Translate this page into:
Wheat germ oil extenuates malathion-pesticide induced hepatic toxicity in male Albino rats
⁎Corresponding author. mialkhalaf@uj.edu.sa (Maha I. Alkhalaf),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Malathion, an organophosphorus and rampantly used insecticide in agriculture, is hepatotoxic agent. In this study, the hepatoprotective capacity of wheat germ oil WGO was tested against malathion-induced liver damage in male Albino rats.
Methods
Rats were divided into four groups as follow: A group of healthy rats were fed on a balanced diet (control group), while other groups of rats were fed on malathion- supplemented basal diet (100 mg/kg basal diet) (malathion group), malathion and WGO (400 mg/kg basal diet) (malathion + WGO group) and wheat germ oil (400 mg/ Kg. basal diet) (WGO group) for 30 days. Oxidative stress OS markers including: Malondialdehyde MDA, superoxide dismutase SOD, catalase and glutathione S-transferase GST were examined. In addition, functional markers enzymes of the liver including aspartate aminotransferase AST, ALP, alanine aminotransferase ALT, alkaline phosphatase ALP, glutamyl transferase γGT and other indices including total proteins, globulin and albumin levels were measured in serum. Liver histological changes were studied by H&E staining.
Results
A marked elevation in OS and markers of liver function and a significant decrease in albumin, globulin, and total protein levels were noted in rats fed on a malathion supplemented diet as compared to those in control rats. Contrarily rats fed on WGO supplemented diet exhibited a significant reversal in all the studied parameters as well as histopathological changes. Findings demonstrate the hepatoprotective potential of WGO against malathion-induced liver toxicity and likely mediated through the attenuation of oxidative stress OS.
Keywords
Malathion
Wheat germ oil
Liver
Oxidative stress
1 Introduction
Pest controlling and increasing agricultural productivity by organophosphorus pesticides has shown harmful effects on humans and animals. Pesticides produce numerous side effects and are considered toxic owing to their capacity to generate reactive oxygen species (ROS) (Zhang et al., 2019). Malathion [S-1,2(bis-ethoxycarbonyl) ethyl-O, O-dimethyl phosphorodithioate] (C10H19O6PS2) is extensively used in agriculture. Malathion is an organophosphate insecticide commonly used to control mosquitoes and other flying insects. It is also used pharmaceutically to eliminate head lice. The principal toxicological effect of malathion is cholinesterase inhibition, primarily due to malaoxon and phosphorus thionate impurities (World Health WOrganization (2015)). However, malathion was found to be high in toxicity and low in specificity, causing widespread damage to agricultural products. Malathion can enter the human body through different routes such as: absorption through the skin, ingestion or inhalation, then it is metabolized to form malaxon, which is substantially more toxic (Alni et al., 2018). Moreover, it is implicated in genotoxicity, hepatic dysfunction, neurobehavioral changes and hematological and immunological abnormalities. Numerous insecticides are hydrophobic compounds that attach to different cellular membranes as bilayers of phospholipids. These properties potentiate them to induce OS and cause cellular damage (Kalender et al., 2010).
Liver is a major susceptible organ to toxic effects of xenobiotics, drugs and numerous chemicals due to its link to the gastrointestinal tract (Arafa et al., 2003). Cells have different mechanisms of minimizing the harmful effects of OS by modulating different enzymatic and non-enzymatic reactions. Nutrients that are rich in phytochemicals are digested through pathways similar to those of pesticides (Panemangalore and Bebe, 2009).
During the wheat grain milling into flour, the byproduct, germ, is separated from starch and bran. The wheat germ, constitutes around 2.5 % of the total weight, provides high nutritional value to the food. Wheat germ oil WGO exerts multiple health benefits because of its antioxidant, antihyperlipidemic, hypo-cholesterolemic and anticancer properties. WGO is rich in nutrients such as dietary fibers, unsaturated fatty acids, proteins, essential amino acids, sterols and flavonoids, altogether accounting for 381 calories of energy/100 ml of which 54 % comes from carbohydrates and 23 % each from proteins and fats (Boukid et al., 2018). In addition, WGO is rich in tocopherol, as compared to other vegetable oils, particularly α-tocopherol (vitamin E), which was considered a potent antioxidant agent protecting organs against oxidative damage by directly scavenging oxidative radicals (Yun et al., 2019). WGO is also abundant in octacosanol (Tolouie et al., 2021), a natural compound that endorses heart health by balancing blood lipid levels within normal range. Moreover, it enhances physical performance and has been used to treat chronic inflammatory reactions, manage cholesterol, delays aging, manage neurological disorders and balance serum biochemical factors (Megahed, 2011). Besides, WGO is high in B complex vitamins that have applications in chemoprevention. Wheat germ oil halts the autoxidation of unsaturated fatty acids and exhibits protection against DNA damage (Yun et al., 2019).
Taking into account the multiple beneficial health effects, here we examined the positive role of WGO against malathion-induced liver toxicity by measuring oxidative stress OS and liver functional markers and also assessing histological damage to liver tissue.
2 Materials and method
2.1 Materials
Malathion (organophosphorus pesticide) (purity = 99.9 %) was obtained from an agrochemical market in the Kingdom of Saudi Arabia, the company of production is (Seed Wood, USA), while WGO was procured from (Now food company, USA).
Kits for measuring aspartate aminotransferase (AST), alanine aminotransferase (ALT), glutamyl transferase (γGT), and alkaline phosphatase (ALP)activities were purchased from (Kamiya Biomedical Co. CA., USA). Malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), glutathione S-transferase (GST), albumin, and total protein were determined using the kits (BioVision USA).
