Translate this page into:
Vitamin D Status and its correlation with Parathyroid Hormone level among population in Riyadh, Saudi Arabia
⁎Corresponding author at: Chair for Biomarkers of Chronic Diseases, Biochemistry Department, College of Science, King Saud University, PO Box, 2455, Riyadh 11451, Saudi Arabia. aldaghri2011@gmail.com (Nasser M. Al-Daghri)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study aimed to assess the relationship between vitamin D [25(OH)D] status and parathyroid hormone (PTH) in Saudi adults to determine the optimum 25(OH)D cut-off in the Saudi adult population. A total of 1093 apparently healthy Saudi subjects (720 women and 373 men) aged 50.7 ± 13.9 years were included in this cross-sectional study. Serum 25(OH)D, PTH, calcium and albumin levels were determined. The over-all prevalence of 25(OH)D deficiency (<50 nmol/) was 42.5% (N = 464). The overall prevalence of severe 25(OH)D deficiency (<25 nmol/L) was 24% (N = 262). In all subjects, there is a significant inverse relationship between serum PTH and 25(OH)D (r = − 0.40; p < 0.0001), but no distinct plateau for serum PTH with rising of serum 25(OH)D levels were observed. Despite the lack of threshold, the rising trend in serum 25(OH)D levels had an equivalent gradual decline in PTH levels in both males and females (p < 0.001). In conclusion, the lack of optimum 25(OH)D cut-off in the present study may suggest a lower threshold for the Saudi population and the current definitions for vitamin D status overestimate the true prevalence of vitamin D deficiency in Saudi Arabia.
Keywords
Vitamin D
Parathyroid hormone
Adults
Saudi Arabia
1 Introduction
Vitamin D [25(OH)D] deficiency has been found to be greatly associated with many systemic disorders (Attar and Siddiqui, 2013) and is a worldwide health problem (Wahl et al., 2012). Populations in Middle-eastern countries, despite abundant sunshine, have lower 25(OH)D levels compared to other regions, particularly women, owing to their cultural and religious adherence to the manner of dressing that covers the entire body and limited sun exposure outside time (Al-Mogbel, 2012). According to several Saudi studies, it was reported that the prevalence of vitamin D deficiency was in the range of 50–100% for both healthy and non-healthy citizens in all age groups from 1984 to 2015 (Fonseca et al., 1984; Ardawi et al., 2011; Nabi et al., 2015). Within this timeframe, the lifestyle of the Saudi population has changed dramatically in the past few decades, leaning to a more sedentary type of life. This has caused an increase in the prevalence of several obesity-related chronic metabolic disorders such as, type 2 diabetes mellitus, cardiovascular diseases (Al-Daghri et al., 2015).
Vitamin D deficiency is a risk factor for secondary hyperparathyroidism which can negatively affect bone metabolism (Dawson-Hughes et al., 1991). Inverse associations between serum 25(OH)D and parathyroid hormone (PTH) concentrations have been established in a few studies (Oliveri et al., 1993; Guillemant et al., 1999; Outila et al., 2001; Harkness and Cromer, 2005; Harinarayan et al., 2007; Hill et al., 2010). Nevertheless, the optimum 25(OH)D status that can elevate serum PTH is still a matter of debate (Bischoff-Ferrari, 2009). This is because some studies reported that not all vitamin D deficient individuals have high PTH (Sahota et al., 2001, 2006; Sai et al., 2011). While various studies have demonstrated the inverse association between serum 25(OH)D and PTH, only a few ones have observed a 25(OH)D concentration at which the level of serum PTH levels have plateaued. These thresholds are essential to characterize the clinical cut offs at which 25(OH)D deficiency was at a level low enough to increase risk for secondary hyperparathyroidism.
In this study, we examined the association between serum 25(OH)D and serum PTH in Saudi Arabian ethnicity to determine at which level of 25(OH)D is considered clinically relevant in terms of bone health among Saudi adults.
