Translate this page into:
Synthesis, characterization and thermal properties of sodium pyruvate thiosemicarbazone and some of its metal complexes
*Corresponding author. Tel.: +964 7901 333 232 a_jasim2006@yahoo.com (Ahmed Jasim M. Al-Karawi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 16 October 2010
Abstract
The Schiff base sodium pyruvate thiosemicarbazone (SPTSC) was synthesized from the condensation of sodium pyruvate and thiosemicarbazide hydrochloride in aqueous medium. This molecule coordinates to a variety of divalent metal ions M2+ (M = Fe, Co, Ni, Cu or Cd) and one type of complexes was obtained with general formulae [M (SPTSC)2]. The modes of bonding and overall geometry of the complexes were determined through physicochemical and spectroscopic methods. These studies revealed octahedral geometry of all prepared complexes. Thermal analyses (TG and DTG) of (SPTSC) and all complexes have been carried out to confirm the final molecular structures of free ligand and its metal complexes and also to study their thermal stability. This study confirms that the complexes have been formed by the coordination of metal ion to the (SPTSC) donor atoms only (there is no coordinate and hydrate solvents). Also the study shows that the prepared complexes have more thermal stability than free ligand (SPTSC) and the thermal stability of (SPTSC) has been enhanced by the coordination to metal ions.
Keywords
Sodium pyruvate
Thiosemicarbazide
Thermal properties
1 Introduction
Pyruvic acid is an organic acid. It is also a ketone, as well as being the simplest alpha-keto acid. The carboxylate ion (anion) of pyruvic acid (CH3COCOO−) is known as pyruvate and is a key intersection in several metabolic pathways (Cody et al.,2000). The coordination behaviour of aliphatic carboxylic acids with aldehyde or ketone groups is interesting due to the presence of multi-coordination sites. The anions of these acids can act as monodentate, bridging bidentate or chelating ligands showing ambidentate character (Raju and Sivasankar, 2009). Various transition metal complexes with bi, tri and tetradentate Schiff bases containing nitrogen, sulfur and oxygen donor atoms play an important role in biological systems and represent interesting models for metalloenzymes, which efficiently catalyze the reduction of dinitrogen and dioxygen (Frausto and Williams, 1991; Kaim and Schwederski, 1996). Furthermore, metal complexes of thiosemicarbazone derivatives have attracted special attention due to their biological activity (Scovill et al., 1982) and medicinal properties (Petering et al., 1964; El Asmy et al., 1987). In this work we reported the synthesis and characterization of sodium pyruvate thiosemicarbazone (SPTSC). This molecule coordinates to a variety of divalent metal ions M2+ (M = Fe, Co, Ni, Cu or Cd) and one type of complexes with octahedral geometry are obtained. Thermal analyses (TG and DTG) of (SPTSC) and the complexes have been carried out to study their thermal stability.
2 Experimental
2.1 Materials and measurements
All reagents were commercially available (Aldrich Chemical Co.) and were used without further purification. All manipulations in the synthesis of (SPTSC) and the complexes were performed in air. Solvents used in the synthesis were distilled from the appropriate drying agent immediately prior to use.
Elemental analysis was performed on a (C.H.N) analyzer, from (CARLO ERBA 1108). Conductivity measurements were made with DMF solution using a Jenway Ltd.4071 digital conductometer. Room temperature magnetic moments were measured with a Magnetic susceptibility balance (Jonson, Mattey catalytic system Division). UV–Vis spectra for the compounds were measured in the region (200–900) nm for (10−3 M) solution in DMF at (RT) by using (UV–Vis) spectrophotometer type Shimadzu, 100. Infrared spectra were recorded as KBr discs using the (8400) (FTIR) Shimadzu spectrophotometer in the range (4000–400) cm−1. 1H NMR spectrum was recorded in DMSO-d6 using a Brucker 300 MHz with a tetramethylsilane (TMS) as an internal standard. Thermal analyses (TGA and DTG) of (SPTSC) and the complexes were carried out by using PerkinElmer thermal analysis. Atomic absorption data were obtained with the aid of a Phoenix-986 AA spectrophotometer.
