Translate this page into:
Wound healing potential of Dodonaea viscosa extract formulation in experimental animals
⁎Corresponding author. sasdag@mcst.edu.sa (Syed Mohammed Basheeruddin Asdaq)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background & objectives
Dodonaea viscosa is one of the natural medications utilized in Saudi Arabia for treatment of several diseases and disorders. Prior investigations have revealed its anti-inflammatory and antioxidant properties. In the present study, the chloroform and methanolic extracts of Dodonaea viscosa were formulated as ointments using the emulsifying base and its wound healing property was determined in animal models.
Materials and methods
The dried leaves of Dodonaea viscosa was macerated in methanol and chloroform solvents to obtain respective solvent extracts. Both the extracts were subjected for phytochemical investigation. A 10% w/w herbal ointment of the extracts were formulated and their physicochemical properties; color and odor, loss on drying, pH, and spreadability were evaluated. Further, both the formulations were tested for stability studies. The wound healing activity of individual herbal formulations were studied in Sprague Dawley rats using incision and excision wound models. The application of ointment was done topically once every day up to the end of the healing of the wound. The epithelization period in the excision wound model and tensile strength in the incision wound model were assessed to determine the wound-healing effects.
Results
Methanolic and chloroform extracts of Dodonaea viscosa leaves have flavonoids and saponins in addition to other phytochemicals. All the evaluated physicochemical properties of both the formulations were within ideal cutoff points and they remained stable. In comparison with control and base groups, methanolic extract formulation took significantly (p < 0.001) less time for epithelization of the excision wound. A significant (p < 0.001) increase in the tensile strength was observed in the incision wound model in group of animals treated with either methanolic or chloroform extract formulations when compared with other groups.
Interpretation & conclusion
It was evident from the results that the extracts had the potential for use as wound healing agents. The wound healing potency of the extracts could be an element of either the individual bioactive constituents or synergistic impact of number of active molecules present in the extract.
Keywords
Dodonaea viscosa
Epithelization
Tensile strength
Excision wound
Incision wound
1 Introduction
The domination of herbal therapy in treating health disorders is well known, as it is practiced in many countries (Parham et al., 2020). This widespread interest in herbal drugs is attributed to the common perception that they are less toxic, economical, and easily available. Earlier studies have reported several plants for their effective use in accelerating the healing of wounds and other skin related problems (Sharma et al., 2021). The potential benefit of herbal extract is obviously due to the presence of medicinally active phytoconstituents (Elakiya et al., 2012). Any substance that may cause stress or trauma may lead to the formation of the wound and their healing is necessary to be accelerated to reduce the patients' discomfort and its consequences. It is well known that treatment of wounds is not economical and sometimes may lead to devastating effect. Therefore, herbal remedies have become the center of attention in the recently published literature. Although wound-healing properties of several plants are already reported, there is still a huge reserve of natural habitat that remains unexplored (Biswas and Mukherjee, 2003). Wound repair has several complicated stages wherein the repair of the tissue involves various overlapping phases i.e. inflammation, cellular proliferation, and remodeling (Clark, 1993). Several efforts for exploring novel agents that promote wound healing and manage other related complications have been proposed by researchers (Kokane et al., 2009). Ethnic use of herbal remedies in the form of different preparations is widespread for the treatment of skin disorders and healing of a variety of wounds (Maver et al., 2015).
One such plant is Dodonaea viscosa (family-Sapindaceae) that is widely distributed in Saudi Arabia. It is called Daidon or Dodanaia in Arabic (Naira et al., 2019). It is traditionally used by the local folklore to treat various diseases and disorders; some of them being antidiabetic, antimalarial, in inflammation, rheumatism, liver disorders, as uterine colic, antipruritic, sore throat, etc. However, the traditional uses of this plant in Saudi Arabia has not been confirmed by scientific studies (Rojas et al., 1992; Meenu et al., 2011). This research was done to evaluate the wound healing effect of methanolic and chloroform extracts of the leaves. We have earlier reported an in-vitro antioxidant and anti-inflammatory activities of this extract (Naira et al., 2019). Anti-inflammatory property is an essential element in wound healing as this is the initial phase of wound healing. Any drug having anti-inflammatory and anti-oxidant properties may exhibit wound healing properties. Hence it was found worthwhile to explore the role of Dodonaea viscose leaves extracts for the possible activity in the healing of wounds in experimental animals.