2 Material and methods
2.1 Study population
A total of 1093 apparently healthy Saudi subjects [720 females (47.5 ± 13.8 years) and 373 males (56.7 ± 11.9 years)] were recruited from randomly selected from public primary health care centers in Riyadh, Saudi Arabia. Written informed consents were obtained before inclusion. Patients that have any chronic conditions such as cardiovascular disease or those using medications including multivitamin and calcium supplements were excluded. The procedures followed were in accordance with the ethical standards of the College of Science, King Saud University, Saudi Arabia. The study was approved with number E/08/2106 by the Ethics Committee of the College of Science, King Saud University, Riyadh, Kingdom of Saudi Arabia. Patients filled a questionnaire including information on socio-demographics and their medical history.
2.2 Blood sample collection and anthropometry measurements
Patients were asked to fast (10 h) prior to blood withdrawal. Anthropometrics were measured by an assigned physician and nurse on duty. Anthropometry included height (cm), weight (kg), body mass index (BMI) and waist/hip circumference (cm) as well as blood pressure (mmHg). Fasting blood samples were directly centrifuged in a non-heparinized tube. Serum was stored at −20 °C until further analysis in the Chair for Biomarkers of Chronic Diseases (CBCD) in King Saud University, Riyadh, KSA.
2.3 Sample analysis
A chemical analyzer (Konelab, Espoo, Finland) was used to measure fasting glucose, calcium, lipid profile, and phosphorous in the collected serum samples. Total 25(OH)D was used using Roche Elecsys Cobas e411 analyzer (Roche Diagnostics, GmbH, Mannheim, Germany) by means of Electrochemiluminescence immunoassay. Different 25(OH)D status were defined as: severe deficiency (<25 nmol/L); deficiency (25–50 nmol/L); sufficiency (50–75 nmol/L) and desirable (>75 nmol/L) (Al-Daghri et al., 2017).
2.4 Data analysis
The SPSS software (version 16.5 Chicago, IL, USA) was used to analyze the data in this study. Mean/standard deviations and median/ interquartile range were used for the Gaussian variables and non-normal variables respectively. Frequencies and percentages (%) were used for data categorization. Logistic regression was used to measure the association between 25(OH)D status and other variables. Significance was considered at p-value < 0.05.
3 Results
3.1 General characteristics of subjects
Demographic characteristics and selected anthropometric parameters of the participants are shown in Table 1. Depends on physical investigations, biochemical tests result, and detailed questionnaires, 1093 participants with an average age of 50.7 ± 13.9 years, consisting of 720 females (47.5 ± 13.8 years) and 373 males (56.7 ± 11.9 years) were included. About 51.0% of patients were obese (BMI > 30 kg/m2). The overall median serum levels of 25(OH)D, PTH, phosphorous, calcium and albumin were 38.2 nmol/L, 2.7 pg/mL, 1.1 mmol/L, and 2.4 mmol/L, respectively. Note: Data presented in Mean ± SD; categorical data is presented as frequencies (%); superscript A indicates significant difference compared to females; p-value significant at <0.05.