2.2 Animals and diet preparation
Forty-eight male Albino rats weighing about 100–110 g (about 2 months in age) were left to acclimatize to the animal house facility for 2 weeks under ambient temperature, humidity, and 12-hr light/dark cycle conditions. The Organization for Economic Cooperation and Development (OECD) guidelines 407 (OPPTS 870.3050; OECD 407) (1992) were followed in the treatment of animals. The ethical approval was granted by Ethics Committee at King Fahd Medical Research Center. Jeddah, KSA. to conduct the study.
2.3 Experimental design
Rats were randomly separated into 4 groups, each group containing 12 animals, which were designated as follows:
G1: Control healthy rats fed on a balanced diet.
G2: Malathion-fed rats − rats fed daily on a basal malathion-supplemented basal diet (MBD) at a dose of 100 mg/kg basal diet for 30 days (Al-Attar et al., 2010).
G3: Malathion and WGO fed rats − rats fed on MBD and WGO (400 mg/ Kg. basal diet) for 30 days according to Askar and Halloull (2018).
G4: Healthy rats fed on a basal diet enriched with WGO (400 mg/ Kg. basal diet) for 30 days.
Rats had free access to these diets and water during the experimental period and at the end rats, were anesthetized using diethyl ether, and sacrificed by cervical dislocation. The serum was separated from blood, obtained from hepatic portal veins, by centrifugation and stored at −20 °C until analysis. Liver tissues were excised and stored in formalin.
2.4 Biochemical analysis
Serum AST, ALT, ALP, and γGT activities were measured as per the manufacturer’s instructions (Kamiya Biomedical Co. CA., USA). Serum total protein and albumin were determined using the colorimetric method using kits as described by the supplier (BioVision USA). Globulin concentrations were calculated by deducting the values of albumin from the values of total protein and expressed as g/dl. MDA, SOD, GST, and catalase CAT activities were determined calorimetrically following the procedure detailed by the manufacturer (BioVision, USA).
2.4.1 Determination of AST & ALT
The AST and ALT activities were estimated by the procedures described by Ferard et al (2005) and by Wilkinson et al (1972) respectively. Briefly, 50 µl of test or standard (2 mmol/l sodium pyruvate) was mixed with AST (2 mmol/l 2-oxoglutarate, 0,1 mol/l L-aspartate; 0,1 mol/l phosphate buffer pH 7,4) or or ALT (0,2 mol/l DL-α-alanine; 2 mmol/l 2-oxoglutarate; 0,1 mol/l phosphate buffer pH 7,4) substrate solutions and incubated at 37 oC for 1 h. Following this, 250 µl of 2,4-dinitrophenylhydrazine (2,4-DNPH; solution of 1 mmol/l in 1 mol/l of HCl) was added and incubated for 20 min at room temperature. Finally, 2.5 ml sodium hydroxide was added and the absorbance (A1) was measured at 510 nm. and again after 10 min (A2). The Optical density OD of the color generated by deamination of glutamate is ΔA450 nm = A2 – A1.
Alkaline Phosphatase (ALP) Assay.
Analysis was done according to the method by (Hviid, 1967). Briefly, 50 µl of test or standard (4 mM p-nitrophenol) was added to test tube containing disodium p-nitrophenyl phosphate substrate in buffer solution (0.8 M, 2-amino, 2-methyl propon-1-ol, pH 10.4). Contents were incubated at 37 °C for 15 min and absorbance was recorded at 510 nm.
Determination of gamma glutamyltransferase (γGT).
The γGT was measured as per the method prescribed by Gjerde and Mørland, 1985. Briefly, 50 µl haemolysed blood was mixed with 10 mM gamma glutamyl-p-nitroanilide and 5 mM glycylglycine at 37 °C for 15 min. Reaction was stopped by 1 mM trichloroacetic acid. The supernatant pH was adjusted to 7.5 and the absorbance of p-nitroaniline thus formed was measured at 540 nm. Good correlation of this method with that of serum GGT activity as established by standard kinetic method was noted (r = 0.982). The GGT activity of blood (y) correlated with serum activity (x): y = 0.415x + 3.8.
Determination of Albumin
Albumin was assayed as per Douman et al (1981) method. Briefly. serum sample (100 µl) or standard albumin solution (4 g/dl), was added to 2 ml bromocresol green solution in 0.075 M succinate buffer (pH 4.2) mixed and absorbance was recorded after 5 min. at 630 nm.
Estimation of Total protein
Total protein was estimated as per method of Parvin et al (1965). Test sample (0.5 ml) or standard (0.1 mM BSA) was added to 1 ml Biuret Reagent and incubated for 10 min. at 37 °C. Absorbance was measured at 550 nm.
Albumin/ Globulin ratio.
Globulin was determined by subtracting the albumin values from total protein value (Walker et al.,1990).
2.4.2 Determination of OSMDA
Liver tissue homogenate was mixed with 175 µL o 20 %TCA) with 1 % butyl-hydroxytoluene and centrifuged at1000 rpm for 10 min. Obtained supernatant (200 µl) was mixed with 160 µL 26 mM Tris (pH 7.4) and 40 µL 0.6 M of HCl buffer with 0.72 mM thio-barbituric acid (TBAand boiled for 10 min and left to cool. Absorbance was measured at 530 nm (6105 spectrophotometer (UK). Results were presented as nmol MDA/mg protein.
SOD activity.