2 Methods
2.1 Collection of plant material
The fresh leaves of Dodonaea viscosa was collected from herbal garden of Rafha region, Saudi Arabia during January 2019 and authenticated through a departmental voucher specimen (MCST#2019/3). The leaves were separated and after drying, pulverization was done to get the coarse powder. The resultant powder was kept in the tightly closed container for their later use.
2.2 Extracts preparation
The coarse powder of leaves was macerated for 24 h with occasional stirring in chloroform or methanol to prepare the respective extracts.
2.3 Phytochemical analysis
Both methanolic and chloroform extracts of Dodonaea viscose leaves were subjected for phytochemical investigation. The presence of alkaloid was tested with the help of Mayer's, Dragendorff's and Wagner's reagents (Salehi-Surmaghi et al., 1992). The proteins present in the extract was detected by Ninhydrin test and Millon's tests (Asdaq et al., 2020). The steroids, triterpenoids, and cardiac glycosides of the extract were checked by a method describe by Siddiqui and Ali, (1997). The presence of carbohydrates in both the extracts were analyzed by Molisch, Fehling’s, Barfoed’s, and Benedict’s tests (Asdaq et al., 2020). The phytochemical analysis of saponins were done by froth test, whereas, tannins were spotted by ferric chloride and lead acetate tests. Finally, zinc hydrochloric acid reduction, alkaline reagent and lead acetate tests were done to identify flavonoids (Wagner et al., 1984).
2.4 Formulation of Ointment:
A 10% w/w herbal ointment was formulated by the fusion method in sufficient quantities of white soft paraffin, liquid paraffin, and emulsifying base.
2.5 Herbal formulation assessments:
The prepared herbal formulation was assessed for physicochemical properties such as color and odor, homogeneity, washability, loss on drying, pH, and spreadability based on published literature (Rajasree et al., 2012; Dwivedi et al., 2017; Kolhe et al., 2018). The odor, color, and homogeneity were visually examined. The ointment was placed in the petridish on the water bath and heated to get the dried weight to determine the loss on drying. A digital pH meter (pH 211/Hanna instrument) was used for recording the pH of the ointment. A known amount of the ointment sample was dissolved in distilled water and kept for 1 h and the pH was measured three times and the average of these three values were taken as an actual measure of pH of the ointment. Further, a known quantity of the ointment was applied on the hand of one of the researchers and washed with water to evaluate the extent of washability. The spreadability was measured in seconds as the time taken by slides to slip from ointment that was placed in the middle of the two slides in line with the load. The extent of spreadability was determined using a formula S = M.L/T, (S: Spreadability; M: load with upper slide; L: glass slides length, and T: time consumed to separate the slides)
2.6 Diffusion study
A known concentration of agar medium was used to carry out the diffusion study. The agar medium was placed in the petridish and allowed to settle down. Once it is set, a circular hole was made in the middle of the petridish and the formulation was poured into it and allowed to diffuse. The total time consumed by the ointment for diffusion was recorded (Paul et al., 2010).
2.7 Skin irritation study
Skin irritation of the prepared formulations was evaluated in rabbits (Ananth et al., 2010). The skin of the rabbits was shaved at three different areas on the dorsal side, each measuring approximately 500 mm2. In the first area, the ointment base was applied while the second area and the third area were used for application of chloroform extract and methanolic extract formulations to check the skin irritation. The skin of the rabbit was observed after 4 h for any symptom of irritation. Redness, swelling or inflammation at the site of application was considered as a positive indicator of irritation.
2.8 Stability studies
Both chloroform extract and methanolic extract formulations were subjected to stability studies at 4 °C, 27 °C, and 37 °C for 3 months (Naira et al., 2009).