Overall
Female
Male
N
1093
720
373
Diabetes mellitus (%)
574 (52.5)
321 (44.6)
253 (67.8)A
Obesity (%)
557 (51.0)
402 (55.8)
155 (41.6)A
Hypertension (%)
257 (23.5)
143 (19.9)
114 (30.6)A
Age (years)
50.7 ± 13.9
47.5 ± 13.8
56.7 ± 11.9A
BMI (kg/m2)
31.2 ± 6.5
32.0 ± 6.8
29.7 ± 5.7A
WHR
1.0 ± 0.1
0.9 ± 0.1
1.0 ± 0.1A
Systolic blood pressure (mmHg)
125.9 ± 16.1
124.4 ± 16.1
128.5 ± 15.8A
Diastolic blood pressure (mmHg)
76.4 ± 10.2
75.7 ± 10.9
77.7 ± 8.8A
Log glucose (mmol/L)
2.1 ± 0.5
2.0 ± 0.5
2.3 ± 0.4A
Sqrt triglycerides (mmol/L)
1.3 ± 0.3
1.3 ± 0.3
1.4 ± 0.3A
Total cholesterol (mmol/L)
5.1 ± 1.1
5.1 ± 1.1
5.2 ± 1.2
HDL cholesterol (mmol/L)
1.0 ± 0.3
1.1 ± 0.3
1.0 ± 0.3A
LDL cholesterol (mmol/L)
3.2 ± 1.0
3.2 ± 0.9
3.1 ± 1.0
Albumin (g/L)
40.7 ± 5.7
39.7 ± 5.3
42.7 ± 6.1A
Corrected calcium (mmol/L)
2.4 ± 0.2
2.3 ± 0.2
2.4 ± 0.2A
Phosphorus (mmol/L)
1.1 ± 0.3
1.1 ± 0.3
1.1 ± 0.3
Log PTH (pg/mL)
2.7 ± 0.5
2.6 ± 0.5
2.8 ± 0.5A
25(OH)D (nmol/L)#
38.2
37.7
38.0
Severe deficiency
262 (24.0)
190 (72.5)
72 (27.5)A
Deficiency
464 (42.5)
266 (57.3)
198 (42.7)
Sufficiency
200 (18.3)
137 (68.5)
63 (31.5)
Desirable
166 (15.2)
126 (75.9)
40 (24.1)
Data were analyzed separately for males and females. On average, males had significantly higher PTH, calcium and albumin concentrations than females did. The concentrations of 25(OH)D (p = 0.31) and phosphorous (p = 0.96) did not differ significantly between males and females. Males were significantly older and had significantly higher blood pressure, glycemic indices and triglycerides than females (p-values < 0.001). Females on the other hand, had a significantly higher obesity indices and HDL-cholesterol than males (p-values < 0.001).
3.2 25(OH)D status
Based on the vitamin D status recommended for use in the Saudi population, patients were categorized into 4 groups. The incidence of insufficiency and 25(OH)D deficiency were 24.0% and 42.5%, respectively; and only 15.2% of the subjects had a circulating 25(OH)D level ≥75 nmol/L. The majority of subjects fell under the 25(OH)D insufficient category; 42.5% (Table 2). Females have a significantly higher 25(OH)D deficiency and insufficiency than males (72.5% versus 27.5% and 57.3 versus 42.7 respectively; p < 0.001). Note: Data presented as coefficient (R); C – Corrected; ** indicates significance at 0.01 level and * indicates significance at <0.05.
Parameters
Total 25(OH)D
Deficient
Insufficient
Sufficient
Desirable
log PTH
−0.08*
0.11
−0.05
0.01
−0.23*
Calcium (mmol/L)
0.23**
0.04
−0.11
0.07
C. Calcium (mmol/L)
−0.04
0.14*
0.04
−0.07
−0.03
Phosphorus (mmol/L)
−0.08*
0.05
0.04
−0.11
−0.11
Albumin (g/L)
0.05
0.23**
0.04
−0.07
0.08
To determine the association between 25(OH)D and other parameters measured such as calcium, phosphorous, albumin and PTH, multinomial logistic regression analysis was done and presented in Table 2. The levels of 25(OH)D were significantly and inversely associated with PTH (r = −0.08; p = 0.02), and phosphorous (r = −0.08; p < 0.05) but not with calcium and albumin. Levels of deficient 25(OH)D status were positively correlated with calcium and albumin levels the (r = 0.23; p < 0.01).
