Translate this page into:
ZnS quantum dots and their derivatives: Overview on identity, synthesis and challenge into surface modifications for restricted applications
⁎Corresponding author. hidourislah@yahoo.co.in (Slah Hidouri)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Among different inert materials, quantum dots have been qualified by their fashion proprieties especially those related to structure and sizes. This review talks about the structural identity and some relevant methods to synthesize quantum dots, there is a challenge in the adaptation of nanoparticles to fulfill the biological, chemical and physical applications which demonstrate the crucial role of the surface. Modifications of the surface, cross stepwise the electronic transition layers of the particles, contribute to the functionalization in order to incorporate atoms in the main structure or immobilize small molecules onto the surface which lead to a restricted application. Finally, quantum dots are revealed to be an attractive material that can be a support for large potential applications in the future.
Keywords
Quantum dots
Pristine structure
Surface engineering
Biocompatible
Applications
1 Introduction
The semiconductor nanoparticles also called quantum dots (QDs) have been characterized by sizes generally inferior to 100 nm. Due to this small size, they exhibit specific properties different from those of the corresponding bulk materials. Moreover, they are characterized by a wide band gap with particular value of 3.66 eV for ZnS at room temperature (Medintz et al., 2005). QDs have particularly interesting fluorescence properties, the emission wavelength depends strongly on the particle size also and the nature of the corresponding phase, it is possible then to excite them in a wide wavelengths range below the wavelength of fluorescence. Their fluorescence performance exceeds those of conventional organic dyes. In addition to that, the quantum yield fluorescence and emission wavelength are greatly influenced by the particle size, which is directly related to their method of preparation, the nature and proportion of the doping element. Regarding these particular proprieties, quantum dots was considered to be among the best candidates for various applications, especially those based on electroluminescent exploration, solar cells, infrared windows, dielectric filters (Fedorov et al., 1991). As applications have been extended to various