SOD activity was assayed as per the method of Beyer and Fridovich (1987). Briefly, 50-µL sample was added to phosphate buffer (pH 7.8), containing L-methionine, 0.3 mM EDTA, and nitro-blue tetrazolium. Contents were mixed and 22.6 µL riboflavin was added and the absorbance measured at 560 nm. The homogenate needed to lower SOD-inhibitable nitro-blue tetrazolium by 50 % defines one unit of SOD activity.
Determination of GST activity.
The GST activity was determined by the method of Vontas et al (2000). Briefly, 100 µl of sample was mixed with 0.1 M potassium phosphate, 5 mM GSH and 1-chloro-2,4-dinitrobenzene (CDNB) (pH 6.2) as a substrate and the absorbance was measured at 340 nm. Specific activity was defined as nmol of formed product per minute per milligram protein.
The CAT activity.
Catalase activity was ascertained by the protocol of Aebi (1974). Hemolysate sample (20 µl) was added to 0.1 mM phosphate buffer (780 µL, pH 7.5) in a cuvette. The reaction was started by adding 0.1 ml H2O2 (500 mM). Decomposition of H2O2 was established by a reduction and the difference in extinction (ΔE240) at 240 nm per unit time was considered to indicate catalase activity. One activity unit is defined as sample that decomposes 1.0 µmole of H2O2 per minute at 25 °C defines the catalase activity.
2.5 Histological analysis
Liver histology was examined by staining the formalin fixed liver tissue sections with hematoxylin and eosin (H&E), followed by examination under tri nuclear light microscopy (Olympus Microscope) and examined by specialist.
2.6 Molecular docking
Auto Dock tools 4.2, ArgusLab; to have three dimensional of native ligand, AutoDock, Hyper Chem and Gaussian; to reduce energy of compound having it in low energy, Pymole; to have three-dimensional figure, discover studio; to have two-dimensional figure and VEGA ZZ (vina dock); to do docking process. Docking was carried out as described previously (Abd-Elfatah et al 2022) and simulative docking was carried out by Solis & Wets local search method and Lamarckian genetic.
2.7 Statistical analysis
Statistical analyses were done using SPSS (IBM Corporation, New York, NY., USA). Intergroup differences were calculated by ANOVA followed by Bonferroni post hoc analysis. Significance at P<0.01 and P<0.05 was considered to be statistically significant.
3 Results
3.1 Liver functional biomarkers
The data on markers of liver function in serum are presented in Table 1. Compared to the basal diet fed control rats, rats fed on malathion supplemented basal diet had significantly elevated AST, ALT, ALP, and γGT activities. On the other hand, rats fed on WGO supplemented basal diet demonstrated a significant decrease in all the above parameters. Malathion and WGO exerted more pronounced effects on ALP levels as compared to other functional markers. No significant difference was found comparing results of WGO group and control rats group. acompared to control; bcompared to malathion group; ccompared to malathion + WGO group *p < 0.05, **p < 0.01. For variables with the same letter in each row, the difference is not statistically significant. Data are presented as mean ± S.D (N=12).
GroupsALT
(U/L)AST
(U/L)ALP
(U/L)γGT
(U/L)
Control
36.2 ± 1.8
46.9 ± 1.1
73.5 ± 15.3
3.6 ± 0.2
Malathion
72.2 ± 2.4a*
86.4 ± 3.9a*
232.9 ± 16.3a**
8.6 ± 0.3a*
Malathion + WGO
53.6 ± 2.3b*
64.9 ± 2.7b*
108.6 ± 17.0b**
4.3 ± 0.1b*
WGO
37.5 ± 1.9c,b*
48.3 ± 1.2c,b*
70.3 ± 12.2c.b**
3.8 ± 0.2c,b*
3.2 Biochemical indices
Serum levels of studied parameters are provided in Table 2, Total protein, albumin and globulin levels were significantly lower in rats fed on malathion supplemented basal diet compared to the basal diet fed control rats. Contrarily, the levels of these parameters were significantly improved following the feeding of rats with a WGO supplemented basal diet compared to those receiving a malathion supplemented diet. Similarly, no recorded significant difference was fount between the WGO rat group and control rats. As expected the albumin to globulin ratios were significantly decreased in malathion group of rats and significantly augmented in WGO group of rats. WGO, wheat germ oil. A/G, albumin to globulin ratio; acompared to control; bcompared to the malathion group, ccompared to malathion + WGO group. For variables with the same letter in each row, the difference is not statistically significant. *p < 0.05, Data were provided as mean ± S.D (N=12).
