Translate this page into:
Electrospray technique for cocrystallization of phytomolecules
⁎Corresponding author. sharvilpatil25@gmail.com (Sharvil Patil) sharvil.patil@bharatividyapeeth.edu (Sharvil Patil)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract

Abstract
Poor aqueous solubility of most of the phytomolecules has restricted their vital biological use. Cocrystal approach can be one of the remedies for the problem. The present work was carried out with an objective to screen the potential of electrospray technique towards cocrystallization of quercetin (QUE). QUE was cocrystallized with caffeine (CAF) and nicotinamide (NIC) as coformers. Saturated methanolic solutions of QUE with either of the coformers (CAF and NIC) in 1:1 ratio were electrosprayed at 40 °C. The technique was successfully used for cocrystallization of QUE with CAF and NIC separately as revealed by Powder X-ray Diffraction, Differential scanning calorimetry and Fourier transform infrared spectroscopy studies. Additionally, the results of saturation solubility study suggested 14 and 11 folds increase in solubility of QUE upon its cocrystallization with CAF and NIC respectively. Herein we propose a new technique for cocrystallization and in turn resolving solubility issues of phytomolecules which can be an alternative to the existing cocrystallisation techniques.
Keywords
Electrospray crystallization
Cocrystals
Quercetin
Caffeine
Nicotinamide
1 Introduction
Several biological activities including anticancer, antimicrobial, antiviral and antioxidant have been reported for most of the phytomolecules such as curcumin, plumbagin, galangin, and quercetin. However, formulation development of many phytomolecules has remained a challenge for researchers owing to the drawback of poor aqueous solubility. It is put onto the record that the fundamental properties of crystalline materials are dependent on the molecular arrangement within the solid, and these properties are greatly influenced upon alteration in the arrangement of molecules (Seddon and Zaworotko, 1999; Gandhi et al., 2016). Thus in order to obtain desired properties of crystals, crystal engineering has remained one of the focus areas of formulation scientists. Cocrystallization of active pharmaceutical ingredient is widely used amongst the several approaches of engineering crystals to address problems such as poor aqueous solubility and stability associated with APIs. According to USFDA guidelines, pharmaceutical co-crystals are crystalline materials consisting of an active pharmaceutical ingredient (API) and a co-former present in the same crystal lattice (FDA, 2011).
The methods proposed for cocrystal synthesis includes solution crystallisation (Friščić, 2012), Liquid assisted grinding (Trask et al., 2005), use of ultrasound (Aher et al., 2012), spray drying (Patil et al., 2014) and twin screw extrusion (Kelly et al., 2012). These methods have drawbacks like the conventional solution crystallization method has been found unsuitable for non-congruent cocrystal pairs, liquid assisted grinding lacks scalability and has issues related to solvent selection and stoichiometric diversity. Solvent free cocrystallization of APIs using twin screw extrusion is unsuitable for thermolabile drugs. Thus researchers are searching new methods for cocrystallization of APIs to mitigate the drawbacks of existing ones. Recently, we have proposed use of electrospray technology for cocrystallization of caffeine and maleic acid which is known to be one of the most difficult pairs for cocrystallization (Patil et al., 2016). However, applicability of this technique for other molecules including phytomolecules is questionable. Thus in the present work we have tried to apply electrospray technique for cocrystallization of quercetin, a well known phytomolecule.
Electrospray technique uses an electric charge for dispersing the liquid into droplets by overcoming the surface tension of the solvents. The process involves formation of Taylor cone at the tip of needle having positive charge through which a mist of ultrafine charged solution droplets is emitted and travel towards collector plate having negative charge. It is well known that the Taylor cone exhibits high energy vibrations which propagate into jet forming mist of droplets (Rulison and Flagan, 1993). Additionally this single step technique can be used in a continuous manner (Wang et al., 2012). The technique is currently being used for the preparation of nanofibres. The high energy vibrations of Taylor cone and rapid solvent evaporation during electrospray were thought to be beneficial in cocrystal synthesis as these factors will increase the rate of crystal nucleation and growth tremendously. Thus objective of the present work was to analyze suitability of electrospray technique for synthesis of cocrystals. Quercetin, 3,3′,4′,5,7-pentahydroxyflavone (QUE) a flavonoid of plant kingdom was taken as a model molecule. QUE has many beneficial pharmacological effects however its in vivo performance is questionable due to poor aqueous solubility leading to unfavorable pharmacokinetics (Karadag et al., 2014; Fang et al., 2011). In the present work cocrystallization of QUE with caffeine and nicotinamide coformers has been attempted using electrospray technique.