fields in biology, chemistry and physics, many new exigencies in the native nanoparticles becomes required. This review focuses on the identity revelation of the structure of ZnS nanomaterials family and explains the duality between a particular propriety and restricted application which reveal a real challenge to contribute in the manipulation of QDs at different levels from the outer layers to the most profound composition of the particles.
2 QDs identity
2.1 Crystal structure of semiconductor QDs II-VI
Semiconductors II-VI metal are classified in chalcogenides periodic group which generally crystallizes in cubic or hexagonal structures related to the arrangement of atoms participating in the structure Fig. 1. The cubic phase is sphalerite (B) which is more stable at room temperature (Fedorov et al., 1991). Crystallographic data of the structures of some semiconductors from the II-VI group are shown in Table 1.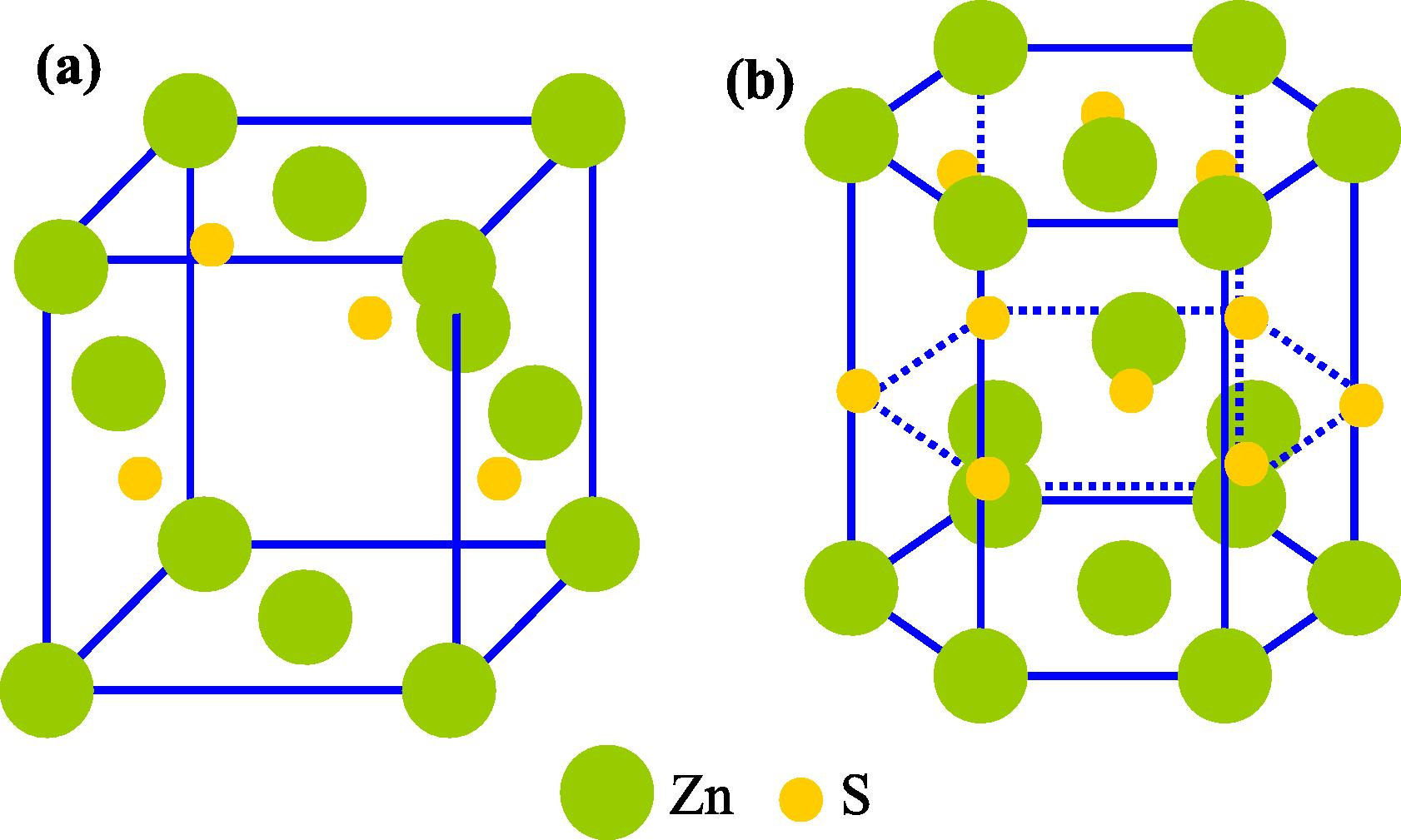
ZnS crystalline structure of the blende phase (a) and wurtzite (b) (Moore and Wang, 2006).
QDs
Structure
Parameters (Å)
CdS
Blende
a = 5820
CdS
Wurtzite
a = 4150; c = 6737
ZnO
Blende
a = 4271
ZnO
Wurtzite
a = 3249, c = 5204
ZnS
Blende
a = 5414
ZnS
Wurtzite
a = 3822; c = 6260
2.2 Signification of sizes and consequences
Physicochemical properties of QDs vary according to their sizes. Indeed, it is possible to modify the width of the band gap by a simple change in the diameter of nanoparticles. In a semiconductor, it is possible to excite an electron (e−) from the valence band (BV) to conduction band (CB) following the absorption of suitable photon energy (hν ⩾ Eg), creating a hole (h+) in BV level (Fig. 2). The electron and the hole cannot move independently because of the Coulomb interaction. Thus, they form a pair of electron-hole called exciton, the pair e− -h+ have a slightly less energy than the value of BC level. Typically the radius of the exciton is large enough but the effective masses of the charge carriers are small compared to hydrogen (Brus, 1984). The decrease in particle size to a few nanometers leads to an increase in the gap and confinement of energy levels into discrete values resulting from the passage of a band structure in a discontinuous structure levels, this phenomenon is called quantum size effect (Bawendi et al., 1990).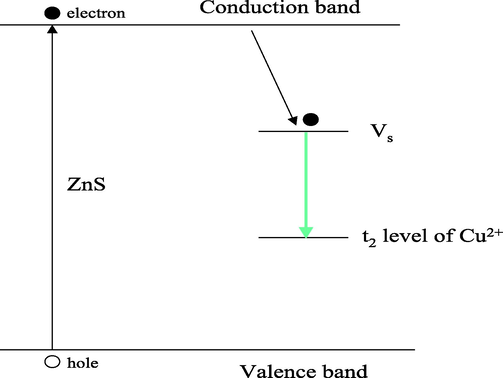
Energy level of ZnS: Cu NPs emission mechanism (Vs: Sulfur vacant band) (Peng et al., 2006).