Groups
Total Protein
(g/dl)Albumin
(g/dl)Globulin
(g/dl)A/G ratio
Control
8.07 ± 0.22
5.05 ± 0.21
2.92 ± 0.16
1.71 ± 0.19
Malathion
4.94 ± 0.22a*
2.06 ± 0.20a*
2.68 ± 0.30a*
0.72 ± 0.18a*
Malathion + WGO
5.82 ± 0.59b*
3.70 ± 0.21b*
2.81 ± 0.42b*
1.75 ± 0.28b*
WGO
8.23 ± 0.53c,b*
5.00 ± 0.23c,b*
2.94 ± 0.54c,b*
1.70 ± 0.16c,b*
3.3 Oxidative stress OS markers
Serum OS markers in control and treatment groups are presented in Fig. 1 a,b,c,d for SOD, Catalase, and GST and MDA respectively. A significant reduction was noted in serum SOD, Catalase, and GST significantly. While MDA levels was significantly increased in malathion supplemented basal diet-fed rats as compared to those in the basal diet fed control rats. Whereas, an improvement was recorded in the OS biomarkers was seen in rats fed a basal diet supplemented with WGO. As there was a significant increase in SOD, catalase, and GST levels and a significant decrease in MDA levels compared to the levels in rats fed on a malathion diet.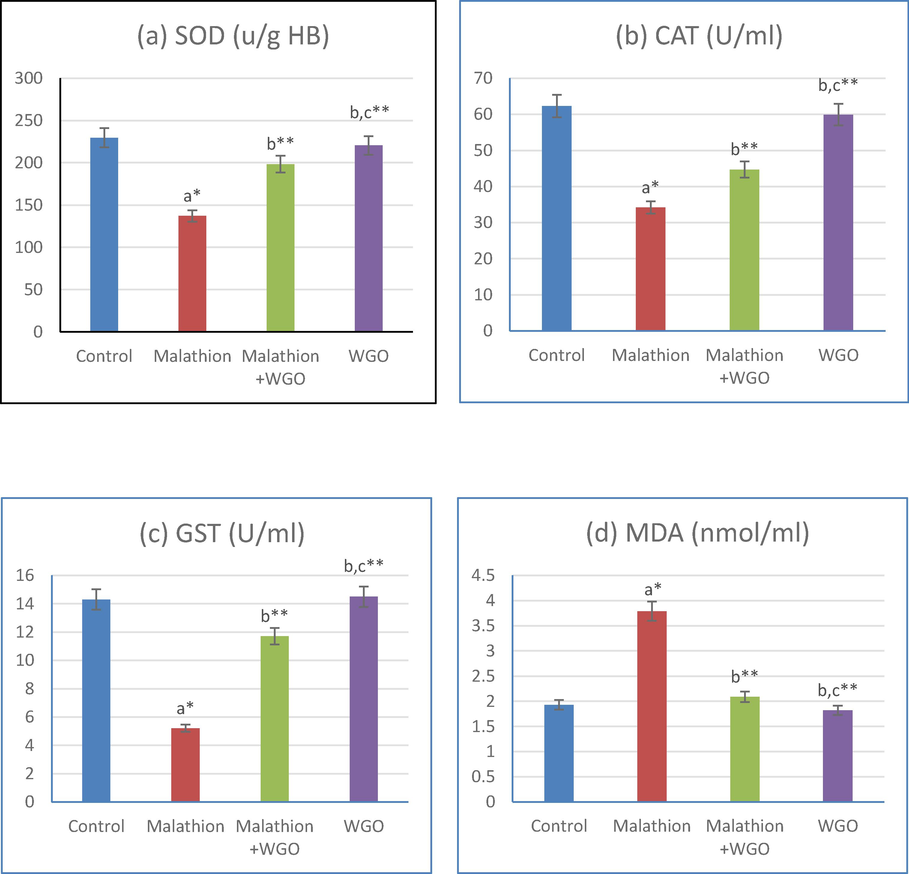
(1-a,b,c,d): acompared to control, bcompared to malathion group. ccompared to malathion + WGO group *p < 0.05, **p < 0.01. Data are mean ± S.D (N=12).
3.4 Liver histology
Histological changes in the liver tissues of the studied groups are shown in Fig. 2. Significant histopathological changes including inflammatory cellular infiltration were noticed in liver tissue sections of rats fed on a malathion diet (B) as matched to the basal diet fed control rats (A) wherein normal histology was noted in the liver tissue. Contrarily, as seen in figure (C) a marked reduction in inflammatory cells was observed in liver tissues of rats fed on a WGO diet. Meanwhile, supplementing WGO to rat diet Figure (D) did not represent any change in liver architecture.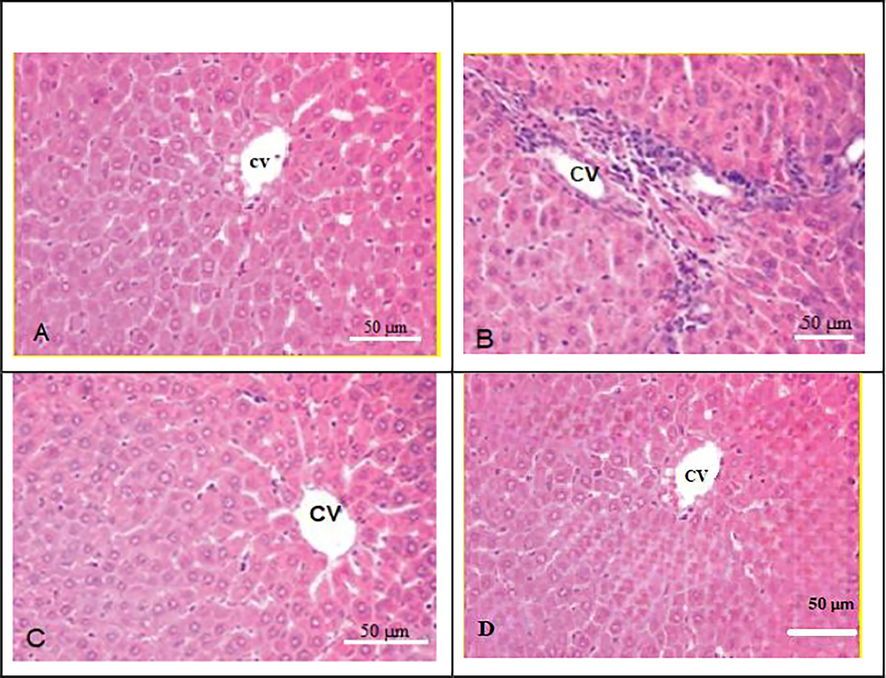
Photomicrograph of liver section from all studied rat groups. (A) control rat group show normal liver architecture;(B) Malathion treated group showed infiltration of inflammatory cells; (C) M+WGO-malathion, and wheat germ oil group, showing a reduction in histological abnormalities and reduced amounts of inflammatory cells, (D)WGO group, show normal liver architecture. CV stands for central vein.
