Translate this page into:
Water purification by adsorption of pigments or pollutants via metaloxide
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The growth in industrialization ultimately enhances the toxic discharges of wastewater containing hazardous dyes from various industrial units as a consequence severe ecological and public health issue exhibit a major challenge to conventional system to decontaminate water. Depending upon experimental constrains various physicochemical and biological treatment process have been made possible to remove pollutant of interest according to their functional abilities. In spite of all the treatment techniques, Adsorption process is considered one of the most excellent option due to the cost-effectiveness, ease of operation, high removal efficiency and regeneration of adsorbents. Over the decades, metal oxides and their composites-based absorbents showed tremendous efficiency in wastewater decontamination by dyes. The ample surface-active sites, tunable surface chemistry, ease to synthesize and functionalization, high accessible surface area, economic viability, and good recyclability make the metal oxide-based nanomaterials potential adsorbents for fast and effective removal of a wide range of heavy metal and metalloid ions. The presence of toxic dye molecules in water system poses a serious threat to aquatic ecosystem. This paper comprehensively reviews the source of contamination, possible health hazards, and adsorption technique by metal oxides to eliminate dyes from wastewater. Also, an environmentally friendly and self-sustainable treatment method should be explored to address this problem for emerging contaminants.
Keywords
Emerging contaminants
Dyes
Adsorption
Nanoparticles
Metal-oxides
Wastewater purification
1 Introduction
The rapid proliferation of worldwide massive population explosion, urbanization, industrial development as a consequence climate change, environmental degradation devastatingly affects the water quality resulting in freshwater crisis globally as water is crucial element for all living creatures (Dutta et al., 2021). The demand for water is increasing day by day due to overpopulation and more pressure on natural resources. According to Rathi and Kumar (2021) approximately 700 organic pollutants had been documented in drinking water as open release of contaminants and natural decay of animal and vegetable waste. Naseem and Durrani (2021) reported that every-three in ten people have no access to clean drinking water, only 3 % ground water is pure available for domestic usage and drinking form estimated by United Nations. As a consequence, several diarrheal deaths had reported yearly due to consumption of contaminated water. However, to meet the increasing demand of freshwater, remediation of potential pollutants from water is essential before blending with freshwater resources to boost decontamination (Naseem and Durrani, 2021).
The amount of several new type pollutants ever increasing in hydrological environments and the accessibility of pure water is shrinking unceasingly so, by 2025 half of the world population will be living in drinking water-deficient areas, to prevent these complications and toxic effects, the water remediation becomes more challenging (Yadav et al., 2021). To understand the problem, several consumers ultimately showed significant contributions in freshwater depletion in which the main culprit polluters are heavy metals and dyes in different industries. According Water Chemistry to recent financial reports, 7 × 105 tons of 10,000 different dyes and pigments are produced worldwide, and its market is skyrocketing each year and has exceeded over US $11 billion by 2008. It is estimated that only 1–2 % of the dyes are utilized for the production and for application purpose 10–15 % of dyes are discharged as effluent stream (Hussain et al., 2020). Water should be decontaminated from toxic and lethal pollutants for drinking purpose. As long as these toxic heavy metals and dyes enter in water stream, it strongly affects the ecosystem, deteriorate aquatic life and complicated to treat it. Therefore, it is essential to remediate these toxic contaminants from water to prevent humans and other living creatures from harmful effects (Patanjali et al., 2019; Naseem and Durrani, 2021). Among them, the increasingly used dyes including methylene blue (MB), rhodamine B (RhB), methyl orange (MO), Congo red (CR), Disperse Violet 26, methyl red, and crystal violet are the most important sources of industrial pollutants originating from different industries such as the textile, cosmetic, leather, food, pharmaceutical, paint and varnish, and pulp and paper industries. Dutta et al. (2021) reported that approximately 7 million tons of dyes are being manufactured per year globally. The water containing dyes can adversely interfere with the photosynthetic activity in the aquatic system. Due to excess amount of metals and aromatic compounds, it negatively pose harmful effects, as creation of mutagenic and teratogenic effect on organisms present in water such as fish. Ultimately these dyes have severe toxic implications on human health, as well as carcinogenic, mutagenic, dermatitis effects, renal disorders and skin allergies. It has been reported that chromium-based dyes are generally complex in structure and cause carcinogenic effects on human health. Thus, the disposal of dyes in the environment contaminates the water bodies, subsequently affecting the water quality, aquatic life, and human health. Therefore, removing water contaminants before surface discharge is extremely important (Nayeri and Mousavi, 2020; Ghani et al., 2021). A traditional wastewater treatment plants alone are not successful in eliminating such massive contaminant groups and therefore additional water treatment is required which is to be cost effective. Since standard primary and secondary treatment plants are unsuccessful at eliminating or degrading these harmful chemicals, a cost-effective tertiary treatment approach is proposed. Many techniques have been adopted for the water-contaminant removal. Different methodologies based on the physical, chemical and biological means have been reported in the past which include, floatation, membrane filtration, ion exchange, precipitation, solvent extraction, oxidation, electrochemistry, phytoremediation and adsorption. Sometime, a combination of methods works to achieve desirable water quality (Iqbal et al., 2022). Therefore, removing water contaminants before surface discharge is extremely important. Among them, the adsorption and photocatalytic degradation/reduction are considered the most suitable methods for removing these water contaminants because of their simplicity, eco-friendly nature, and capability of producing high quality water. Adsorption is a successful approach for Contaminants removal globally, because it is low installation expense, high performance and has easy operational design. Emerging pollutants have been removed from wastewaters using various adsorbents like activated carbons, improved bio chars, Nano adsorbents, hybrid adsorbents, and others (Rathi and Kumar, 2021; Iqbal et al., 2022; Elwakeel et al., 2020). Nanomaterials are widely used as adsorbents as well as catalysts for removing water contaminants. The properties of the nanomaterials are absolutely different from their bulk equals because of their large surface area-to-volume ratio, which has novel optical, thermal, electrical, and magnetic properties. In this regard, nanomaterials are extensively used in numerous fields such as medicine, materials manufacturing, energies, electronics, environmental remediation, and so forth. However, designing environmentally benign nanomaterials that have specific properties like low preparation cost, specific selectivity, high stability, efficient recovery, and excellent recyclability by tailoring their size and shape is one of the urgently needed challenges for practical applications. In the last decades, the most extensively applied nanomaterials in the wastewater treatment process are zerovalent metal nanoparticles (NPs; such as Ag, Fe, and Zn), carbon nanotubes (CNTs), and metal oxide NPs (TiO2, ZnO, and iron oxides). Among the adsorbents discussed, metal oxides have been reported to hold immense potential for adsorptive remediation of waste water. Because of their small sizes and large surface area-to-volume ratios, nanomaterials have strong adsorption as well as excellent photocatalytic capacities (Boruah et al., 2019; Nagpal and Kakkar, 2018).
2 Water contamination by dyes
Municipal and industrial wastewater from waste sources, which typically comprise urine, feces, and agricultural and industrial wastewater sources, as well as residential compositions and organic and inorganic compounds, can all be classified as wastewater. Large microorganisms such as viruses, bacteria, and protozoa, as well as harmful chemicals radionuclides, heavy metals, and trace elements, can be found in the wastewater (Naseem and Durrani, 2021). Zinc (Zn), nickel (Ni), mercury (Hg), Lead (Pb), cadmium (Cd), arsenic (As), chromium (Cr), and copper (Cu), are the most common heavy metals. Despite the fact that certain heavy metals may be identified in small amounts, they are nevertheless dangerous. The heavy metal contaminated wastewater finds its way into the environment, threatening human health and the ecosystem (Adnan et al., 2020).
