Translate this page into:
Vitexin and an HMG-Co A reductase inhibitor prevent the risks of atherosclerosis in high-fat atherogenic diet fed rats
⁎Corresponding author. scilei@sina.com (Xiubing Lei)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Vitexin, a flavone is known for its anti-oxidative and anti-inflammatory activities. The aim of this study was to investigate the effect of vitexin in HFAD induced atherosclerosis risks in rats. Atherosclerosis risks were induced in Albino Wistar rats by administering HFAD for 45 days. Vitexin (three dose levels) and pravastatin were administered to HFAD animals for 30 days, starting day 16 onwards. Serum lipids (TC, LDL, HDL, Atherogenic index- (AI), HDL/TC-ratio-%HTR), adhesion molecules-inflammatory mediators (MCP-1, VCAM-1, ICAM-1, IL-1β, IL-6, TNF-α), anti-atherogenic markers (PON1 and HT activities) aortic nitrative-oxidative stress (nitrotyrosine, SOD, GPx, CAT), liver HMG-CoA-reductase activity and endothelial function (using thoracic aorta in organ chamber) were assessed. Administration of Vitexin and pravastatin (selective HMG-CoA reductase inhibitor) both alone and in-combination have corrected HFAD-induced increase in serum TC, LDL, AI, MCP-1, VCAM-1, ICAM-1, IL-1β, IL-6, TNF-α, aortic nitro tyrosine and liver HMG-CoA-reductase activity. Vitexin and pravastatin have also amended HFAD-induced reduction in serum HDL, %HTR, PON1, HT, aortic SOD, GPx, CAT and endothelial function. Vitexin and pravastatin may be considered as a possible anti-atherogenic agents, which may reduce the risk of atherosclerosis and its associated conditions, like ischemic-cerebrovascular disease, coronary Heart Disease and peripheral vascular disease.
Keywords
Flavone
Vitexin
Pravastatin
HMG-CoA
Oxidative stress
Inflammation
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- SOD
total superoxide dismutase
- PON 1
paraoxonase 1
- GPx
glutathione peroxidase
- HFAD
high fat atherogenic diet
- HT
homocysteine thiolactonase
- CAT
catalase
- HMG-CoA reductase
3-hydroxy-3-methylglutaryl-coenzyme A
- MCP-1
monocyte chemoattractant protein-1
- IL-6
interleukin-6
- IL-1β
interleukin-1β
- TNF-α
tissue necrotic factor
- VCAM-1
vascular cell adhesion protein 1
- ICAM-1
intercellular adhesion molecules-1
- AI
atherogenic index
- TC
total cholesterol
Abbreviations
1 Introduction
Atherosclerosis, characterized by lipid deposition, immune cell infiltration and plaque formation is a gradual process responsible for ischemic stroke and several heart conditions. Atherosclerosis majorly stems through chronic inflammation of the arterial wall, constituting the most common cause for stroke in middle aged population (Verde and De Pietro, 2019). Adaptive and innate originated immune cell infiltrates are observed in the arteries during the development of this disease. Moreover, in the adventitia artery, tertiary lymphoid organs could be observed as the disease advances (Hu et al., 2016). Change in the lifestyle and social environment are accompanying the rise in the incident rates of metabolic disorders and also an increase in the rate of cardiovascular disease due to progressing atherosclerosis. Progression and onset of atherosclerosis is closely related with dyslipidemia indicated by hypertriglyceridemia, hypocholesterolemia and hypo alpha lipoproteinemia (Hirayama, 2016).
Cholesterols’ such as low-density lipoprotein (LDL), high-density lipoprotein (HDL) and value of total cholesterol (TC) have been widely used as quantitative markers of lipids to evaluate cardiovascular risks (Hirayama, 2016). Induction of regression and limiting progression in atherosclerosis, with the aid of medical therapy has been associated extensively with marginalizing the risk of a cardiovascular events (Raggi et al., 2016). Development of insulin resistance, cardiovascular disease and atherosclerosis, can be attributed to hyperlipidemia (Li et al., 2016a,b). The progression of atherogenesis is followed majorly by the lipoperoxidation induced oxidative damage, therefore, in the atherosclerosis development, oxidized LDL is a major causative agent (Choi et al., 1862).
Flavonoids are a main class of phenolic compounds and secondary plant metabolites, having a wide array of reported pharmacological activities (Harborne and Williams, 2000). Vitexin (apigenin-8-C-glucoside), also known as ‘Mujingsu’ in China, is a c-glycosylated flavone found in various Chinese medicinal plants such as, hawthorn, pigeon pea, bamboo, chasteberry in seeds, fruits, flowers, etc. Vitexin has received greater attention, due to its wide spectrum of pharmacological interventions. Vitexin has been reported to downregulate lipogenesis and up regulate fatty acid oxidation and lipolysis in NAFLD (Non-alcoholic fatty liver disease) mice (Inamdar et al., 2019). Inhibition of the lipogenic genes were observed with vitexin administration in the diabetic mice (Kang et al., 2015a,b). Vitexin has also been reported in reducing the acetyl CoA carboxylase gene expression in adipose tissue of HFAD fed mice (Peng et al., 2019). Hepatoprotective effect of vitexin has been observed in colitis- induced liver injury by inhibiting the activation of NFκB (Duan et al., 2020). Vitexin prevents oxidative stress and improves histopathological structures in CCl4 induced liver damage (Manikya et al., 2012). Vitexin pharmacological interventions further includes its anti-cancer, anti-inflammatory, anti-oxidant, anti-Alzheimer's, anti-nociceptive, anti-hypertensive, anti-hypoxia and ischemia injury, anti-depressant-like actions and anti-viral, activities (He et al., 2016; Peng et al., 2019)
In the present work, effect of vitexin (in three different doses) as well as pravastatin (a selective 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor) administration both in combination and alone on lipid profile, AI, endothelial function, anti-atherogenic markers (paraoxonase 1 (PON1) and homocysteine thiolactonase (HT) activities), aortic nitrative-oxidative stress, adhesion molecules- inflammatory markers, liver HMG-CoA reductase activity, in high fat atherogenic diet fed (HFAD)animals, were studied.