2.2 Synthetic procedures
2.2.1 Preparation of thiosemicarbazide hydrochloride
Thiosemicarbazide hydrochloride was prepared using literature procedure (Mark et al., 1985). A typical preparation consisted of adding 4 ml of concentrated HCL to a slurry of 4.4 g thiosemicarbazide in 80 ml absolute ethanol. The slurry was stirred overnight and the white powdery product was isolated by filtration and washed several times with absolute ethanol.
2.2.2 Preparation of sodium pyruvate thiosemicarbazone (SPTSC)
This preparation is slightly modified from literature preparation (Mark et al., 1985). A solution of sodium pyruvate (2.2 g, 20 mmol) in 20 ml distilled water was added dropwise with stirring to solution of thiosemicarbazide hydrochloride (2.55 g, 20 mmol) in 20 ml distilled water. The stirring was continuous for 1 h at room temperature and the white microcrystalline product was isolated by filtration, washed with distilled water several times and finally recrystallized from distilled water to give 2.85 g from pure product (checked by TLC, eluent; 3:1 benzene, methanol; Rf = 0.6). Yield 79%, m.p. = 208–210 °C. Elemental analysis (calculated value in parentheses): C 25.33(25.0), H 3.21(3.64), N 21.52(21.88). 1H NMR data (ppm): 2.05 (CH3, s, 3H), 8.6 (NH2, d, 2H; JHH = 31 Hz), 10.63 (NH, s, 1H).
2.2.3 Synthesis of the complexes
The preparation of all complexes is essentially the same and so a generic description will be presented. To a hot solution of (SPTSC) 2 mmol in water (25 ml) was added 1 mmol of metal salt (FeCl2·4H2O, Co(NO3)2·6H2O, Ni(NO3)2·6H2O, CuCl2·2H2O, Cd(NO3)2·4H2O). The solution was stirred under reflux and a microcrystalline solid was deposited over the course of 1 h. The solid was removed by filtration washed with hot water, then diethyl ether and finally dried in vacuo. 1H NMR data of [Cd(SPTSC)2] (ppm): 2.1 (CH3, s, 3H), 7.85 (NH2, d, 2H; JHH = 30 Hz), 9.8 (NH, s, 1H). All attempts to grow crystals suitable for X-ray crystallography were unsuccessful. Elemental analysis data, color and yield for the complexes are given in Table 1.
Compound
Color
Yield (%)
m.p. (°C)
Found (calcd.) %
Λ/(S cm2 mol−1)
M
C
H
N
(SPTSC)
White
79
208–210
–
25.33(25.0)
3.21(3.64)
21.52(21.88)
–
[Fe(SPTSC)2]
Dark brown
48
323–325
12.88(14.85)
25.98(25.55)
3.02(3.19)
22.88(22.35)
8.9
[Co(SPTSC)2]
Red
80
320–323
14.30(15.54)
25.03(25.34)
3.84(3.17)
22.54(22.17)
8.5
[Ni(SPTSC)2]
Green
92
322–326
13.87(15.50)
24.94(25.35
3.97(3.17)
22.64(22.18)