Dyeing industries release significant amounts of wastewater into water bodies, and water pollution caused by these operations is a major problem. The inclusion of chromium in complex dyes makes them carcinogenic. Furthermore, these dyes show resistance to degradation due to their xenobiotic and complex characteristics (Nagpal and Kakkar, 2018). Dye effluent composition varies, and it may be exceedingly complicated and poisonous. There is a variety of dye compounds that depend on the type of raw material used in the industry. High suspended particles and hazardous compounds are seen in some dye effluents. Though, some of them include only high levels of residual dyes, non-biodegradable compounds formaldehyde-based color fixing agents, and hydrocarbon-based softeners. Textile dyeing has been linked to the introduction of 72 hazardous compounds into water bodies. Synthetic dyes, for example, are widely utilized in industries, particularly the textile sector, and are generated from organic or inorganic compounds. The most common dyes are direct, acid, basic, reactive, mordant, metal complex, vat, Sulphur, and disperse. Workers participating in the manufacture of textiles or paper may be in danger of being exposed to hazardous chemicals and associated health hazards due to the toxicity of dyes (Oyewo et al., 2020; Sahoo and Prelot, 2020).
3 Dyes, sources, classification, and ecotoxicological effects
The presence of heavy metals in water can be harmful because these are non-biodegradable and also can be carcinogenic. These can cause health issues to living organisms if found in a high amount (Qasem et al., 2021; Yadav et al., 2021). The harmful effects of wastewater pollution and pathogens on agriculture, animals, and humans have made wastewater treatment necessary in recent years. To preserve the environment from contamination, wastewater treatment on a governmental and personal level must be considered. For water purification from diverse impurities, wastewater treatment might include chemical, biological, and physical techniques. Nanotechnology may help with catalysis, reactivity, electrostatics, adsorption, and changeable pore volume, as well as optical electronics and sensors with high aspect ratios and hydrophilic and hydrophobic interactions. The wastewater treatment through nanotechnology-based methods is better because of the efficiency, flexibility, adaptability with high performance and low cost. This technique can also be used to restore the unique sources of water along with the clean-up process at a low cost (Naseem and Durrani, 2021). All these factors such as high specific surface area, surface charge, well-developed internal pore construction, the volume of the pore, the presence of surface functional groups, and distribution of pore size affect the efficient removal of dye through absorbent (Nayeri and Mousavi, 2020). The material that is capable of providing color to a substrate through chemical or physical binding is defined as a dye. The production of color is attributed to the existence of chromophores in the dye, to which auxochromes are connected.
There are 2 major sources of dyes. Natural dyes are obtained from plants, animals, and insects however other synthetic dyes are made from organic compounds. These dyes are used in daily life and are discharged into the environment along with the inorganic, organic compounds that are used in industries. The effluents that contain harmful compounds cause harm to the environment. As a result, numerous physical, chemical, and biological treatments are required to safeguard the environment from these harmful dye effluents produced in water. Physicochemical properties, Chemical structure, provenance, and uses may all be used to classify dyes. The severely hazardous industrial effluents are also included in this category and act as carcinogenic compounds to human health and the environment (Dutta et al., 2021; Weidner and Ciesielczyk, 2019). The water bodies are polluted by the discharge of contaminated water by the consuming or producing units of dyes and constitute a possible threat particularly to the human health, plants and animals, and in general to aquatic biota. Even in tiny concentrations, the presence of colors in wastewater is very unpleasant and unwanted. The azo dyes are used in food products, in their molecular structure has aromatic centers. The aromatic amines, benzene sulphonic acids, and benzidines which are the metabolic and degradation products of these food dyes are mutagens, carcinogens, and DNA adducts that cause the death of cells. Furthermore, dyes can color water, limiting transparency (sunlight penetration) and aeration of water bodies, thus impacting the effectiveness of critical photosynthesis and, as a result, drastically lowering dissolved oxygen (DO) levels in the water. Dye effluents discharged into bodies of water have both direct and indirect effects on aquatic ecosystems. DO depletion, Dyestuff leaching from soil to groundwater, and decreased reoxygenation potential are the direct effects and cause the reduction in the light penetration on water. These effects inhibit the process of photosynthesis which gives a red signal to the aquatic flora and fauna and aesthetic issues with water downstream. Micro toxicity, genotoxicity, and death of aquatic organisms are imposed by colored allergens, allergic reactions, immune system depression, bladder cancer in humans, hyperactivity in children, and the deadly process of water eutrophication are just a few of the indirect effects caused by dye effluents (Hussain et al., 2020). Excessive heavy metal intake through the food chain or polluted water harms the neurological and reproductive systems and destroys critical organs such as the lung, liver, and kidney variety of illnesses is caused by this including Parkinson's and Alzheimer's. Metalloid and heavy metal buildup over time can be mutagenic and carcinogenic. Furthermore, they obstruct biological functioning by displacing vital nutritional elements. At trace levels, heavy metals such as Cu, Mn, Co, Zn, Fe, and others are essential for biological functions. However, if they are accumulated more than the allowed levels, they might cause health problems and hazardous consequences. Manganese is a necessary heavy metal for the human body, but too much of it can be neurotoxic, resulting in neurological illnesses such as postural instability and Parkinson's disease (Gupta et al., 2021) (Fig. 1).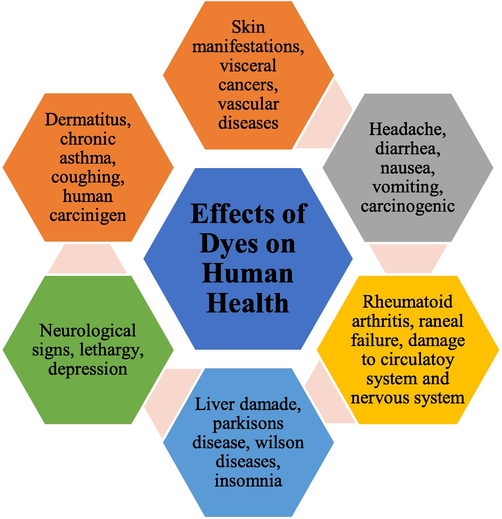
Effects of dyes on human health.
4 Available techniques for remediation of dyes
Purification of dyestuff-contaminated water is critical from both a water purification and reusability standpoint (Hussain et al., 2020). The discharge limit has prompted the development of improved color removal technologies. Different techniques have been described such as Coagulation, flocculation, several biological (e.g., MBR, ASP, CW, SBR, and MBBR) and advanced physicochemical (e.g., adsorption, anodic oxidation, Fenton reaction, filtration, ozonation, photocatalysis, and UV/H2O2 for the purification of dye-contaminated wastewater. Suspended and attached growth systems are used in biological procedures to remove dyes utilizing anaerobic and aerobic or facultative microorganisms. Various coagulants are used in the flocculation and coagulation process to destabilize the charged colloidal and suspended contaminants. The free radical reactions, solid–liquid separation, sieving process, attraction–repulsion, electrochemical reactions, and catalytic oxidation are all examples of advanced physicochemical processes (Lin et al., 2020; Wu et al., 2014; Gupta and Saleh, 2013). The treatment of industrial dye effluent is carried out by using the adsorption technique which is a very basic and straightforward method. The movement of dye molecules from the liquid phase to the solid surface of various adsorbents is involved in this process. Nanofiltration (NF) Reverse osmosis (RO), and microfiltration (MF) membrane technologies have all proved successful in the treatment of dye–laden water. However, pore obstruction and membrane fouling are still significant problems throughout these operations. Active radicals, primarily hydroxyl radicals, are produced in AOPs and play an essential role in the degradation of dye chemicals which are persistent in nature. However, their practical applicability is limited by their high cost/energy needs and the generation of hazardous by-products. Furthermore, biological processes such as MBR, ASP, MBBR, and SBR, have demonstrated high dye removal performance. However, biological methods' high dye removal efficiency is eclipsed by their drawbacks, such as poor removal rates, space requirements, and inefficiency in treating stubborn dye components. Overall, the adsorption process is advantageous for treating dye wastewater in comparison to existing filtration, biological, and advanced oxidation treatment processes because of its low cost, ease of operation, recycling of the adsorbents, high efficiency, suitability for the treatment of persistent dye compounds, and practical application (Dutta et al., 2021; Patanjali et al., 2019).