2 Materials and methods
2.1 Drugs and chemicals
Vitexin was obtained from Shaanxi Green Bio-Engineering Co., Ltd., Shaanxi, China. Pravastatin was obtained from Hubei Ocean Biotechnology, China. The colorimetric assay kits for serum TC, LDL, VLDL, HDL, were purchased from Innovita Biological Technology Co., Ltd. China. The ELISA kits for checking the serum inflammatory markers like interleukin-1β (IL-1β), interleukin-6 (IL-6), tissue necrotic factor-α (TNF-α) along with ELISA kits for serum vascular cell adhesion protein (VCAM-1), intercellular adhesion molecule (ICAM-1), monocyte chemoattractant protein (MCP-1), PON 1, total superoxide dismutase (TSOD), HT, Aortic nitrotyrosine level, glutathione peroxidase (GPx), catalase activity and liver HMG-CoA reductase activity were purchased from Shanghai Transhold Tech. Dev. Co. Ltd., Shanghai, China. All other chemicals were purchased from Sigma-Aldrich, Hong Kong, China. The Chemicals used were highly pure with AR grade and were prepared freshly just before use.
2.2 Animals
Male Wistar rats (weight 150–180 g) were obtained from animal house of Medical College of Panzhihua University, Panzhihua City, Sichuan Province, 617000, China. All animals were handled and treated as per the guidelines mentioned for the Care and Use of Laboratory Animals, approved by the Research Review and Ethics board of Medical College of Panzhihua University, Panzhihua City, Sichuan Province, 617000, China. All the animals were housed at temperature 23 °C, relative humidity 60%, and 12:00 h light/dark cycles. Water and foods (normal-fat diet or high fat atherogenic diet) were free available to the animals.
2.3 Induction of atherosclerosis risks using high fat atherogenic diet (HFAD)
Standard laboratory chow diet was purchased from Hunan AHL Food Co., Ltd., Yuhua District, Hunan, China. HFAD was prepared by mixing lard (28%), casein (25%), sucrose (20%), corn starch (13.12%), L-cysteine (0.38%), soybean oil (2%), cellulose (5%), cholesterol (1.3%), cholic acid (0.5%), vitamin mix (1%), mineral mix (3.5%), tert-butylhydroquinone (0.006%), choline bitartrate (0.25%), in the equal amount of standard laboratory chow diet (Matsuzaka et al., 2012). Male Wistar rats were allowed to consume HFAD freely for 45 days. In the treatment groups, respective treatments were also administered for total 30 days, starting from day 16 to till the end of study, i.e. day 45.
2.4 Experimental protocol
In total seven groups were employed in this study where 10 animals were present in each group (n = 10).
Group I- (Normal Control): Animals were orally administered (10 mL/kg) vehicle (5% DMSO, 90% saline, 5% Tween-80) daily for 45 days and standard laboratory chow diet (Normal fat diet) was available to the animals throughout the study.
Group II- (HFAD): Animals were provided with HFAD for 45 days, 10 mL/kg, p.o., vehicle (5% DMSO, 90% saline, 5% Tween-80) was also administered to HFAD animals. HFAD was available to the animals throughout the study (45 days).
Group III- (HFAD + Vitexin 5: HFAD): Animals, were administered orally with vitexin 5 mg/kg dissolved in its vehicle (5% DMSO, 90% saline, 5% Tween-80), daily for 30 days (from day 16 to day 45). HFAD was available to the animals throughout the study (45 days).
Group IV- (HFAD + Vitexin 10): HFAD animals, were administered orally with vitexin 10 mg/kg daily for 30 days (from day 16 to day 45). HFAD was available to the animals throughout the study (45 days).
Group V- (HFAD + Vitexin 20): HFAD animals, were administered orally with vitexin 20 mg/kg, daily for 30 days (from day 16 to day 45). HFAD was available to the animals throughout the study (45 days).
Group VI- (HFAD + Pravastatin): HFAD animals were orally administered with pravastatin 10 mg/kg, daily for 30 days (starting from day 16 to day 45). HFAD was available to the animals throughout the study (45 days).
Group VII- (HFAD + vitexin 20 + Pravastatin): HFAD animals were orally administered with vitexin 20 mg/kg, p.o. and pravastatin 10 mg/kg, p.o., daily for 30 days (starting from day 16 to day 45). HFAD was available to the animals throughout the study (45 days).
2.5 Collection of biological samples
2.5.1 Blood samples
Samples of Blood were collected on day 0, 15 and 30 from retro-orbital plexus of the eye of 12 h fasted rats. Overnight fasted animals were euthanized on day 45th (at the end of the study) under anesthesia (pentobarbital sodium, 30 mg/kg, i.p.) and blood was collected via cardiac puncture. Collected blood was then allowed to clot for 1 h at room temperature, and then later centrifuged (Medical Analytical Instrument Factory, Shanghai, China) for 10 min at 3,000 rpm. Serum formed was then separated and collected in Eppendorf tubes, stored at −20 °C for biochemical analyses.
2.5.2 Liver samples
After the blood collection on day 45, livers of animals were isolated and were kept in liquid nitrogen. After which it was stored at a temperature of −80 °C for further analysis of HMG-CoA reductase activity.
2.5.3 Aortic samples
Thoracic aorta was excised out after blood collection on day 45. It was then cleaned from the adhering tissues and then carefully cut into rings (3–4 mm). Aortic rings were then utilized for the assessments of endothelial function in organ chamber and for aortic biochemical assessments.
2.6 Assessment of serum lipid profiles
Serum total cholesterol, HDL and LDL levels were assessed enzymatically on days 0, 15, 30 and 45, using commercially available assay kits, in accordance with the manufacturer’s instructions. The AI and HTR% were calculated for all four time points. Where AI = (TC-HDL)/HDL and % HTR = (HDL/TC) × 100
2.7 Assessment of serum adhesion molecules, chemokines and cytokines
Also, Atherosclerosis depicts chronic inflammation from the initiation to the progression (Libby et al., 2002). Serum MCP-1, VCAM-1, ICAM-1, IL-1β, IL6 and TNF alpha levels were evaluated using commercially available ELISA kits as per the manufacturer’s instruction.