6.5
[Cu(SPTSC)2]
Dark green
82
240–242
15.18(16.56)
25.80(25.03)
3.76(3.13)
22.14(21.90)
10.4
[Cd(SPTSC)2]
White
94
305–307
23.44(25.99)
22.89(22.20)
2.15(2.78)
20.01(19.43)
7.6
3 Results and discussions
Sodium pyruvate thiosemicarbazone (SPTSC), Scheme 1 was prepared by the aqueous reaction between sodium pyruvate and thiosemicarbazide hydrochloride in mole ratio of (1:1). The Schiff base ligand was characterized by elemental analyses, FTIR, UV–Vis and H NMR spectroscopy. The reactions of SPTSC with a variety of divalent metal ions (M = Fe, Co, Ni, Cu or Cd), in a 2:1 stoichiometric ratio, respectively, gave rapid deposition of microcrystalline solids, and despite numerous attempts, we have been unable to produce crystals suitable for X-ray structural analysis. However, the color, melting points, elemental analysis and molar conductivity values, Table 1, of these materials indicate that they all are neutral complexes with the formulation [M(SPTSC)2], Scheme 2. Thermal analyses (TG and DTG) have been carried out for the free ligand (SPTSC) and all its metal complexes in order to study the thermal properties and stability of all of them.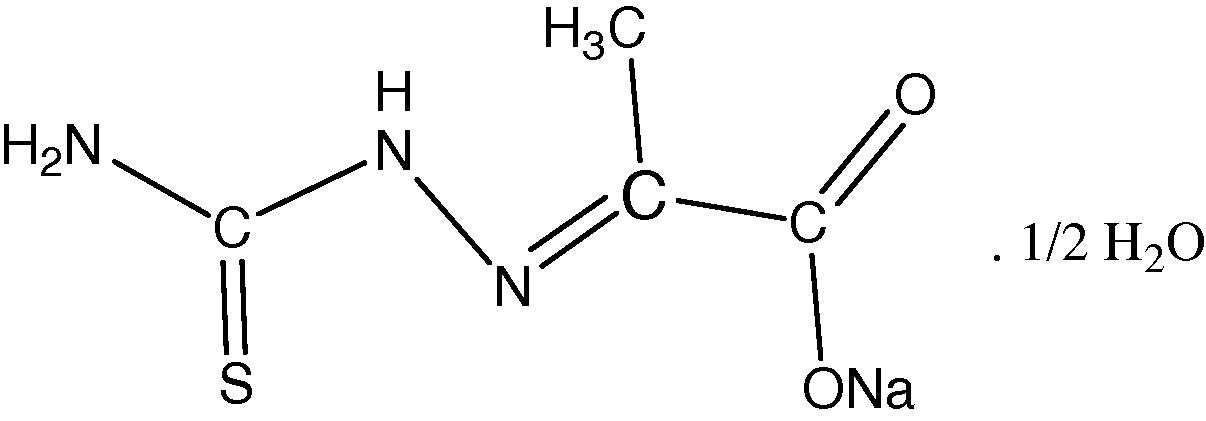
General structural formulae of (SPTSC).
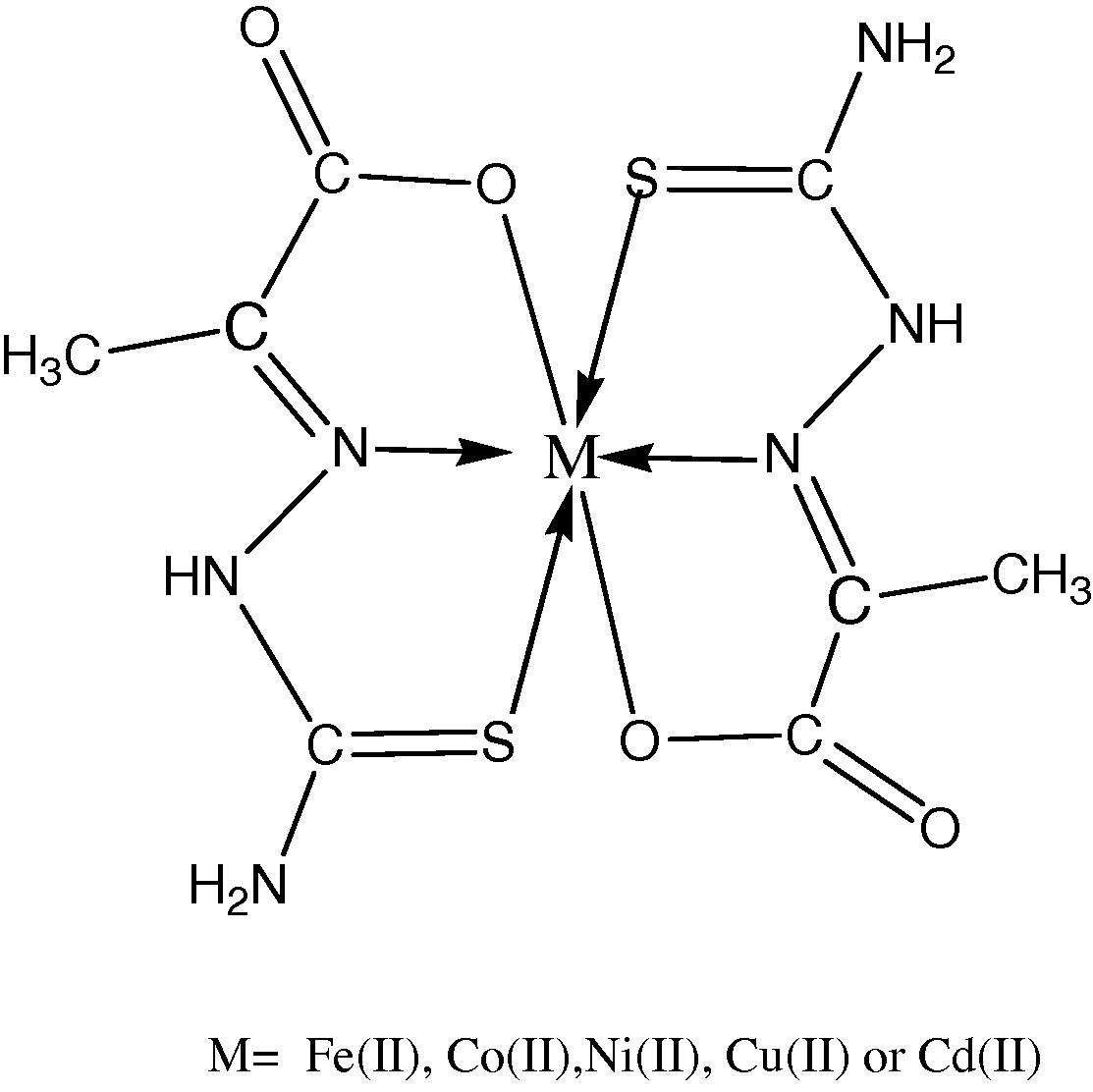
Proposed structures of the complexes.
The molar conductivity data of all complexes in DMSO, Table 1 correspond to their nonelectrolytic nature (Geary, 1971). The room temperature magnetic moment data of Fe(II), Co(II), Ni(II) and Cu(II) complexes are 5.1, 4.6, 2.9 and 1.86, respectively. These data indicate the high spin nature of these complexes. Due to the completely filled (d) orbitals the cadmium complexes are diamagnetic.
The 1H NMR spectrum of (SPTSC) in DMSO-d6 Fig. 1A, showed three characteristic signals, singlet at 2.05 ppm which could be assigned to protons of methyl group (CH3), doublet at 8.6 ppm, this signal may be attributed to the protons of NH2 group. The third signal was observed at 10.63 ppm as a singlet which can be assigned to the proton of NH group. The 1H NMR spectrum of [Cd(SPTSC)2] in DMSO-d6 Fig. 1B showed no change in the number of signals compared with the spectrum of free ligand (slightly different in the signals positions). This indicates that the coordination of SPTSC to the metal ions has occurred through sulfur atom as thione (i.e. there is no deprotonation of hydrazide nitrogen site and thiolization of C⚌S group) (Al-Karawi et al., 2009).![1H NMR spectra A1 and A2 for SPTSC, B for [Cd(SPTSC)2].](/content/185/2012/24/1/img/10.1016_j.jksus.2010.08.006-fig3.png)