3.5 Molecular docking analysis
The liver is a key organ in the metabolism of toxicological chemicals, which ultimately results in organ damage. Malathion is one of many pesticides made of an organophosphate chemical that has been known to harm the liver. Insecticides like Malathion are frequently employed in public health procedures as well as in agriculture. The main indicators for identifying Malathion hepatotoxicity are liver enzyme activity. Our discovery of elevated liver function indicators in Malathion is supported by the fact that altered liver function is symptomatic of hepatic injury and damage to the hepatocyte membranes of liver enzymes. According to the aforementioned statistics, Malathion primarily damages the liver and frequently results in hepatic injury. Malathion is also transformed into a more toxic intermediary product. Since the WGO also contains vitamin E, unsaturated fatty acids like −linoleic and oleic acids, octacosanols, and lutathione, it is abundant with antioxidants such flavonoids, carotenoids, phenolic acids, and tocopherols (Ma et al.,2005). Furthermore, WGO's positive benefits on liver functionality demonstrate its safety and lack of negative effects due contains vitamin E. In this study, WGO considerably decreased the rise in hepatic enzyme activity caused by Malathion. These results are in line with the research, which showed that the dietary supplemented WGO (vitamin E) reduced the harmful effects of Malathion. These beneficial effects of WGO are most likely owing to tocopherol, phenolic acids, flavonoids, and vitamin E constituents. In order to cure liver toxicity caused by organophosphates, WGO seems to be a promising hepatoprotective agent (Mannhold, 2008). Following exposure to Malathion, a considerable drop in total protein and albumin has been noted, which may indicate liver injury. In the current investigation, lower levels of albumin, total protein and globulin were consistently seen. These results might be the result of changes in protein metabolism brought on by Malathion negative effects on hepatocytes. However, the elevated levels of albumin, globulin, and total protein in response to WGO food supplementation highlight its hepatoprotective properties. Pesticide-induced cytotoxicity has been linked to the harmful effects of organophosphorus insecticides on antioxidant defense mechanisms (Hayam et al.,2023). Against Malathion, WGO demonstrated a considerable antioxidant activity. we confirmed the experimental study by theoretical one, by using docking process, we find that; vitamin E which included in WGO is more linked by amino acid which are in liver rates proteins than malathion, this emphasized by the number of bonds between vitamin E and liver proteins than malathion with liver proteins, also by binding energy between vitamin E and amino acid of liver proteins than of malathion with amino acid of liver proteins, as follow: Auto Dock and vina dock tools were used to ascertain the toxicity of Malathion and confirmation of reduction of toxicity by using WGO. Molecular docking was undertaken to identify the Malathion toxicity was established with molecular docking of crystal structure of proteins.. The interaction of Malathion with liver proteins like (PDB code: 4RT7) and its interaction with amino acids with binding energy −5.6_Kcal/mol) are shown in Fig. 3A: The interaction of vitamin E with liver proteins like (PDB code: 4RT7) and its interaction with amino acids with binding energy −8.1 (kcal/mol) shown in Fig. 3B: Comparison between connection of Malathion with liver rate and vitamin E with liver rate; we found the number of bond and binding energy between vitamin E with liver rate is bigger than connection with malathion with liver rate, this means the capability of vitamins E WGO to reduce the toxicity of malathion on liver rate.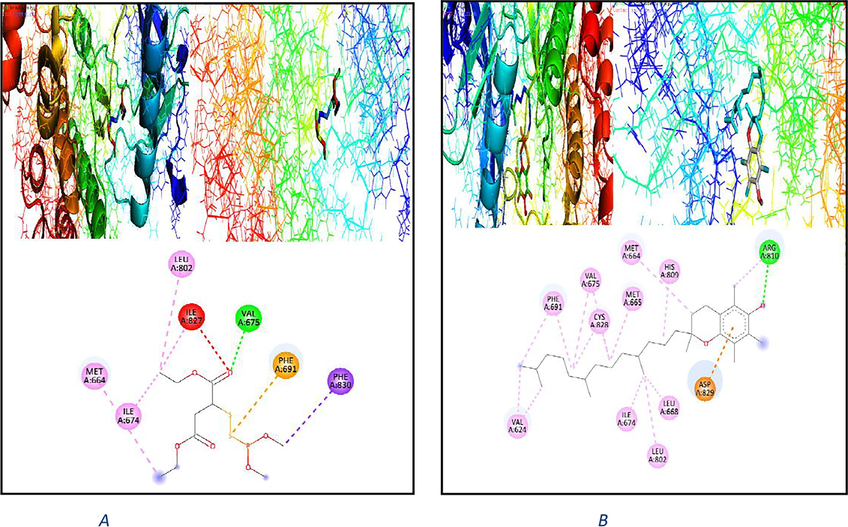
2D & 3D plot interaction of malathion (3A) and plot interaction of vitamin E WGO (3B) with liver rat protein (PDB code: 4rt7) receptor.
