Translate this page into:
Vitexin attenuates cisplatin-induced renal toxicity by reducing oxidative stress and inflammation
⁎Corresponding authors at: Department of Zoology, Government College University, Faisalabad, Pakistan (A. Ashraf); Department of Zoology, College of Science, King Saud Unicersity, Riyadh 11451, Saudi Arabia (S. Mahboob). asmabinm@gmail.com (Asma Ashraf), mushaid@ksu.edu.sa (Shahid Mahboob)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cisplatin (CP) is one of the most effective chemotherapeutic drugs used to treat different tumors. Vitexin (VIT) is a natural flavonoid having various pharmacological activities along with its curative effects. The present research was planned to evaluate the therapeutic potential of VIT on cisplatin-induced renal damage in male albino rats. Twenty-four male albino rats were divided into four equal groups. These groups were treated with CP (10 mg/kg injection on the first day of trial) administered group, cotreated (CP; 10 mg/kg + VIT; 10 mg/kg) and only VIT (10 mg/kg orally till the end of the trial) treated group and a control. CP administration significantly decreased the activities of catalase (CAT), superoxide dismutase (SOD), peroxidase (POD), glutathione S-transferase (GST), glutathione (GSH), and glutathione reductase (GSR). The levels of thiobarbituric acid reactive substances (TBARS) and hydrogen peroxide (H2O2) were increased. CP-treatment significantly increased the levels of urea, creatinine, kidney injury molecule-1 (KIM-1), and neutrophil gelatinase-associated lipocalin (NGAL) while considerably reducing the creatinine clearance. The results demonstrated that CP significantly increased the inflammation markers, including tumor necrosis factor-α (TNF-α), nuclear factor kappa B (NF-κB), Interleukin-1β (IL-1β), Interleukin-6 (IL-6) levels and cyclooxygenase-2 (COX-2) activities and histopathological damages. However, co-treatment with VIT efficiently minimized the CP-induced biochemical, inflammatory, and histopathological impairments in rat kidneys. The study's outcomes indicated the significant curative efficacy of VIT to overcome CP-induced nephrotoxicity in male albino rats.
Keywords
Cisplatin
Nephrotoxicity
Vitexin
Flavonoid
Curative potential
1 Introduction
Continuous advancements in chemotherapy have increased life expectancy up to five years in about 82% of cancer patients (Gatta et al., 2014). Platinum-based anticancer medicines like cisplatin (CP) are being used to treat head, neck, lung, testicular, bladder, and ovarian cancer. CP has become the most extensively used anticancer drug due to its practical therapeutic effects against cancer (Ijaz et al., 2020a). However, residual effects and medicinal resistance are the main challenges due to CP and other metal-based anticancer drugs. Especially, CP-induced nephrotoxicity had become a significant problem (Dasari and Tchounwou, 2014).
Oxidative stress, inflammation, and apoptosis are the main pathways involved in cell damage. Multiple studies have shown that CP-induced nephrotoxicity is linked with reactive oxygen species (ROS) (Abdellatief et al., 2017). For several years, efforts have been made to produce derivatives of CP with decreased side effects which resulted in the formation of oxaliplatin and carboplatin with a narrow therapeutic spectrum (Kruger et al., 2015). The other method to reduce the nephrotoxic effects of CP is extensive hydration to wash out CP from the kidneys (Dasari and Tchounwou, 2014).
Flavonoids are significant natural plant chemicals; that commonly exist in fruits, vegetables, and certain beverages. Flavonoids have shown numerous pharmacological properties to cure several diseases, i.e., Alzheimer's, atherosclerosis, and cancer (Ovando et al., 2009). Flavonoids are linked with an extensive range of health-improving effects. They are an essential component of pharmacological, medicinal, nutritional, and beautifying applications due to their anti-mutagenic,anti-carcinogenic, antioxidant, and anti-inflammatory properties and their proficiency to control critical functions in cellular enzymes (Walker et al., 2000).