2.9 Wound healing activity
The wound healing activity of the prepared formulations was tested using standard wound-healing models namely, the excision wound and the incision wound model. The research committee of the College of Pharmacy, AlMaarefa University approved the research protocol with its reference number MCST/(AU)-COP 1920/RC dated 15/02/2020. Healthy male and female Sprague Dawley rats were used for the study. Ether was used to anesthetize the animals.
2.9.1 Excision wound model
The Sprague Dawley rats were divided into five groups with six animals in each group. After anesthetizing the animals with anesthetic ether, the skin was removed from the dorsal region around 5 cm away from the ear and 1 cm from the vertebral area to get a total wound area of around 500 mm2. Animals from the group I was applied with emulsifying base, group II was treated with standard ointment (nitrofurazone 0.2% (w/w) ointment), while animals from group III were used as control (without any treatment), finally, group IV and V animals were applied with chloroform and methanol formulations, respectively. The application of ointment was done topically once every day up to the end of the healing of the wound. The area of the wound was measured every other day using an mm-scale graph sheet. Scar falling is an indicator for completeness of epithelisation and the days taken to reach this point was taken as a period of epithelization (Ananth et al., 2010).
2.9.2 Incision wound model:
Five groups of animals were used as described in the excision wound model. Their treatment protocol was also similar to the one that is given in the excision wound model. Two paravertebral straight incisions of 6 cm were made on either side of the vertebral column. A cotton swab dipped in saline was used to achieve homeostasis. The wound was sutured at an equal distance of 1 cm. The tensile strength was measured after ten days of treatment. The tensile strength was measured using the continuous water flow technique and was expressed as weight (g) required to break the wound (Ananth et al., 2010).
2.10 Statistical analysis
Statistical analysis was done by one-way analysis of variance (ANOVA) that is subsequently supported by Dunnett’s comparison testto elucidate the level of significance. All values are expressed as mean ± SEM and p < 0.05 was considered significant.
3 Results
3.1 Phytochemical analysis
The initial investigation on identification of phytochemicals showed the presence of flavonoids, saponins and tannins in both methanolic and chloroform extracts of Dodonaea viscosa leaves. The details of the phytochemicals identified are given in Table 1.
Sl No
Phytochemicals
Methanolic extract
Chloroform extract
1
Alkaloids
++
–
2
Carbohydrates
++
–
3
Steroids, triterpenoids and glycosides
++
++
4
Saponins
++
++
5
Tannins
++
++
6
Reducing sugar
–
++
7
Steroids
++
++
8
Proteins and Amino acids
–
–
9
Flavonoids
++
++
3.2 Physiochemical properties of extracts
The maceration process to prepare ointments yielded a sufficient amount of the extracts. The physicochemical properties, stability, skin irritation of the ointment prepared by the fusion method are shown in Table 2. Physicochemical properties exhibited satisfactory results falling within an acceptable range. The spreadability was 11 s and the pH was 6.8. Both the formulations were stable at various temperature conditions. No skin irritation was noticed in the experimental conditions.
Sl No
Parameter
Methanolic extract formulation
Chloroform extract formulation
1
Color
Pale green
Green
2
Odor
Characteristic
Characteristic
3
Taste
Slightly bitter
bitter
4
Loss on Drying
9.2%
9.4%
5
Spreadability(Seconds)
11
14
6
Diffusion study
0.75 cm
0.9 cm
7
pH
6.8
6.9
8
Stability
Stable at 400,240 and 370
Stable at 400,240 and 370
9
Skin irritation study
No skin irritation was observed
No skin irritation was observed
10
Washability
Satisfactory
Satisfactory
11
Homogeneity
Good
Good
3.3 Excision wound model
As shown in Table 3, the healing of wounds was significantly hastened in groups of animals treated with chloroform and methanolic extracts compared with control. The reduction in wound area in the excision wound model was recorded over 21 days. The wound was measured on every alternate day. The results of our study have shown that both chloroform and methanolic extract formulations increased the speed of epithelization and complete epithelization was obtained in significantly less number of days compared to both control (p < 0.001) and base (p < 0.01) groups. The period of epithelization in the excision wound model in the case of methanolic extract was around 12 days while the chloroform extract formulation showed a period of epithelization at about 14 days (Fig. 1). The period of epithelization was quicker in the formulation groups than the standard ointment group but there was no significant difference between the standard and the formulation groups. Values are expressed Mean ± SEM of six readings, * p < 0.1, ** p < 0.01, *** p < 0.001 compared to control, †p < 0.1, ††p < 0.01 compared to base, Me:Methanolic extract formulation, Ch: Chloroform extract formulation.