5 Adsorption mechanism and theory
A surface phenomenon called adsorption is when the adsorbent surface is the only thing that matters, and the adsorbate should not enter the adsorbent's structure. An excellent adsorbent should also be non-hazardous and not attach to pollution particles. It should be easy to regenerate and have a high selectivity for contaminants at low concentrations (Iqbal et al., 2022; Rathi and Kumar, 2021). In the process of removing pollutants using adsorbents, one of the two primary adsorption methods, physical or chemical, is utilized. The forces of van der Waals, which have a low adsorption heat, bind target molecules to pores in the walls of high-level adsorbents in physical adsorption. The target pollutant is exposed to a chemical covalent reaction that connects to specific places in the adsorbent, and the heat produced is substantially higher than adsorption and is about equivalent to the heat of the reaction (Nayeri and Mousavi, 2020). Various adsorption methods are used to adsorb dye from polluted water onto the surface of an adsorbent. p–p interactions, hydrogen bonding, van der Waals forces, Electrostatic attraction, hydrophobic interaction, and acid-base reactions are all important factors in the adsorption of water contaminants on adsorbents. The Rhodamine dye adsorption on the surface of the adsorbent is governed by electrostatic and intermolecular interactions. In addition, the ion exchange method includes ions being exchanged between a liquid (dye solution) and a solid phase (adsorbent). The adsorption process is defined by the binding of ions to different surface functional groups accessible on the adsorbent's surface, as well as electrostatic contact between the adsorbent and adsorbate surfaces. The adsorption mechanism, is explained by the production of hydrogen bonds between Acid Orange 7 dye and adsorbents. Positively charged molecules interact with negatively charged surfaces in p–p bonding/p-effects/p-interactions (noncovalent) systems, which are comparable to electrostatic interactions. Furthermore, the adsorption process might employ many mechanisms at the same time. Electrostatic interactions, p–p interactions, and intermolecular H-bonding, for example, control the adsorption of Coomassie Brilliant Blue R 250 dye on the surface of adsorbents (Dutta et al., 2021). The transfer of mass and adsorption of a carbon molecule from a fluid phase (liquid or gas phase) to a solid surface is the basic principle of carbon adsorption (adsorbent). Activated carbon is manufactured in such a way that it produces very porous carbon molecules with a large interior surface area. Inorganic, organic, and even metals are caught and retained by the porous structure described above. Adsorption occurs when a pollutant has low solubility in the waste, when the pollutant has a greater carbon affinity than the trash, or when both. Adsorption is generated by the fact that the surface particles of the adsorbent retain unequal or residual attractive forces that are not in the same condition as the particles in the bulk adsorbent, which have all of the forces balanced together. The energy of the particles near the adsorbent's surface is substantially higher than that of the particles in the middle. Surface energy is the extra energy per unit of surface area that is required for the attraction of adsorbate to its adsorbent surface. At a given setting, the degree of adsorption tends to increase as the surface area of the adsorbent per unit mass rises. The adsorption enthalpy and entropy are two more important factors to consider when it comes to adsorption (Awad et al., 2020; Iqbal et al., 2022).
Chemical adsorption and Physical adsorption are the two forms of adsorption based on how the adsorbate adsorbs to the adsorbent surface. Chemical adsorption is also known as chemisorption, in which the adsorbate is bound to the adsorbent surface by strong covalent bonds. Physical adsorption is also known as physisorption, in which the adsorbate binds to the adsorbent surface by weak forces such as electrostatic attraction and van der Waals forces (Rathi and Kumar, 2021).
Because adsorption is a surface phenomenon, the surface chemistry of modified and natural materials has a significant impact on its efficiency. The nature of the precursor and the manner of surface alteration determine the surface functions. A review of the influence of chemical changes on the performance of adsorbents was published by Abegunde et al. Chemically changing materials (metal oxides) refers to the process of customizing a material's surface by manipulating its physical, chemical, and biological properties. These qualities set it apart from the originals and allow it to be used for the intended purpose (Iqbal et al., 2022) (See Fig. 2).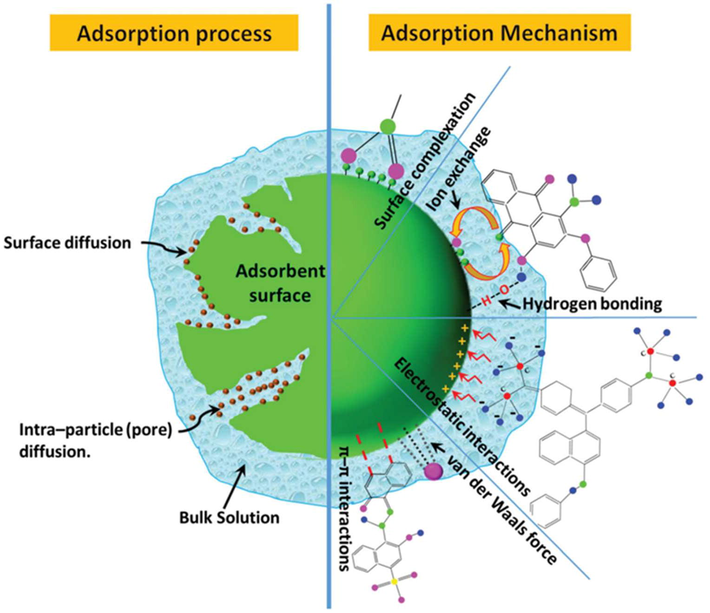
Adsorption mechanism and process for dye removal (Source Dutta et al., 2021).
6 Factors affecting dyes adsorption
In the adsorptive removal of dyes, critical parameters such as solution pH, starting dye concentration, duration, adsorbent dosage, and temperature play a crucial effect. It's worth noting that a rise in dye concentration has beneficial benefits up to a point. Furthermore, the accessible active sites on the adsorbent's surface are closely connected to the growing propensity of adsorption for high amounts of dye pollutants. Due to the presence of unsaturated active sites in the adsorbent, the improved adsorption capacity is initially accelerated. The adsorption of dye decreases dramatically when the surface of the adsorbent becomes saturated. Sodium hydroxide treated saw-dust has an adsorption capability of 55.86 mg/g with an initial concentration of 100 mg/L for brilliant green dye according to Mane and Babu (2011). Because of the surface charge of the adsorbent and the change in the ionization level, a change in pH might impact the potential interactions between dye molecules and adsorbents. Low and high solution pH, in general, encourage anionic and cationic dye adsorption, respectively (Hussain et al., 2020). It is reported an adsorption capacity of 1093 mg g1 for Acid Blue 25 at pH 2 and 600 mg/l for Rhodamine 6G at pH 7. The determination that whether the process is endothermic or exothermic, temperature changes can also assist. In an exothermic process, the adsorption capacity drops as the temperature rises, but in an endothermic process, the adsorption capacity rises as the temperature rises. Increases in adsorbent dosage have a beneficial effect on dye adsorptive removal in general, owing to the increase in active sorption sites. A greater dosage, on the other hand, may produce congestion in the adsorbent's active areas. Fe2O3chitosan–bamboo saw-dust composite to achieve an adsorption capacity of 217.39 mg/g for bromothymol blue at a dosage of 0.5 g/L. Lastly, a rise in contact duration may have both detrimental and good impacts on dye removal via adsorptive action. A further increase in the reaction time does not affect the adsorption after equilibrium is reached between the active sites of the adsorbent and the dye molecules. On the other hand, an adsorption capacity of 1057.90 mg/g for MO dye was attained in 120 min on a nano-dimensional Co/Cr-codoped ZnO adsorbent, demonstrating that increasing reaction time improves the adsorption process (Dutta et al., 2021).
