Translate this page into:
Characterization and fermentation optimization of novel thermo stable alkaline protease from Streptomyces sp. Al-Dhabi-82 from the Saudi Arabian environment for eco-friendly and industrial applications
⁎Corresponding author. naldhabi@ksu.edu.sa (Naif Abdullah Al-Dhabi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study a novel thermo stable alkaline protease producing Streptomyces sp. Al-Dhabi-82 was isolated from the soil. The effect of fermentation period on enzyme production by Streptomyces sp. Al-Dhabi-82 was optimized in submerged fermentation. Protease activity was found to be maximum after 5 days of incubation (129.5 ± 7.1 U/ml) and depleted after 6 days of incubation (113.8 ± 4.1 U/ml). Enzyme production increases with the increase in pH up to 9.0 (136.2 ± 3.6 U/ml) and enzyme production depleted significantly at pH 11.0 (67 ± 2.9 U/ml). Maximum production of protease was observed at 40 °C (164 ± 11.1 U/ml). Among the evaluated carbon sources, maltose significantly influenced on protease production (212 ± 14.8 U/ml). The optimum amount of protease (269 ± 10.4 U/ml) produced by Streptomyces sp. Al-Dhabi-82 was observed in the production medium containing yeast extract. Enzyme production was maximum in the presence of 0.15% Ca2+. The specific activity of crude enzyme was 26 U/ mg protein and it increased as 276 U/mg protein after chromatography separation. The molecular weight of purified protease obtained from sephadex G-75 gel filtration chromatography was estimated to be 37 kDa using SDS-PAGE. This enzyme showed high activity at pH 9.0, and lost about 16% activity at pH 10. The optimum temperature for Streptomyces sp. Al-Dhabi-82 protease was 40 °C. The extracellular alkaline protease from Streptomyces sp. Al-Dhabi-82 hydrolyzed chicken feather completely.
Keywords
Actinomycetes
Streptomyces sp.
Alkaline protease
Optimization
1 Introduction
Proteases are very important group of industrial enzymes and are used extensively in various industries including leather, textiles, detergents, cheese, meat tenderization, baking, dehairing, brewery, organic synthesis and waste water treatment (Sun et al., 2019). These enzymes are used in the recovery of silver ions from used photographic film and in digestive aids (Schmid and Verger, 1998). Alkaline proteases are important group of enzymes that specifically hydrolyze proteins into peptides and amino acids and involved catalysis of peptide synthesis. Proteases account more than 60% of the Global enzyme market and are widely used in leather, textiles, pharmaceuticals and detergents (Pastor et al., 2001). Many microorganisms, including yeast, bacteria, actinomycetes, fungi and plant produced various alkaline proteases. Microbial alkaline proteases can meet the required market demand for various industrial processes due to short doubling time than animals and plants. The most of the proteases produced by microbial species for commercial applications are extracellular origin, and these enzymes showed stability towards wide physical and chemical changes in the environment (Moreira et al., 2001). Most alkaline proteases such as commercial preparations of Esparase, alkaline proteases, Savinase, Subtilisin Carlsbery are produced by bacteria mainly from the genus Bacillus. Proteases from actinomycetes showed unique activity however report on protease production by Streptomyces is limited (Ramesh et al., 2009). Keratinolytic proteases have great potential in the degradation of keratin waste from leather and poultry industries. The important application of keratinolytic enzyme is to hydrolyze keratin from the leather industry, conversion of wastes in textile industry, cosmetics, medicines and also drug delivery. Microbial proteases have been used widely for various industrial processes. However, alkaline proteases find great applications in various industries, including detergent industry however, limitations were reported in detergent formulations (Joo et al., 2003). Hence, the demand for potent active proteases with good stability over a range of temperature, pH and mineral ions and various organic solvent continue to initiate the search for novel proteolytic enzymes (Rajkumar et al., 2011). Proteases are widely used in leather processing industries, in the process of dehairing, thus totally eliminating the application of chemicals (Vijayaraghavan and Vincent, 2012; Vijayaraghavan et al., 2012). Due to the heavy demand of proteolytic enzymes in the leather processing industry, there is a continuous search for novel proteases.