On the other hand, the interaction of malathion with another liver proteins like (PDB code: 4xuf) and its interaction with amino acids with binding energy −4.7 (kcal/mol) shown in Fig. 4A. The interaction of vitamins E WGO with another liver protein like (PDB code: 4xuf) and its interaction with amino acids with binding energy −8.3 (kcal/mol) shown in Fig. 4B. 4xuf-A-chain-h/A1/A/ALA‘642/OD1– with hydrogen bond length 3.5 Ao.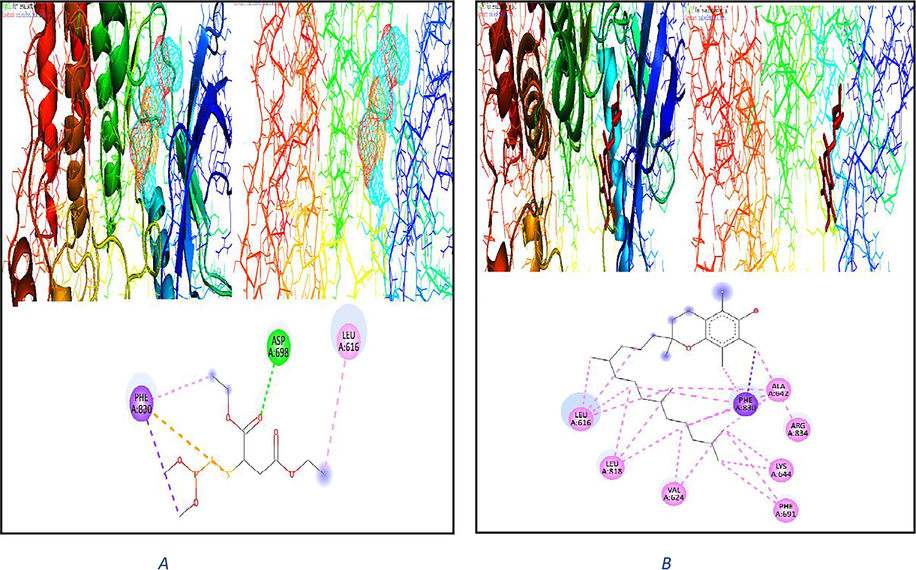
2D & 3D plot interaction of malathion (4A) and plot interaction of vitamin E WGO (4B) with liver rat protein (PDB code: 4xuf) receptor.
Also, we see; in a comparison of the connections between malathion and liver rate, we find that there are more bonds and binding energies between vitamin E and liver rate than between malathion and liver rate. This indicates that vitamin E WGO has the ability to lessen the toxicity of malathion on liver rate. All proteins can be chosen depending on the (X, Y, Z) cavity, rmsd value, crystallography, technique, resolution, and other protein-related factors. It is evident that vitamin E WGO might unleash the harmful effect in the liver as well. Rats or mice that have been exposed to a chemical or medication candidate are often used in toxicity tests. The characteristics and pharmacokinetic behavior of the substances under investigation are significantly influenced by their molecular structure.
4 Discussion
The liver is a primary organ of metabolic detoxifying activities and is therefore susceptible to insults from xenobiotics and toxicological substances eventually leading to organ damage. Organophosphate compounds such as malathion is a widely used pesticides and have been reported to cause hepatic damage (Arafa et al., 2003). Malathion is a widely used insecticide and acaricide in agriculture, veterinary, and even public health practices. Malathion exposure is linked to OS, metabolic and immune disorders, and inflammation (Lasram et al., 2014).
Liver enzyme activities are key biomarkers for the detection of hepatotoxicity of malathion. Elevated functional biomarkers and altered liver function are indicative of hepatic injury. In the present study, increased markers of liver function such as ALT, ALP, and AST in serum in the malathion exposed rats could reflect derailed plasma membrane permeability due to cellular necrosis, degeneration, and tissue damage. Malathion has been reported previously to increase serum ALT and AST levels (Jalili et al., 2019).
Likewise, damage to hepatocyte membranes and increased liver enzymes that are found in the cytosolic regions are detected in the bloodstream of malathion-fed rats Further high levels of ALT have been shown to result from increased lysosomal mobilization and necrosis due to malathion toxicity (Etim et al., 2006). These data confirm our finding of increased markers of liver function in malathion-fed rats. The above experimental data substantiate that the liver is the primary target of malathion and thus it often causes hepatic injury (Al-Othman et al., 2012). As proposed by Kalender et al. (2010), malathion is converted into a more toxic intermediary product, which is primarily implicated in the production of ROS, which in turn leads to lipid peroxidation and secretion of inflammatory cytokines. The robust generation and secretion of these pro-inflammatory cytokines are responsible for subsequent inflammation, fibrogenesis, and hepato-steatosis (Cui et al., 2016).
The WGO is an excellent source of antioxidants, including flavonoids, carotenoids, phenolic acids, and tocopherols in addition to unsaturated fatty acids such as α-linoleic and oleic acids, octacosanols, and glutathione)Vaher et al., 2010(. Moreover, WGO is shown to be safe and had no side effects based on its favorable effects on liver functional enzymes (Lin et al., 2004). In this study, WGO significantly reduced malathion-induced increase in hepatic AST, ALT, and ALP enzyme activity levels. These findings are in line with studies, where the dietary supplementation WGO minimized the adverse effects of nitrates on liver function (Mohamed and Anwar, 2010). These favorable effects of WGO presumably are attributed to its flavonoids, carotenoids, phenolic acids, and tocopherol content. Therefore, WGO appears to be a promising hepatoprotective mediator in the treatment of organophosphate-induced liver toxicity.
A significant reduction in albumin, and total protein following malathion exposure has been reported suggesting liver damage (Al-Attar, 2010). Consistently, decreased total protein, albumin, and globulin was noted in this study. These findings could be due to alterations in protein metabolism in the liver resulting from the harmful effects of malathion on hepatocytes. Whereas the elevated levels of total protein, albumin, and globulin levels in response to WGO dietary supplementing underscore its hepatoprotective effects. OS is postulated to be a major underlying mechanism in malathion-induced hepatotoxicity. This is evident from the observation that malathion-treated rats demonstrated a derailed pro- and antioxidants balance resulting in OS. It is shown that rats exposed to malathion exhibited hepatotoxicity due to elevated hepatic OS and fulminant hepatic failure (Nili-Ahmadabadi et al., 2013).