3.3 Association between PTH and 25(OH)D
Fig. 1 shows the mean PTH level according to vitamin status in all subjects. The level of serum PTH were altered in all categories of 25(OH)D (p < 0.003). PTH level in the deficient category was significantly higher than other categories (p < 0.003 for all comparisons). There was a comparatively modest reduction in PTH up to 50 nmol/L and a sharp reduction above 50 nmol/L (Fig. 2).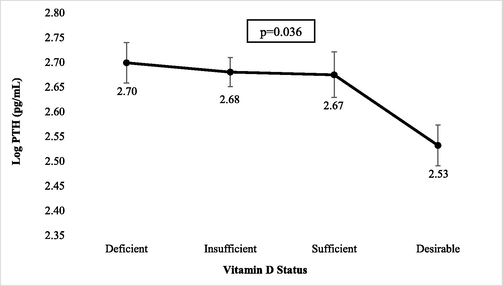
Mean PTH level according to 25(OH)D status in all subjects.
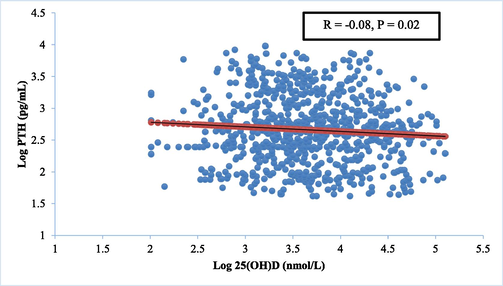
Relationship between serum 25(OH)D and intact iPTH values in the whole population studied.
Log linear regression model was used to give the best fitting curve to describe the association of serum 25(OH)D with PTH and used to take up the 25(OH)D concentration at which iPTH level theoretically reached the plateau value. However, this relationship failed to identify a plateau for serum PTH with rising serum 25(OH)D levels. Such correlation persisted even after adjustments for age, or BMI and there was no 25(OH)D cut offs value at which a change between 25(OH)D and PTH relationship had been detected.
4 Discussion
The present study showed that majority of adults living in Riyadh city, Saudi have either 25(OH)D deficiency and/or insufficiency. Females have a significantly higher prevalence of 25(OH)D deficiency than males (Siddiqui and Kamfar, 2007). For cultural and religious reasons, Saudi females are entirely covered in black, loose clothes (abaya) whenever they leave home. This is likely the main factor contributing to widespread vitamin deficiency among the Saudi female population.
Similar results were also reported in Saudi females in different regions of the kingdom (Al Faraj and Al Mutairi, 2003). However, some investigators suggested that, though veils can reduce sun exposure, this was not a main contributing issue for 25(OH)D deficiency in Saudi females (Sedrani et al., 1983). Other study found no difference in 25(OH)D in non-veiled and veiled Bangladeshi women (Islam et al., 2006). The results in our study showing that 25(OH)D deficiency in girls owing to short time exposure to sun.
Furthermore, majority of the participants in this study were either obese or overweight. Obesity is also more widespread among the Saudi females population than the males. It is known that more body fat hinders the absorption of UV light by the skin, which may in turn impair the 25(OH)D synthesis by the body. Also, obesity decreases the bio-availability of 25(OH)D due to increasing its sequestration in fat tissue.
There is still no agreement on optimal serum 25(OH)D level and a functional description should be approved as serum 25(OH)D level change with sun exposure, diet and season. The suggested cut-off of 25(OH)D is the level at maximum reduction of PTH (McKenna and Freaney, 1998, Heaney, 1999). In line with earlier studies (Souberbielle et al., 2001; Pepe et al., 2005), this study gave a significant inverse relationship between serum 25(OH)D and PTH independent of BMI and age and that 25(OH)D sufficiency can sustain normal serum PTH levels.