Streptomyces species produce multiple proteases in the culture medium. Many studies have been carried out on the proteolytic enzymes of various mesophilic actinomycetes (Al-Dhabi et al., 2016, 2019a, 2019c). The organism such as, Streptomyces thermonitrificans produced maximum protease activity with novel properties. In addition, alkaline protease has also been purified from Nocardiopsis sp. NCIM 5124 (Mohamedin, 1999). Hence the production of novel proteolytic enzymes from actinomycetes should be studied to meet industrial demand. Actinomycetes synthesized various groups of industrially useful proteolytic enzymes, especially Streptomyces sp. produces multiple proteases in the culture medium, which is generally regarded as safe for various industrial processes (Sun et al., 2019; Al-Dhabi et al., 2018a, 2018b, 2019b, 2019d; Arasu et al., 2017). The species such as, S. griseus and S. thermovulgaris were used for the production of proteases (De Azeredo et al., 2006; Arasu et al., 2013, 2019a, 2019b, 2019c; Arokiyaraj et al., 2015). Proteases such as, serine protease from Streptomyces fradiae and Streptomyces fradiae have been studied for its enzyme action and structure (Boovaragamoorthy et al., 2019;Gurusamy et al., 2019; Roopan et al., 2019; Valsalam et al., 2019; Ilavenil et al., 2015; Balachandran et al., 2015). Also, alkaline proteases have been purified and characterized from various species namely, Streptomyces gulbargensis, Streptomyces sp., Streptomyces clavuligerus and Streptomyces viridifacens (Dastager et al., 2008). In this study, an attempt was made to use Streptomyces sp. Al-Dhabi-82 for enhanced production of alkaline protease. Medium composition of culture medium influences effectively on protease production in Streptomyces sp. Al-Dhabi-82. The purified protease from Streptomyces sp. Al-Dhabi-82 showed potent activity and stability at various conditions. Hence this enzyme could be considered for various eco-friendly applications.
2 Materials and methods
2.1 Chemicals and reagents
The isolation medium and nutrients medium used for the isolation and characterization of the actinomcyetes strains were procured from Sigma, USA. The antibiotics used in the experiments were procured from Himedia, India.
2.2 Isolation of Streptomyces sp. isolates
Soil samples were collected from the Jazan region of Saudi Arabia. For isolation of Streptomyces, 1% soil suspension was appropriately diluted and was placed on starch casein agar plates. Antibiotics such as nystatin and nalidixic acid were added to reduce microbial growth. The potent strain Streptomyces sp. Al-Dhabi-82 was purified by repeated streaking on starch casein agar plates.
2.3 Screening of actinomycetes isolates for protease production
Skimmed milk agar medium was used for the production of proteases. 1% skimmed milk was supplemented with minimal medium and the actinomycetes isolates were streaked on the culture medium and incubated for 5 days at 28 °C. A clear zone appears around the actinomycete isolates if the strain is protease positive (Mitra and Chakrabartty, 2005).
2.4 Morphological and cultural characteristics of Streptomyces sp. Al-Dhabi-82
The actinomycete isolate was subjected to morphological, cultural characteristics and biochemical analysis. Also, 16S rDNA sequencing was performed to identify the potent actinomycete (Korn-Wendisch and Kutzner, 1991) and identified as Streptomyces sp. Al-Dhabi-82.
2.5 Protease production from Streptomyces sp. Al-Dhabi-82
In this study Streptomyces sp. Al-Dhabi-82 was grown in basal medium for protease production. Basal medium consists of: glucose 0.5 g/l; KNO3, 0.6 g/l; peptone, 10 g/l; MgSO4. 7 H2O, NaCl, 5 g/l; CaCl2, 1.0 g/l and K2HPO4, 0.5 g/l. To the basal medium 1% casein (w/v) was added. To this medium inoculum was added and incubated for 7 days. The cell free culture was obtained after centrifugation and assayed for protease activity.
2.6 Optimization of fermentation conditions for protease production
2.6.1 Effect of fermentation period on enzyme production
Effect of incubation time was analyzed by incubating the culture for 6 days, then enzyme activit was assayed.
2.6.2 Effect of medium pH on enzyme production
The effect of pH on enzymes production was performed by varying pH of the medium from 6 to 11, then enzyme assay was carried out.