The toxic effect of organophosphorus pesticides on the antioxidant defense systems was considered an alternative mechanism of pesticide-induced cytotoxicity (Gupta, 2011). Glutathione plays an important role in malathion detoxification via conjugation reactions. The binding of glutathione to malathion or its metabolite may serve as a key pathway for its detoxification (Ajith et al., 2007). Malathion is reported to decrease GST, SOD, and catalase enzyme activities and increase MDA levels in rat liver (Khalifa and Alkhalaf, 2020).
In addition, Goel et al. (2005) observed that highly reactive hydroxyl radicals, react with unsaturated fatty acids to generate malondialdehyde. Inline, a significant reduction in SOD, catalase, and GST enzyme activities and a significant elevation in MDA were found in malathion diet-fed rats in this study. WGO showed a significant antioxidant potential against malathion-induced OS in the current study as the rats fed on WGO supplemented diet had decreased MDA and increased antioxidants. Positive effects of WGO in attenuating OS have been reported previously (Shokry et al., 2020). For example, WGO was able to prevent OS-induced DNA damage through its antioxidant activity and this effect was attributed to the vitamin E content of WGO (Paranich et al., 2000). WGO has been shown to enhance antioxidant protection by increasing blood and liver enhancing lipid metabolism (Singh et al., 2006). In addition, WGO is rich in Vitamin E which is an antioxidant and possesses free radical scavenging activity. It is proposed that vitamin E downregulates oxidative DNA damage by neutralizing increased ROS (Zou et al., 2018). These favorable effects were found to be due to alpha and gamma tocotrienols and carotenoids, including zeaxanthin, lutein, and beta-carotene content of WGO (Leenhardt et al., 2008). Wheat germ oil could also facilitate the tocopherol-mediated redox system and suppress eicosanoid synthesis, which stimulates lipid peroxidation (Field et al., 2008).
The obtained results in this study are in compliance with those of Brandolini and Hidalgo (2012) which have reported antioxidant nature of WGO due to its vitamin E content, a potent scavenger of peroxyl radicals s which abrogates free radical-induced damages to cell membranes. Moreover, consumption of WGO resulted in an increased vitamin E content in the major organs of the body (Ceylan et al., 2020) further substantiating the findings in the study.
Histological analysis of liver revealed marked pathological changes in malathion exposed rats indicating tissue injury. Malathion has previously been shown to damage liver and kidney cell organization accompanied by thin subcapsular infiltrations and diffused parenchymatous deterioration of liver cells (Handy et al., 2002). Pathophysiological changes found in malathion diet-fed rats were significantly reversed in rats fed on the WGO diet demonstrating the capacity of WGO to prevent tissue damage. These positive effects also correlate with the improved markers of liver function in these rats. These beneficial effects of WGO could be attributed to its antioxidant potential as observed in the study.
5 Conclusion
This study found that malathion is potent hepatotoxic and causes liver injury in rats. Supplementation of WGO abrogated the malathion-induced toxicity, improved liver function, and prevented liver damage. These beneficial effects could be attributed to the ability of WGO to blunt OS. On the other hand, supplementing WGO alone did not affect normal liver enzymes, OS biomarkers or histological appearance of the liver. Collectively, these data underline the antioxidant potential of WGO and suggest the beneficial effects of its supplementing in our daily diet.
Funding.
This work was funded by the University of Jeddah, Jeddah, Saudi Arabia, under grant No. (UJ-23-DR-187). Therefore, the authors thank the University of Jeddah for its technical and financial support.
CRediT authorship contribution statement
Maha I. Alkhalaf: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation. Fawzia A. Alshubaily: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis, Data curation.
References
- Acrylamide Levels in Some Food Products in Egyptian Market and Protective Effect of Extra Virgin Olive Oil. Egypt. J. Food. Sci.. 2022;50(1):15-32.
- [Google Scholar]
- Catalase. In: Bergmeyer H.U., ed. Methods of Enzymatic Analysis. Vol 2 vols. New York: Academic Press; 1974. p. :673-684.
- [Google Scholar]
- Zingiber officinale Roscoe alone and in combination with α-tocopherol protect the kidney against cisplatin-induced acute renal failure. Food Chem. Toxicol.. 2007;45:921-927.
- [Google Scholar]
- Al-Attar, A.M. (2010): Physiological and histopathological investigations on the effects of -lipoic acid in rats exposed to malathion. J. Biomed. Biotechnol. 2010.
- Detection of toxic shock syndrome toxin (TSST) gene among staphylococcus aureus isolated from patients and healthy carriers. Avicenna J. Clin. Microbiol. Infect.. 2018;5:14249.
- [Google Scholar]
- Al-Othman, M.; Al-Othman, A. A.; El-Desoky, Z.E.; Yusuf, G.; K. & Aboul-Soud, A.M. (2012): Ameliorative effect of α-tocopherol and selenium on effects of malathion on plasmatic biochemical indices and lesions in the liver of rats. Curr. Pharm. Anal. 8, 214–218.
- No additive effect between Helicobacter pylori infection and portal hypertensive gastropathy on inducible nitric oxide synthase expression in gastric mucosa of cirrhotic patients. Dig. Dis. Sci.. 2003;48:162-168.