1H NMR spectra A1 and A2 for SPTSC, B for [Cd(SPTSC)2].
In order to study the binding mode of the Schiff base ligand (SPTSC) to the metal ion in complexes, the IR spectrum of the free ligand was compared with the spectra of the complexes. The IR spectrum of (SPTSC) Fig. 2A shows band in the regions 3406 cm−1 and 1734 cm−1 which are assigned to the υ(NH) and υ(C⚌O) vibrations, respectively. The spectrum also shows band with double peaks in the range of (3292–3282) cm−1, which could be attributed to the asymmetrical and symmetrical stretching vibrations of NH2 group. The uasym and usym stretching of carboxylate ion (COONa) were observed at 1626 cm−1 and 1340 cm−1, respectively (Raju and Sivasankar, 2009). The bands due to u(C⚌N) and u(C⚌S) vibrations are located in the regions 1516 and 1271 cm−1, respectively. The (C⚌S) group is less than (C⚌O) group and has a considerably weaker band; in consequence the band is not intense. Identification is therefore difficult and uncertain. Spectra of compounds in which the (C⚌S) group is attracted to a nitrogen atom show an absorption band in the general (C⚌S) stretching region. In addition several other bands in the broad region of 1515–700 cm−1 can be attributed to vibrations involving interaction between (C⚌S) stretching and (C–N) stretching (Silverstien et al., 2005). All the IR spectra of complexes show bands around (3427–3379 cm−1) which are due to stretching vibrations of NH group. The uasym and usym stretching of NH2 group were observed in all spectra of complexes, Table 2. Also the spectra show bands in the range (1228–1207 cm−1) which could be assigned to stretching vibration of C⚌S group. The stretching vibration of (C⚌N) group is found to shift to higher wave numbers in the spectra of all complexes in comparison with free ligand, suggesting the coordination of the azomethane nitrogen atom to the central metal ions, in agreement with previous studies (Rejani et al., 1999; Al-Karawi, 2009). The uasym and usym stretching of carboxylate group in all the complexes spectra are comparable with that of (SPTSC), which suggests the ionic nature of pyruvate ion in the complexes.![FTIR Spectra of (A) SPTSC, (B) [Co(SPTSC)2], (C) [Cd(SPTSC)2].](/content/185/2012/24/1/img/10.1016_j.jksus.2010.08.006-fig4.png)
FTIR Spectra of (A) SPTSC, (B) [Co(SPTSC)2], (C) [Cd(SPTSC)2].