7 Application of metal oxide nanoparticle in wastewater treatment
Due to their particular features, such as highly active sites and the ability to combine with other sorbents, metal oxide-based adsorbents are also favored on a wide scale. Due to their unique features, such as low concentrations, and huge surface areas metal oxide nanoparticles offer a lot of potential for cleaning polluted water (Naseem and Durrani, 2021). To reach their full potential, chemical modification may be needed in some cases. Polymeric metal oxides, carbonaceous metal oxides, and surfactant-supported metal oxides might all result from a change in composition. Surface modifications using materials such as carbon nanotubes, activated carbon, and natural poly with carbonaceous components such as functionalization, oxidation, and heat treatment are utilized to improve the performance of metal oxides as an adsorbent. The surface areas of bare metal oxides are typically small, whereas carbonaceous materials have large surface areas. Better outcomes can be obtained by using the combination of these two. Metal oxides represent a very little risk to the environment. Secondary contamination is eliminated due to the decreased solubility of these chemicals. As a result, they are very desired for use as sorbents to remove water pollutants such as dyes, heavy metals, and other contaminants (Iqbal et al., 2022). Due to their increased surface activity and porosity, metal–organic framework and polymer-based adsorbents have also been investigated as adsorbents in the treatment of dye-contaminated water (Dutta et al., 2021). Adsorbents made of metal oxides Adsorbents such as carbon-based nanomaterial, ceramics, zeolites, polymers, composites, agro-wastes, hybrid materials, and metal oxides have all been employed extensively for the adsorptive removal of a wide range of contaminants from polluted water. For a variety of industrial applications, including wastewater treatment, highly efficient, ecologically friendly, multifunctional, economically feasible, and recyclable adsorbents are in great demand. Metal oxide nanoparticles are intriguing prospects for quick, selective, and effective adsorptive removal of metalloid ions and heavy metals due to their, numerous non-toxicity, economic feasibility variable size and shape, surface active sites, and plentiful availability. Transition metal oxide nanomaterials are potential adsorbents due to their abundant availability via natural resources, ease of synthesis, and functionalization. The adsorption process is aided by the presence of hydroxyl groups on the surface of varying metal oxide nanoparticles. Amphoteric properties are seen in metal oxide nanoparticles. The displacement of a proton or the attachment to the negatively charged oxygen functionalities over the metal oxide causes the absorbance of the heavy metal ions depending on the pH. Furthermore, for surface modification/functionalization of metal oxide nanoparticles, the hydroxyl groups serve as linkers and anchoring sites (Lu et al., 2016; Oyewo et al., 2020). Bidentate ligands such as oxalate, acetylacetonate, ethylenediamine, and others can adsorb heavy metal ions by replacing the hydroxyl ions on the metal oxide surface. Even at low concentrations, metal oxide-based nanomaterials have been shown to be very effective adsorbents for heavy metal and metalloid ions. Several contaminants, such as 0.1–1.5 ppm arsenic, can be found in contaminated natural water at low amounts. Furthermore, the adsorption of heavy metal and metalloid ions by metal oxide-based nanomaterials is accelerated by charge-induced contact, ion exchange, complexation, and proton transfer. Furthermore, these materials hold promise for the continuous metal ions removal by absorption through the creation of a fixed-bed column paving the way for scaling-up and practical application in wastewater purification (Gupta et al., 2021).
Elimination of dyes: Paper, clothing, rubber, food, printers, tannery, textiles, cosmetic goods, and dyeing sectors are just a few of the businesses that employ dyes. Disposal of dye in the environment would give the water body an overwhelming tint, reducing sunlight penetration and hurting marine life. Anthraquinone dyes, Azo dyes, Indigoid dyes, Nitro dyes, Nitroso dyes, and Triaryl methane dyes are the different types of dyes based on their chemical structure. Chemical oxidation, coagulation, adsorption, membrane separation process, microbiological degradation, and electrochemical are all ways of removing colors, each with its own set of limitations. The pH, adsorbent dose, starting concentration of adsorbate, and temperature all had an impact on dye removal in adsorption (Naseem and Durrani, 2021; Iqbal et al., 2022, Gupta et al., 2021).
Elimination of heavy metal: pollution is among the most pressing environmental issues today. Toxic metals are poured into the water from several sectors. In reality, they can be cancer-causing or toxic posing serious personal health risks as well as marine environmental concerns. Chromium, lead, copper, mercury, arsenic, cadmium, and zinc, are some of the heavy metals. Heavy metal removal is influenced by a number of parameters, including adsorbent dose, contact duration, pH, initial adsorbate concertation, and temperature (Saleem and Zaidi, 2020; Gupta and Saleh, 2013).
Elimination of emerging contaminants: Emerging toxins is becoming more harmful as time goes on. Organic molecules such as fertilizers, pharmaceuticals, and personal care products, as well as surfactants, cleaning products, fire retardants, polymers, wood preservatives, hormones, food ingredients, antiseptics, and other organic chemicals, have all been found in natural wastewater streams produced by industrial and human sectors. Adsorption is a popular method for removing emerging pollutants because it has a cheap initial cost, is exceedingly effective, and is simple to use. Modified biochar, activated carbons, composite adsorbents, nano adsorbents, and other adsorbents have also been employed to extract water and wastewater from Emerging pollutants, according to research. Bisphenol-A, Salicylic acid, Naproxen, Triclosan, Ketoprofen, diclofenac, Ibuprofen, Acetaminophen, Hydrocodone, Androstenedione, Caffeine, Atrazine, Carbamazepine, Estrone, Dilantin, Estriol, Fluoxetine, Ethynyl estradiol, and others are some of the growing contaminants (Rathi and Kumar, 2021).
8 Various adsorbents for dyes removal in wastewater treatment
The most often employed nanomaterials in water/wastewater treatment are categorized into three groups based on their shape, size, and chemical properties:
-
carbon-based nano adsorbents
-
metal oxide-based nano adsorbents
-
polymer-based nano adsorbents. Below are descriptions and examples of each type of nano adsorbent (Ghani et al., 2021).
8.1 Carbon-based nano adsorbents
Carbon-based nano adsorbents such as multiwalled carbon nanotubes (MWCNTs, graphene, and carbon nanotubes (CNTs) have been widely used in wastewater treatment adsorption techniques. These nano adsorbents have high removal effectiveness of pollutants (i.e., heavy metals) from wastewater due to their unique properties of nontoxicity, specific surface areas, large pore volumes, the presence of oxygen-containing surface, and non-corrosive. One-dimensional carbon-based nano adsorbent material called CNT. A number of CNTs have been used extensively in the adsorption process to remove heavy metals such as lead, mercury, nickel, copper, chromium, cadmium, arsenic, and copper from aqueous solutions. Because of its high surface area, CNT's multilayered and hollow structure can contribute to high adsorption efficiency. Strong oxidizing chemicals such as KMnO4 and H2SO4 are used to introduce carboxyl and hydroxyl functional groups to CNTs' surfaces. The binding capacity of heavy metals ions has been boosted by the functionalized carbon nanotubes and developed active spots on CNTs for the adsorption of heavy metals by electrostatic bonding. Graphene is another carbon-based nano adsorbent that is widely used in wastewater treatment. Graphene is a two-dimensional material made up of a single atomic layer of SP2 hybridized carbon atoms packed tightly in a honeycomb crystal lattice. Graphene is the world's strongest and lightest substance. With a particular surface area of 2620 m2/g, graphene has Young's modulus of 1 TPa and inherent strength of 130 GPa. Graphene is not suitable for use in water applications due to its water-repellant and hydrophobic nature properties. As a result, due to the presence of oxygenous functional groups that contribute to the hydrophilicity qualities of that nanoparticle, manufacturing graphene oxide (GO) would be the ideal option for overcoming this issue. A graphene oxide-MgO nanohybrid for removing lead ions from an aqueous solution in their research. According to the findings, external mass transfer and intraparticle diffusion control led to adsorption via graphene oxide -MgO processes, and the Langmuir model also suggests monolayer adsorption has happened. In a nutshell, carbon-based nano adsorbents used in heavy metal adsorption have varied adsorption methods in aqueous solutions depending on the physical, electrostatic interaction, and chemical (Ghani et al., 2021; Rathi and Kumar, 2021).
8.2 Metal oxide
Metal precursors are used to making nanoparticles called Metal oxide nanoparticles (MONPs). In different fields of science such as chemistry, material science, and physics these nanoparticles are of great importance. These can have a wide range of structural geometries, as well as an electrical structure that can have an insulator, semiconductor, or metallic properties. Because of their well-known localized surface plasmon resonance properties, these nanoparticles exhibit unique optoelectrical properties (Naseem and Durrani, 2021).