Treatment
50% wound contraction(days)
Period of epithelization (days)
Control
12.16 ± 0.39
20.66 ± 0.99
Standard
7.33 ± 0.49**
15.33 ± 0.92**
Base
8.5 ± 0.8*
17.83 ± 0.94
Formulation (Me)
6.66 ± 0.78***
12.16 ± 0.94***††
Formulation (Ch)
7.5 ± 0.8***
13.83 ± 0.5***†
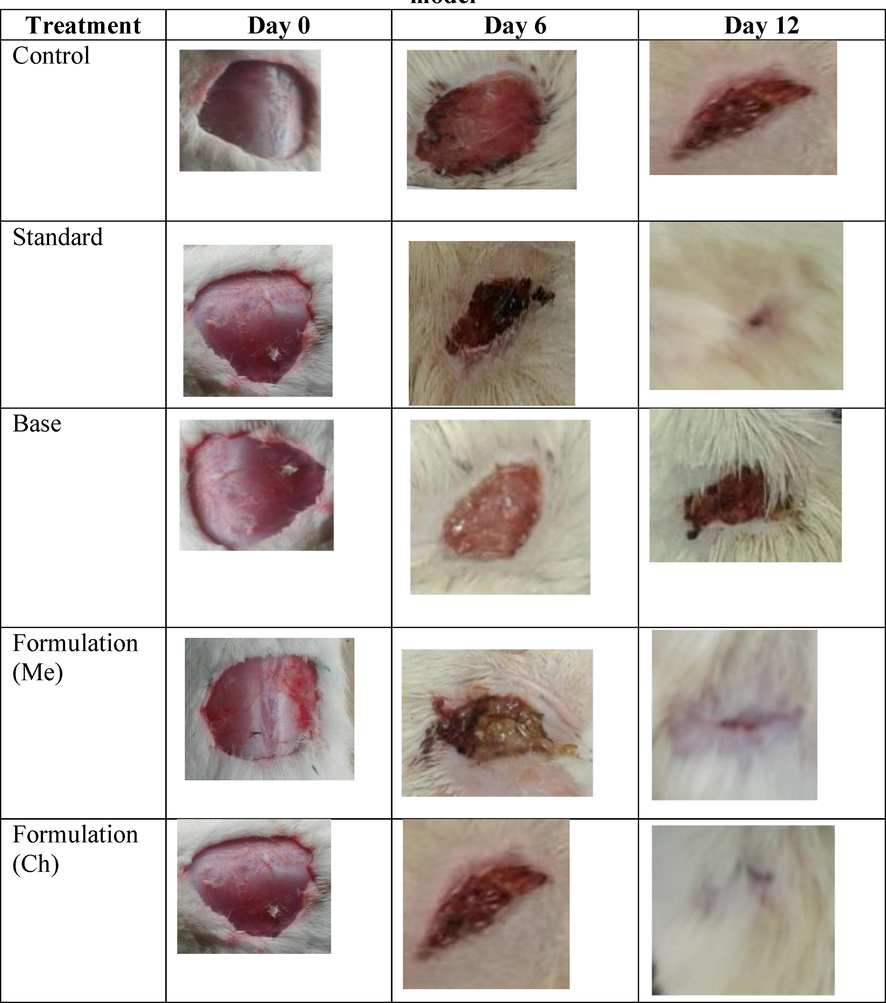
Comparison of wound healing activity in different groups of excision model.