- [Google Scholar]
- A compendium of wheat germ: Separation, stabilization and food applications. Trends Food Sci. Technol.. 2018;78:120-133.
- [Google Scholar]
- Wheat germ oil nanoemulsion for oil stability of the cooked fish fillets stored at 4° C. J. Food Sci. Technol.. 2020;57:1798-1806.
- [Google Scholar]
- Shared genetic effects between hepatic steatosis and fibrosis: A prospective twin study. Hepatology. 2016;64:1547-1558.
- [Google Scholar]
- The protective effect of aloe vera juice on lindane induced hepatotoxicity and genotoxicity. Pak. J. Pharm. Sci.. 2006;19:337-340.
- [Google Scholar]
- Feeding wheat germ meal and wheat germ oil reduced azoxymethane-induced aberrant crypt foci in fisher 344 male rats. Int J Cancer Res. 2008;4:127-136.
- [Google Scholar]
- Determination of gamma glutamyltransferase in completely haemolysed blood samples. Scand. J. Clin. Lab. Invest.. 1985;45(7):661-664.
- [Google Scholar]
- Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem. Biol. Interact.. 2005;156:131-140.
- [Google Scholar]
- Toxicology of organophosphate and carbamate compounds. Academic Press; 2011.
- Chronic diazinon exposure: pathologies of spleen, thymus, blood cells, and lymph nodes are modulated by dietary protein or lipid in the mouse. Toxicology. 2002;172:13-34.
- [Google Scholar]
- An automated alkaline phosphatase assay with phenolphthalein monophosphate as substrate. Clin. Chem.. 1967;13(4):281-289.
- [Google Scholar]
- Resveratrol attenuates malathion-induced liver damage by reducing OS. Journal of Laboratory Physicians. 2019;11(03):212-219.
- [Google Scholar]
- Malathion-induced hepatotoxicity in rats: the effects of vitamins C and E. Food Chem. Toxicol.. 2010;48:633-638.
- [Google Scholar]
- Effects of black seed and thyme leaves dietary supplements against malathion insecticide-induced toxicity in experimental rat model. J. King Saud Univ.. 2020;32:914-919.
- [Google Scholar]
- Association of inflammatory response and oxidative injury in the pathogenesis of liver steatosis and insulin resistance following subchronic exposure to malathion in rats. Environ. Toxicol. Pharmacol.. 2014;38:542-553.
- [Google Scholar]
- Wheat germ supplementation of a low vitamin E diet in rats affords effective antioxidant protection in tissues. J. Am. Coll. Nutr.. 2008;27:222-228.
- [Google Scholar]
- Wheat germ policosanol failed to lower plasma cholesterol in subjects with normal to mildly elevated cholesterol concentrations. Metabolism. 2004;53:1309-1314.
- [Google Scholar]
- Predictive model of blood-brain barrier penetration of organic compounds. Acta Pharm. Sin.. 2005;26:500-512.
- [Google Scholar]
- Molecular Drug Properties: Measurement and Prediction. Wiley-VHC Verlag GmbH & Co.; 2008. KGaA:Weinheim, Germany, 30
- Study on stability of wheat germ oil and lipase activity of wheat germ during periodical storage. Agric Biol JN Am. 2011;2:163-168.
- [Google Scholar]
- Efficacy of wheat germ oil in counteracting of some biochemical hazards induced by sodium nitrate in rats. Isot. Radiat. Res.. 2010;42:211-227.
- [Google Scholar]
- On the biochemical and molecular mechanisms by which malathion induces dysfunction in pancreatic islets in vivo and in vitro. Pestic. Biochem. Physiol.. 2013;106:51-60.
- [Google Scholar]
- Short-and long-term exposure to low levels of pesticide and flavonoid mixtures modify endogenous antioxidants in tissues of rats. J. Environ. Sci. Heal. Part B. 2009;44:357-364.
- [Google Scholar]
- The effect of wheat germ oil on the antioxidant system of animals. Likars’ Ka Sprav. 2000:40-44.
- [Google Scholar]
- Pregabalin induced reproductive toxicity and body weight changes by affecting caspase3 and leptin expression: Protective role of wheat germ oil. Life Sci.. 2020;260:118344
- [Google Scholar]
- Policosanol inhibits cholesterol synthesis in hepatoma cells by activation of AMP-kinase. J. Pharmacol. Exp. Ther.. 2006;318:1020-1026.
- [Google Scholar]
- Argon and nitrogen cold plasma effects on wheat germ lipolytic enzymes: Comparison to thermal treatment. Food Chem.. 2021;346:128974
- [Google Scholar]
- Phenolic compounds and the antioxidant activity of the bran, flour and whole grain of different wheat varieties. Procedia Chem.. 2010;2:76-82.
- [Google Scholar]
- Standardization of clinical enzyme assays: a reference method for aspartate and alanine transaminases. J. Clin. Pathol.. 1972;25(11):940.
- [Google Scholar]
- World Health WOrganization. (2015). WHO Specifications and Evaluations for Public Health Pesticides: Alpha-Cypermethrin. Geneva. World Health Organization.
- Structural variation and microrheological properties of a homogeneous polysaccharide from wheat germ. J. Agric. Food Chem.. 2019;66:2977-2987.
- [Google Scholar]
- Enantioselective metabolism of four chiral triazole fungicides in rat liver microsomes. Chemosphere. 2019;224:77-84.
- [Google Scholar]
- Effect of roasting on physico-chemical properties, antioxidant capacity, and oxidative stability of wheat germ oil. LWT. 2018;90:246-253.
- [Google Scholar]