Metal oxides represent a very little risk to the environment. Secondary pollution is eliminated due to the lower solubility of these substances. As a result, they are very desired for use as sorbents to remove water pollutants such as dyes, heavy metals, and other contaminants. Crystal structure, size, surface area, and shape are just a few of the qualities of interest that have been the focus of the investigation. Because iron possesses four unpaired electrons in its 3d outer shell, iron oxides or their magnetic composites have attracted the interest of a number of researchers owing to their highly distinctive features, such as superparamagnetic and a significant magnetic moment. Functionalized magnetic nanoparticles are the most effective because they contain a large number of active sites that inhibit aggregation (Iqbal et al., 2022).
Manganese oxide, nickel oxides, zinc oxide, aluminum oxides, magnesium oxides, titanium oxide, zirconium oxides, and iron oxides, are metal oxide-based nanoparticles with sizes ranging from 1 to 100 nm. Because of their high adsorption capacity and huge surface areas, these metal oxide-based nanoparticles are attractive nano adsorbents for removing heavy metals from aqueous systems (Ghani et al., 2021). In the visible electromagnetic spectrum zone, alkali nanoparticles and noble metals such as Au, Cu, and Ag, exhibit a broad absorption band. The angle, size, and shape of metal nanoparticles production play a vital role in today's state-of-the-art materials. Metal oxides with nanoscale dimensions exhibit a number of unique features, including a high removal capacity and heavy metal selectivity. They have tremendous promise as heavy metal adsorbents. Nanosized iron oxides, magnesium oxides, manganese oxides, titanium oxides, ZnO, aluminum oxides, and zirconium oxides are examples of metal oxide-based nanomaterials. Metal oxide nanoparticles are used in a variety of ways (Qasem et al., 2021) (Fig. 3).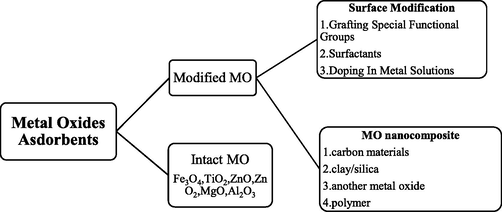
Schematic view of metal oxide-based adsorbents.
Zinc oxide nanoparticles as a disinfectant: Due to its great chemical stability and outstanding photocatalytic activity in the removal of water contaminants, ZnO is considered a good photocatalyst. At ambient temperature, ZnO has a large bandgap (3.37 eV) and a high exciton binding energy (60 meV). Nanowires, nanosheets, nanorods, nanobelts, and complex hybrid structures are among the ZnO nanostructures that may be created. Because of its physical and chemical properties, such as excellent electrochemical stability, super oxidative capacity, and low toxicity, ZnO is a potential material for conducting the photocatalytic activity. Among other metal oxides, ZnO is the earliest and most extensively used material for heterogeneous photocatalysis. There are different advantages of the photocatalytic approach, but the photocatalytic effectiveness and creation of photocurrent are hampered by a fast recombinant photo-excited carrier in ZnO (Saleem et al., 2022; Dutta et al., 2021).
Copper oxide nanoparticles: A semiconductor with a monoclinically organized structure is called Copper (II) oxide. Solar energy efficiency, Superconductivity at high temperatures, relative stability, antibacterial activity, and cheap cost are only a few of the chemical and physical properties it possesses. Because the size of the particle reduces the surface-to-volume ratio, the number of reactive sites rises, using CuO nanoparticles with a narrow size distribution for these applications will further boost the nanoparticles' chemical reactivity. The discovery of the copper hydroxide/oxide layer on Cu nanotube surfaces has served a key role in improving the electrocatalytic activity and stability of the hydro catalyst (Sahoo and Prelot, 2020).
Silver oxide nanoparticles: High purity, ultra-high purity, and transparent silver oxide nanoparticles are available in dispersed and coated forms. Different techniques have been used to make Ag2O nanoparticles, which may be classed as solid, gas, or liquid-phase processes. Physical and chemical production techniques for Ag2O nanoparticles are well known. A silver oxide nanoparticle aggregation was created by Jiang et al. Under both artificial and natural light, the synthesis demonstrated excellent photocatalytic activity. The results show that methyl orange decomposes fully in 40 min when exposed to near-infrared light and in 120 s when exposed to sunshine, artificial ultraviolet light, or artificial visible light (Boruah et al., 2019).
Titanium oxide nanoparticles: Titanium oxide (TiO2) nanoparticles have been the most extensively studied metal oxides in recent decades. Titanium dioxide is referred to as the most exceptional photocatalyst mainly due to its reasonable price, photostability, chemical and biological stability, and high photocatalytic activity. Because TiO2 nanoparticles have low selectivity, they can degrade a wide range of pollutants, including chlorinated pesticides, organic compounds, polycyclic aromatic hydrocarbons, dyes, arsenic, phenols, cyanide, and heavy metals. Titanium oxide is also employed in food color, taste enhancer additions, disinfectants and white pigments, and the breakdown of organic molecules, among other things (Dutta et al., 2021).
Iron Oxides Nanoparticles: Iron oxide nanoparticles have been widely used to remove heavy metals in recent years due to their ease of use and availability. Iron oxide-based nanomaterials possess favorable properties such as high surface area, high tensile strength, improved membrane properties, and small particle oxide nanoparticles have been synthesized using different methods. their different properties such as high porosity, strong magnetic response, specific surface area, and iron-based nanomaterials have lately demonstrated a remarkable sorption potential, resulting in an amazing sorption capacity. Because of their high BET surface area, super magnetic characteristics, and large pore volume, iron-based nanomaterials have gotten a lot of interest in recent years. The synthesized Fe3O4 nanoparticles have been shown to have a good dye removal performance in a large range of pH levels. Angamuthu et al. employed a nanomaterial made from a Fe3O4 mesoporous carbon shell to decompose methylene blue dye. This manufactured nanomaterial had remarkable catalytic activity for the breakdown of methylene blue dye (Naseem and Durrani, 2021).
Nickel oxide: The lead and zinc removal by using nickel oxide nano powder has been reported, with improved high adsorption capacity and catalytic activity of 63.7 mg/g and 50.5 mg/g, accordingly. According to the authors, lead removal by nickel oxide corresponded to the Langmuir isotherm and pseudo-second-order kinetic models, whereas the Freundlich isotherm and pseudo-first-order kinetic model for the zinc removal. The adsorption process was fitted to the Langmuir model and pseudo-first-order reaction, and equilibrium was attained at 2-hour. The capacity to remove nickel, chromium, and copper, from an aqueous solution was also demonstrated by the green production of nickel oxide from extracts of lemon juice. With pseudo-second-order kinetic models and Langmuir isotherm, the findings revealed that adsorption was pH-dependent and characterized. According to a recent study chromium-doped nickel oxide nanoparticles have a high potential for heavy metal removal, including lead, cadmium, and copper. The formation of hydroxide on the surface nanoparticle is responsible for cation removal and high adsorption capacity in aqueous solution by chromium-doped nickel oxide, and the adsorption kinetics and isotherm models were well fitted using pseudo-second-order kinetic and Freundlich isotherm models, respectively (Ghani et al., 2021).
Magnesium Oxide: As the MgO can be manufactured cheaply has a huge surface area, and a large number of active sites, it has long been utilized for dye removal created MgO powder with particle sizes ranging from 38 to 44 nm and a specific surface area of 153.7 m2/g. The produced MgO powder successfully removed anthraquinone and azo dyes, namely Reactive red 198, with maximal adsorption capacities of123.5 and 166.7 mg/g, respectively. Surfactants have also been used as structure guiding agents in the synthesis of MgO, which helps to improve adsorption efficiency. The production of nanostructured MgO was done by utilizing cetyltrimethylammonium ammonium bromide. Mesoporous MgO nanoplates produced using Dioctyl sulfosuccinate sodium surfactant had maximal adsorption capacities of 471–588 mg/g, 180 mg/g, and 350 mg/g, for Congo red, Sudan III, and Methyl orange, respectively (Table 1).