2.8 Assessment of serum anti-atherogenic markers
Serum PON1 and HT activities were evaluated using commercially available ELISA kits as per the manufacturer’s instruction.
2.9 Assessment of aortic nitrative-oxidative stress
Aortic nitrotyrosine level, GPx levels, T-SOD, and catalase activity were determined using commercially available ELISA kits as per the manufacturer’s instruction.
2.10 Assessment of endothelial function using organ chamber studies
Organ chamber study was performed as described in previous papers (Li et al., 2016a,b). The contractile response was elicited by the addition of phenylephrine (1 μM) and when the plateau of contraction was reached, Ach or SNP was added to the organ bath for inducing endothelium‐dependent or ‐independent relaxation.
2.11 Assessment of liver HMG-CoA reductase activity
Liver portion weighing 0.5 g was homogenized and centrifuged in PBS (0.25 g/mL) at pH 7.2 at 4000 rpm for 10 min, at 4 °C. The supernatant was then collected, 1 mL of which was extracted with 10 mL of 95% ethanol for each mouse at 60 °C, on three occasions. This was followed by vacuum filtration. The residues formed were dissolved in PBS to adjust the solution at certain concentration. These liver homogenate samples were then used to measure its HMG-CoA reductase activity using its assay kit as mentioned in the manufacturers’ instruction.
2.12 Histopathological studies
After the end of the study, day 45, histopathlogical studies were conducted as described in previous papers (Lee et al., 2015). Morphological alterations were recognized in the HFAD rats using high-power microscope (Nikon ELWD 0.3/OD 75; Japan) and photographs (400×) were taken and compared with the control group and treatment group
2.13 Statistical analysis
The results are expressed in the form of mean ± standard deviation. The statistical analysis of the experimental data was done using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Data for endothelial function was analyzed by Newman–Keuls test. P values < 0.05 were accepted as statistically significant.
3 Results
3.1 Effect on body weight
All the animals have shown gradual increase in body weight. As compared to normal-fat diet animals, the rats fed on HFAD for 45 days have not shown any significant difference in body weight (data not shown).
3.2 Effect on serum lipid profiles, AI and %HTR
As compared to normal control animals, the rats fed on HFAD for 45 days have showed significant increase in serum total cholesterol, LDL and AI along with reduction in serum HDL and %HTR. This was significantly corrected by the administration of vitexin (10 and 20 mg/kg) and pravastatin (10 mg/kg). Combination of vitexin (20 mg/kg) and pravastatin (10 mg/kg) have shown better effect on serum lipid profiles, AI and % HTR as compared to both the treatments when administered alone. The effect of vitexin (5 mg/kg) on lipid profile of the animals was insignificant (Table 1). Results are expressed as mean ± S.D; a p < 0.05 vs Normal control group; b p < 0.05 vs HFAD group; c p < 0.05 vs HFAD + treatment groups (Vitexin 10 or Vitexin 20 or Prava). HFAD-high fat atherogenic diet; Vitexin 10- Vitexin 10 mg/kg; Vitexin 20-Vitexin 20 mg/kg; Prava-Pravastatin 10 mg/kg.
Normal Control
HFAD
HFAD + Vitexin 5
HFAD + Vitexin 10
HFAD + Vitexin 20
HFAD + Prava
HFAD + Vitexin 20 + Prava
Serum total cholesterol (mg/dl)
Day 0
90 ± 10.8
94 ± 6.58
91 ± 5.46
93 ± 7.44
89 ± 5.34
95 ± 4.75
92 ± 3.68
Day 15
95 ± 11.4
380 ± 26.6 a
379 ± 22.7 a
370 ± 29.6 a
375 ± 22.5 a
382 ± 19.1 a
378 ± 15.1 a
Day 30
99 ± 11.8
350 ± 24.5 a
353 ± 21.1 a
300 ± 24b
250 ± 15b
255 ± 12.7b
180 ± 7.2b, c
Day 45
105 ± 12.6
330 ± 23.1 a
320 ± 19.2 a
250 ± 20b
200 ± 12b
180 ± 9b
129 ± 5.16b, c
Serum LDL (mg/dl)
Day 0
30 ± 3.6
28 ± 1.96
25 ± 1.5
32 ± 2.56
34 ± 2.04
33 ± 1.65
29 ± 1.16
Day 15
40 ± 4.8
280 ± 19.6 a
285 ± 17.1 a
289 ± 23.1 a
278 ± 16.6 a
290 ± 14.5 a
288 ± 11.5 a
Day 30
43 ± 5.16
250 ± 17.5 a