2.6.3 Effect of culture medium temperature on enzyme production in Streptomyces sp. Al-Dhabi-82
The effect of culture medium temperature for enzyme production was carried out by varying temperatures. Enzyme assay was performed as described earlier.
2.6.4 Effect of carbon sources on enzyme production
Effect of carbon source on enzyme production was performed with glucose, lactose, fructose, maltose, arabinose, starch, xylose and trehalose at 1% level with the basal medium. After that enzyme assay was performed.
2.6.5 Effect of nitrogen sources on enzyme production
Effect of various nitrogen sources on the enzyme activity was performed with nitrogen sources at 1% level. It was incubated for 6 days at 175 rpm on Orbital shaker. After that enzyme assay was performed as described previously.
2.6.6 Effect of ions on enzyme production
The ions such as, Ca2+, Mg2+, Mn2+, Cu2+ and Hg2+ (0.1%, w/v) was added with the basal medium. It was incubated for 6 days at 175 rpm on Orbital shaker. After that enzyme assay was performed as described previously.
2.7 Purification of alkaline protease
In this study, alkaline proteases activity by Streptomyces sp. Al-Dhabi-82 were purified and quantified by the method of Laemmli (1970).
2.8 Characterization of enzymes
2.8.1 Effect of pH, temperature and ions on enzyme activity and stability
Effect of pH, temperature and different ions (Mg2+, Mn2+, Cu2+, Hg2+, Fe2+, Zn2+, Na+, Co2+ and Ca2+) was evaluated by following the standard methodology.
2.9 Degradation of keratinous wastes using proteolytic enzymes from Streptomyces sp. Al-Dhabi-82
In this study feather degradation efficacy of alkaline protease from Streptomyces sp. Al-Dhabi-82 was evaluated. Erlenmeyer flask (250 ml) containing 1.0 gm feather with 100 ml buffer (sodium phosphate buffe, pH 8.0, 0.1 M) was autoclaved for 20 min at 121 °C. The feather degradation step was initiated by applying 500 U of alkaline protease with the Erlenmeyer flask. To the control flask, enzyme was not added.
3 Results
3.1 Isolation and screening of actinomycetes protease production
Analyses of the actinomyces strains in the various soil samples for the production of proteases were performed. The highest level of the protease producing actinomycetes was isolated. The microscopic observations revealed that this organism is Gram-positive, non-acid fast, filamentous and branched. Biochemical tests revealed that this was catalase positive and showed positive reaction to nitrate reduction, starch hydrolyzing ability, negative response in Voges-Proskauer and indole production. Based on microscopic observations, staining properties and 16S rDNA sequencing, this organism was identified as, Streptomyces sp. Al-Dhabi-82. The selected organism showed 19 mm zone on skimmed milk agar plates.
3.2 Optimization of protease production
3.2.1 Physiochemical factors on protease activity
The effect of fermentation period on enzyme production by Streptoymyces sp. Al-Dhabi-82 was optimized in submerged fermentation. Protease activity was high after 5 days (129.5 ± 7.1 U/ml) and depleted after 6 days of incubation (113.8 ± 4.1 U/ml) (Table 1a). Effect of pH on alkaline protease activity was performed for 5 days at various pH values (6.0 – 11.0). In this study the production of proteases increases with the increase in pH up to 9.0 (136.2 ± 3.6 U/ml) and enzyme production depleted significantly at pH 11.0 (67 ± 2.9 U/ml) (Table 1b). The production of protease increase with the increase in fermentation temperature up to 40 °C (164 ± 11.1 U/ml) (Table 1c).