3.4 Incision wound model
Table 4 explains the effect of methanolic and chloroform extract formulations on tensile strengths in the incision wound model. Significantly higher tensile strength was noticed with both the chloroform extract and the methanolic extract formulations when compared to the control (p < 0.001) and the base (p < 0.01) groups. The tensile strength of methanolic extract was more than double (569 Vs 270) than the control group while the tensile strength of chloroform formation was almost two times that of the control group (48% Vs 270). Values are expressed Mean ± SEM of six readings; * p < 0.1, ** p< 0.01, *** p< 0.001 compared to control, †p< 0.1, ††p< 0.01 compared to base, Me:Methanolic extract formulation, Ch: Chloroform extract formulation
Treatment
Tensile strength (g)
Control
269.83 ± 7.42
Standard
451.5 ± 11.16**
Base
330.5 ± 7.5
Formulation (Me)
568.83 ± 7.77***††
Formulation (Ch)
485.33 ± 9.6***†
4 Discussion
A wound is formed due to a rupture of a living tissue that causes anatomical and physiological alterations. The process of healing of this ruptured area is sometimes complicated that eventually may result in the restructuring and restoring of the original tissue (Clark, 1991). The traditional system of medicine reports for several natural products and herbal preparations for assisting the repairing processes of various kinds of wounds (Kumar et al., 2007). Some of the ethnopharmacological claims directly promote wound repair, while, some remedies work through enhancing a few different properties (analgesic, antimicrobial, cell reinforcement, mitigating) that would help in injury healing. Plant constituents like saponins, phenolics, and flavonoids are ascribed to expedite the stages of wound healing (Dash and Murthy, 2011; Choudhary, 2006). The literature survey has revealed that several plants like Ficusre ligiosa, Lantana camaraetc that possess analgesic, anti-inflammatory, and antioxidant activities have also exhibited wound healing property (Pastar et al., 2014; Kesarvani and Khan, 1999).
Healing of wound involves several stages that include inflammatory processes, contraction of wounds, and lastly epithelization. Agents that relieves pain and attenuate inflammation with free radical scavenging potential and additional anti-microbial effects are known to show synergistic wound-healing effect. Several natural substances are reported to expedite the healing process of the wound by these combinational activities. Recently, novel technologies such as natural and synthetic skin grafts, bioengineered alternative tissue products are available for healing wounds (Kadam, 2016). However, these are very expensive, require more care, and cannot attain fully functional skin. Therefore novel biodegradable, biocompatible, and economical components need to be identified for the healing of wounds.
In our study, we noticed that methanolic extract formulation diffuse readily than the chloroform extract, however, the difference was not significant. Nevertheless, diffusion characteristic of both the formulations indicate that the formulation can pass through the medium to the site of action effectively. Hence both formulations demonstrated significant wound healing activity in excision and incision wound models. In the excision wound model, epithelization was the major outcome variable to determine the extent of the wound healing potential of the extract. Epithelialization is only a progression of steps to cover the open epithelial surface. It is an essential part of wound healing for the successful closure of the wound. In the vast majority of the injuries, the epithelization interaction is weakened. Therefore it is important to increase the steps that are associated with initiating, maintaining, and completing the epithelization process to effectively close the wound (Pirzada et al., 2010). The formulations that are tested in this study were able to diminish the repairing time significantly due to the presence of its bioactive materials including flavonoids and saponins.
In the incision wound model, the tensile strength of the wounds that were treated with formulations significantly increase by two folds. This enhancement in tensile strength is an indicator of the wound healing potential of herbal extracts. This is probably due to the ability of the extract to increase the concentration and stabilization of the collagen fibers. Different phytoconstituents have been accounted for in the literature, for example, quercetin, kaempferol, rutin, isorhamnetin, dodoneasides A and B, citronellal, α-spinasterol, geraniol, fraxetin, syringic corrosive, cleomiscosin A, cleomiscosin C (Wollenweber and Roitman, 2007; Riaz et al., 2012; Dimbi et al., 1985; Rojas et al., 1996).