Trigonella foenum-graecum (Fenugreek) is an annual leguminous herb. Seeds of this plant have a higher content of polyphenolic flavonoids (Kaviarasan et al., 2004), which have shown a stimulating effect on the reproductive system (Jamalan et al., 2016). Vitexin, one of the essential bioactive flavonoids extracted from Fenugreek seeds (Khole et al., 2014), has anticancer, anti-inflammatory, antioxidant, and anti-diabetic effects (He et al., 2016). This experiment was planned to evaluate the protecting potential of vitexin against cisplatin-induced renal damage in male albino rats considering the above-stated facts.
2 Material and methods
2.1 Chemicals
CP and VIT were purchased from Sigma-Aldrich (Germany).
2.2 Animals
The experiment was carried out on 24 male albino rats, having 150–200 g weight. The rats were kept in standard laboratory conditions at room temperature (25-27℃), and suitable moisture with 12 h light/dark cycle was provided. They were provided with regular feed and tap water. Rats were handled in compliance with the European Union of animals care and experimentation (CEE Council 86/609) guidelines.
2.3 Experimental layout
Four experimental groups of 24 rats (having six rats in each group) were made. The first group was given with normal saline and considered as control. The second group was treated with an injection of CP intraperitoneally (10 mg/kg) on the first day of treatment. The third group was administered with both vitexin (10 mg/kg) orally for seven days and an injection of CP (10 mg/kg) on the first day of treatment. The fourth group was treated with vitexin alone (10 mg/kg) during the experiment (once a day). All the rats were anesthetized on the eighth day of the experiment with diethyl ether, decapitated, and sterile syringes were used to take trunk blood for biological estimation of serum profile. After dissection, both the kidneys were separated; one was packed in zipper bags and stored at −80 °C for biochemical analysis. The other was preserved in a 10% formalin buffer solution for histological examination.
2.4 Biochemical analysis
The activity of CAT was determined with the help of the Aebi (1984) procedure. Protocol of Kakkar et al. (1984) was followed for the estimation of SOD activity. Chance and Maehly (1955) procedure was applied for the assessment of POD activity. The protocol of Moron et al. (1979) was used for the evaluation of GSH activity. The protocol was followed to assess the GST activity as described by Younis et al. (2016). Protocol of Carlberg and Mannervik (1975) was followed for the determination of GSR activity.
2.5 Estimation of H2O2 and TBARS
The methodology of Pick and Keisari (1981) was used for the estimation of H2O2. To estimate the TBARS level was assessed as described by Iqbal et al. (1996).
2.6 Biological study of serum and urine
The standard diagnostic kits were used for the estimation of urea, creatinine, and creatinine. Urinary KIM-1 and Serum NGAL were determined according to the manufacturer's command using KIM-1 Quantikine ELISA Kit and NGAL Quantikine ELISA Kit (R and D Systems China Co. Ltd., Changning, China).
2.7 Inflammatory markers assessment
Commercially available kits were used to assess the inflammatory markers of the hepatic tissues. TNF-α, NF-κB, IL-6, IL-1β levels, and COX-2 activity were determined with a rat ELISA kit (Shanghai-YL-Biotech. Co. Ltd., China). Analyses were completed by following the manufacturer’s instructions through ELISA Plate-Reader (BioTek, Winooski-VT, USA).
2.8 Histopathological study
The samples were fixed with a mixture of 20% formaldehyde, 10% glacial acetic acid, and 70% absolute alcohol. After fixation, samples were embedded in paraffin and fixed in blocks. Thin slices (3–4 µm) were cut down, stained with Hematoxylin/eosin, fixed on slides, and analyzed under the light microscope (Nikon Eclipse E100 LED, Tokyo, Japan) at 40X.
2.9 Statistical study
The data were presented as means ± SEM. One-way ANOVA followed by Tukey’s test was applied by using Minitab software for comparison between groups. The level of significance was considered as p < 0.05.