Fermentation period (Days)
Enzyme activity (U/ml)
1
0 ± 0
2
37 ± 2.2
3
68 ± 1.5
4
84 ± 6.1
5
129 ± 7.1
6
113.8 ± 4.1
pH
Enzyme activity (U/ml)
6
14.1 ± 2.2
7
27.8 ± 1.9
8
106.3 ± 2.2
9
136.2 ± 3.6
10
123 ± 3.2
11
67 ± 2.9
Temperature (°C)
Enzyme activity (U/ml)
20
13 ± 3.2
25
48 ± 2.8
30
69 ± 2.1
35
96 ± 2.2
40
164 ± 11.1
45
144 ± 5.9
50
101 ± 4.6
3.2.1.1 Effect of nutritional factors on protease production
The result on the ability of Streptomyces sp. Al-Dhabi-82 on protease production by utilizing various carbon sources are given in Table 2a. Among the evaluated carbon sources, maltose significantly influenced on protease production (212 ± 14.8 U/ml). The optimum level of maltose for the production of protease was 1.5%. Streptomyces species produces less protease (163 ± 21.4 U/ml) by utilizing lactose. The optimum amount of protease (269 ± 10.4 U/ml) produced by Streptomyces sp. Al-Dhabi-82 was observed with yeast extract. Among various concentrations of yeast extract, 1.5% (w/v) significantly enhanced protease production (Table 2b and 2c).
Carbon source (1%)
Enzyme activity (U/ml)
Glucose
187 ± 12.8
Lactose
163 ± 21.4
Fructose
201 ± 10.4
Maltose
212 ± 14.8
Starch
183 ± 6.4
Trehalose
194 ± 7.6
Control
158 ± 13.4
Nitrogen source (1%)
Enzyme activity (U/ml)
Beef extract
182 ± 12.1
Yeast extract
269 ± 10.4
Peptone
253 ± 4.6
Glycine
178 ± 5.3
Ammonium sulphate
174 ± 11.3
Ammonium nitrate
156 ± 9.3
Urea
150 ± 4.9
Control
161 ± 11.2
Ionic sources (0.1%)
Enzyme activity (U/ml)
Ca2+
241 ± 3.8
Cu2+
41 ± 6.9
Mg2+
172 ± 5.4
Mn2+
169 ± 3.8
Hg2+
32.5 ± 8.7
Control
165 ± 5.6
3.2.1.2 Molecular properties of alkaline protease from Streptomyces sp. Al-Dhabi-82
In this study, crude protease was fractionated initially using ammonium sulphate, dialysis and purified by gel permeation chromatography. The ammonium sulphate precipitation fractionated proteolytic enzymes showed 1.3 fold purification (Fig. 1).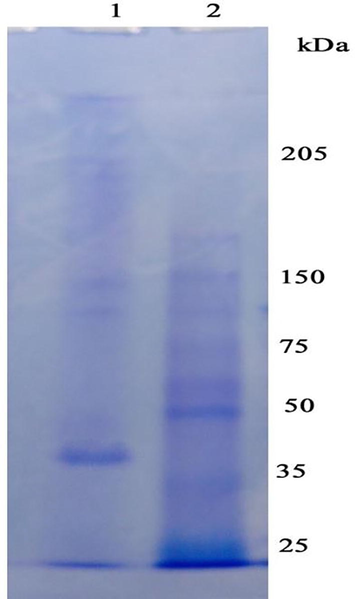
Protease enzyme analysis using SDS-PAGE 2. (Lane 1: purified enzyme; Lane 2: protein molecular weight marker).
3.3 Characterization of protease from Streptomyces sp. Al-Dhabi-82
The effect of pH on Streptomyces sp. Al-Dhabi-82 protease was studied. This enzyme showed high activity at pH 9.0 (Figs 2a and 2b). However, this enzyme showed very broad activity at alkaline pH range. The effect of temperature on protease activity from Streptomyces sp. Al-Dhabi-82 was shown in Fig. 3a. Streptomyces sp. Al-Dhabi-82 protease activity was high at 40 °C. At 70 °C, protease lost about 60% of its activity. At 40 °C, protease lost only 20% enzyme activity within 20 min of incubation (Fig. 3b). Among the metal ions tested Mg2+, Mn2+, Co2+ and Ca2+ activated protease activity, while other ions inactivated enzymes (Table 3).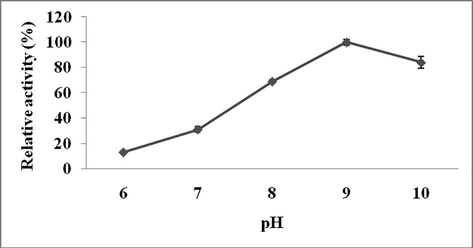
Effect of pH on enzyme activity. Purified enzyme was individually assayed to reveal optimum pH value.