2 Materials and methods
2.1 Materials
Quercetin (QUE), Caffeine (CAF) and nicotinamide (NIC) were purchased from Sigma Aldrich, Bengaluru, India. The analytical-grade solvents used for the studies were obtained from the commercial sources.
2.2 Methods
2.2.1 Electrospray process
Saturated methanolic solutions of QUE with either of the coformers (CAF and NIC) were prepared separately in 1:1 ratio. The prepared solutions of QUE/CAF and QUE/NIC were electrosprayed using E-spin nano (PECO-Chennai, India) equipment. The parameters used for the whole process include syringe (10 mL), Flow rate-2 mL/hr, temperature-40 ± 1 °C, Voltage-15 kV with a distance between needle tip and collector being 25 cm. The cocrystals recovered upon electrospraying of solutions are abbreviated as QUECAF and QUENIC.
2.3 Characterization
2.3.1 Powder X-ray Diffraction (PXRD)
Powder X-ray diffraction (PXRD) patterns for QUE, CAF, NIC, QUECAF and QUENIC were recorded using X-ray diffractometer (PW 1729; Philips, Almelo, Netherlands) having Cu Kα radiation of 1.542 Å and operating at a voltage of 40 kV. Samples were scanned from 3° to 30° at 2θ.
2.3.2 Differential Scanning Calorimetry (DSC)
DSC thermograms of QUE, CAF, NIC, QUECAF and QUENIC were recorded using DSC 821e (Mettler-Toledo, Greifensee, Switzerland). Samples (5–10 mg) were heated in hermetically sealed aluminium pan with a heating rate of 10 °C/min over a range of 25–350 °C under a nitrogen atmosphere (flow rate 50 ml/min).
2.3.3 Fourier transform infrared spectroscopy (FTIR)
KBR discs were prepared containing about 2–3 mg of QUE, CAF, NIC, QUECAF and QUENIC separately. IR spectra were recorded from 4000 to 400 cm−1 with a Fourier transform infrared spectrometer (FTIR-8400; Shimadzu Corporation, Kyoto, Japan) equipped with a diffuse reflectance accessory (DRS-8000; Shimadzu Corporation, Japan).
2.3.4 Saturation solubility
Saturation studies were carried out for QUE, QUECAF and QUENIC. The crystals in amounts that exceeded its solubility were separately transferred to screw capped vials containing 5 mL 1:1v/v ethanol/water mixture separately. Magnetic stirrer was used to stir the contents of vials at room temperature for 24 h. Preliminary experiments suggested this duration to be sufficient to reach equilibrium, after which no improvement in solubility was observed. After 24 h, samples were filtered through a Whatman paper (0.45-μm) diluted with distilled water and analyzed for QUE content at 360 nm using a spectrophotometer (Shimazdu-1601, UV–vis spectrophotometer, Shimadzu Corp, Kyoto, Japan).
3 Results
In the preliminary studies, electrospraying of methanolic solutions containing QUE/CAF and QUE/NIC (1:1 ratio) was attempted at different voltages below 15 kV. However as stated previously the surface tension forces of solvent must be overcome by the electrical forces to initiate the electrospray process. The voltages below 15 kV were insufficient to initiate electrospray involving formation of mist of droplets through Taylor cone. Thus the present work has been performed at 15 kV. Further the distance between the needle tip and plate collector was kept 25 cm so as achieve complete evaporation of methanol till the API molecules reach the collector plate. Since, the objective of the present work was to screen suitability of electrospray technique towards cocrystal synthesis, the electrospraying was carried out at a voltage of 15 kV with a needle aperture of 23G (0.6 mm) keeping needle tip and collector 25 cm apart.