3 Results
3.1 Protective effect of vitexin on antioxidant enzyme activity
The CP-administered group showed a significant (p < 0.05) reduction in antioxidant enzyme activities such as CAT, POD, SOD, GSR, GSH, and GST compared to the control group. Cotreatment of CP + VIT exhibited significantly (p < 0.05) amplified antioxidant activity as compared to CP-treated rats. The treatment of vitexin alone showed regular activity of antioxidant enzymes near to control (Table 1). Vales sharing different superscripts are significantly different from each other.
Groups
CAT (U/mg protein)
SOD (nanomole)
POD (U/mg protein)
GST (mg/dl)
GSR (Nm NADPH oxidized/min/mg tissues)
GSH (nM/min/mg protein)
Control
8.73 ± 0.34a
6.48 ± 0.11a
7.19 ± 0.09a
24.84 ± 0.29a
4.54 ± 0.06a
17.33 ± 0.29a
CP (10 mg/kg)
4.29 ± 0.16b
2.99 ± 0.07c
3.44 ± 0.13c
14.14 ± 0.38c
2.11 ± 0.12b
8.28 ± 0.46c
CP (10 mg/kg) + VIT (10 mg/kg)
8.41 ± 0.23a
5.95 ± 0.10b
6.58 ± 0.06ab
21.66 ± 0.32ab
4.25 ± 0.07c
15.13 ± 0.30ab
VIT (10 mg/kg)
8.88 ± 0.11a
6.44 ± 0.13ab
7.13 ± 0.09b
24.73 ± 0.51b
4.56 ± 0.03a
17.45 ± 0.25b
3.2 Protective effect of vitexin on the level of TBARS and H2O2
A remarkable elevation (p < 0.05) in TBARS and H2O2levels was found in the CP-treated rats when matched with the control group. Rats administered with CP + VIT showed reduced TBARS and H2O2levels when compared to CP-treated rats. However, vitexin alone administration maintained the TBARS and H2O2 levels as in the control rats (Table 2). Vales sharing different superscripts are significantly different from each other.
Groups
TBARS (nM/min/mg protein)
H2O2 (µM/min/mg protein)
Control
13.80 ± 0.32a
1.49 ± 0.09a
CP (10 mg/kg)
26.66 ± 0.26c
3.84 ± 0.14c
CP (10 mg/kg) + VIT (10 mg/kg)
16.74 ± 0.28b
1.86 ± 0.07b
VIT (10 mg/kg)
14.08 ± 0.26ab
1.62 ± 0.19ab
3.3 Protective effect of vitexin on renal function markers
CP-treatment significantly (p < 0.05) escalated the creatinine, urea, KIM-1, and NGAL, while a considerable decline (p < 0.05) was witnessed in creatinine clearance when compared to the control group. Co-treatment of CP + VIT showed a remarkably lowered creatinine level, urea, KIM-1, and NGAL, and a considerable (p < 0.05)rise in creatinine clearance. Group of rats treated with VIT alone exhibited average values of urinary markers as in the control group (Table 3). Vales sharing different superscripts are significantly different from each other.
Groups
Urea (mg/dl)
Creatinine (mg/dl)
Creatinine clearance (ml/min)
KIM-1 (ng/day)
NGAL (mg/ml)
Control
17.90 ± 0.16a
1.54 ± 0.08a
1.4 ± 0.03a
0.37 ± 0.02a
0.52 ± 0.03a
CP (10 mg/kg)
39.28 ± 1.70b
4.58 ± 0.10b
0.54 ± 0.05c
1.51 ± 0.03b
1.90 ± 0.04b
CP (10 mg/kg) + VIT (10 mg/kg)
23.37 ± 1.20c
2.01 ± 0.07c
1.26 ± 0.03ab
0.85 ± 0.04c
0.81 ± 0.03c
VIT (10 mg/kg)
18.36 ± 0.58d
1.59 ± 0.12a
1.38 ± 0.05b
0.35 ± 0.05a
0.49 ± 0.02a
3.4 Protective effect of vitexin on inflammatory markers
CP-treatment substantially (p < 0.05) elevated the inflammatory parameters; TNF-α, NF-κB, IL-1β, IL-6 levels, and COX-2 activities in contrast to the control group. While VIT administration considerably (p < 0.05) decreased the levels of these inflammatory parameters in the cotreated rats compared to the CP-treated group. No increase in inflammatory markers was noted in the VIT alone administered group (Table 4). Vales sharing different superscripts are significantly different from each other.