3 Brief summary of synthesis methods of QDs
3.1 Solvo-hydrothermal synthesis
Hydrothermal synthesis is generally carried out, from a metal salt solution between 100 and 250 °C and under particular pressure. The synthesis is hydrothermal when water is used as solvent, if the solvent is organic, it is solvothermal, the advantage of this method is to allow the well-crystallized nanoparticles. Its major disadvantage is associated to the poor control of the morphology and particle size. For instance, Ma and Lou have synthesized zinc sulfide manganese doped nanoparticles (ZnS: Mn) by adopting the hydrothermal method (Ma and Lou, 2011), the protocol consists of preparing a zinc acetate aqueous solution at room temperature, which will be slowly injected into an aqueous solution of sodium sulfide at 150 °C (Zhang et al., 2010). The synthesis shows the formation of blended phase of zinc with an average particle size estimated by the Scherrer equation to 4.3 nm. The analysis carried out by UV–Visible spectroscopy demonstrates that ZnS-NPs, shows a blue fluorescence after excitation with 281 nm. The orange fluorescence is observed for ZnS: Mn under an excitation at 335 nm, the most intense emission is centered at 581 nm is attributed to the cation Mn2+.
3.2 Synthesis in aqueous phase
Synthesis in aqueous medium has been developed by Weller and it has been used for the synthesis of quantum dots such as ZnS (Li et al., 2010), ZnSe, (Lan et al., 2007). For instance, the ZnS doped QDs are generally obtained by the chemical precipitation method with controlled parameters such as temperature, the pH of the solution and the concentrations of the precursors both oxides and salts (Xue et al., 2016). These parameters have significant effects on the physical and structural characteristics of nanomaterials such as particle size, their particle size distribution and shape. Sulfur precursors can be thioacetamide (Vacassy et al., 1998), thiourea (Lee et al., 2004) and /or sodium sulfide (Na2S). Commonly used ligands are thioglycolic acid (TGA) (Huang and Han, 2010), 3-mercaptopropionic acid (MPA) and/or (3-mercaptopropyl) trimethoxysilane (MPS) or L-cysteine (Cys) (Koneswaran and Narayanaswamy, 2009). It was reported that the doped ZnS prepared by aqueous method generally have cubic blended structure. They have a diameter of several nanometers, and fluorescence emission is centered about 590 nm for ZnS doped with Mn2+, and about 500 nm in the case of ZnS doped with Cu2+ at some percentage of quantum efficiency of photo-luminescence (Yang et al., 2002).
For further investigation of preparation of ZnS:Mn QDs, the authors observed that the photoluminescence spectra were influenced by the ratio [S2−]/[Zn2+], the concentration of Mn2+ ions (1–20%) and temperature (70–110 °C). QDs obtained has a zinc blende cubic structure and a diameter around 3 nm, the highest luminescence was observed with the ratio of [S2−]/[Zn2+] equal to 0.7 (Ren et al., 2008).
Regarding their attractive proprieties, N-doped semiconductor (Ni, Mn, Cu) by their higher luminescence efficiencies (Lommens et al., 2006), were used in many biological applications such as imaging and detection. The transition metal or rare earth ions could be semiconductors of II-VI doping such as ZnS, CdS, ZnSe and CdSe. However, the effectiveness of specific doping depends on the similarity of the chemical properties (ionic radius, the valence state) between the host, the doping element and the synthesis conditions.
The model of the incorporation of the Mn2+ ions within the crystal lattice ZnS is due to the same charge and similar sizes of Mn2+ ions and the Zn2+ ions, which improves the properties of doped QDs (Bhargava and Gallagher, 1994). The use of manganese ion as doping ions for various II-VI semiconductors can be explained by the easy insertion in the crystal lattice of the host material which can be confirmed by the electron paramagnetic resonance technique (EPR). In addition, this model can be generalized for all doped ZnS QDs with manganese that have a high radiative lifetime and high emission efficiency (Cruz et al., 2006).
The doped ZnS QDs have sufficient fluorescence emission ability. The general mechanism can be explained by the model given in case of Mn-doped ZnS QDs, similar to ZnSe (Kim et al., 2006). The fundamental state of Mn2+ is 6A1, and the first excited state is 4T1 then, the high energy gap of the most common transition described in studies of QDs photoluminescence doped with Mn, was noted 4T1-6A1 which was combined with the low energy photon provided by the majority of the II-VI semiconductors and this photoluminescence 4T1-6A1 transition state that occurs at high quantum yields (Beaulac et al., 2008).