The presence of these flavonoids, tannins, phenolic acids, and so on has been accounted for to build the recuperating capability of plants. The anti-oxidant and anti-inflammatory properties of the extract may also be the contributing factors for its wound healing activity. In our earlier study (Naira et al., 2019), we reported the presence of nine chemical components in HPTLC chromatogram with affirm peaks of flavonoids kaempferol and rutin in methanolic extract. The phytochemical investigation done in this study additionally showed presence of flavonoids, saponins and tannins in both, methanolic and chloroform extracts. Along these lines, wound healing potency observed in this study is attributed to free radical-scavenging action of flavonoids and the antimicrobial properties of other phytoconstituents present in the extract, and the enhanced process of wound healing could be a due to either the individual or the synergistic effects of bioactive molecules.
The extract showed wound healing effect similar to nitrofurazone, a known antibiotic used to prevent infection in the wounds. The excellent effect of the crude extract similar to a standard pure antibiotic warrants (Table 3 & 4) further investigation on the isolated chemical constituents from the extracts. These further studies may help to identify chemical constituent(s) responsible for the wound healing activity. It will also provide insights to understand whether a single chemical constituent is responsible for the effect or a group of chemical constituents showed the wound healing activity due to additive/synergistic effects.
For treatment and prevention of chronic diseases, herbal or traditional medicines are practiced in several countries including Saudi Arabia. An integrative approach of treatment that combines modern medicines with traditional medicines is being discussed in both developing and developed countries (Asad et al., 2021). The results of this study will serve as a tool to understand the potential of Dodonaea viscosaas as a wound-healing agent and may give a positive direction for its possible clinical use after necessary regulatory steps.
5 Conclusions
In conclusion, herbal ointments prepared using the methanolic extract and chloroform extract of the leaves of Dodonaea viscosa was found to be effective in the healing of wounds. Isolation of active constituents from these extracts will help in discovering novel medicinal products for a rapid wound healing effect. The wound healing potency of the extracts could be an element of either the individual bioactive constituents or synergistic impact of number of active molecules present in the extract.
Acknowledgment
The authors would like to thank the Research Center at King Fahd Medical City, Riyadh, for their financial support provided for the manuscript. The authors are also thankful to AlMaarefa University for providing support to do this research.
Funding
Walaa F. Alsanie would like to acknowledge Taif university for support No. TURSP 2020/53.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of Wound Healing Potential of Bauhinia purpurea Leaf Extracts in Rats. Indian J.. Pharm. Sci.. 2010;72(1):122-127.
- [Google Scholar]
- Pharmacokinetic and pharmacodynamic interaction of Rosuvastatin calcium with guggulipid extract in rats. Saudi J. Biolog. Sci. 2021
- [CrossRef] [Google Scholar]
- Role of Daucus carota in Enhancing Antiulcer Profile of Pantoprazole in Experimental Animals. Molecules. 2020 Jan;25(22):5287.
- [Google Scholar]
- Plant medicines of Indian origin for wound healing activity: a review. Int J low Extrem Wounds.. 2003;2(1):25-39.
- [Google Scholar]
- Evaluation of the ethanolic extract of Ficusreligiosa on the incisionand excision wound in rats. Planta Indica.. 2006;2(3):17-19.
- [Google Scholar]
- Regulation of fibroplasia in cutaneous wound repair. Am. J. Med. Sci.. 1993;306(1):42-48.
- [Google Scholar]
- Clark RAF. Basics of wound repair. J. Derma Sur. Onco.1991; 1524.
- Wound healing effects of Ageratum conyzoides Linn. Inter J. Pharma. Bio. Sci.. 2011;2(2):369-383.
- [Google Scholar]
- Triterpentrïdes De Dodonaea Viscosa. Bulletin des Sociétés Chimiques Belges.. 1985;94(2):141-148.