Groups
NF-κB (ng/g tissue)
TNF-α (ng/g tissue)
IL-1β (ng/g tissue)
IL-6 (ng/g tissue)
COX-2 (ng/g tissue)
Control
17.7 ± 0.62 a
6.95 ± 0.31 a
23.8 ± 0.53 a
5.59 ± 0.28 a
21.0 ± 0.81 a
CP (10 mg/kg)
81.2 ± 2.54b
19.6 ± 1.19b
79.3 ± 1.95b
16.9 ± 0.38b
68.2 ± 1.79b
CP (10 mg/kg) + VIT (10 mg/kg)
28.9 ± 1.24c
9.18 ± 0.63c
30.5 ± 1.31c
11.4 ± 0.46c
29.8 ± 0.72c
VIT (10 mg/kg)
17.3 ± 0.63 a
6.58 ± 0.29 a
23.0 ± 0.73 a
5.46 ± 0.29 a
20.5 ± 0.98 a
3.5 Protective effect of vitexin on histology of renal tissues
Microscopic analysis of kidneys showed that the treatment of rats with CP caused severe damage in renal parenchyma. CP made some tubular dilation in the cortex and focal epithelial cell damage throughout constrained areas, while in the outer medulla, it persuaded chronic and noticeable cytolysis of epithelial cells. Capillaries that interacted with tubules were dilated and dilated, and pyknotic nuclei were observed in the inner medulla. The co-treatment of CP + VIT showed more minor damage and significant recovery in the tubules of the renal parenchyma. (Fig. 1).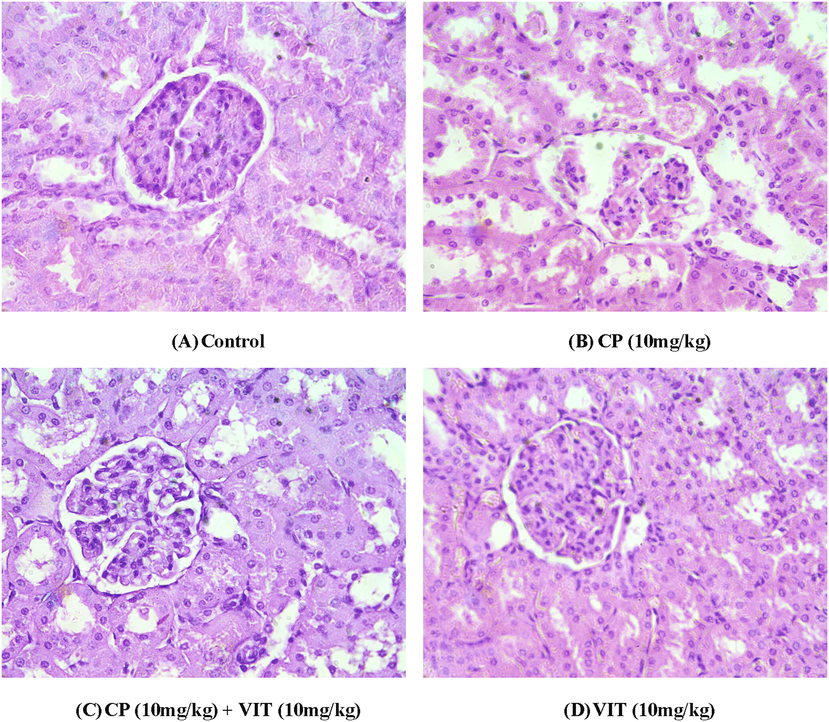
Histopathological analysis of the various groups of kidney tissues. (A) a microphotograph of kidney section of control group rats, revealing normal histological structure of glomeruli and renal tubules. (B) a microphotograph of kidney section of CP (10 mg/kg) treated rats, demonstrating significant degenerative changes, granular deposits in their lumens and desquamation of the kidney epithelium. (C) a microphotograph of VIT (10 mg/kg) + CP (10 mg/kg) administered rats displaying degenerative alterations in renal epithelium and granular deposits in their lumens. (D) a microphotograph of section of kidney showing mild glomerulo-nephrosis associated with comparatively normal kidney lining epithelium in rats treated with VIT alone. (H&E, 400X).