In case of switching Mn by Cu, the fluorescence of ZnS doped with Cu2+ is located at green range with a maximum emission around 510 nm, it is due to the recombination between the deep vacant of sulfur and the t2 levels of Cu2+, Fig. 2 (Porambo and Marsh, 2009).
3.3 Sol-gel method
Sol-gel process is a relatively new approach in inorganic chemistry that has been developed by the French chemists Livage and Sanchez (1989). The synthesis protocol is based on obtained solid phase by polymerization of molecular precursors at room temperature. Two routes are possible, depending both on precursor involved and the solvent nature. The aqueous pathway wherein the precursor is an inorganic salt aqueous solution and the second called organic when the precursor is an organometallic compound dissolved in an organic solvent such as ethanol. Whatever the pathway, aqueous or organic, the mechanism is divided into two steps:
A hydrolysis reaction in which the water molecule gives rise to a reactive group M-OH releasing a molecule of alcohol ROH (R 1 reaction):
3.4 Method polyol
The polyol method consists of direct precipitation of the metal particles or the corresponding oxides, within a polyalcohol. The syntheses are carried out under reflux at a temperature less or equal to the boiling temperature of the solvent. The particularity of this process can be resumed in the double-role played by the polyol as a solvent and size controller. The polyol is an appropriate medium for nucleation and growth of the α-diols such as ethylene glycol EG (1,2-ethanediol), propylene glycol and PEG (1,2-propanediol) or derived compounds from intermolecular dehydration as DEG diethylene glycol (dihydroxydiéthyléther). Feldmann and Metzmacher (2001) are among the first to prepare ZnS nanocrystals by the polyol process (diethylene glycol, DEG) by the reaction of zinc acetate and thiourea which shows the importance of the stoichiometric proportions, then a slight excess of sulfur directly influences the particle size.
3.5 Method of co-precipitation
Co-precipitation is a simultaneous precipitation of poorly soluble compounds in a liquid medium. The final product passes through the precipitation of intermediate precursor obtained by hydrolysis of ionic salts (Uzunoua and Kissurski, 1993). The temperature, precursor concentration and pH were the main physico-chemical parameters necessary to obtain precipitates. On the other hand, the nature of the anion, the ionic force and pH are parameters which strongly act on the morphology (size and shape) and size monodispersity of the particles formed (Verdier et al., 2005). It is thus possible to control the size, shape, composition and homogeneity of the nanomaterials obtained.
With this method, various works carried out for ZnS semiconductor synthesis and applications, Xang et al. (2006) synthesized ZnS nanoparticles in aqueous phase using zinc acetate and thioacetamide as precursors and PVP as surfactant and the aqueous solution of zinc acetate was added. The formation of the cubic phase of ZnS is confirmed by XRD and the crystallite size estimated by the Scherrer formula at an average size of 5 nm, micrographs done by TEM shows the particles shape and size which were spherical around 5 nm.
4 Challenge in applications
As the synthesis methods engineering grow in the field of QDs, a continuous evolution arise regarding the flexibility of the chemical formula of the nanocrystals, the synthesis lead to great number of combination to get the right phases either in simple or in composites forms. Many application has been done regarding the methods of synthesis, (Table 2) sometimes it need many modifications of the particles at different level from the external electronic layers to the profound atoms of the crystal, some modifications seems to be at the earliest times regarding the synthesis other are post synthesis which describe a challenge in the adaptation to the application of these nanomaterials.
QDs
Applications
References
ZnS
Luminance
Shanmugam et al. (2014)
Graphene/ZnO
Electrochemical capacitors
Ezeigwe et al. (2015)
CdTe
Biosensor
Ebrahim et al. (2015)
CdTe
Acetylcholinesterase biosensor
Dua et al. (2008)
CdSe
Electrochemiluminescence biosensor
Li et al. (2012)
CdSeTe/ZnS
Electrogenerated chemiluminescence
Liang et al. (2010)
CdTe
Biosensor for the Detection of Sequence-Specific Oligonucleotides
Zhang et al. (2012)
PbS
Solar Cell Applications
Sun et al. (2013)
CdSe-ZnS
Color-Saturated Green-Emitting QD-LEDs
(Jonathan et al., 2006)
CdSe-ZnS
Electronic wave functions in semiconductor
Louis (1986)
ZnS
Quantum Dot Solar Cells
Kim et al. (2015)
ZnTe/ZnSe
Photovoltaic Properties
Bang et al. (2010)
InAs/ZnSe
Solar cell (QDSSC) application
Lee et al. (2015)
CdSe/ZnSe
Potential biomedical applications
Sukanya et al. (2015)
CdSe/ZnSe
Solar Cells
Jamshidi et al. (2015)
ZnSe
Optoelectronic and Biomedical Material
Kumara and Singh (2009)
ZnSe
Electrochemical chlorophenols sensor
Li et al. (2013)
ZnSe doped Mn
Electrochemical
Weaver and Gamelin (2012)
CdSe and CdTe
Electrochemical
Amelia et al. (2012)
4.1 The role of ligands in the synthesis and adaptation to the applications
Structuring agents (ligands) as organic molecules play a crucial role in the stability of the nanocrystals, they surround the surface of the nanocrystals by steric repulsion between particles, allowing them suspended in a suitable solvent, a lack of ligands leads to particle aggregation. The affinity of ligands with particle surface depends on the interaction of their functional groups and their chain length with the surface.