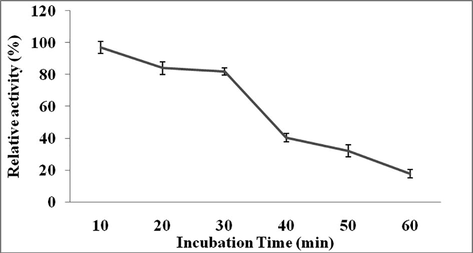
Effect of pH on enzyme stability from Streptomyces sp. Al-Dhabi-82 at various incubation times. Purified protease was incubated with buffer at pH 9.0 for 60 min at 10 min interval and relative enzyme activity was assayed.
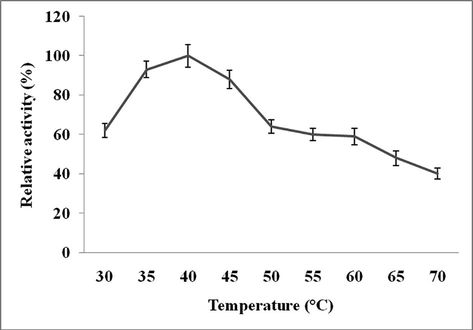
Effect of temperature on enzyme activity. Enzyme assay was performed at various temperatures (30–70 °C) individually, and relative activity was measured.
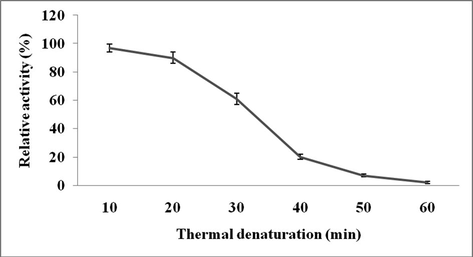
Effect of temperature on enzyme stability. The purified enzyme was individually pre-incubated at for 60 min and enzyme assay was performed by standard method.
Ions (10 mm)
Relative activity (%)
Mg2+
108.4 ± 6.7
Mn2+
102.1 ± 6.1
Cu2+
63 ± 3.4
Hg2+
27 ± 1.2
Fe2+
18 ± 1.0
Zn2+
37 ± 4.2
Na+
96 ± 4.8
Co2+
103 ± 5.0
Ca2+
118 ± 5.7
Control
100 ± 0.0
3.4 Degradation of keratinous waste by protease from Streptomyces sp. Al-Dhabi-82
The application of alkaline protease for feather degradation is very much efficient and eco-friendly. The extracellular alkaline protease from Streptomyces sp. Al-Dhabi-82 hydrolyzed chicken feather completely. Results also revealed that residual activity of Streptomces sp. Al-Dhabi-82 protease decreased over time (Fig. 4).
Degradation of chicken feather by alkaline protease from Streptomyces sp. (a) control, (b) hydrolyzed feather by proteases.
4 Discussion
Actinomycetes produce various bioactive compounds including enzymes. In this study, 45 actinobacteria strains isolated and screened for alkaline protease. Many bacteria produce alkaline protease and screened for various applications. Several workers used various screening plate media for the determination of alkaline protease (Kasana and Yadav, 2007; Vijayaraghavan et al., 2013). In this study, the isolated Actinomycetes utilized skimmed milk. Among the 45 actinobacteia, the strain Al-Dhabi-82 showed potent activity on substrate agar plate. It showed 14 mm zone of hydrolysis and was found to be high than other isolates. In the case of actinomycetes, colour of substrate mycelium and aerial mycelium are mainly considered as important characters for actinomycetes classification. The colour of the selected strain was grey and this kind of grey coloured actinomycete strains has been reported previously from the soil sample (Kim et al., 1998). The selected actinobacterium was Gram-positive, filamentous type and the colour of mycelium was light ash. Based on these characteristic features, the selected strain belonged to Streptomyces sp. Based on the cultural and molecular characteristics, the selected actinomycete was identified as Streptomyces sp. Al-Dhabi-82. The process parameters were optimized to enhance the production of protease. In our study, protease production was found to be high after 5th day of incubation at 28 ± 2 °C. The results of the present finding on fermentation period confirmed the synthesis of protease by Streptomyces rimosus and Streptomyces cyanens at the end of log phase (Yang and Wang, 1999; Petinate et al., 1999). Culture medium pH critically influenced on protease production. The present finding exhibited enhanced protease production at pH 9.0 after which enzyme activity declined sharply. It has been previously reported that the pH value 9.0 enhanced the production of alkaline protease in Streptomyces pulvereceus MTCC 8374 (Nadeem et al., 2008). Temperature is one of the significant factors influenced on alkaline protease production. Enzyme production was maximum at 40 °C. This result was in good agreement with previous studies on other Streptomyces sp. (Yeoman and Edwards, 1994). In this study supplementation of maltose showed enhanced protease level. It was reported that the carbon sources such as fructose and lactose enhanced protease production (Sen and Satyanarayana, 1993). In our study, yeast extract (1.5%) enhanced protease production. Yang and Lee (2001) reported the influence of yeast extract on protease production from Streptomyces sp. Also, supplementation of Ca2+ greatly enhanced the production of protease. In general, supplementation of cation induces the secretion of proteases and also stabilizes enzyme activity (Yang and Wang, 1999).