Compound
(uasym) usym
υ(NH)
υ(C⚌N)
υ(C⚌S)
υ(M–O)
υ(M–N)
υ(M–S)
NH2
(SPTSC)
(3292) 3282
3406
1516
1271
–
–
–
[Fe(SPTSC)2]
(3292) 3182
3392
1575
1220
554
480
410
[Co(SPTSC)2]
(3290) 3184
3392
1573
1224
545
490
400
[Ni(SPTSC)2]
(3284) 3182
3385
1566
1228
551
484
405
[Cu(SPTSC)2]
(3315) 3182
3427
1583
1207
511
424
410
[Cd(SPTSC)2]
(3290) 3173
3379
1583
1217
540
450
420
UV–Vis spectrum of Schiff base ligand (SPTSC) Fig. 3, exhibited an intense absorption peak at (33,003 cm−1) related to the (π–π∗) transition. The (n–π∗) transition may be obscured by this absorption band. The UV–Vis spectra of all prepared complexes showed hypsochromic shift of ligand band except Cd(II) complex which caused bathochromic shift, Table 3. The appearance of new medium intensity bands at lower wave numbers were observed either merged or slightly separated from the intra ligand (π–π∗) band. These bands were mainly attributed to charge transfer transition. Further bands with very low extinction coefficients appeared in the visible and near IR region. These bands were attributed to ligand field (d–d) transitions (Lever, 1968; Figgis, 1966; Sutton, 1969). The electronic spectrum of Fe(II) complex showed the (π–π∗) transition plus two additional bands which could be attributed to CT and spin allowed transition in octahedral geometry (Lever, 1968; Figgis, 1966; Sutton, 1969; Lokesh and Sulekh, 2006). The electronic spectrum of the Co(II) complex recorded in DMSO solution displayed three peaks at (27,700 cm−1), (19,723 cm−1) and (14,749 cm−1). These bands may be assigned to the CT, (4T1g(F) → 4A2g(F)) and (4T1g(F) → 4T2g(F)) transitions, respectively (Lever, 1968; Figgis, 1966; Sutton, 1969; El Asmy et al., 2005). The position of these bands suggests an octahedral environment around the cobalt (II) atom. The spectrum of Ni(II) complex displayed absorption bands at (26,525 cm−1) and (15,380 cm−1). These bands could be attributed to the spin allowed transitions (3A2g → 3T1g(P)) and (3A2g → 3T1g(F)), respectively, (Lever, 1968; Figgis, 1966; Sutton, 1969; El Asmy et al., 2005). The position of these bands confirms an octahedral geometry of nickel (II) complex. The electronic spectrum of Cu(II) complex shows band at (31,847 cm−1) which should be assigned to the CT. The spectrum also displayed a broad band in the range (14,285–13,947 cm−1). This band corresponded to the transition (2B1g → 2A1g). The position of these bands confirm the distorted octahedral Cu(II) complex (Lever, 1968; Figgis, 1966; Sutton, 1969). The electronic spectrum of Cd(II) complex exhibited bands at (32,362 cm−1) and (24,390 cm−1), which could be attributed to the ligand band (π–π∗) and CT transitions, respectively. No ligand field transitions are observed because of filled d–orbital. Since the d10 configuration affords no crystal field stabilization, the stereochemistry depends on size and polarizing power of the MII cation and the steric requirement of the ligand (Figgis, 1966).![UV–Vis spectra of (1) SPTSC, (2) [Co(SPTSC)2], (3) [Cu(SPTSC)2].](/content/185/2012/24/1/img/10.1016_j.jksus.2010.08.006-fig5.png)
UV–Vis spectra of (1) SPTSC, (2) [Co(SPTSC)2], (3) [Cu(SPTSC)2].