3.1 Powder X-ray Diffraction
PXRD pattern of QUE showed characteristic peak of 2θ at 10°, 10.7°, 13.5°, 16.5°, 17.8°, 21.7°, 24.4°, 25.1°, 26° and 27.7° (Fig. 1). PXRD pattern of CAF showed characteristic peaks at 12° and 12.2° 2θ whereas NIC showed peaks at 11.16°, 14.6°, 18.8°, 22°, 25.69°, and 27.18° 2θ. PXRD pattern of QUECAF showed distinct peaks at 9.5°, 11.25°,12.39°, 12.93°, 14.56°, 16.29°, 21.19°, 23.18°, 26.18° and 26.85° 2θ which were in accordance with the literature (Smith et al., 2011) whereas QUENIC showed peaks at 6.65°, 7.43°, 11.13°, 11.81°, 12.58°, 14.86°, 16.14°, 19°, 24.61° and 25.39° 2θ revealing their purity and existence in crystalline form (Leyssens et al., 2012; Patil et al., 2015). Additionally, the distinct peaks observed in PXRD patterns of QUENIC when compared to PXRD patterns of QUE and NIC alone suggested formation of cocrystals of QUE with NIC. To the best of our knowledge, cocrystals of QUE with NIC have been reported for the first time. In order to confirm the results of PXRD, DSC analysis was performed.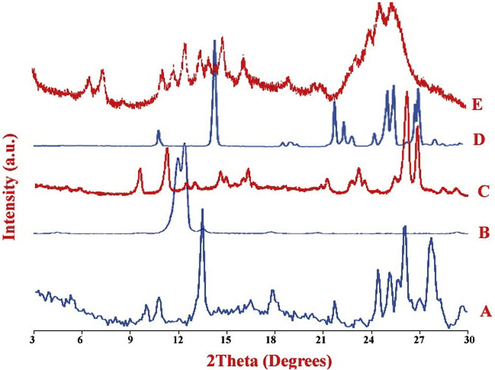
PXRD patterns of (A) QUE, (B) CAF, (C) QUECAF, (D) NIC and (E) QUENIC.
3.2 Differential Scanning Calorimetry
DSC thermograms for CAF and NIC showed sharp, characteristic melting endothermic peaks at 238 °C and 129.16 °C respectively. DSC thermograms of QUECAF showed melting endotherm at 243.6 °C matching with the reported value (Smith et al., 2011) whereas QUENIC cocrystals showed melting endotherm at 232.72 °C (Fig. 2). DSC can also be used to confirm cocrystal formation by analyzing the melting endotherms. Generally the melting point value of cocrystals falls between the melting point of API and a conformer (Schultheiss and Newman, 2009) which is the case in the current work suggesting formation QUENIC cocrystals.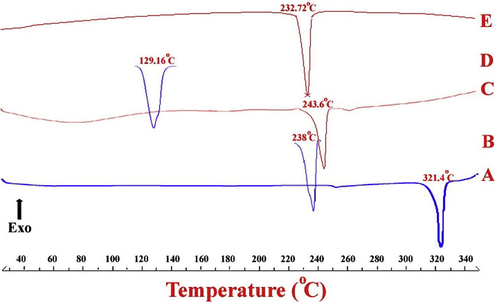
DSC thermograms of (A) QUE, (B) CAF, (C) QUECAF, (D) NIC and (E) QUENIC.