In the present study, even though there is a significant inverse association between 25(OH)D and PTH, there were no clear cut offs points for 25(OH)D concentration at which point the PTH concentration plateaued. These results were in line with some other studies (Harinarayan et al., 2007; Ho-Pham et al., 2011; Ardawi et al., 2012), but not all (Saliba et al., 2011). However, a more recent study done in the Saudi population proposed that the recommended cut-off for 25(OH)D relative to bone health is 30 nmol/l (AlQuaiz et al., 2019). The study mentioned used >2000 Saudi adults who were all vitamin D deficient as compared to the present study where only less than half were deficient. This larger sample size of vitamin D deficient Saudis in the study of AlQuaiz et al. (2019) was crucial as not only did it have enough statistical power but it also had enough participants in the lower spectrum of 25(OH)D, hence finally detecting an arbitrary cut-off which was lower than the international recommendations. We previously investigated the absence of correlation between PTH and 25(OH)D in 25(OH)D deficient Saudi adults (Al-Daghri et al., 2010). This is in agreement with other latest studies using the same ethnic group (Hanley et al., 2010; Ardawi et al., 2012). More studies are required at a large scale to determine whether the recommended cut-off from the study of AlQuaiz et al. (2019) are indeed applicable to the Saudi population.
The present study has various strengths points including large highly homogeneous sample, such that the results can be applicable to the general Saudi population. Furthermore, both serum intact PTH and 25(OH)D concentration were measured in a blinded manner and independently by in King Saud University, Saudi Arabia.
The authors acknowledge some limitations in this study. The cross-sectional design prevents assessment of causality of the association. As mentioned previously, while the sample was robust, the number of cases with severe vitamin D deficiency might not be enough to detect a meaningful cut-off despite an apparent inverse association with PTH.
5 Conclusions
In conclusion, vitamin D deficiency and/or insufficiency remains common in Riyadh, Saudi Arabia, with 57% of men and 42% of having 25(OH)D levels within 25–50 nmol; 72% of women and 27.5% of men have 25(OH)D < 25 nmol/L. Low serum 25(OH)D concentrations were associated with high PTH but no clear 25(OH)D threshold at which PTH concentrations plateaued was seen. More studies using a larger number of individuals with severe deficiency may provide better insights as to the optimal cut-off of vitamin D for use in the Saudi population.
Acknowledgements
The authors are grateful to the International Scientific Partnership Program (ISPP) at King Saud University for funding this research through ISPP #0111.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Severe hypovitaminosis D is widespread and more common in non-diabetics than diabetics in Saudi adults. Saudi Med. J.. 2010;31(7):775-780.
- [Google Scholar]
- Sensitivity of various adiposity indices in identifying cardiometabolic diseases in Arab adults. Cardiovasc. Diabetol.. 2015;14:101.
- [Google Scholar]
- Vitamin D status correction in Saudi Arabia: an experts' consensus under the auspices of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases (ESCEO) Arch. Osteoporos.. 2017;12(1):1.
- [Google Scholar]
- Vitamin D status among adult Saudi females visiting Primary Health Care Clinics. Int. J. Health Sci. (Qassim). 2012;6(2):116-126.
- [Google Scholar]
- Vitamin D deficiency and chronic low back pain in Saudi Arabia. Spine. 2003;28(2):177.
- [Google Scholar]
- Vitamin D cut-off point in relation to parathyroid hormone: a population-based study in Riyadh city, Saudi Arabia. Arch. Osteoporos.. 2019;1491:22.
- [Google Scholar]
- Vitamin D status in relation to obesity, bone mineral density, bone turnover markers and vitamin D receptor genotypes in healthy Saudi pre- and postmenopausal women. Osteoporos. Int.. 2011;22(2):463-475.
- [Google Scholar]
- High prevalence of vitamin D deficiency among healthy Saudi Arabian men: relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporos. Int.. 2012;23(2):675-686.
- [Google Scholar]
- Vitamin D deficiency in patients with systemic lupus erythematosus. Oman Med. J.. 2013;28(1):42-47.
- [Google Scholar]
- Vitamin D: what is an adequate vitamin D level and how much supplementation is necessary? Best Pract. Res. Clin. Rheumatol.. 2009;23(6):789-795.
- [Google Scholar]
- Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann. Intern. Med.. 1991;115(7):505-512.