246 ± 14.7 a
210 ± 16.8b
180 ± 10.8b
160 ± 8b
140 ± 5.6b, c
Day 45
50 ± 6
260 ± 18.2 a
250 ± 15 a
170 ± 13.6b
145 ± 8.7b
90 ± 4.5b
65 ± 2.6b, c
Serum HDL (mg/dl)
Day 0
60 ± 7.2
58 ± 4.06
57 ± 3.42
61 ± 4.88
62 ± 3.72
59 ± 2.95
63 ± 2.52
Day 15
63 ± 7.56
33 ± 2.31 a
32 ± 1.92 a
30 ± 2.4 a
31 ± 1.86 a
32 ± 1.6 a
33 ± 1.32 a
Day 30
62 ± 7.44
32 ± 2.24 a
31 ± 1.86 a
39 ± 3.12b
44 ± 2.64b
45 ± 2.25b
50 ± 2b, c
Day 45
65 ± 7.8
35 ± 2.45 a
33 ± 1.98 a
44 ± 3.52b
53 ± 3.18b
54 ± 2.7b
65 ± 2.6b, c
Atherogenic Index (AI)
Day 0
0.5 ± 0.06
0.62 ± 0.04
0.59 ± 0.03
0.52 ± 0.04
0.43 ± 0.02
0.61 ± 0.03
0.46 ± 0.01
Day 15
0.50 ± 0.06
10.5 ± 0.73 a
10.8 ± 0.65 a
11.3 ± 0.90 a
11.0 ± 0.66 a
10.9 ± 0.54 a
10.4 ± 0.41 a
Day 30
0.59 ± 0.07
9.93 ± 0.69 a
10.3 ± 0.62 a
6.69 ± 0.53b
4.68 ± 0.28b
4.66 ± 0.23b
2.6 ± 0.10b, c
Day 45
0.61 ± 0.07
8.42 ± 0.59 a
8.69 ± 0.52 a
4.68 ± 0.37b
2.77 ± 0.16b
2.33 ± 0.11b
0.98 ± 0.03b, c
HDL/TC ratio (%HTR)
Day 0
66.6 ± 8
61.7 ± 4.31
62.6 ± 3.75
65.5 ± 5.24
69.6 ± 4.17
62.1 ± 3.10
68.4 ± 2.73
Day 15
66.3 ± 7.95 a
8.68 ± 0.60 a
8.44 ± 0.50 a
8.10 ± 0.64 a
8.26 ± 0.49 a
8.37 ± 0.41 a
8.73 ± 0.34 a
Day 30
62.6 ± 7.51
9.14 ± 0.64 a
8.78 ± 0.52 a
13 ± 1.04b
17.6 ± 1.05b
17.6 ± 0.88b
27.7 ± 1.11b, c
Day 45
61.9 ± 7.42
10.6 ± 0.74 a
10.3 ± 0.61 a
17.6 ± 1.40b
26.5 ± 1.59b
30 ± 1.5b
50.3 ± 2.01b, c
3.3 Effect on serum adhesion molecules, chemokines and cytokines
As compared to normal control animals, the rats fed on HFAD for 45 days have showed significant increase in serum MCP-1, VCAM-1, ICAM-1, IL-1β, IL6 and TNF alpha. This increase in serum MCP-1, VCAM-1, ICAM-1 IL-1β, IL6 and TNF alpha of HFAD animals was significantly corrected by the administration of vitexin (10 and 20 mg/kg) and pravastatin (10 mg/ kg). Combination of vitexin (20 mg/kg) and pravastatin (10 mg/kg) have shown better effect on adhesion molecules chemokines and cytokines as compared to both the treatments when administered alone. Except the serum levels of IL-6 and TNF alpha, effect of vitexin 5 mg/kg was insignificant on MCP-1, ICAM-1, VCAM-1, IL-1β (Table 2). Results are expressed as mean ± S.D; a p < 0.05 vs Normal control group; b p < 0.05 vs HFAD group; c p < 0.05 vs HFAD + treatment groups (Vitexin 10 or Vitexin 20 or Prava). HFAD-high fat atherogenic diet; Vitexin 10- Vitexin 10 mg/kg; Vitexin 20-Vitexin 20 mg/kg; Prava-Pravastatin 10 mg/kg.
Normal Control
HFAD
HFAD + Vitexin 5
HFAD + Vitexin 10
HFAD + Vitexin 20
HFAD + Prava
HFAD + Vitexin 20 + Prava
Serum MCP-1 (pg/mL)
101 ± 3.03
139 ± 4.17 a
137 ± 4.11 a
125 ± 3.75b
116 ± 3.48b
114 ± 3.42b
104 ± 3.12b, c
Serum ICAM-1 (pg/mL)
5.2 ± 0.15
7.9 ± 0.23 a
7.8 ± 0.23 a
7.2 ± 0.21b
6.3 ± 0.18b
6.4 ± 0.19b
5.5 ± 0.16b, c
Serum VCAM-1 (ng/mL)
40 ± 1.2
54 ± 1.62 a
53 ± 1.59 a
49 ± 1.47b
47 ± 1.41b
46 ± 1.38b
42 ± 1.26b, c
Serum IL-1β (pg/mL)
97 ± 2.91
186 ± 5.58 a
178 ± 5.34 a
152 ± 4.56b
132 ± 3.96b
135 ± 4.05b
107 ± 3.21b, c
Serum IL-6 (pg/mL)
50 ± 1.5
145 ± 4.35 a
132 ± 3.96b
120 ± 3.6b
103 ± 3.09b
98 ± 2.94b
75 ± 2.25b, c
Serum TNF-α (pg/mL)
7.2 ± 0.21
43 ± 1.29 a
32 ± 0.96b
26 ± 0.78b
18 ± 0.54b
19 ± 0.57b
10 ± 0.3b, c
Serum PON1 activity (nm/mL/min)
145 ± 4.35
69 ± 2.07 a
66 ± 1.98b
89 ± 2.67b
99 ± 2.97b
101 ± 3.03b
120 ± 3.6b, c
Serum HTLase activity (nm/mL/min)
240 ± 7.2
155 ± 4.65 a
152 ± 4.56b
180 ± 5.4b
201 ± 6.03b
199 ± 5.97b
215 ± 6.45b, c
3.4 Effect on serum anti-atherogenic markers
As compared to normal control animals, the rats fed on HFAD for 45 days have showed significant reduction in serum PON1 and homocysteine thiolactonase (HT) activities. This reduction was significantly corrected by the administration of vitexin (10 and 20 mg/kg) and pravastatin (10 mg/ kg). Combination of vitexin (20 mg/kg) and pravastatin (10 mg/kg) have shown better effect on serum PON1 and HT as compared to both the treatments when administered alone. The effect of vitexin 5 mg/kg on serum PON1 and HT of the HFAD animals was insignificant (Table 2).