Sr no.
Adsorbent
Dye
Surface area (m2/g)
Adsorption capacity (mg g−1)
Reference
1.
MgO nanoplates
Congo Red
198
297
Nagpal and Kakkar, 2018
2.
Al2O3 microspheres
Congo Red
317
690
Nagpal and Kakkar, 2018
3.
ZnO- Al2O3
Congo Red
201
397
Nagpal and Kakkar, 2018
4.
NiO-Al2O3
Congo Red
157
357
Nagpal and Kakkar, 2018
5.
CeO2 nanospheres
Congo Red
53.3
942.7
Weidner and Ciesielczyk, 2019
6.
Al2O3
0.55
0.14
Weidner and Ciesielczyk, 2019
7.
TiO2 nanoparticles
Reactive Red 195
–
87
Saleem and Zaidi, 2020
8.
CuO-ZnO nanofibers
Congo red dye
–
126.4 Langmuir
Saleem and Zaidi, 2020
9.
ZnO
Acid black 26
–
52.63
Saleem and Zaidi, 2020
10.
PbO-AC
Methyl orange
–
333.33
Nayeri and Mousavi, 2020
11.
SnO2-AC
Malachite green
–
142.87
Nayeri and Mousavi, 2020
12.
CuO-AC
Acid blue 129
–
65.36
Nayeri and Mousavi, 2020
13.
ZnO nanorods-AC
Crystal violet
–
113.64
Nayeri and Mousavi, 2020
14.
CeO2
Cr(VI)
Pb(II)72
–
Sahoo and Prelot, 2020
15.
Hydrous amorphous Fe oxides
Pb(II)
600
–
Sahoo and Prelot, 2020
16.
Ca(OH)2/Na2FeO4 modified fly ash
Orange II
–
24.8
Dutta et al., 2021
17.
Iron oxide–alumina
CR
–
416.66
Dutta et al., 2021
18.
MgFe2O4
Methyl green (MG) and basic fuchsin (BF)
–
1.231
Dutta et al., 2021
19.
Fe3O4/CeO2
Azo dye (Acid Black 210 (AB210))
–
93.08
Dutta et al., 2021
20.
Ni–MgO
Methyl blue
–
367
Dutta et al., 2021
21.
SiO2/carbon (corn cobs) nanocomposite
U(VI) Cr(VI) MB
–
U(VI) Cr(VI) MB
Yadav et al., 2021
22.
FeOOH/CuO and cellulose (Bamboo) composite
As(III)
–
76.1
Yadav et al., 2021
23.
ZnO-NRs-AC
BCG, EY
855.18
57.80 (BCG), 61.73 (EY) mg g−1
Iqbal et al., 2022
24.
MCB
Cu(II), Pb(II), Zn(II)
57.80 (BCG), 61.73 (EY) mg g−
47.64 (Cu), 37.99 (Pb), 22.30 (Zn) mg g−1
Iqbal et al., 2022
25.
Cobalt ferrite, magnetic silica
AB1 dye and Cr (VI)
–
Cobalt ferrite, magnetic silica
Iqbal et al., 2022
26.
CoFe2O4
RR-133 & MB dye
–
278 (RR), 34 (MB) mg g − 1
Iqbal et al., 2022
27.
Fe3O4
RhB & MB
–
35.96
Iqbal et al., 2022
28.
Mesoporous maghemite
Methyl orange
93
385
Nagpal and Kakkar, 2018
29.
MgO powder
Reactive red 198
153.7
123.5
Nagpal and Kakkar, 2018
30.
Maghemite nanoparticles
Brilliant cresyl blue
81.62
200
Nagpal and Kakkar, 2018
Aluminum oxides: The most frequent activated alumina low-temperature metastable polymorph called γ-Al2O3. On the surface, t has hydroxyl groups. Boehmite (-AlOOH) undergoes an isomorphous transition around 400–700 °C, resulting in the creation of γ-Al2O3 with the same shape as the parent boehmite nanostructures. By merely changing the reaction temperatures, different morphologies were achieved. The impact of reaction temperature, shape, and size of boehmite nanostructures in increasing the potentialities of γ -Al2O3 as an adsorbent for dye removal was highlighted in the study. Congo red has a maximum adsorption capacity of 416 mg/g on the as-synthesized -Al2O3 nanorods. The impact of surface treatment of alumina nanofiber films with NaOH and HCl on the adsorption capacity of Methyl orange (MO) dye in order to better understand the mechanism of anionic dye adsorption. Electrostatic repulsion existed between the negatively charged alumina nanofilm surface and the anionic MO dye molecules when treated with NaOH; hence, no MO dye adsorption by the alumina nanofilm was detected, however treatment with HCl resulted in greater absorption of MO dye by the nanofilm. This can be explained by the anionic dye molecules' electrostatic attraction to the positively charged nanofilm surface (Nagpal and Kakkar, 2018).
8.3 Metals and metal-oxide-based nanomaterials for water disinfection
The disinfection of water through different metals, as well as metal oxides, has shown to be effective antimicrobials. Silver compounds and silver ions are the most often utilized metals for disinfection because of their better germicidal effects and stronger antibacterial activity. Because of their antibacterial qualities, silver nanoparticles are widely used in a variety of goods. The antimicrobial activity of zinc oxide nanoparticles is boosted against a wide spectrum of microorganisms, including Lactobacillus helveticus and Escherichia coli. For the study, an enhanced antibacterial filter was created using a granule substance containing red mud as the raw material. A cationic polyelectrolyte layer was successfully created using nano ZnO as a modifier. The red mud granule material enhanced with nano-ZnO and polyelectrolyte diallyl dimethylammonium chloride (PDDA) has a high SSA and abundant adsorption sites, exhibiting remarkable antibacterial characteristics. This nano ZnO-polyelectrolyte diallyl dimethylammonium chloride modified red mud granule material is easy to manufacture, low in cost, easy to separate, and performs exceptionally well, which is beneficial in reducing microbial contamination of water bodies as well as the risk of disinfection byproducts. Furthermore, the titanium dioxide-iron (III) oxide nanocomposite was discovered to be an effective microbial disinfectant, capable of eliminating Escherichia coli through photocatalytic activity. Metals and metal oxide nanoparticles inactivate microbial cells primarily by causing physical damage to the cells (Saleem and Zaidi, 2020).
8.4 Polymer-based
To solve the drawbacks of traditional adsorbents, a variety of organic–inorganic hybrid polymers with increased adsorption capacity, thermal stability, and recyclability have been created. Polymeric-based nano adsorbents with a large specific surface area, porous structure, and functional groups on the surface have been shown to bind well to organic dyes and heavy metal ions such as zinc, arsenic, lead, and cadmium wastewater. Different types of polymers have been reviewed and demonstrated that polymer-based absorbents have good potential to remove various kinds of heavy metal ions from wastewaters and aqueous solutions based on materials used (i.e., dendrimers, chitosan, cellulose), methods of preparation of a good nano adsorbent, adsorption process, and mechanism. The effect of chitosan-alginate nanoparticles in mercury removal was explored. The highest adsorption capacity of 217.39 mg/g was attained with a pH of 5 and a contact period of 90 min with an initial ion concentration of 4 mg/L in this investigation. Chitin nanofibrils, a chitosan derivative, to remove a wide spectrum of metal ions including nickel, cadmium, copper, lead, and chromium. To construct three ion-selective nanofibers modified with chelating groups of ethylenediamine (EDA), ethylene glycol (EG), or diethylenetriamine, polyacrylonitrile was used as the basis polymer (DTA). Zn (II), Pb (II), and Cu (II) have adsorption capacities of 7.2, 8.8, and 6.1 mmol/g, respectively, which are higher than other materials due to their wide surface area and ha high degree of surface functionalization as a result of the increased number of chelating groups available for metal adsorption. Heavy metal ion uptake has been examined using biopolymer-based nano adsorbents like cellulose. Due to the presence of functional groups on the surface of the nano papers, that cellulose nanofibrils containing phosphate groups adsorbed Cu (II) ions effectively from an aqueous solution (Ghani et al., 2021).