In this study, alkaline protease from Streptomyces sp. Al-Dhabi-82 was purified various methods. During protease purification, the active gel filtration fractions were pooled and lyophilized. The specific activity of protease purified from Streptomyces species was 276 U/mg and this has been higher than other extracellular alkaline protease from Streptomyces megasporus strain SDP4 and Streptomyces sp. MAB18 (Manivasagan et al., 2013; Moreira et al., 2003).. In our study the molecular weight of alkaline protease was found to be 37 kDa. The molecular weight of protease from Thermus aquaticus YT-1 was 38 kDa and it was 43 kDa in the case of Bacillus circularns MTCC 7942 (Patil et al., 2014; Ol dzka et al., 2003). Alkaline protease activity was maximum at 40 °C and was stable up to 30 min at this temperature. Enzyme activity was maximum at pH 9.0 and was highly stable at this pH value for 40 min and further declined. Metal ions play significant role on enzyme activity. In our study, protease activity was enhanced by adding Ca2+ ions with the reaction mixture. It was previously reported that most of alkaline protease from microbial origin are depend on Ca2+ ion for its activity and further inhibited by various heavy metals (Ol dzka et al., 2003). The effect of purified alkaline protease on feather degradation was analyzed. The alkaline protease form Streptomyces sp. Al-Dhabi-82 degraded more than 90% keratin from the feather. This digestibility efficacy was found to be high than that of previous report. This clearly indicated that additional enzymes were not required for the hydrolysis of feather meal (Boeckle et al., 1995).
5 Conclusion
A novel extracellular alkaline protease producing Streptomyces sp. Al-Dhabi-82 was isolated, screened and characterized. The process parameters were optimized to enhance the production of proteases. Enzyme obtained from Streptomyces sp. Al-Dhabi-82 hydrolyzed chicken feather completely. This Streptomyces sp. Al-Dhabi-82 strain and enzymes may further be exploited for various industrial applications.
Acknowledgments
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (11-BIO1873-02).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring, Saudi Arabia. Extremophiles. 2016;20:79-90.
- [Google Scholar]
- Chemical profiling of Streptomyces sp. Al-Dhabi-2 recovered from an extreme environment in Saudi Arabia as a novel drug source for medical and industrial applications. Saudi J. Biol. Sci.. 2019;26:758-766.
- [Google Scholar]
- Chemical constituents of Streptomyces sp. strain Al-Dhabi-97 isolated from the marine region of Saudi Arabia with antibacterial and anticancer properties. J. Infect. Public Health 2019
- [CrossRef] [Google Scholar]
- Environmental friendly synthesis of silver nanomaterials from the promising Streptomyces parvus strain Al-Dhabi-91 recovered from the Saudi Arabian marine regions for antimicrobial and antioxidant properties. J. Photochem. Photobiol., B. 2018;189:176-184.