3.5 Effect on aortic nitrosative-oxidative stress
As compared to normal control animals, the rats fed on HFAD for 45 days have showed significant increase in aortic nitro tyrosine level and significant decrease in T-SOD, GPx and CAT activities. This impairment was significantly corrected by the administration of vitexin (10 and 20 mg/kg) and pravastatin (10 mg/kg). Combination of vitexin (20 mg/kg) and pravastatin (10 mg/kg) have shown better effect on HFAD induced aortic nitrosative-oxidative stress as compared to both the treatments when administered alone. However, the effect of vitexin 5 mg/kg on aortic nitrosative-oxidative stress of HFAD animals was insignificant (Fig. 1a–d).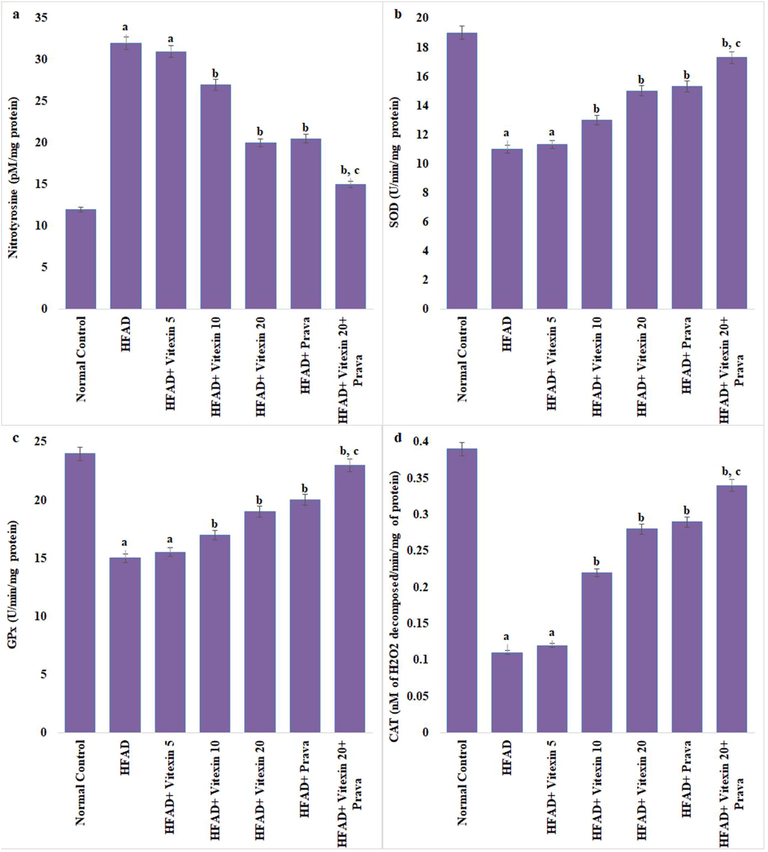
Effect on aortic nitrative-oxidative stress. a. Nitrotyrosine; b. Superoxide dismutase (SOD); c. Glutathione peroxidase (GPx); d. Catalase (CAT). Results are expressed as mean ± S.D. a p < 0.05 vs Normal control group; b p < 0.05 vs HFAD group; c p < 0.05 vs HFAD + treatment groups (Vitexin 10 or Vitexin 20 or Prava). HFAD-high fat atherogenic diet; Vitexin 10- Vitexin 10 mg/kg; Vitexin 20-Vitexin 20 mg/kg; Prava-Pravastatin 10 mg/kg.
3.6 Effect on endothelial function
The HFAD rats have showed significant inhibition of acetylcholine-induced relaxation in thoracic aorta. The administration of vitexin (10 and 20 mg/kg) and pravastatin (10 mg/ kg) in combination or alone, significantly and dose dependently corrected the HFAD induced endothelial dysfunction induced in HFAD animals. There was no effect of any of the treatment on endothelial independent relaxation in aortic tissue (Fig. 2a and b).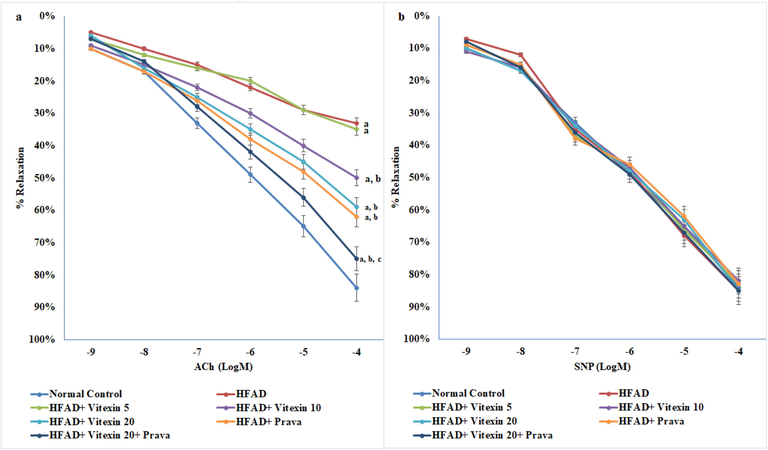
Effect on endothelial function using organ chamber studies. a. Endothelium-dependent relaxation; b. Endothelium-independent relaxation. Results are expressed as mean ± S.D. a p < 0.05 vs Normal control group; b p < 0.05 vs HFAD group; c p < 0.05 vs HFAD + treatment groups (Vitexin 10 or Vitexin 20 or Prava); HFAD-high fat atherogenic diet; Vitexin 10- Vitexin 10 mg/kg; Vitexin 20-Vitexin 20 mg/kg; Prava-Pravastatin 10 mg/kg; ACh- acetylcholine; SNP- sodium nitro prusside.
3.7 Effect on liver HMG-CoA reductase activity
As compared to normal-fat diet animals, the rats fed on HFAD for 45 days have showed significant increase in liver HMG-CoA reductase activities. This increase was significantly corrected by the administration of vitexin (10 and 20 mg/kg) and pravastatin (10 mg/kg). Combination of vitexin (20 mg/kg) and pravastatin (10 mg/kg) have shown better effect on HFAD increased liver HMG-CoA reductase activities as compared to both the treatments when administered alone. The effect of vitexin 5 mg/kg on liver HMG-CoA reductase activities of HFAD animals was insignificant (Fig. 3).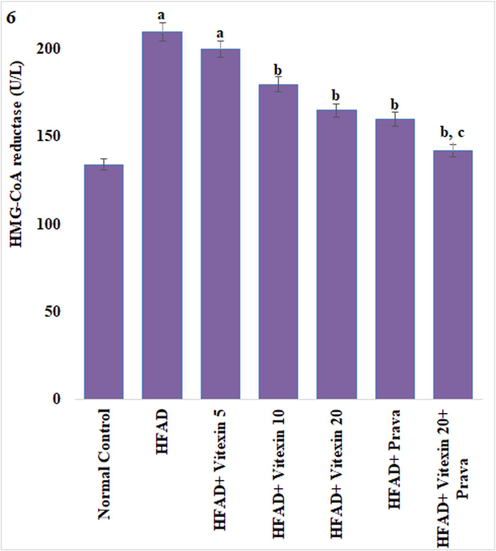
Effect on liver β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) Reductase Activity. Results are expressed as mean ± S.D. a p < 0.05 vs Normal control group; b p < 0.05 vs HFAD group; c p < 0.05 vs HFAD + treatment groups (Vitexin 10 or Vitexin 20 or Prava). HFAD-high fat atherogenic diet; Vitexin 10- Vitexin 10 mg/kg; Vitexin 20-Vitexin 20 mg/kg; Prava-Pravastatin 10 mg/kg.