3.3 Fourier transform infrared spectroscopy
QUE showed characteristic FTIR band at 3459 cm−1 (free-OH bond vibration) the stretching vibration of C⚌O group showed bands at 1731 cm−1, 1671 cm−1 and 1619 cm−1. Additionally, an aromatic group of QUE showed bands at 1550 cm−1, 1520 cm−1 and 1469 cm−1 along with C-O-C vibration at 1320 cm−1 and 1165 cm−1 (Ni et al., 2015). CAF showed characteristic peaks at 1659 cm−1 (C⚌O stretch), 1240 cm−1 and 1359 cm−1 (C-N stretch). QUECAF showed red shift in the band assigned to free-OH bond vibration which appeared at 3351 cm−1 and red shift in the bands observed for C⚌O stretch which appeared at 1743, 1697 and 1649 cm−1. Thus QUECAF involved hydrogen bond interaction between phenolic O—H-----Naromatic and phenolic O—H-----O⚌C supramolecular H-bonds (Fig. 3).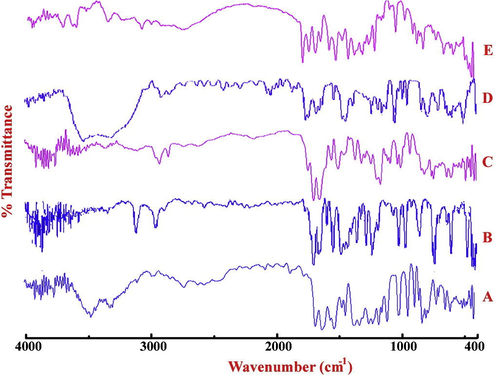
FTIR spectrum of (A) QUE, (B) CAF, (C) QUECAF, (D) NIC and (E) QUENIC.
NIC showed the bands at 3367 and 3161 cm−1 corresponding to the asymmetric and symmetric stretching vibrations of the −NH2 group which are blue shifted to 3422 cm−1 and 3180 cm−1 in QUENIC. Additionally, the bands at 1699 cm−1 and 1620 cm−1 due to C⚌O stretch vibrations are blue shifted 1705 cm−1 and 1659 cm−1 respectively. Further appearance of two new bands at 2924 cm−1 and 2854 cm−1 in QUENIC indicates the hydrogen-bonding interaction between phenolic O—H and N aromatic (Wang et al., 2013).
3.4 Saturation solubility
We tried to determine solubility of QUE in water however the amount dissolved was below the detection level of the method used. Thus 1:1 ethanol/water mixture was used for the assessment of solubility of QUE, QUECAF and QUENIC as described previously (Smith et al., 2011). The solubility of QUE alone was found to be 0.284 ± 0.11 mg/mL whereas that of QUECAF and QUENIC was 4.102 ± 0.21 and 3.12 ± 0.32 mg/mL respectively. Thus the solubility of QUE was found to be increased by 14 and 11 folds when formulated as cocrystals with CAF and NIC. Such drastic increment in solubility of QUE could be attributed to the increase in surface area available for dissolution of drug upon solubilization of coformer. The electrospray technique has been reported to produce nanopharmaceuticals (Radacsi et al., 2012). Thus generation of nano cocrystals of QUECAF and QUENIC by electrospray technique could be another reason for improvement in solubility of QUE.
4 Discussion
It is well known fact that the crystal habit of a molecule depends on the hydrogen-bonding propensity of a solvent. During nucleation and crystal growth, on one hand the solvated molecules must get desolvated during their integration in the crystal lattice while at the same time on the other hand, the adsorbed solvent molecules on the growing crystal surface must be replaced by the incoming solute molecules in order to get integrated into the crystal lattice (Gu et al., 2001). The solvent-solute interaction involving van der Waals force and hydrogen bonding govern these aspects of crystal growth. In the present work methanol was used as a solvent for cocrystallization of QUE. Methanol has hydrogen bond acceptor (HBA)/hydrogen bond donor (HBD) value >0.5 revealing its ability to form hydrogen bonds. In electrospray process the methanolic solutions containing QUE and either of the coformer form a Taylor cone involving high shear mixing which subsequently propagates into mist. During such transformation positive charge is generated on the solvent molecules (present at Taylor cone) which are then pulled towards negatively charged collector plate. Such pull of solvent molecules along with its components (API and coformer) increases the area for evaporation of solvent molecules which ultimately induces supersaturation and subsequent crystallization of API and coformers together forming a cocrystal. Additionally, since nanosize droplets are generated during the electrospray process complete solvent evaporates till it reaches the collector plate raising the chances of cocrystallization. Thus the technique can be extended for resolving solubility issues of other phytomolecules so as to extract their biological activities. Our group has been currently working on the same.
5 Conclusion
In the present work electrospray technique has been successfully used for cocrystallization of quercetin. Cocrystals of quercetin with nicotinamide were reported for the first time. The solubility of quercetin was found be increased by 14 and 11 folds upon its cocrystallization with caffeine and nicotinamide respectively. Thus an electrospray technique can be used for continuous manufacturing of cocrystals.
