be due to various compounds, including phenolics, terpenoides, flavonoids and alkaloids present in plants, of these compounds may jointly or independently contribute to produce adult emergence inhibition, and adulticidal effect against Japanese encephalitis vector, C. tritaeniorhynchus. Undoubtedly, plant-derived toxicants are a valuable source of potential insecticides. These and other naturally occurring insecticides play a prominent role in mosquito control programs in the future (Mordue and Blackwell, 1993). The results of this study may contribute to a great reduction in the application of synthetic insecticides, which in turn increase the opportunity for natural control of various medicinally important vectors by botanical chemicals. Since these are often active against a limited number of species including specific target insects, less expensive, easily biodegradable to non-toxic products, and potentially suitable for use in mosquito control programme (Alkofahi et al., 1989), they could lead to the development of new classes of possible safer insect control agents. Plant allelochemicals may be quite useful in increasing the efficacy of biological control agents because plants produce a large variety of compounds that increase their resistance to insect attack (Berenbaum, 1988; Murugan et al., 1996; Senthil Nathan et al., 2005).
Our results agree with some previous studies, such as the aqueous leaves extract of Calotropis procera reported that the 50% of adult emergence inhibition (EI50) was shown at 277.90 and 183.65 ppm for An. arabiensis and Cx. quinquefasciatus, respectively, and the pupal stage was not affected till a concentration of 5000 ppm (Elimam et al., 2009b). Similar study was conducted by Elimam et al. (2009a) and they reported that aqueous extracts from leaves of Ricinus communis showed 50% of adult emergence inhibition (EI50) was 374.97 and 1180.32 ppm against 3rd instar larvae of Anopheles arabiensis and Cx. quinquefasciatus and the extract showed oviposition deterrent effect against both species. The larvicidal, growth inhibitor and repellent actions of Dalbergia sissoo oil was evaluated against A. stephensi, A. aegypti and C. quinquefasciatus under laboratory conditions and observed no adult emergence was observed at 4 ml/m2 (Ansari et al., 2000). Deformities produced in pupae further reduced adult emergence from 78% in the control to 75% at 6.25 ppm, 72% at 12.5 ppm, 70% at 25 and 50 ppm, and 66% at 100 ppm and the successful adult emergence from eggs treated was 78% in the control, which was reduced to 45%, 40%, 22%, 17%, and 10% at 6.25, 12.5, 25, 50, and 100 ppm, respectively was observed in methanol extract of Azadirachta indica on the development and growth index of C. quinquefasciatus (Sharma et al., 2009).
The effects of the tested extract, adult emergence and adulticidal activity of the mosquitoes are remarkably greater than those reported for other plant extracts in the literature. For example at the highest concentration, 50% inhibition of the emergence of the adult mosquitoes was observed by the use of the ethyl acetate fractions of Calophyllum inophyllum seed and leaf, Solanum suratense and Samadera indica leaf extracts and the petrol ether fraction of Rhinocanthus nasutus leaf extract on C. quinquefasciatus, A. stephensi and A. aegypti (Muthukrishnan and Puspalatha, 2001). Similarly 88% of the adult mortality was observed by the use of Pelargonium citrosa leaf extracts at 2% concentration against A. stephensi (Jeyabalan et al., 2003).