It is interesting to mention that the ligands are not linked to the surface, but they are in constant exchange between the free and the bound forms which allows the particles to grow during synthesis. In general, the ligands have growth control ability, several types of ligands can be used in the syntheses, and their relative affinity to the surface is the parameter which scientists use to modify the reactivity of different crystal faces to obtain particular sizes and shapes of nanocrystals (Peng et al., 2000). The ligands may also play a direct role in the reaction mechanism, leading to different crystal structures.
Nevertheless, ligands have a capacity to burn off the ability of the nanocrystals surface, the dangling bonds due to surface atoms can create loads that cannot be compensated by neutral ligands. In addition, the ligands form an insulating layer that can be used to confine the charges in the nanocrystal and protect their fluorescence even without shell. Thus, the ligands are essential in the synthesis of the nanocrystals and their applications.
4.2 Keys in chemical and biological orientation for applications
As many requirements for possible uses of inert materials in biological applications, the biocompatibility of the nanomaterials is a fundamental propriety, when the initial phase is toxic for biological leaving process, it is necessary to perform an activation of the surface to react with a target molecule in the restricted application, Fig. 3 show some specific small molecules used to the activation or for the functionalization of the surface.
Particular modification for restricted application.
Furthermore, the engineering of biohybrid QDs is based on immobilization of the biomolecules onto the surface. The biocompatibility of these nanomaterials is considered to be the most important condition for biological application, and then it can be inspired from the biosynthesized nanomaterials by living cells which lead to natural biocompatible nanoparticles.
For instance, and not limited to that, ZnS-QDs complexes with AgInS2, characterized by a higher level of luminescence and very small size less than 5 nm allow the possibility to be coated with multiple carboxylate groups and attached to baculovirus that can cross the outer membrane and label the cell consequently this evidence have a wide uses in genetic therapy of cells (Regulacio et al., 2013). The enhancement of the luminescent ZnS QD by doping the main phase using various elements as metals, transition metals and halides can serve for imaging of the positive cells especially used for cancer identification cells and life cell imaging. The application was based on conjugated ZnS using folic acid to detect cancer in lung fibroblast cell (Manzoor et al., 2009). Nanoparticles may be functionalized, also, in aqueous medium to fit tightly to biological molecules such as peptides, proteins, and DNA (Fig. 4).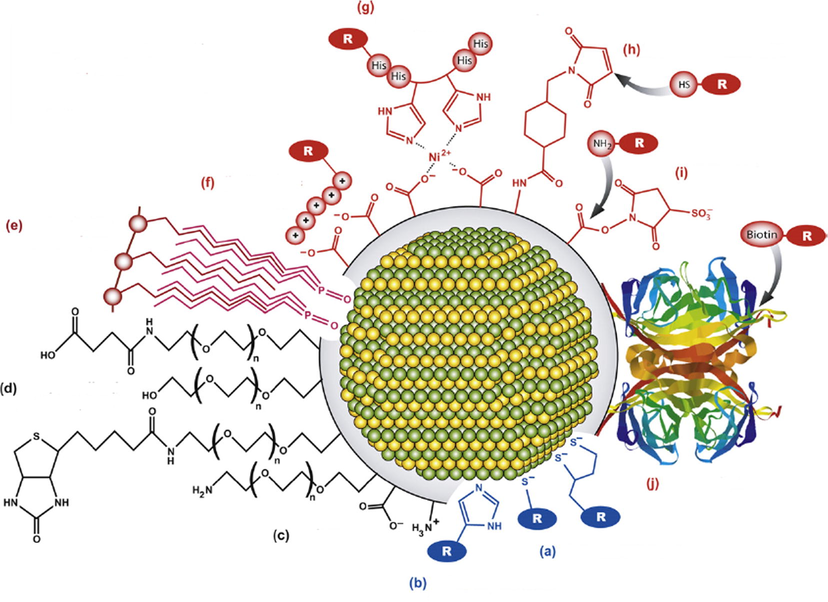
Overview on general modifications of the QDs surface from (Zhou et al., 2015) with modifications, (a) thiol modifications, (b) imidazole, polyimidazole, (c) amine or carboxyl groups, (d) functionalized PEG, (e) hydrophobic interactions, or ligand, (f) electrostatic association, (g) nickel mediated assembly, (h) Maleimide activation, (i) active ester formation, (j) Streptavidin-Biotin-labeling.