8.5 Nanocomposite
The water and wastewater treatment by fabricating new nanocomposites is a successful method. To generate various types of nanocomposites, a new technique in nano adsorbent modification by combining nanoparticles with polymer/metal/carbon-based has been developed. There have been numerous types of nanocomposites produced recently, including inorganic-polymer, organic-polymer, and magnetic nanocomposites, all of which benefit from distinct nanomaterials. For wastewater treatment applications, the creation of hybrid nanocomposite adsorbents offers incredible benefits in terms of physicochemical stability and magnetic properties. Different results indicate that nanocomposite adsorbents have excellent regeneration capabilities and the reusability and lifetime of nanocomposite adsorbents have also been reported. This acts as a critical feature in a cost-effective heavy metals removal method from wastewater (Ghani et al., 2021).
9 Latest advances in the adsorption process (nanotechnology)
Nanotechnology offers a lot of promise for improving the efficiency of water purification and disinfection. Nanotechnology has been used in water treatment and remediation, where different nanomaterials can cleanse water by converting hazardous molecules into less dangerous chemicals, adsorbing heavy metals, dyes, and other contaminants, inactivating and removing pathogens, and, among other methods (Saleem and Zaidi, 2020). Nanomaterials are effective in removing heavy metals, inorganic and organic pollutants, and microbes from wastewater. Metal oxides are the most diverse class of materials, with features that encompass practically every aspect of solid-state physics and material science. There are just a few review papers in the literature that particularly discuss metal oxide nanoparticles and their use in wastewater treatment. The primary use of nanomaterials is to solve serious water and wastewater issues. A nanometer is a trillionth of a meter in size and is referred to as a “nanomaterial.” Pharmaceuticals, Environmental detection, pharmaceuticals, biomedicine, electronics optoelectronics, cosmetics, and the apparel industry all employ nanomaterials. The addition of these small nanomaterials causes a number of physical changes, including an increase in the volume to surface-area ratio and the influence of quantum characteristics on particle size (Naseem and Durrani, 2021). The ability of metal oxides in nanoforms, known as “NMOs” (nano metal oxides), such as oxides of zinc, aluminum, iron, manganese, titanium, copper, and magnesium to sequester waste contaminants from aqueous systems has been extensively investigated. Their structures manage overall performance through variable efficiency to enhance adsorption efficiency. The increased adsorption effectiveness of NMOs is attributed to alterations in the active sites, which result in chemical modifications and surface functionalization of metal oxides. Different support and functional groups can be used to make these changes (Iqbal et al., 2022).
The removal of all types of pollutants along with the emerging contaminants while removing any drawbacks through the developments in the adsorption process. Only new contaminants from medications, personal care items, pesticides, and other sources are being targeted at this time. Metal-organic frameworks adsorbent are considered to be attractive technologies for the removal of emerging contaminants among all the innovations identified in water treatment processes, as they have many characteristics that distinguish them in water treatment: easy-to-use cavities, large specific surface area, can be replicated on a large scale and a few are water-stable and so on. Furthermore, metal–organic frameworks' adsorbents are referred to as multifunctional wastewater treatment systems since they may be used not only to remove emergent pollutants but also as harmful chemical detectors. Hybrid technologies focus on procedures that combine chemical or biological treatment with adsorption to enhance efficiency. A mix of physical treatments such as gamma rays and ultrasound, as well as adsorption on activated carbon, might be used in the newest wastewater treatment. Magnetic carbon material can assure the rapid and precise removal of contaminants from wastewater, and magnetic separation may be regarded as a novel water treatment approach due to its low cost and ease of operation. Separation is accomplished by generating an external magnetic field that aids in segregation and cleaning, as well as re-dispersion of the compounds. In fact, the ability to recycle magnetic carbon material increases the potential of adopting these products in large-scale wastewater treatment (Rathi and Kumar, 2021).
Because of the high volumes of pollutants released during the treatment of organic pigments, it has gotten a lot of attention across the world. The major goal of this research is to develop an ecologically safe, low-cost adsorbent for water filtration that has high efficiency and selective adsorption. Three typical organic pigments, acid orange 7 (AO7), methyl blue (MB), and alizarin red (AR), were chosen as anionic, cationic, and azo pigments, respectively, from an inorganically modified mesoporous biochar produced from sorghum straw. Fe3O4 particles effectively adhered to the surface of biochar following modification, according to the characterization results. Although the Langmuir isotherm successfully reflected the improved adsorption behaviors of the three pigments on the modified biochar, the as-prepared materials demonstrated a higher selective adsorption impact on the cationic pigments. Furthermore, all of the pigments studied had endothermic adsorption mechanisms that were regulated by entropy. The selective adsorption of MB is ascribed to its smaller molecular dimensions, lower molecular weight, and low molecular electrostatic potential; nevertheless, the improved adsorption affinity is related to the interaction of the benzene ring of pigment molecule with hydrogen bond and n-p interaction (Lin et al., 2020).
10 Bio-sorbents
Bio-sorbents such as Penaeus indicus shrimp, wheat flour, Spirulina platensis, cellulose, Graham flour, and Ganoderma lucidum have made significant progress in the field of color removal from contaminated water. FD&C Red No. 40 and Acid Blue 9 were successfully adsorbed on the bio-sorbent produced from Spirulina platensis. The biomass of Spirulina platensis as seen using scanning electron microscopy (SEM). At an initial solution pH of 2 and a contact period of 100 min, the biosorption capacity of Acid Blue 9 and FD&C Red No. 40 dyes was found to be 1653.0 and 400.3 mg/g, respectively. The shell of the Penaeus indicus shrimp was also shown to be an excellent adsorbent for Acid Blue 25, and dye biosorption was found to correspond well with the pseudo-second-order model. The best conditions for dye adsorption are pH 2 and 0.1 g/L dosage, giving in a 1093 mg/g adsorption capacity. For the elimination of MB, crystal violet, MG, and RhB colors from polluted water in 30 min, neem sawdust had adsorption capacities of 2.120, 2.197, 2.038, and 1.480 mg/g (Dutta et al., 2021).
11 Regeneration of adsorbents
After the adsorption process, the adsorbents must be regenerated in order to be reused, which gives economic benefits. Microwave regeneration, thermal regeneration, extraction, electrochemical regeneration, enhanced oxidation, and using supercritical fluids are some of the methods that may be utilized to rejuvenate PC. Thermal regeneration consumes a lot of energy to keep the temperature above 850 °C, and some carbon materials are lost due to attrition, burn-off, and washout during the process. The advanced oxidation method, which is based on the breakdown of organic contaminants by free hydroxyl radicals, has the benefit of being both simple and straightforward to implement on a large scale in situ. External energy, such as UV light irradiation or temperature input, is used to boost catalytic activity (Gupta and Saleh, 2013). There are many different types of regeneration processes, such as adsorption/desorption cycles, thermal regeneration, flocculent precipitate production, and so on. By dissolving the adsorbent in a solution containing the adsorbate, the CNT/CoFe2O4 adsorbent was regenerated from the adsorbed Sulfamethoxazole. In a 0.05 M NaNO3 solution containing SMX, 50 mg of CNT/CoFe2O4 is added. After the adsorption achieves equilibrium, the drying process is finished by heating to 80 °C and then to 300 °C for use in the next adsorption experiment. Furthermore, the Cr/Co - Codoped ZnO nanoparticles are regenerated by enabling them to absorb anionic contaminants and then centrifuge with HNO3 solution to create a precipitate. As a result, after adding NaOH to the production of Zn(OH) and obtaining the flocculent precipitate, centrifugation is used to acquire it. Finally, to regenerate ZnO nanoparticles, the precipitate is dried and calcined at 200 °C. The sequential cycles of the adsorption/desorption experiment are a good means of renewing the adsorbents, taking all approaches into account. Recovering the silica-coated Fe3O4 magnetic nanosphere from adsorbed Congo red dye took five consecutive cycles (Awad et al., 2020). The leaching of sorbed metal ions with bases, chelating agents, acids, and salts is used to desorb metal ions. Desorption may be accomplished using a mix of salts and bases in some instances High concentrations of bases and acids are incompatible with some sorbents (oxides such as Si, Al, and Fe as well as carbonates of Mg and Ca) because they can dissolve or corrode sections of the sorbents, altering their structure or preventing their regeneration and reuse. The search for an appropriate eluent for the novel created materials requires more research; the volume, kind, regeneration kinetics, and concentration of the eluent must all be investigated further (Elwakeel et al., 2020).