The present study results agree with Howard et al. (2009) who had reported that 50% inhibition of adult emergence (IE50) of all larval instars obtained with <0.4 g of neem bark chippings of A. indica in 1 L of distilled water. The hexane extract obtained from leaves of Eucalyptus citriodora tested at lowest concentration viz. 10 ppm, 73% larvae of An. stephensi failed to emerge as adult mosquito while in Cx. quinquefasciatus and Ae. aegypti only 10% and 6% larvae failed to emerge (Singh et al., 2007). Wiesman and Chapagain (2006) reported that the high total saponins compound isolated from methanol extracts of fruit mesocarp of Balanites aegyptiaca showed inhibition of 50% of the larval population from emerging adults (EC50 at 0.0014% (w/v) against A. aegypti. The neem formulation, Neem Azal produced an overall mortality or inhibition of emergence of 90% (EI(90), when 3(rd) instar larvae were treated) at 0.046, 0.208 and 0.866 ppm in An. stephensi, Cx. quinquefasciatus and Ae. aegypti, respectively (Gunasekaran et al., 2009). The extracts of Syzygium aromaticum were less toxic to the larvae; however their influence on development was remarkable, causing complete inhibition of adult emergence at 200 and 600 ppm concentrations of the methanol and ether extracts, respectively against Culex pipiens (El Hag et al., 1999). Three limonoids, namely limonin, nomilin and obacunone, isolated from the seeds of Citrus reticulata showed that the EC50 for inhibition of adult emergence were 6.31, 26.61 and 59.57 ppm for obacunone, nomilin and limonin, respectively on 4th instar larvae of mosquito C. quinquefasciatus (Jayaprakasha et al., 1997). Similar result was obtained in the root extract of Valeriana jatamansi which exhibited adulticidal activity of 90% lethal concentration against adult An. stephensi, An. culicifacies, Ae. aegypti, Anopheles albopictus, and Cx. quinquefasciatus and were 0.14, 0.16, 0.09, 0.08, and 0.17 and 0.24, 0.34, 0.25, 0.21, and 0.28 mg/cm2, respectively (Dua et al., 2008). Nathan et al. (2005) considered pure limonoids of neem seed, testing for biological, larvicidal, pupicidal, adulticidal, and antiovipositional activity, A. stephensi and the larval mortality was dose-dependent with the highest dose of 1 ppm azadirachtin evoking almost 100% mortality, affecting pupicidal and adulticidal activity and significantly decreased fecundity and longevity of A. stephensi. Choochote et al. (2005) also reported that the in testing for adulticidal activity, the hexane-extracted Curcuma aromatica (LC50: 1.60 microg/mg female) was found to be slightly more effective against female A. aegypti than volatile oil (LC50: 2.86 microg/mg female). The highest adulticidal effect was established from Piper sarmentosum, followed by Piper ribesoides and Piper longum, with LD50 values of 0.14, 0.15 and 0.26 microg/female, respectively (Choochote et al., 2006). In testing for adulticidal activity, the crude seed extract of celery, Apium graveolens, exhibited a slightly adulticidal potency on A. aegypti with LD50 and LD95 values of 6.6 and 66.4 mg/cm2, respectively, (Choochote et al., 2004). The unsaponifiable portion and volatile oil of Thymus capitatus showed the highest adulticidal potency (LC50 = 0.0070 and 0.0076 mg/cm2, respectively against Culex pipiens (Mansour et al., 2000). This result is also comparable to earlier reports of Dua et al. (2010) who observed that the adulticidal activity of the essential oil of Lantana camara was evaluated against different mosquitoes species on 0.208 mg/cm2 impregnated papers, and the KDT50 and KDT90 values of the essential oil were 20, 18, 15, 12 and 14 min and 35, 28, 25, 18 and 23 min against Ae. aegypti, Cx. quinquefasciatus, An. culicifacies, An. fluvialitis and An. stephensi with their percent mortality of 93.3%, 95.2%, 100%, 100% and 100%, respectively. Also the result agrees with the finding of Halim (2008) who have reported the insecticidal activity of Zingiber officinale against the adult emergency of A. pharoensis 3rd stage was evaluated at the concentrations of 100%, 70%, 50%, 25%, 5%, 2%, 1%, 0.9%, 0.7%, 0.5% and 0.3% showed 100% larval mortality rate and at 0.2% and 0.1% caused mortality of 66.7%, respectively.
All the toxins used in vector control pose some hazards to the user and also to the aquatic environment (Kreutzweiser, 1997). Hence this research is mainly focused on finding newer insecticides which will be more effective, biodegradable and also easily available at low cost. In our observation, acetone extract of A. marmelos, hexane, extracts of A. lineata, the methanol of C. hirsutus, E. prostrata, and T. erecta possessed higher EI and adulticidal activity than the other solvent extracts.
In conclusion, the present study clearly proved that the efficacy of plant leaves extracts of A. lineata and T. erecta can be suggested as an adult emergence inhibition and adulticidal activity against C. tritaeniorhynchus activity. The results reported that the efficacy for controlling mosquitoes and mortality properties of natural product extracts. Since it is considered environmentally safe, the isolation and purification of crude extract of leaf hexane of A. lineata and methanol of T. erecta are in progress.
Acknowledgements
The authors are grateful to C. Abdul Hakeem College Management, Dr. S. Mohammed Yousuff, Principal, Dr. K. Abdul Subhan, Associate Professor and HOD of Zoology Department, and Dr. Sait Sahul Hameed, Reader in Zoology for their help and suggestion.