Compound
(u cm−1)
Extinction coefficient
Assignments
μeff (BM)
L Mol−1 cm−1
(SPTSC)
33,003
2672
π–π∗
–
[Fe(SPTSC)2]
36,764
1086
π–π∗
5.1
27,777
460
CT
17,543
150
5T2g → 5A1g
14,925
150
5T2g → 5Eg
[Co(SPTSC)2]
36,231
3142
π–π∗
4.6
27,700
500
CT
19,723
50
4T1g(F) → 4A2g(F)
14,749
40
4T1g(F) → 4T2g(F)
[Ni(SPTSC)2]
33,222
2400
π–π∗
2.9
26,525
170
3A2g → 3T1g(P)
15,380
150
3A2g → 3T1g(F)
[Cu(SPTSC)2]
35,971
1983
π–π∗
1.86
31,847
1666
CT
14,285–13,947
50
2B1g → 2A1g
[Cd(SPTSC)2]
32,362
887
π–π∗
Diamagnetic
24,390
60
CT
3.1 Thermal analyses
The content of a component in a complex changes with its composition and structure. Thus, the content of such component can be determined based on the mass losses of these components in the thermogravimetric plots of the complex (Hatakeyama and Liu, 1998; Al Shihri, 2004). Therefore, the thermogravimetric analysis (TGA and DTGA) (50–900 °C) for prepared complexes was recorded to distinguish between the coordinate and hydrate solvents and to give an insight into the thermal stability of the studied complexes. In order to study the thermal stability of the prepared complexes, the TG plot of the free ligand was compared with the TG plots of prepared complexes. The TG plot of (SPTSC) Fig. 4 showed that it was decomposed in three successive steps. These decomposition steps occurred in the temperature range 150–350 °C. The first decomposition peak occurred at 150 °C with a percentage weight loss of 3.7%. This decomposition step was due to a loss of H2O molecule. The other decomposition peaks may be attributed to degradation of thiosemicarbazide and sodium pyruvate moieties, Table 4.![Thermogravimetric analysis TG and DTG of (A) SPTSC, (B) [Co(SPTSC)2], (C) [Ni(SPTSC)2], (D) [Cd(SPTSC)2], (E) [Cu(SPTSC)2].](/content/185/2012/24/1/img/10.1016_j.jksus.2010.08.006-fig6.png)
Thermogravimetric analysis TG and DTG of (A) SPTSC, (B) [Co(SPTSC)2], (C) [Ni(SPTSC)2], (D) [Cd(SPTSC)2], (E) [Cu(SPTSC)2].
Compound
Stage
TG – temperature range (°C)
TG mass loss (%)
Residue
Found
(Calcd.)
(SPTSC)
I
150–180
3.7
(4.7)
C4H6N3O2NS
II
210–230
35.9
(34.9)
C3H6N3S
III
250–350
37.3
(39.0)
C2H3N
[Co (SPTSC)2]
I
330–400
65.2
(64.5)
Co N2S2
II
430–520
5.6
(6.6)
Co S2
III
575–680
5.6
(7.5)
Co S
[Ni(SPTSC)2]
I
320–400
68.5
(67.8)
NiNS2
II
520–600
3.8
(3.3)
NiS2
III
680–760
5.6
(7.5)
NiS
[Cu (SPTSC)2]
I
230–280
45.2
(45.6)
C6H6N2O4Cu
II
310–380
20.3
(20.0)
C2N2O2Cu
III
400–440
8.2
(8.8)
CuNO2
[Cd (SPTSC)2]
I
320–360
48.7
(50.6)
Cd N2O2S2
II
450–650
14.4
(12.6)
Cd S2
The curves obtained for [Co(SPTSC)2], [Ni(SPTSC)2] and [Cd(SPTSC)2] Fig. 4 showed no decomposition peaks below 300 °C. This highly confirms that the complexes have been formed by the coordination of metal ion to the (SPTSC) donor atoms only (i.e. there is no coordinate and hydrate solvents). Also the curves showed thermal stability up to 330 °C for [Co(SPTSC)2], 320 °C for [Ni(SPTSC)2] and [Cd(SPTSC)2] Fig. 4. This leads to say that the prepared complexes have more thermal stability than free ligand (SPTSC) and the thermal stability of (SPTSC) has been enhanced by the coordination to metal ions. The final decomposition steps of the complexes are attributed to complete decomposition of the complexes leaving MS residue (M = Co, Ni) and CdS2 for Cd(II) complex. The details of degradation data of SPTSC and all prepared complexes are illustrated in Table 4.