4.3 Pathway in physical applications
The orientation in physical application using QDs derive from the flexibility of these particular structure having the ability to be reinforced or doped by other materials in order to be more suitable for application, some polymers are grafted to the main phase of the QDs it is the case of carboxylated colyether that have been grafted to Zinc Oxide to improve nanoparticles dispersion and enhance the thermal stability, so it found a flame retardant ability (Díez-Pascual and Díez-Vicente, 2014). The main characteristic to participate in physical application is the flexibility of the main backbone of the QDs to be a host matrix for a guest other phases metallic even polymers. On behind of the size, the different electronic layers of the particles have to be crossed in order to touch the more profound component of the particle (Fig. 5). In energy storage application, nanocomposites based on ZnO/graphene display a capacitive performance around 236 F/g with an average energy density of 11.80 W h/kg, this particular nanomaterial has been qualified to be a supercondensator regarding the electric conductivity of graphene coupled with the ZnO good electroactive property (Ezeigwe et al., 2015).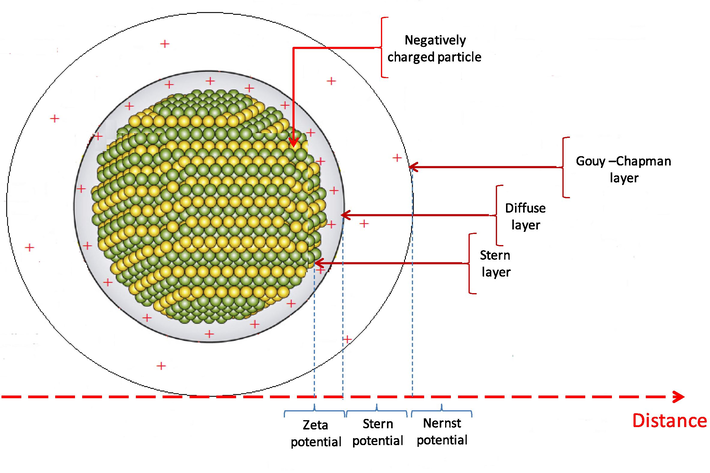
Electronic transition layers of the particles.
In case of element intercalation some nanocomposites were not possible structurally which is a tributary to the diameter of the doped atoms, the intercalation of Ni was not allowed to the main structure of ZnS, however, Mn and Cu were well incorporated based on shifting of emission wavelength detection, to explain the selectivity of atoms incorporation, the model of atomic radius that should be small enough than Zn to be incorporated in the structure.
5 Conclusion
The synthesis of QDs pursues a large combination derived from the II-VI metallic salts and huge anions can be used to build semiconductor nanomaterials. The nanocrystalline structure leads to the formation of specific surface which has become a veritable theater for many modifications after the final formation of the QDs and even under the genesis of the primary nanomaterials species. The implantation of exogenous atoms and polymers orientate the destination of the synthesized materials which is the window for different applications. There are still many challenges to overcome the obstacles of practical utilization (e.g. stability, dispersibility problems, cost and performance), which gives to this field more attention in the research and without doubt, the star of the practical contribution of QDs will rise soon.
Conflict of interest
The authors declare that there is no conflict of interest about the publication of this document
References
- Electrochemical properties of CdSe and CdTe quantum dots. Chem. Soc. Rev.. 2012;41:5728-5774.
- [Google Scholar]
- ZnTe/ZnSe (Core/Shell) Type-II quantum dots: their optical and photovoltaic properties. Chem. Mater.. 2010;22:320-332.
- [Google Scholar]
- The quantum mechanics of larger semiconductor clusters. Rev. Phys. Chem.. 1990;41:477-496.