References
- A method of computing the effectiveness of an insecticide. J. Eco. Entomol.. 1925;18:265-266.
- [Google Scholar]
- Search for new pesticides from higher plants. In: Arnason J.T., Philogene B.J.R., Morand P., eds. Insecticides of Plant Origin. Washington, DC: American Chemical Society; 1989. p. :25-43.
- [Google Scholar]
- Larvicidal and repellent actions of Dalbergia sissoo Roxb (F. Leguminosae) oil against mosquitoes. Bioresour. Technol.. 2000;73:207-211.
- [Google Scholar]
- Allelochemicals in insect–microbe–plant interactions: agents provocaterurs in the coevolutionary arms race. In: Barbosa P., Lotourneau D.K., eds. Novel Aspects of Insect–Plant Interactions. New York: Wiley; 1988. p. :97-123.
- [Google Scholar]
- Potential of crude seed extract of celery, Apium graveolens L., against the mosquito Aedes aegypti (L.) (Diptera: Culicidae) J. Vector Ecol.. 2004;29(2):340-346.
- [Google Scholar]
- Chemical composition and anti-mosquito potential of rhizome extract and volatile oil derived from Curcuma aromatica against Aedes aegypti (Diptera: Culicidae) J. Vector Ecol.. 2005;30(2):302-309.
- [Google Scholar]
- Adulticidal activity against Stegomyia aegypti (diptera: culicidae) of three Piper spp. Rev. Inst. Med. trop. S Paulo. 2006;48(1):33-37.
- [Google Scholar]
- Larvicidal activity of Tagetes patula essential oil against three mosquito species. Bioresour. Technol.. 2005;96(11):1235-1240.
- [Google Scholar]
- Insecticidal activity of Valeriana jatamansi (Verbenaceae) against mosquitoes. J. Am. Mosq. Control Assoc.. 2008;24:315-318.
- [Google Scholar]
- Adulticidal activity of essential oil of Lantana camara leaves against mosquitoes. Indian J. Med. Res.. 2010;131:434-439.
- [Google Scholar]
- Toxic and Growth Retarding Effects of Three Plant Extracts on Culex pipiens Larvae (Diptera: Culicidae) Phytother. Res.. 1999;13:388-392.
- [Google Scholar]
- Oviposition-deterrent, ovicidal, and repellent activities of indigenous plant extracts against Anopheles subpictus Grassi (Diptera: Culicidae) Parasitol. Res.. 2009;1051567:1576.
- [Google Scholar]
- Laboratory study on larvicidal activity of indigenous plant extracts against Anopheles subpictus and Culex tritaeniorhynchus. Parasitol. Res.. 2009;104(6):1381-1388.
- [Google Scholar]
- Studies on effects of indigenous plant extracts on filarial vector Culex tritaeniorhynchus Giles. Parasitol. Res.. 2010;107:167-176.
- [Google Scholar]
- Efficacy of leaves extract of Calotropis procera Ait (Asclepiadaceae) in controlling Anopheles arabiensis and Culex quinquefasciatus mosquitoes. Saudi J. Biol. Sci.. 2009;16:95-100.
- [Google Scholar]
- Larvicidal, adult emergence inhibition and oviposition deterrent effects of foliage extract from Ricinus communis L Against Anopheles arabiensis and Culex quinquefasciatus in Sudan. Trop. Biomed.. 2009;26(2):130-139.
- [Google Scholar]
- Does antiviral therapy have a role in the control of Japanese encephalitis? Antiviral Res.. 2008;78(1):140-149.
- [Google Scholar]
- Larvicidal activity of Tagetes minuta (marigold) toward Aedes aegypti. J. Am. Mosq. Control Assoc.. 1991;7(2):282-286.
- [Google Scholar]
- Larvicidal & emergence inhibitory activities of NeemAzal T/S 1.2 per cent EC against vectors of malaria, filariasis & dengue. Indian J. Med. Res.. 2009;130(2):138-145.
- [Google Scholar]
- Efficacy of Zingiber officinale on third stage larvae and adult fecundity of Musca domestica and Anopheles pharoensis. J. Egypt Soci. Parasitol.. 2008;38(2):385-392.
- [Google Scholar]
- Laboratory evaluation of the aqueous extract of Azadirachta indica (neem) wood chippings on Anopheles gambiae s.s. (Diptera: Culicidae) mosquitoes. J. Med. Entomol.. 2009;46(1):107-114.