- [Google Scholar]
- Green biosynthesis of silver nanoparticles produced from marine Streptomyces sp. Al-Dhabi-89 and their potential applications against wound infection and drug resistant clinical pathogens. J. Photochem. Photobiol., B 2019111529
- [Google Scholar]
- Characterization of silver nanomaterials derived from marine Streptomyces sp. Al-Dhabi-87 and its in vitro application against multidrug resistant and extended-spectrum beta-lactamase. Clinical Pathogens. Nanomater.. 2018;8(5)
- [Google Scholar]
- Bioactivity assessment of the Saudi Arabian Marine Streptomyces sp. Al-Dhabi-90, metabolic profiling and its in vitro inhibitory property against multidrug resistant and extended-spectrum beta-lactamase clinical bacterial pathogens. J. Infect. Public Health. 2019;12:549-556.
- [Google Scholar]
- Green chemical approach towards the synthesis of CeO2 doped with seashell and its bacterial applications intermediated with fruit extracts. J. Photochem. Photobiol., B. 2017;172:50-60.
- [Google Scholar]
- One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol., B. 2019;190:154-162.
- [Google Scholar]
- Antibacterial and antifungal activities of polyketide metabolite from marine Streptomyces sp. AP-123 and its cytotoxic effect. Chemosphere. 2013;90(2):479-487.
- [Google Scholar]
- Synthesis and characterization of ZnO nanoflakes anchored carbon nanoplates for antioxidant and anticancer activity in MCF7 cell lines. Mater. Sci. Eng., C. 2019;102:536-540.
- [Google Scholar]
- Essential oil of four medicinal plants and protective properties in plum fruits against the spoilage bacteria and fungi. Ind. Crops Prod.. 2019;133:54-62.
- [Google Scholar]
- Green synthesis of silver nanoparticles using aqueous extract of Taraxacum officinale and its antimicrobial activity. South Indian J. Biol. Sci.. 2015;2:115-118.
- [Google Scholar]
- Antimicrobial and cytotoxic properties of Streptomyces sp. (ERINLG-51) isolated from SouthernWestern Ghats. South Indian J. Biol. Sci.. 2015;1:7-14.
- [Google Scholar]
- Characterization of a keratinolytic serine proteinase from Streptomyces pactum DSM 40530. Appl. Environ. Microbiol.. 1995;61:3705-3710.
- [Google Scholar]
- Clinically important microbial diversity and its antibiotic resistance pattern towards various drugs. J. Infect. Public Health 2019
- [CrossRef] [Google Scholar]
- Proteolytic activity from an Alkali-Thermotolerant Streptomyces gulbargensis sp. nov. Curr. Microbiol.. 2008;57:638-643.
- [Google Scholar]
- A low-cost fermentation medium for thermophilic protease production by Streptomyces sp 594 using feather meal and corn steep liquor. Curr. Microbiol.. 2006;53:335-339.
- [Google Scholar]
- Environmental friendly synthesis of TiO2-ZnO nanocomposite catalyst and silver nanomaterials for the enhanced production of biodiesel from Ulva lactuca seaweed and potential antimicrobial properties against the microbial pathogens. J. Photochem. Photobiol., B. 2019;193:118-130.
- [Google Scholar]
- Growth and metabolite profile of Pediococcus pentosaceus and Lactobacillus plantarum in different juice. South Indian J. Biol. Sci.. 2015;1:1-6.
- [Google Scholar]
- Oxidant and SDS-stable alkaline protease from Bacillus Clausii I-52: production and some properties. J. Appl. Microbiol.. 2003;95:267-272.
- [Google Scholar]
- Isolation of a psychrotrophic Exiguobacterium sp. SKPB5 (MTCC7803) and characterization of its alkaline protease. Curr. Microbiol.. 2007;54:224-229.
- [Google Scholar]
- Characterization of the leupeptin inactivating enzyme from Streptomyces exofoliatus SMF13 which produces leupeptin. Biochem. J.. 1998;331:539-545.
- [Google Scholar]
- Korn-Wendisch, F., Kutzner, H.J., 1991. The Family Streptomycetaceae, The Prokaryotes, A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications, vol. 41. Springer Verlag, Berlin, New York, 965–968.
- Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature. 1970;227:680-685.