4 Discussion
In the current study, CP administration showed a substantial decline in antioxidant enzyme activities of CAT, POD, SOD, GSR, GSH, and GST. In contrast, a remarkable elevation in the level of TBARS and H2O2 was observed. CP induces an immune response and reactive oxygen species-mediated tissue damage (Abdellatief et al., 2017). Amplified generation of these reactive oxygen species reduces antioxidant enzymes (Ashraf et al., 2020; Ijaz et al.,2020b; Latif et al., 2020; Qamar et al., 2020) activity (CAT, SOD, POD, GSH) and increases lipid peroxidation in the kidneys. The evaluation of lipid peroxides and H2O2 in our samples helps validate renal irregularities. These results are inconsistent with the results shown in an earlier study where the level of oxidative stress markers was investigated four days, followed by CP treatment in rats (Darwish et al., 2017). In our study, improvement in antioxidant enzyme activities and decrease in TBARS and H2O2 levels due to the co-treatment of VIT with CP apprised the defensive potential of VIT in CP instigated renal injuries in rats. As in many other flavonoids, VIT inhibits ROS production and shows its antioxidant potential, which may be the main reason for increasing antioxidant enzyme activity and reducing the TBARS and H2O2 levels.
The toxicity induced by CP administration exhibited considerable escalation in urea and creatinine, while a significant decline was observed in creatinine clearance. Renal toxicity persuaded by CP can be described by considerably reduced kidney functions indicated by increased blood urea and serum creatinine (Farooqui et al., 2017). Creatinine is a metabolite that is excreted entirely in the urine through glomerular filtration, and a rise of its level in the blood is a sign of reduced renal function. Along with this, the augmented urea, creatinine, and decreased creatinine clearance are the markers for severe oxidative damage to the kidneys (Khan et al., 2010). This study revealed that VIT maintained the serum and urine profile towards normal conditions, which depicts the reno-protective role of VIT.
CP administration raised the KIM-1 and NGAL levels in treated rats. KIM-1 and NGAL are the prominent biomarkers of AKI (Lei et al., 2018). KIM-1 is nearly not expressed in the healthy renal tissue, but it is expressed during the early stages of nephrotoxic injury (Luo et al., 2016). NGAL is a cytosolic protein discharged in the urine, blood, and renal/proximal–distal tubule due to nephrotoxicity, kidney parenchymal damage, and renal ischemia (Mori et al., 2005). It is usually discharged into the blood to greater extents after damage and evacuated through the urine (Yim, 2015). Abdelsalam et al. (2018) stated that substantial elevation was observed in renal KIM-1 and NGAL levels in platinum-based drugs induced nephrotoxicity. VIT administration substantially decreased the KIM-1 and NGAL expressions when cotreated with CP. Our studies are in line with the findings of Wang et al. (2019), who reported the curative nature of VIT against lipopolysaccharide-induced acute kidney injury in rats.