- [Google Scholar]
- Luminescence in colloidal Mn2+-doped semiconductor nanocrystals. J. Solid State Chem.. 2008;181:1582-1589.
- [Google Scholar]
- Optical properties of manganese-doped nanocrystals of ZnS. Phys. Rev. Lett.. 1994;72:416.
- [Google Scholar]
- Electron–electron and electronhole interactions in small semiconductor crystallites: the size dependence of the lowest excited electronic state. J. Chem. Phys.. 1984;80:4403-4409.
- [Google Scholar]
- The effect of ultraviolet irradiation on the photothermal, photoluminescence and photoluminescence excitation spectra of Mn-doped ZnS nanoparticles. Thin Solid Films. 2006;499:104-109.
- [Google Scholar]
- Development of nanocomposites reinforced with carboxylated poly(ether ether ketone) grafted to zinc oxide with superior antibacterial properties. ACS Appl. Mater. Interfaces.. 2014;6:3729-3741.
- [Google Scholar]
- Development of acetylcholinesterase biosensor based on CdTe quantum dots/gold nanoparticles modified chitosan microspheres interface. Biosens. Bioelectron.. 2008;24:475-479.
- [Google Scholar]
- CdTe quantum dots as a novel biosensor for serratia marcescens and lipopolysaccharide. Spectrochim. Acta Part A.. 2015;150:212-219.
- [Google Scholar]
- One-step green synthesis of graphene/ZnO nanocomposites for electrochemical capacitors. Ceram. Int.. 2015;41:715-724.
- [Google Scholar]
- Effect of structure transformation on the work hardening characteristics of Sn – 0.5 wt% Zn. Phys. Status Solidi A 1991:126.
- [Google Scholar]
- Polyol mediated synthesis of nanoscale MS particles (M = Zn, Cd, Hg) Mater. Chem.. 2001;11:2603.
- [Google Scholar]
- One-step synthesis of water-soluble ZnSe quantum dots via microwave irradiation. J. Mater. Lett.. 2010;64:1099-1101.
- [Google Scholar]
- Efficiency enhanced colloidal mn-doped type II core/shell ZnSe/CdS quantum dot sensitized hybrid solar cells. J. Nanomater.. 2015;2015:1-9.
- [Google Scholar]
- Influences of surface capping on particle size and optical characteristics of ZnS: Cu nanocrystals. J. H. Chun Mater. Sci. Eng. B.. 2006;13:113-117.
- [Google Scholar]
- Kim, J.-Y., Yang, J., Yu, J.H., Baek, W., Lee, C., Son, H.J., Hyeon, T., Ko, M.J., 2015. Highly efficient copper-indium-selenide quantum dot solar cells: suppression of carrier recombination by controlled ZnS overlayers. 24, 9(11), 11286–11295.
- L-Cysteine-capped ZnS quantum dots based fluorescence sensor for Cu2+ ion. Sens. Actuators B: Chem.. 2009;139(1):104-109.
- [Google Scholar]
- Wurtzite ZnSe quantum dots: synthesis, characterization and PL properties. Optoelectron. Biomed. Mater.. 2009;1:59-69.
- [Google Scholar]
- A facile method for the preparation of bifunctional Mn:ZnS/ZnS/Fe3O4 magnetic and fluorescent nanocrystals. Beilstein J. Nanotechnol.. 2015;6:1743-1751.
- [Google Scholar]
- Synthèse photo-assistée de ZnSe fortement fluorescent (S) quantum dots en solution aqueuse. J. Mater. Chem.. 2007;17:2661-2666.
- [Google Scholar]
- Effects of synthesis temperature on particle size/shape and photoluminescence characteristics of ZnS: Cu nanocrystals. Mater. Lett.. 2004;58:342-346.
- [Google Scholar]
- Synthesis of colloidal InAs/ZnSe quantum dots and their quantum dot sensitized solar cell (QDSSC) application. Opt. Mater.. 2015;49:230-234.
- [Google Scholar]
- Highly photoluminescent and stable aqueous ZnS quantum dots. Eng. Chem. Res.. 2010;49:578-582.
- [Google Scholar]
- Electrochemiluminescence biosensor based on CdSe quantum dots for the detection of thrombin. Electrochim. Acta. 2012;65:1-6.