3.8 Histopathological results
H &E staining in the induction group showed microvascular depots of fat, with morphological alterations showing undefined contours, congestion and necrotic changes. Cell evidenced cellular ballooning were observed in the livers of high fat diet group with vitexin (5 mg/kg). Morphological alterations also showed alterations of peripheral nuclei, fragmented cells and characteristic of cellular necrosis. Treatment with vitexin (5, 10, 20 mg/kg) and pravastatin (10 mg/kg) have shown better effect in prevention of the morphological alterations caused by the HFAD (Fig. 4).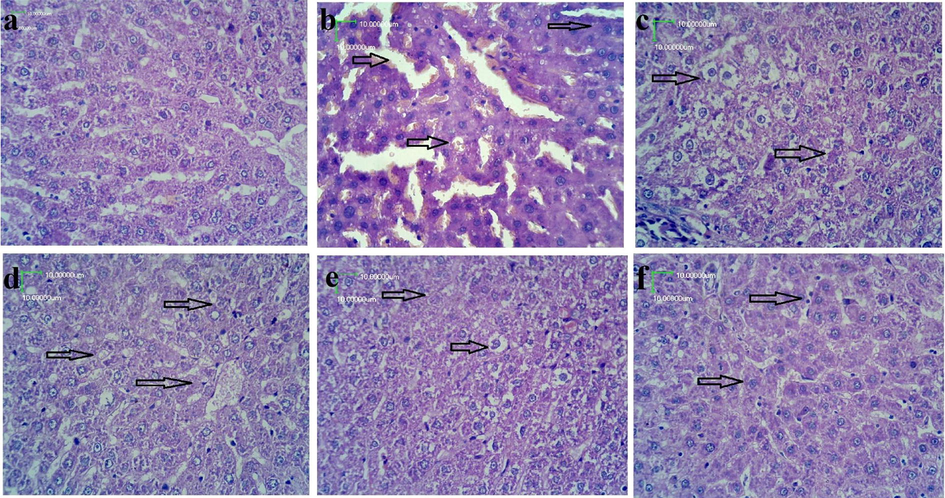
Effect on liver structure (H & E staining) 400×. a: Control group showing normal morphological characteristics. b: high fat atherogenic diet group animals showing microvascular depots of fat with morphological alterations of undefined contours along with congestion and necrotic changes. c: HFAD + vitexin (5 mg/kg) treated animal groups showing reduced fat depots with reduced congestion. d: HFAD + vitexin (10 mg/kg) treated animal groups, showing, reduction in the cytoplasmic ballooning and fragmented cells. e: HFAD + vitexin (20 mg/kg) treated animal group showing, largely reduced morphological alterations of peripheral nuclei, undefined contours, cellular ballooning and reduction in the fat depots. f: HFAD + vitexin(20 mg/kg) + pravastatin (10 mg/kg) treated animal groups showing near normal cells with reduction in morphological alterations induced by HFAD.
4 Discussion
The results of this study showed that HFAD administration for 45 days has resulted in significant increase in serum TC, TG, LDL, AI, %HTR, MCP-1, VCAM-1, ICAM-1, IL-1β, IL6, TNF alpha, aortic nitro-tyrosine level and liver HMG-CoA reductase activity along with significant reduction in serum PON1, HT, aortic T-SOD, GPx, CAT activity and endothelial function. The results of the present study are in parallel to previously published reports (Blankenberg et al., 2003; Cho et al., 2012; Farid et al., 2010; Gu et al., 2015; Kosaka et al., 2005; Kruzliak et al., 2015; Li et al., 2016a,b; Misra et al., 2019; Paudel et al., 2016; Subramanian et al., 2009; Varatharajalu et al., 2010; Yang et al., 2015)
Exacerbation of obesity related conditions like endothelial dysfunction and atherosclerosis can be linked with increased fat in the diet, significantly altering cellular metabolism, leading to insulin resistance, dyslipidemia, increased cytokine expression and secretion from skeletal muscle and adipose tissue (Farid et al., 2010; Misra et al., 2019). Elevated serum cholesterol in itself is a developmental cause of atherosclerosis. Adhesion molecules and chemokines, including ICAM-1, VCAM-1 and MCP-1, are important atherosclerotic pathogenic marker, upregulated in smooth muscle cells, macrophages and endothelial cells during atherosclerosis. High fat diet is reported to increase macrophage accumulation in adipose tissue which produces cytokines causing inflammatory changes in the liver, blood vessels and other parts of the body, which play a role in formation of atherogenesis (Subramanian and Chait, 2009).
PON1 is an enzyme with potent antioxidant properties. It prevents LDL oxidation and detoxifies homocysteine thio-lactone, known as causative agents of atherosclerosis. Serum PON and HT activity decreases in atherosclerotic diseases and higher activities may provide an indication for anti-atherogenic effect (Varatharajalu et al., 2010). The HDL associated PON-1 enzyme protects serum lipids from oxidation (Farid et al., 2010). Activity of paraoxonase, lactonase protects against homocysteinylation, thus it is considered a potential contributing factor to prevent atherosclerosis (Locsey et al., 2013).