12 Future prospect
However, the expense of manufacturing nano adsorbents must be taken into account in any industrial effluent treatment if they are to be used commercially. Despite this, it is an expensive process to separate nanoparticles after adsorption and disposing of them. In addition, the cost-effectiveness of the used adsorbent in terms of reusability should be addressed. For safe and effective reuse, spent adsorbents must be regenerated and activated by treating them with acid or alkali. Furthermore, the cost-effectiveness and reuse of spent/exhausted adsorbents or safe disposal must be taken into account. Dye-loaded wasted adsorbents may be used to make charcoal, fuel cells, and energy generation. They can also be used in landfills. Similarly, energy and economic concerns must be examined to overcome the challenges and constraints in commercializing the treatment technique. Furthermore, the development of cost-effective methods and large-scale testing needed more research for the deployment of metal oxide nanomaterials.
The latest research trend is to develop cost-effective and eco-friendly adsorbents from the trash. However, the major challenge is to dispose of such adsorbents after the adsorption process and avoid environmental risks. Adsorption onto ACs has been described as a viable industrial-scale approach. Additional study on the adsorption of metal ions from low traces and effective regeneration procedures is required. Industrial applications' economic viability is also critical. There is also much research to be done in order to discover effective low-level detection methods as well as complete pollution elimination using appropriate treatment methods. Of course, the end-user must evaluate the overall economics of an adsorption water treatment system.
The outstanding adsorbents are created by researchers that can offer superior outcomes. Metal oxides, on the other hand, require further refinement in commercial applications due to their superior sorption capacities when compared to traditional adsorbents. For enhancing CMMO adsorption for water contaminants, scientists are investigating the creation of high-performance sorbents utilizing inexpensive and suitable synthesis techniques. However, there is a need to further investigate the risk of incorporated metals being released under various adsorption conditions, such as time and pH. The release of these metals during subsequent adsorption/desorption cycles is also a significant consideration. The authors also propose a comprehensive desorption study to improve desorption conditions (thermodynamics, eluent concentration, equilibrium parameters, desorption kinetics, optimum pH, and desorption temperature). Furthermore, the impact of several industrial factors on the sorption/desorption behavior of these materials, such as ultrasonic power and microwave radiation, must be investigated.
13 Conclusion
The threat to human life, flora, and animals is rising as environmental toxins continue to rise. The necessity of the hour is that by keeping the environmental benignity and economic viability in mind, seek techniques for the effective removal of the harmful contaminants. The problem of wastewater is arising day by day. Dyeing industries release significant amounts of wastewater into water bodies, and water pollution caused by these operations is a major problem. The wastewater treatment through nanotechnology-based methods is better because of the efficiency, flexibility, adaptability with high performance and low cost. There is need of adapting best treatment techniques at personal and governmental level. Nanotechnology has been used in water treatment and remediation, where different nanomaterials can cleanse water by converting hazardous molecules into less dangerous chemicals, adsorbing heavy metals, dyes, and other contaminants, inactivating and removing pathogens, and, among other methods. Through the adsorption of metal oxide, the removal of harmful contaminants has demonstrated enormous promise. Metal oxides with porous architectures, a wide surface area, thermal stability, a large number of active sites, and low toxicity have demonstrated excellent adsorption and remediation efficacy. To address the growing problem of water shortage, the study of the interface between inorganic contaminants and adsorbents is gaining popularity as a wastewater treatment tool.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mitigation of pollutants by chitosan/metallic oxide photocatalyst: a review. J. Cleaner Prod.. 2020;261:121190
- [CrossRef] [Google Scholar]
- Adsorption of organic pollutants by nanomaterial-based adsorbents: An overview. J. Mol. Liquids. 2020;301:112335.
- [CrossRef] [Google Scholar]
- Magnetic metal/metal oxide nanoparticles and nanocomposite materials for water purification. In: Nanoscale Materials in Water Purification. Elsevier; 2019. p. :473-503.
- [CrossRef] [Google Scholar]
- Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv.. 2021;2(14):4497-4531.
- [Google Scholar]
- Perspectives regarding metal/mineral-incorporating materials for water purification: with special focus on Cr (vi) removal. Mater. Adv.. 2020;1(6):1546-1574.
- [CrossRef] [Google Scholar]
- The role of nano adsorbents and nanocomposite adsorbents in the removal of heavy metals from wastewater: A review and prospect. Pollution. 2021;7(1):153-179.
- [Google Scholar]
- Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterials. Coord. Chem. Rev.. 2021;445:214100.
- [CrossRef] [Google Scholar]
- Sorption of pollutants by porous carbon, carbon nanotubes and fullerene-an overview. Environ. Sci. Pollut. Res.. 2013;20(5):2828-2843.
- [CrossRef] [Google Scholar]
- Contamination of water resources by food dyes and its removal technologies. In: Eyvaz M., Yüksel E., eds. Water Chemistry. IntechOpen; 2020.
- [Google Scholar]
- Recent advances in adsorptive removal of wastewater pollutants by chemically modified metal oxides: A review. J. Water Process Eng.. 2022;46:102641.
- [CrossRef] [Google Scholar]
- Selective adsorption of organic pigments on inorganically modified mesoporous biochar and its mechanism based on molecular structure. J. Colloid Interface Sci.. 2020;573:21-30.
- [CrossRef] [Google Scholar]
- An overview of nanomaterials for water and wastewater treatment. Adv. Mater. Sci. Eng.. 2016;2016:1-10.
- [CrossRef] [Google Scholar]
- Use of metal oxides for the adsorptive removal of toxic organic pollutants. Sep. Purif. Technol.. 2018;211:522-539.
- [CrossRef] [Google Scholar]
- The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: a review. Environ. Chem. Ecotoxicol.. 2021;3:59-75.
- [CrossRef] [Google Scholar]
- Dye removal from water and wastewater by nanosized metal oxides-modified activated carbon: a review on recent researches. J. Environ. Health Sci. Eng.. 2020;18(2):1671-1689.
- [Google Scholar]
- Metal oxide-cellulose nanocomposites for the removal of toxic metals and dyes from wastewater. Int. J. Biol. Macromol.. 2020;164:2477-2496.
- [CrossRef] [Google Scholar]
- Nanotechnology for water treatment: A green approach. In: Green Synthesis, Characterization and Applications of Nanoparticles. Elsevier; 2019. p. :485-512.
- [CrossRef] [Google Scholar]
- Removal of heavy metal ions from wastewater: A comprehensive and critical review. NPJ Clean Water. 2021;4(1):1-15.
- [CrossRef] [Google Scholar]
- Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ. Pollut.. 2021;280:116995.
- [CrossRef] [Google Scholar]
- Adsorption processes for the removal of contaminants from wastewater: the perspective role of nanomaterials and nanotechnology. In: Nanomaterials for the Detection and Removal of Wastewater Pollutants. Elsevier; 2020. p. :161-222.
- [CrossRef] [Google Scholar]
- Developments in the application of nanomaterials for water treatment and their impact on the environment. Nanomaterials. 2020;10(9):1764.
- [CrossRef] [Google Scholar]
- Removal of hazardous oxyanions from the environment using metal-oxide-based materials. Materials. 2019;12(6):927.
- [CrossRef] [Google Scholar]
- Metal oxide heterostructures for water purification. J. Nanomater.. 2014;2014:1-2.
- [CrossRef] [Google Scholar]
- Adsorptive potential of modified plant-based adsorbents for sequestration of dyes and heavy metals from wastewater-A review. J. Water Process Eng.. 2021;42:102148.
- [CrossRef] [Google Scholar]