- [Google Scholar]
- A sensitive electrochemical chlorophenols sensor based on nanocomposite of ZnSe quantum dots and cetyltrimethylammonium bromide. Anal. Chim. Acta. 2013;804:76-83.
- [Google Scholar]
- Fabrication of nearinfrared-emitting CdSeTe/ZnS core/shell quantum dots and their electrogenerated chemiluminescence. Chem. Commun.. 2010;46:2974-2976.
- [Google Scholar]
- Sol-gel encapsulation for controlled drug release and biosensing. Solid State Chem.. 1989;32:633.
- [Google Scholar]
- Photoluminescence properties of Co2+-doped ZnO nanocrystals. J. Lumin.. 2006;118:245-250.
- [Google Scholar]
- Electronic wave functions in semiconductor clusters: experiment and theory. Phys. Chem.. 1986;90:2555-2560.
- [Google Scholar]
- The dilute magnetic and optical properties of Mn-doped ZnO nanowires. Journal of Nanomaterials 2011:5. Article ID 464538
- [CrossRef] [Google Scholar]
- Bio-conjugated luminescent quantum dots of doped ZnS a cyto-friendly system for targeted cancer imaging. Nanotechnology. 2009;20:065-102.
- [Google Scholar]
- Quantum dot bioconjugates for imaging. labelling and sensing. Nat. Mater.. 2005;4:435-446.
- [Google Scholar]
- Growth of anisotropic one-dimensional ZnS nanostructures. J. Mater. Chem.. 2006;16:3898-3905.
- [Google Scholar]
- Synthesis and photoluminescence of ZnS: Cu nanoparticles. Opt. Mater.. 2006;29:313-317.
- [Google Scholar]
- Synthesis and photoluminescent properties of doped ZnS nanocrystals capped by poly(vinylpyrrolidone. Opt. Mater.. 2009;31:1631-1635.
- [Google Scholar]
- Aqueous synthesis of highly luminescent AgInS2/ZnS quantum dots and their biological applications. Nanoscale. 2013;5:2322-2327.
- [Google Scholar]
- Hydrothermal preparation and properties of nanocrystalline ZnS:Mn. J. Mater. Sci.: Mater. Electron.. 2008;19:1-4.
- [Google Scholar]
- Luminance behavior of Ce3+ doped ZnS nanostructures. Spectr. Chim. Acta Part A: Mol. Biomol. Spectrosc.. 2014;118:557-563.
- [Google Scholar]
- Synthesis of water dispersible CdSe/ZnSe quantum dots in aqueous media for potential biomedical applications. Der Pharma Chem.. 2015;7:271-281.
- [Google Scholar]
- PbS quantum dots embedded in a ZnS dielectric matrix for bulk heterojunction solar cell applications. Adv. Mater.. 2013;25(33):4598-4604.
- [Google Scholar]
- Cobalt-iron hydroxide carbonate as a precursor for the synthesis of high-dispersity spinel mixed oxides. Chem Mater.. 1993;5:576.
- [Google Scholar]
- Mechanosynthesis of zinc ferrite in hardened steel vials: influence of ZnO on the appearance of Fe(II) Solid State Chem.. 2005;178:3243.
- [Google Scholar]
- Photoluminescence brightening via electrochemical trap passivation in ZnSe and Mn2+ doped ZnSe quantum dots. Am. Chem. Soc.. 2012;134:6819-6825.
- [Google Scholar]
- Preparation and characterization of the ZnS nanospheres with narrow size distribution. Opt. Mater.. 2006;28:1080.
- [Google Scholar]
- Synthesis, field emission properties and optical properties of ZnSe nanoflowers. App. Surf. Sci.. 2016;365:69-75.
- [Google Scholar]
- Photoluminescence of Cu+-doped and Cu2+-doped ZnS nanocrystallites. J. Phys. Chem. Solids. 2002;63:639-643.
- [Google Scholar]
- Aqueous synthesis of ZnSe nanocrystals by using glutathione as ligand: the pH-mediated coordination of Zn2+ with glutathione. J. Phys. Chem. C. 2010;114:11087-11091.
- [Google Scholar]
- One-pot synthesized DNA–CdTe quantum dots applied in a biosensor for the detection of sequence-specific oligonucleotides. Chem. Eur.. 2012;18:8296-8300.
- [Google Scholar]
- Toward biocompatible semiconductor quantum dots: from biosynthesis and bioconjugation to biomedical application. Chem. Rev.. 2015;115:11669-11717.
- [Google Scholar]