Peroxynitrite, a highly potent oxidant formed during the reaction of NO and superoxide anion, reacts with protein to form nitro tyrosine. Nitro tyrosine is used as a marker in in-vivo peroxynitrite mediated oxidative/nitrate stress and superoxide anion mediated NO inactivation. Excessive release of oxygen radicals, if not controlled by the body’s own defense anti-oxidant SOD, GPx and catalase enzymes, can lead to oxidant stress. Aortic nitrative and oxidative stress has been implicated and considered as an important risk factor in atherosclerosis (Misra et al., 2019; Wang et al., 2016)
At the onset of atherosclerosis, the damage to the endothelium leads to deposition of harmful cholesterol in the artery wall, thus compromising endothelial function. Thus, endothelial function is considered as an onset marker of atherosclerosis (Yang et al., 2015). As progressive damage to the endothelial system continues, it eventually leads to endothelial dysfunction. Genetic factors, factors involving diet such as high fat diet and high meat consumption, along with lifestyle factors such as tobacco use and increased oxidative stress are all implicated as promoters of atherosclerosis (Li et al., 2016a,b).
The main enzyme involved in the metabolism of TC is HMG-CoA reductase. Cholesterol synthesis is facilitated by HMG-CoA reductase by catalyzing mevalonate synthesis from HMG-CoA and further by generating TC from squalene. An overall decrease in the HMG-CoA can reduce generation of TC effectively (Gu et al., 2015). Inhibitors of HMG-CoA reductase like pravastatin are in main stay for the management of early risks and late life symptoms of atherosclerosis.
Administration of vitexin and pravastatin both alone and in combination, has significantly corrected HFAD induced impairments in levels of serum lipids, AI, adhesion molecules, chemoattractant-inflammatory mediators, anti-atherogenic markers, aortic nitroso-oxidative stress, endothelial function and liver HMG-CoA reductase activity. A very recent study has reported vitexin (10 mg/kg) have significant effect on serum lipids (TC, TG, LDL, HDL) and many other parameters of high fat diet induce obese mice (Tuso et al., 2015). In our study vitexin on two different doses (10 mg/kg and 20 mg/kg) have resulted into significant correction of serum lipids, and the effect of 20 mg/kg dose were almost similar to those observed by pravastatin 10 mg/kg. Further, administration of vitexin (20 mg/kg) in combination of pravastatin resulted in to highest lipid lowering effect.
Zhang colleagues (2017) has reported pre-treatment with vitexin may be responsible for inhibition of the ox-LDL-induced overexpression of IL-1β, IL-6, TNF-α, E-selectin, ICAM1 and VCAM1 in HUVECs. Vitexin has been shown to protect rat chondrocytes in ER stress-induced increase in the expression of inflammatory cytokines, IL-6 and TNF-α (Kang et al., 2015a,b). Vitexin promoted the expression of SOD, while reducing the ox-LDL-induced oxidative stress, along with inhibition of ROS and MDA (Xie et al., 2018). The harmful effects of HF diet on hepatic MDA, GSH, GPx, glutathione reductase and SOD activity were found to be reduced by the beta vulgaris L. extract rich in vitexin (Zhang et al., 2017). Also, vitexin administration alleviated oxidative stress and inflammatory parameters in brain and lungs’ insult (Bustos et al., 2018; Jiang et al., 2018; Lu et al., 2018). Vitexin exerted significant increase in endothelium dependent relaxations due to acetylcholine on isolated rat thoracic aortic ring preparation in chronic myocardial ischemia/reperfusion injury rats (Che et al., 2016). It is important to note here that, no previous study has been found which has studied the role of vitexin on the levels of serum anti-atherogenic factors (PON1, HT), aortic nitrative-oxidative stress, HMG-CoA reductase activity. Overall this is the first study which formally assess the effect of vitexin on serum lipid profile, AI, atherogenic markers, adhesion-inflammatory cytokines, chemokines, endothelial dysfunction, aortic nitrative-oxidative stress markers in HFAD rats.
Pravastatin has been used as positive control in the present study. Pravastatin, an HMG-CoA reductase inhibitor is already known for anti-atherosclerotic properties. Serum total cholesterol, low density lipoprotein-cholesterol, triglyceride in abdominal aorta were decreased. A 10 mg/kg dose of pravastatin was administered which has provided benefits on all the parameters studied in the present study (Wu et al., 2013). When given in combination, Vitexin has significantly enhanced the beneficial effect of pravastatin in all the parameters studied, may be due to HMG-CoA reductase inhibitory activity of vitexin, as observed in our study.
This study suggests that vitexin may be considered as potentially efficacious agent in HFAD induced risk of atherosclerosis due to its anti-hyperlipidemic, anti-atherogenic, anti-inflammatory, endothelium protectant and HMG-CoA reductase inhibitory activity.
5 Conclusion
Vitexin and pravastatin administration both alone and in combination has significantly prevented HFAD induced impairments in levels of serum lipids, AI, adhesion molecules, chemoattractant-inflammatory mediators, anti-atherogenic markers, aortic nitroso-oxidative stress, endothelial function and liver HMG-CoA reductase activity. This suggests that vitexin may be considered as a possible anti-atherogenic agent to minimize atherosclerosis risks, Thus, vitexin may be found useful in atherosclerosis and atherosclerosis associated conditions, such as Coronary Heart Disease, ischemic cerebrovascular disease and peripheral vascular disease. Further research is mandated to find the complete potential of vitexin in atherosclerosis condition.
6 Authors’ contributions
We declare that this work was done by the authors named in this article and all liabilities pertaining to claims relating to the content of this article will be borne by the authors.
XL and YY conceived and designed the study, YY collected and XL analyzed the data, both XL and YY wrote the manuscript. Both the authors read and approved the manuscript for publication.
7 Role of funding source
None.
Acknowledgments
Authors are thankful to Medical College of Panzhihua University, Panzhihua City, Sichuan Province, 617000, China, for providing research facilities to conduct present research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Flavonoids as protective agents against oxidative stress induced by gentamicin in systemic circulation. Potent protective activity and microbial synergism of luteolin. Food Chem. Toxicol.. 2018;118:294-302.
- [Google Scholar]
- Vitexin exerts cardioprotective effect on chronic myocardial ischemia/reperfusion injury in rats via inhibiting myocardial apoptosis and lipid peroxidation. Am. J. Transl. Res.. 2016;8:3319-3328.
- [Google Scholar]
- Randomized controlled trial of Sajabalssuk (Artemisia princeps Pampanini) to treat pre-diabetes. Eur. J. Integr. Med.. 2012;4:e299-e308.