- [Google Scholar]
- Evaluation of wound healing, anti-microbial and antioxidant potential of Pongamia pinnata in wistar rats. J. Traditional Complementary Med.. 2017;7(1):79-85.
- [Google Scholar]
- Wound healing efficacy of a polyherbal ointment used to treat incisions, excisions and burn wounds in albino rats. Asian J Trad Med.. 2012;7(4)
- [Google Scholar]
- Novel expansion techniques for skin grafts. Indian J. Plast. Surg.. 2016;49(01):5-15.
- [Google Scholar]
- An experimental study of wound healing by topical application of turmeric (Curcuma longa) paste on rats. Indian J. Surg.. 1999;61(1):22-32.
- [Google Scholar]
- Evaluation of wound healing activity of root of Mimosa pudica. J. Ethnopharmacol.. 2009;124(2):311-315.
- [Google Scholar]
- Evaluation of polyherbal ointment for wound healing activity in Wistar rats. J. Drug Delivery Ther.. 2018;8(6-s):26-31.
- [Google Scholar]
- Ethnopharmacological approaches to wound healing-Wound healing activity of Wattakakavolubilis leaf Exploring medicinal plants of India. J. Ethnopharmacol.. 2007;114(2):103-113.
- [Google Scholar]
- A review of herbal medicines in wound healing. Int. J. Dermatol.. 2015;54(7):740-751.
- [Google Scholar]
- Evaluation of antihyperglycemic activity of Dodonaeaviscosa leaves in normal and STZ-diabetic rats. Inter. J. Pharm. Pharmaceu. Sci.. 2011;3(3):69-74.
- [Google Scholar]
- Wound healing activity of the hydro alcoholic extract of Ficus religiosa leaves in rats. Internet J. Altern. Med.. 2009;6:2-7.
- [Google Scholar]
- Alsuwayt BHPTLC Analysis and In Vitro Biological Activity of DodonaeaViscosa. Pharmacophore.. 2019;10(6):1-8.
- [Google Scholar]
- Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants (Basel).. 2020;9(12):1309.
- [Google Scholar]
- Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care. 2014;3(7):445-464.
- [Google Scholar]
- Wound healing evaluation of chloroform and methanolic extracts of Mimosa pudica roots in rats. Int. J. Biol. Med. Res.. 2010;1(4):223-227.
- [Google Scholar]
- Antifungal activity of Dodonaeaviscosa Jacq extract on pathogenic fungi isolated from superficial skin infection. Pak. J. Pharma. Sci.. 2010;23(3):337-340.
- [Google Scholar]
- Formulation and evaluation of antiseptic polyherbal ointment. Int. J. Pharmacy Life Sci.. 2012;3(10)
- [Google Scholar]
- Phytochemical screening, free radical scavenging, antioxidant activity and phenolic content of Dodonaeaviscosa. J SerChem Soc.. 2012;77(1):423-435.
- [Google Scholar]
- Screening for antimicrobial activity of crude drug extracts and pure natural products from Mexican medicinal plants. J. Ethnopharmacol.. 1992;35(3):275-283.
- [Google Scholar]
- Smooth muscle relaxing compounds from Dodonaeaviscosa. Planta Med.. 1996;62(02):154-159.
- [Google Scholar]
- Survey of Iranian plants for saponins, alkaloids, flavonoides and tannins IV. DARU. 1992;2:1-11.
- [Google Scholar]
- Medicinal plants and their components for wound healing applications. Futur J Pharm Sci. 2021;7(1)
- [CrossRef] [Google Scholar]
- Siddiqui, A.A., Ali, M., 1997, Practical pharmaceutical chemistry. Ist edition. C B S Publishers and Distributors, New Delhi, Pp-126-131.
- Plant Drug Analysis. Berlin, Heidelberg: Springer Berlin Heidelberg; 1984.
- New reports on surface flavonoids from Chamaebatiaria(Rosaceae), Dodonaea (Sapindaceae), Elsholtzia (Lamiaceae), andSilphium (Asteraceae) Natural Product Commun.. 2007;2:385-389.
- [Google Scholar]