Acknowledgments
We thank the Dept. of Chemistry, college of science, Al-Mustansiriya University for supplying us with some chemicals. Also we wish to thank Prof. Rosiyah Yahya, Faculty of Science polymer, Dept. of Chemistry, University of Malaya for her kind help by carrying out the thermal analyses (TG and DTG) for the ligand and its complexes.
References
- Synthesis, characterization and thermal analysis of some new transition metal complexes of a polydentate Schiff base. Spectrochim. Acta, Part A. 2004;60:1189.
- [Google Scholar]
- Synthesis and characterization of a new N2S2 Schiff base ligand and its complexes with nickel(II), copper(II) and cadmium(II) including the kinetics of complex formation. Transition Met. Chem.. 2009;34:891-897.
- [Google Scholar]
- Synthetic, structural and kinetic studies on the binding of cyclohexane-1,2-bis(4-methyl-3-thiosemicarbazone) to divalent metal ions (Co, Ni, Cu, Zn or Cd) Dalton Trans.. 2009;564:570.
- [Google Scholar]
- Primordial carbonylated iron–sulfur compounds and the synthesis of pyruvate. Science. 2000;289:1337.
- [Google Scholar]
- Ligational, corrosion inhibition and antimicrobial properties of 4-phenyl-1-benzenesulphonyl-3-thiosemicarbazide. Transition Met. Chem.. 1987;12:428-431.
- [Google Scholar]
- Spectral, magnetic, thermal and electrochemical studies on phthyloyl bis(thiosemicarbazide) complexes. J. Coord. Chem.. 2005;58(18):1735.
- [Google Scholar]
- Introduction to Ligand Field. New York: John Wiley and sons; 1966. first ed
- The Biological Chemistry of the Elements. Oxford: Clarendon press; 1991.
- The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev.. 1971;7:81.
- [Google Scholar]
- Handbook of Thermal Analysis. UK: Wiley Chichester; 1998.
- Bioinorganic Chemistry: Inorganic Elements in the Chemistry of Life. New York: Wiley; 1996.
- Inorganic Electronic Spectroscopy (second ed.). New York: Elsevier; 1968.
- Physicochemical and biological characterization of transition metal complexes with nitrogen donor tetradentate novel macrocyclic ligand. Transition Met. Chem.. 2006;31:368.
- [Google Scholar]
- Dynamics of spin-state interconversion and cooperativity for ferric spin-crossover complexes in the solid state. 4. Pyruvic acid thiosemicarbazone complex. Inorg. Chem.. 1985;24:3450.
- [Google Scholar]
- The Anti-tumor activity of 2-keto-3-ethoxybutyraldehyde bis(thiosemicarbazone) and related compounds. Cancer Res.. 1964;24:367.
- [Google Scholar]
- Spectral, thermal, and X-ray studies on some new bis and tris-hydrazine and hydrazinium metal pyruvates. J. Therm. Anal. Calorim.. 2009;98:371-376.
- [Google Scholar]
- Nickel (II), copper (I) and copper (II) complexes of bidentate heterocyclic thiosemicarbazones. J. Braz. Chem. Soc.. 1999;10(3):184-188.
- [Google Scholar]
- 2-Acetylpyridine thiosemicarbazones. 4. Complexes with transition metals as antimalarial and antileukemic agents. J. Med. Chem.. 1982;25:1261.
- [Google Scholar]
- Spectrophotometric Identification of Organic Compounds (seventh ed.). New York: John Wiley and sons; 2005.
- Electronic Spectra of Transition Metal Complex (first ed.). New York: McGraw-Hill publ. Co. Ltd.; 1969.