In the present study, CP administration raised inflammatory-markers NF-κB, TNF-α, IL-1β, IL-6, and COX-2 activities in treated rats. NF-κB activation is fundamental in the expression of proinflammatory cytokines like IL-1β, TNF-α, IL-6, and COX-2 that are concerned with acute inflammatory responses and other disorders linked with elevated ROS production (Rehman et al., 2014). COX-2 is an inductive form of COX and an additional critical inflammation marker, playing a critical biological role in inflammation (Subbaramaiah and Dannenberg, 2003). In this analysis, the level of inflammatory-markers TNF-α, NF-κB, IL-6, IL-1β, and the activity of COX-2 was escalated in kidney tissues of CP-treated groups. In line with the findings of Rehman et al. (2014), the present work confirmed that the CPadministration showed significant elevation of pro-inflammatory cytokines, including TNF-α, NF-κB, IL-6, IL-1β, and COX-2. Our results solidify the inflammatory role of CP on renal tissues. Cotreatment of VIT with CP substantially reduced the level of inflammatory markers TNF-α, NF-κB, IL-6, IL-1β, and COX-2 activities in treated rats. Our findings are in line with Raghu and Agrawal (2016), who reported the anti-inflammatory actions of vitexin. These findings indicate the anti-inflammatory role of VIT.
Histological study of kidneys showed that CP persuaded some tubular dilation and focal epithelial cell destruction throughout restricted areas in the cortex. In contrast, it induced chronic and marked cytolysis of epithelial cells and pyknotic nuclei in the outer medulla. Capillaries that interacted with tubules were dilated, and dilation of tubules was also observed in the inner medulla. Animals treated with CP showed damage in renal tissues in the form of tubular dilation and lesions, which is in line with a previous study conducted by Saifi et al. (2018).VIT reduced tissue damages and inflammation in renal tissues due to the inhibitory effects of vitexin on inflammatory cytokine generation (Rosa et al., 2016), subsequently improved renal histology.
5 Conclusion
Our research showed the therapeutic capability of vitexin against cisplatin-induced renal injuries. Vitexin can restore the antioxidant enzyme activity, renal functional markers, regulated the inflammatory markers, and histological architecture. In conclusion, it is proposed that VIT has a therapeutic consequence over CP-induced nephrotoxicity due to its free radical scavenging ability.
Acknowledgements
The authors (SM and KAG) express their sincere appreciation to the Researchers Supporting Project number (RSP- 2021/93) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ameliorative effect of parsley oil on cisplatin-induced hepato-cardiotoxicity: a biochemical, histopathological, and immunohistochemical study. Biomed. Pharmacother.. 2017;86:482-491.
- [Google Scholar]
- Urinary biomarkers for early detection of platinum-based drugs induced nephrotoxicity. BMC Nephrol.. 2018;19(1)
- [CrossRef] [Google Scholar]
- Assessment of dietary selenium sources in commercial male broiler breeders: effects on semen quality, antioxidant status and immune responses. Pak. Vet. J.. 2020;40:13-18.
- [Google Scholar]
- Vitamin E mitigates cisplatin-induced nephrotoxicity due to reversal of oxidative/nitrosative stress, suppression of inflammation, and reduction of total renal platinum accumulation. J. Biochem. Mol. Toxic.. 2017;31(1):e21833.
- [Google Scholar]
- Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol.. 2014;740:364-378.
- [Google Scholar]
- Protective effect of Nigella sativa oil on cisplatin induced nephrotoxicity and oxidative damage in rat kidney. Biomed. Pharmacother.. 2017;85:7-15.
- [Google Scholar]
- Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5—a population-based study. Lancet Oncol.. 2014;15(1):35-47.
- [Google Scholar]
- A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia. 2016;115:74-85.
- [Google Scholar]
- Casticin alleviates testicular and spermatological damage induced by cisplatin in rats. Pak. Vet. J.. 2020;40:234-238.