- [Google Scholar]
- Exposure to sunlight and vitamin D deficiency in Saudi Arabian women. Postgrad. Med. J.. 1984;60(707):589-591.
- [Google Scholar]
- Vitamin D status during puberty in French healthy male adolescents. Osteoporos. Int.. 1999;10(3):222-225.
- [Google Scholar]
- Vitamin D in adult health and disease: a review and guideline statement from Osteoporosis Canada. CMAJ. 2010;182(12):E610-E618.
- [Google Scholar]
- High prevalence of low dietary calcium, high phytate consumption, and vitamin D deficiency in healthy south Indians. Am. J. Clin. Nutr.. 2007;85(4):1062-1067.
- [Google Scholar]
- Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos. Int.. 2005;16(1):109-113.
- [Google Scholar]
- Lessons for nutritional science from vitamin D. Am. J. Clin. Nutr.. 1999;69(5):825-826.
- [Google Scholar]
- Vitamin D status and parathyroid hormone relationship in adolescents and its association with bone health parameters: analysis of the Northern Ireland Young Heart’s Project. Osteoporos. Int.. 2010;21(4):695-700.
- [Google Scholar]
- Vitamin D status and parathyroid hormone in a urban population in Vietnam. Osteoporos. Int.. 2011;22(1):241-248.
- [Google Scholar]
- Hypovitaminosis D is common in both veiled and nonveiled Bangladeshi women. Asia Pac. J. Clin. Nutr.. 2006;15(1):81-87.
- [Google Scholar]
- Secondary hyperparathyroidism in the elderly: means to defining hypovitaminosis D. Osteoporos. Int.. 1998;8(Suppl. 2):S3-S6.
- [Google Scholar]
- High prevalence of vitamin D deficiency and cancer in Saudi Arabian populations: can we hypothesize a link? Med. Hypotheses. 2015;85(2):117-119.
- [Google Scholar]
- Seasonal variations of 25 hydroxyvitamin D and parathyroid hormone in Ushuaia (Argentina), the southernmost city of the world. Bone Miner.. 1993;20(1):99-108.
- [Google Scholar]
- Vitamin D status affects serum parathyroid hormone concentrations during winter in female adolescents: associations with forearm bone mineral density. Am. J. Clin. Nutr.. 2001;74(2):206-210.
- [Google Scholar]
- Vitamin D status as the major factor determining the circulating levels of parathyroid hormone: a study in normal subjects. Osteoporos. Int.. 2005;16(7):805-812.
- [Google Scholar]
- Hypovitaminosis D and ‘functional hypoparathyroidism’-the NoNoF (Nottingham Neck of Femur) study. Age Ageing. 2001;30(6):467-472.
- [Google Scholar]
- Vitamin D insufficiency and the blunted PTH response in established osteoporosis: the role of magnesium deficiency. Osteoporos. Int.. 2006;17(7):1013-1021.
- [Google Scholar]
- Relationship between vitamin D, parathyroid hormone, and bone health. J. Clin. Endocrinol. Metab.. 2011;96(3):E436-E446.
- [Google Scholar]
- The relationship between serum 25(OH)D and parathyroid hormone levels. Am. J. Med.. 2011;124(12):1165-1170.
- [Google Scholar]
- Sunlight and vitamin D status in normal Saudi subjects. Am. J. Clin. Nutr.. 1983;38(1):129-132.
- [Google Scholar]
- Prevalence of vitamin D deficiency rickets in adolescent school girls in Western region, Saudi Arabia. Saudi Med. J.. 2007;28(3):441-444.
- [Google Scholar]
- Vitamin D status and redefining serum parathyroid hormone reference range in the elderly. J. Clin. Endocrinol. Metab.. 2001;86(7):3086-3090.
- [Google Scholar]
- A global representation of vitamin D status in healthy populations. Arch. Osteoporos.. 2012;7:155-172.
- [Google Scholar]