- [Google Scholar]
- Limonoids from Citrus reticulata and their moult inhibiting activity in mosquito Culex quinquefasciatus larvae. Phytochemistry. 1997;44(5):843-846.
- [Google Scholar]
- Studies on effects of Pelargonium citrosa leaf extracts on malarial vector, Anopheles stephensi Liston. Bioresour. Technol.. 2003;89(2):185-189.
- [Google Scholar]
- Larvicidal potential of medicinal plant extracts against Anopheles subpictus Grassi and Culex tritaeniorhynchus Giles (Diptera: Culicidae) Parasitol. Res.. 2009;104:1163-1171.
- [Google Scholar]
- Non-target effects of neem-based insecticides on aquatic Invertebrates. Ecotoxicol. Environ. Safety. 1997;36:109-117.
- [Google Scholar]
- Liu, S.Q., Shi, J.J., Cao, H., Jia, F.B., Liu, X.Q., Shi, G.L., 2000. Survey of pesticidal component in plant. In Entomology in China in 21st Century, Proceedings of Conference of Chinese Entomological Society ed. Dianmo, Li Beijing, China: Science & Technique Press, pp. 1098-1104.
- Screening of Asteraceae (Compositae) plant extracts for larvicidal activity against Aedes fluviatilis (Diptera:Culicidae) Mem. Inst. Oswaldo. Cruz.. 1997;92(4):565-570.
- [Google Scholar]
- Botanical biocides. 4 Mosquitocidal activity of certain Thymus capitatus constituents. J. Nat. Toxins. 2000;9(1):49-62.
- [Google Scholar]
- Antipupational effect of neem oil and neem seed kernel extract against mosquito larvae of Anopheles stephensi (Liston) J. Ent. Res.. 1996;20:137-139.
- [Google Scholar]
- Effects of plant extracts on fecundity and fertility of mosquitoes. J. Appl. Entomol.. 2001;125:31-35.
- [Google Scholar]
- Effects of neem limonoids on malarial vector Anopheles stephensi Liston (Diptera: Culicidae) Acta. Trop.. 2005;96:47-55.
- [Google Scholar]
- Isolation of the insecticidal components of Tagetes minuta (Compositae) against mosquito larvae and adults. J. Am. Mosq. Control Assoc.. 1995;11(3):307-310.
- [Google Scholar]
- A laboratory diagnosis of Japanese encephalitis using monoclonal antibodies and correlation of findings with the outcome. J. Med. Virol.. 1989;29:221-223.
- [Google Scholar]
- Forecasting mosquito abundance to prevent Japanese encephalitis. Curr. Sci.. 2003;84(9):1172-1173.
- [Google Scholar]
- A new insecticidal protolimonoid from Aegle marmelos. Nat. Prod. Res.. 2004;18(2):117-122.
- [Google Scholar]
- Insecticidal activity of callus culture of Tagetes erecta. Fitoterapia. 2004;75(1):62-64.
- [Google Scholar]
- The toxicity and physiological effect of neem limonoids on Cnaphalocrocis medinalis (Guene´e), the rice leaffolder. Pest. Biochem. Physiol.. 2005;81:113-122.
- [Google Scholar]
- Anti-juvenile activity of Azadirachta indica extract on the development and morphometry of filaria vector, Culex quinquefasciatus (Diptera: Culicidae) Say. Parasitol. Res.. 2009;105(5):1193-1203.
- [Google Scholar]
- Studies on mosquito larvicidal properties of Eucalyptus citriodora Hook (family-Myrtaceae) J. commun. Dis.. 2007;39(4):233-236.
- [Google Scholar]
- WHO, 1981. Instructions for determining the susceptibility or resistance of adult mosquitoes to organochlorine, organophosphate and carbamate insecticides: diagnostic test. Geneva: WHO/VBC/81–807.
- WHO, 2005. Communicable disease tool kit, Sudan. World Health Organization, WHO/CDS/2005.26.
- Larvicidal activity of saponin containing extracts and fractions of fruit mesocarp of Balanites aegyptiaca. Fitoterapia. 2006;77(6):420-424.
- [Google Scholar]
- In vitro antifilarial effects of three plant species against adult worms of subperiodic Brugia malayi. J. Ethnopharmacol.. 2001;78(1):79-84.
- [Google Scholar]