- [Google Scholar]
- Methanolic extract of fraxinus xanthoxyloides attenuates cisplatin-induced reproductive toxicity in male albino rats. Pak. Vet. J.. 2020;40:489-493.
- [Google Scholar]
- Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate mediated hepatic injury. Redox Rep.. 1996;2(6):385-391.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Polyphenol-rich extract of fenugreek seeds protect erythrocytes from oxidative damage. Plant Foods Hum. Nutr.. 2004;59(4):143-147.
- [Google Scholar]
- Prevention of CCl4-induced nephrotoxicity with Sonchus asper in rat. Food Chem. Toxicol.. 2010;48(8-9):2469-2476.
- [Google Scholar]
- Bioactive constituents of germinated fenugreek seeds with strong antioxidant potential. J. Funct. Foods.. 2014;6:270-279.
- [Google Scholar]
- Neutrophils: between host defence, immune modulation, and tissue injury. PLoSPathog.. 2015;11(3):e1004651.
- [Google Scholar]
- Study of oxidative stress and histo-biochemicalbiomarkers of diethyl phthalate induced toxicity in a culturable fish, Labeo rohita. Pak. Vet. J.. 2020;40:202-208.
- [Google Scholar]
- Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci. Rep.. 2018;8:1-9.
- [Google Scholar]
- Evaluation of KIM-1 and NGAL as early indicators for assessment of gentamycin-induced nephrotoxicity in vivo and in vitro. Kidney Blood Press. Res.. 2016;41:911-918.
- [Google Scholar]
- Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J. Clin. Investig.. 2005;115(3):610-621.
- [Google Scholar]
- Levels of glutathione, glutathione reductase and glutathione-s-transferase activities in rat lungs and liver. Biochim. Biophys. Acta.. 1979;582:67-71.
- [Google Scholar]
- Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages induction by multiple nonphagocytic stimuli. Cell Immunol.. 1981;59(2):301-318.
- [Google Scholar]
- Effect of grape seed extract on tibial dyschondroplasia incidence, liver weight, and tibial angiogenesis in chickens. Pak. Vet. J.. 2020;40:187-194.
- [Google Scholar]
- Evaluation of in-vitro and in-vivo anti-inflammatory activities of apigenin and vitexin. J. Pharm. Sci. Res.. 2016;8:1349-1352.
- [Google Scholar]
- Alleviation of hepatic injury by chrysin in cisplatin administered rats: probable role of oxidative and inflammatory markers. Pharmacol. Rep.. 2014;66(6):1050-1059.
- [Google Scholar]
- Vitexin reduces neutrophil migration to inflammatory focus by down-regulating pro-inflammatory mediators via inhibition of p38, ERK1/2 and JNK pathway. Phytomedicine. 2016;23(1):9-17.
- [Google Scholar]
- Protective effect of Nanoceria on cisplatin-induced nephrotoxicity by amelioration of oxidative stress and pro-inflammatory mechanisms. Biol. Trace Elem. Res.. 2018;189(1):145-156.
- [Google Scholar]
- Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends pharmacol. Sci.. 2003;24(2):96-102.
- [Google Scholar]
- Structural determinations of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell.. 2000;6:909-919.
- [Google Scholar]
- Vitexin alleviates lipopolysaccharide-induced acute kidney injury via triggering AMPK/FOXO3a signaling pathway in newborn rats. Lat. Am. J. Pharm.. 2019;38:558-564.
- [Google Scholar]
- Neutrophil gelatinase-associated lipocalin and kidney diseases. Child. Kidney Dis.. 2015;19(2):79-88.
- [Google Scholar]
- Protective effects of Fraxinus xanthoxyloides (wall.) leaves against CCl4 induced hepatic toxicity in rat. BMC Comp. Alter. Med.. 2016;16:407-420.
- [Google Scholar]