- [Google Scholar]
- Effect of vitexin on alleviating liver inflammation in a dextran sulfate sodium (DSS)-induced colitis model. Biomed. Pharmacother.. 2020;121:109683
- [Google Scholar]
- An atherogenic lipid profile with low serum paraoxonase-1 activity during nematode infection in rats. Eur. J. Clin. Invest.. 2010;40:984-993.
- [Google Scholar]
- Characterization and function of the 3-hydroxy-3-methylglutaryl-CoA reductase gene in Alisma orientale (Sam.) Juz. and its relationship with protostane triterpene production. Plant Physiol. Biochem. PPB. 2015;97:378-389.
- [Google Scholar]
- A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia. 2016;115:74-85.
- [Google Scholar]
- Clinical application of methods for the qualitative evaluation of lipid abnormalities. Rinsho Byori. 2016;64:219-225.
- [Google Scholar]
- Preparation of single cell suspensions from mouse aorta. Bio-Protocol. 2016;6(11)
- [CrossRef] [Google Scholar]
- Vitexin alleviates non-alcoholic fatty liver disease by activating AMPK in high fat diet fed mice. Biochem. Biophys. Res. Commun.. 2019;519:106-112.
- [Google Scholar]
- Vitexin reverses the autophagy dysfunction to attenuate MCAO-induced cerebral ischemic stroke via mTOR/Ulk1 pathway. Biomed. Pharmacother.. 2018;99:583-590.
- [Google Scholar]
- Effects of mung bean (Vigna radiata L.) ethanol extracts decrease proinflammatory cytokine induced lipogenesis in the KK-Ay diabese mouse model. J. Med. Food. 2015;18:841-849.
- [Google Scholar]
- Effects of Mung Bean (Vigna radiata L.) ethanol extracts decrease proinflammatory cytokine-induced lipogenesis in the KK-Ay diabese mouse model. J. Med. Food.. 2015;18:841-849.
- [Google Scholar]
- Endothelial endoplasmic reticulum and nitrative stress in endothelial dysfunction in the atherogenic rabbit model. Acta Histochem.. 2015;117:762-766.
- [Google Scholar]
- Histologic and metabolic derangement in high-fat, high-fructose, and combination diet animal models. Sci. World J.. 2015;2015(306326):1-9.
- [Google Scholar]
- Amino acid and biogenic amine profile deviations in an oral glucose tolerance test: a comparison between healthy and hyperlipidaemia individuals based on targeted metabolomics. Nutrients. 2016;8:379.
- [Google Scholar]
- Inhibition of aberrant microRNA-133a expression in endothelial cells by statin prevents endothelial dysfunction by targeting GTP cyclohydrolase 1 in vivo. Circulation. 2016;134:1752-1765.
- [Google Scholar]
- Relationship between serum paraoxonase and homocysteine thiolactonase activity, adipokines, and asymmetric dimethyl arginine concentrations in renal transplant patients. Transplant. Proc.. 2013;45:3685-3687.
- [Google Scholar]
- Vitexin attenuates lipopolysaccharide-induced acute lung injury by controlling the Nrf2 pathway. PLoS One. 2018;13:e0196405
- [Google Scholar]
- Manikya, Kumari, K., Ganga, Rao, B., Padmaja, V., 2012. Role of vitexin and isovitexin in hepatoproctective effect of Alysicarpus monilifer Linn. against CCl4 induced hepatotoxicity. Phytopharmacology 3, 273–285. https://pdfs.semanticscholar.org/9609/cb8831c19f360e247aec8a8178bab5f75c87.pdf.
- Analysis of serum changes in response to a high fat high cholesterol diet challenge revealsmetabolic biomarkers of atherosclerosis. PLoS One. 2019;14:e0214487
- [Google Scholar]
- Chungtaejeon, a K orean fermented tea, prevents the risk of atherosclerosis in rats fed a high-fat atherogenic diet. J. Integr. Med.. 2016;14:134-142.
- [Google Scholar]
- Vitexin ameliorates high fat diet-induced obesity in male C57BL/6J mice via the AMPKα-me diated pathway. Food Funct.. 2019;10:1940-1947.
- [Google Scholar]
- Non-invasive imaging of atherosclerosis regression with magnetic resonance to guide drug development. Atherosclerosis. 2016;251:476-482.
- [Google Scholar]
- The effect of dietary cholesterol on macrophage accumulation in adipose tissue: implications for systemic inflammation and atherosclerosis. Curr. Opin. Lipidol.. 2009;20:39-44.
- [Google Scholar]
- A plant-based diet, atherogenesis, and coronary artery disease prevention. Perm J.. 2015;19:62-67.
- [Google Scholar]
- Betaine protects chronic alcohol and omega-3 PUFA-mediated down-regulations of PON1 gene, serum PON1 and homocysteine thiolactonase activities with restoration of liver GSH. Alcohol. Clin. Exp. Res.. 2010;34:424-431.
- [Google Scholar]
- A neural network approach to classify carotid disorders from heart rate variability analysis. Comput. Biol. Med.. 2019;109:226-234.
- [Google Scholar]
- Geraniol improves endothelial function by inhibiting NOX-2 derived oxidative stress in high fat diet fed mice. Biochem. Biophys. Res. Commun.. 2016;474:182-187.
- [Google Scholar]
- Pravastatin inhibits plaque rupture and subsequent thrombus formation in atherosclerotic rabbits with hyperlipidemia. Chem. Pharm. Bull. (Tokyo). 2013;61:121-124.
- [Google Scholar]
- Vitexin alleviates ER-stress-activated apoptosis and the related inflammation in chondrocytes and inhibits the degeneration of cartilage in rats. Food Funct.. 2018;9:5740-5749.
- [Google Scholar]
- Rosiglitazone via PPARγ-dependent suppression of oxidative stress attenuates endothelial dysfunction in rats fed homocysteine thiolactone. J. Cell. Mol. Med.. 2015;19:826-835.
- [Google Scholar]
- Vitexin alleviates ox-LDL-mediated endothelial injury by inducing autophagy via AMPK signaling activation. Mol. Immunol.. 2017;85:214-221.
- [Google Scholar]