- [Google Scholar]
- Manivasagan, P., Venkatesan, J., Sivakumar, K., Kim, S., 2013. Production, characterization and antioxidant potential of protease from Streptomyces sp. MAB18 using poultry wastes. BioMed. Res. Int. 2013, Article ID 496586. doi.org/10.1155/2013/496586
- An extracellular protease with depilation activity fromStreptomyces nogalator. J. Sci Ind Res.. 2005;64(12):978-983.
- [Google Scholar]
- Isolation, identification and some cultural conditions of a protease producing thermophilic Streptomyces strain grown on chicken feather as a substrate. Int. Biodeter. Biodegrad.. 1999;43:13-21.
- [Google Scholar]
- Partial characterization of prote-ases from Streptomyces clavuligerus using an inexpensive medium. Brazil. J. Microbiol.. 2001;32:23-28.
- [Google Scholar]
- Moreira, K.A., Porto, T.S., Teixeira, M., Porto, A., Lima, Filho, J.L., 2003. New alkaline protease from Nocardiopsis sp.: partial purification and characterization. Process Biochem. 39, 67–72.
- Effect of medium composition on commercially important alkaline protease production by Bacillus licheniformis N-2. Food Technol. Biotechnol.. 2008;46:388-394.
- [Google Scholar]
- Ol dzka, G., D browski, S., Kur, J., 2003. High-level expression, secretion, and purification of the thermostable aqualysin I from Thermus aquaticus YT-1 in Pichia pastoris. Protein Expr. Purif. 29, 223–229.
- Protease obtention using Bacillus subtilis 3411 and amaranth seed meal medium at different aeration rates. Braz. J. Microbiol.. 2001;32:6-9.
- [Google Scholar]
- Detergent compatible, organic solvent tolerant alkaline protease from Bacillus circulans MTCC 7942: purification and characterization. Prep. Biochem. Biotechnol.. 2014;10:12-16.
- [Google Scholar]
- Influence of growth medium in protease and pigment production by Streptomyces cyanens. Mem. Inst. Oswaldo Cruz. Rio de Jenerio.. 1999;94:173-177.
- [Google Scholar]
- Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel. Inter. Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691-4695.
- [CrossRef] [Google Scholar]
- Characterization of a thermostable alkaline protease produced by marine Streptomyces fungicidicus MML1614. Bioprocess Biosyst. Eng.. 2009;32:791-800.
- [Google Scholar]
- CuO/C nanocomposite: synthesis and optimization using sucrose as carbon source and its antifungal activity. Mater. Sci. Eng., C. 2019;101:404-414.
- [Google Scholar]
- Lipases: interfacial enzymes with attractive applications. Angewandte Chem. Int.. 1998;37:1608-1633.
- [Google Scholar]
- Optimization of alkaline protease production by thermophilic Bacillus licheniformis S-40. Indian J. Microbiol.. 1993;33:43-47.
- [Google Scholar]
- Purification and biochemical characteristics of the extracellular protease from Pediococcus pentosaceus isolated from Harbin dry sausages. Meat Sci.. 2019;156:156-165.
- [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol., B. 2019;191:65-74.
- [Google Scholar]
- De-hairing protease production by an isolated Bacillus cereus strain AT under solid-state fermentation using cow dung: Biosynthesis and properties. Saudi J. Biol. Sci.. 2013;21:27-34.
- [Google Scholar]
- Cow dung: a potential biomass substrate for the production of detergent-stable dehairing protease by alkaliphilic Bacillus subtilis strain VV. SpringerPlus. 2012;1:76.
- [CrossRef] [Google Scholar]
- Cow dung as a novel, inexpensive substrate for the production of a halo-tolerant alkaline protease by Halomonas sp. PV1 for eco-friendly applications. Biochem. Eng. J.. 2012;69:57-60.
- [Google Scholar]
- Effect of culture media on protease and oxytetracycline production with mycelium and protoplasts of Streptomyces rimosus. World J. Microbiol. Biotechnol.. 2001;17:403-410.
- [Google Scholar]
- Protease and amylase production of Streptomyces rimosus in submerged and solid state cultivation. Bot. Bull. Acad. Sin.. 1999;40:259-265.
- [Google Scholar]
- Protease production by Streptomyces thermovulgaris grown on rapemeal-derived media. J. Appl. Bacteriol.. 1994;77:264-270.
- [Google Scholar]