Conflicts of interest
The authors report no conflicts of interest.
References
- Ultrasound assisted cocrystallization from solution (USSC) containing a non-congruently soluble cocrystal component pair: Caffeine/maleic acid. Eur. J. Pharm. Sci.. 2012;41:597-602.
- [Google Scholar]
- Bovine serum albumin nanoparticle promotes the stability of quercetin in simulated intestinal fluid. J. Agric. Food Chem.. 2011;59(11):6292-6298.
- [Google Scholar]
- Food and Drug Administration, Guidance for Industry: Regulatory Classification of Pharmaceutical Co-crystals, Silver Spring, 2011.
- Supramolecular concepts and new techniques in mechanochemistry: cocrystals, cages, rotaxanes, open metal-organic frameworks. Chem. Soc. Rev.. 2012;41(9):3493-3510.
- [Google Scholar]
- Ultrasound-assisted preparation of novel ibuprofen-loaded excipient with improved compression and dissolution properties. Drug Dev. Ind. Pharm.. 2016;42(10):1553-1563.
- [Google Scholar]
- Polymorph screening: influence of solvents on the rate of solvent-mediated polymorphic transformation. J. Pharm. Sci.. 2001;90(11):1878-1890.
- [Google Scholar]
- Quercetin nanosuspensions produced by high-pressure homogenization. J. Agric. Food Chem.. 2014;62(8):1852-1859.
- [Google Scholar]
- Monitoring ibuprofen–nicotinamide cocrystal formation during solvent free continuous cocrystallization (SFCC) using near infrared spectroscopy as a PAT tool. Int. J. Pharm.. 2012;426:15-20.
- [Google Scholar]
- Importance of solvent selection for stoichiometrically diverse cocrystal systems: caffeine/maleic acid 1:1 and 2:1 cocrystals. Cryst. Growth Des.. 2012;12(3):1520-1530.
- [Google Scholar]
- Quercetin loaded nanostructured lipid carrier for food fortification: preparation, characterization and in vitro study. J. Food Process Eng.. 2015;38:93-106.
- [Google Scholar]
- Exploring the potential of electrospray technology in cocrystal synthesis. Ind. Eng. Chem. Res.. 2016;55:8409-8414.
- [Google Scholar]
- Liquid crystalline phase as a probe for crystal engineering of lactose: carrier for pulmonary drug delivery. Eur. J. Pharm. Sci.. 2015;68:43-50.
- [Google Scholar]
- Generation of 1:1 carbamazepine: nicotinamide cocrystals by spray drying. Eur. J. Pharm. Sci.. 2014;62:251-257.
- [Google Scholar]
- Electrospray crystallization for nanosized pharmaceuticals with improved properties. Cryst. Growth Des.. 2012;12:3514-3520.
- [Google Scholar]
- Scale-up of electrospray atomization using linear arrays of Taylor cones. Rev. Sci. Instrum.. 1993;64(3):683-686.
- [Google Scholar]
- Pharmaceutical cocrystals and their physicochemical properties. Cryst. Growth Des.. 2009;9(6):2950-2967.
- [Google Scholar]
- Seddon, K.R., Zaworotko, M.J., 1999. Crystal Engineering: The Design and Application of Functional Solids; NATO-ASI Series, Kluwer, Dordrecht, the Netherlands, vol. 539, pp. 553–560.
- Cocrystals of quercetin with improved solubility and oral bioavailability. Mol. Pharm.. 2011;8(5):1867-1876.
- [Google Scholar]
- Pharmaceutical cocrystallization: engineering a remedy for caffeine hydration. Cryst. Growth Des.. 2005;5:1013-1021.
- [Google Scholar]
- Pharmaceutical cocrystals of diflunisal with nicotinamide or isonicotinamide. Org. Process Res. Dev.. 2013;17:1413-1418.
- [Google Scholar]
- Production and characterization of carbamazepine nanocrystals by electrospraying for continuous pharmaceutical manufacturing. J. Pharm. Sci.. 2012;101(3):1178-1188.
- [Google Scholar]







