Translate this page into:
Virulence of four Beauveria bassiana (Balsamo) (Asc., Hypocreales) isolates on rose sawfly, Arge rosae under laboratory condition
*Corresponding author. Tel.: +98 1316690817; fax: +98 1316690282 jjalali@guilan.ac.ir (Jalal Jalali Sendi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 21 April 2014
Peer review under responsibility of King Saud University.

Abstract
Rose sawfly Arge rosae (Hym; Argidae) is the most serious pest of the flowers of Rosaceae family. In order to evaluate the potential of entomopathogenic fungi for controlling this pest, fourth instar larvae were maintained under controlled conditions and exposed to entomopathogenic fungi, Beauveria bassiana. The bioassay was carried out by immersion method with concentrations 2 × 104, 2 × 105, 2 × 106, 2 × 107, and 2 × 108 conidia/ml of Tween-80 solution (0.03%) in distilled water. Each concentration was replicated four times with ten insects in each replicate. The results showed relatively high efficacy of these isolates on this insect pest. In case of IRAN 403C isolate, values of LC50 and LT50 were obtained as 5.54 × 105 conidia/ml and 3.92 days at 2 × 108 conidia/ml, respectively. Results showed that IRAN 403C isolate caused the highest mortality in larvae in comparison with other isolates with a mean of 70% mortality using 107 conidia/ml.
Keywords
Entomopathogen
Rose sawfly
Beauveria bassiana
Mortality
Bioassay
1 Introduction
Synthetic insecticides can leave potentially toxic residues in food products and can be deleterious to non-target organisms in the environment. Problems with synthetic chemical insecticides have created worldwide interest in the development of alternative to these chemicals. A promising alternative for pest control is the use of entomopathogenic fungi (EPF) as biological insecticides. Using fungal biocontrol agents and selected insecticides can potentially reduce the use of chemical insecticides and their subsequent residual side effects in agriculture. These organisms were the first group to be considered as biological control agents (BCAs). Indeed, fungal entomopathogens have been widely investigated as biological control agents of pest insects in attempts to improve the sustainability of crop protection (Roy et al., 2010). In appropriate conditions, entomopathogenic fungi (EPF) are capable of causing epizootics among the pests, which have proved them to be considered for improvement of IPM programs (Carruthers and Soper, 1987).
More than 700 species of fungi belonging to 90 genera are pathogenic to insects (Inglis et al., 2001; Roberts and Humber, 1981), and are capable of infecting all developmental stages of a broad range of pests in stores, greenhouses and field conditions (Barbarin et al., 2012). There has been an extensive research on numerous species of Deuteromycete fungi (e.g. Culicinomyces spp., Beauveria spp., Metarhizium spp. and Tolypocladium spp.) for use as microbial agents to control insect pests in agriculture (Shah and Pell, 2003).
The white muscardin fungus, Beauveria bassiana (Balsamo) Vuillemin is the most studied entomopathogenic fungi applied against agricultural insect pests. They act as active agents in many plant protection products currently in use or under development worldwide. This entomopathogen fungus is widely distributed and it has the largest host ranges among the fungi. It has been detected from over 700 species and is also present in the soil as a saprophyte (Wraight et al., 2000).
Unlike bacteria and viruses, which must be consumed, toxicity from entomopathogenic fungi most often occurs from the contact of the fungal conidia with the host cuticle. This entomopathogen is able to penetrate the host integument and then develop within the host hemocoel. The process of infection begins with the attachment and germination of the conidia in the cuticle. Penetration involves both mechanical and enzymatic actions (Zimmermann, 2007). When the penetrated fungal hyphae reach the hemocoel, they encounter potential cellular and humeral immune responses of the host. Successful attack and proliferation within a host depend on the ability of the fungus to enter the hemocoel and to overcome or avoid host’s immune responses (Gillespie et al., 1998).
Rose sawfly Arge rosae (Hym; Argidae) is the most serious pest of the flowers of Rosaceae family. This pest causes severe damages at larval stages; initially they feed in groups on parenchyma of leaves and on flowers, but later segregate and continue feeding on the leaves of host individually. Terminal branches remain leafless and considerable damage is exerted to flowering organs. Adults cause significant damage to plants by laying eggs in tissues of young branches.
To date, there are no published studies on the efficiency of entomopathogenic fungi against rose sawfly, A. rosae. Due to high risk of chemical insecticides in gardens and parks to human and other organisms, evaluating a safer method for the control of this important pest is worth studying.
2 Materials and methods
2.1 Insect rearing
The fifth instar larvae of rose sawfly were collected from infested rose shrubs in the Guilan province, northern Iran. They were reared in growth chamber under controlled condition (24 ± 1 °C, 75 ± 10% RH and 16:8 h (L:D) photoperiods) on fresh rose leaves. Adult sawflies were placed in wooden cages of 40 × 20 cm and were provided with fresh rose branches for egg laying and cotton wool soaked in 10% honey for feeding. Hatched larvae were transferred to new jars and were provided with fresh rose leaves.
2.2 Fungal isolates
Four isolates of B. bassiana were obtained from the fungal culture collection maintained by the Plant Pest and Diseases Research Institute, Tehran, Iran. Detailed information about the isolates are given in Table 1. Sunn pest with signs of fungal infection were collected from overwintering sites. Each collected pest was stored in a plastic tube for further analysis. Each tube was opened in a sterile dish at the laboratory, and a small portion of the infected tissue was transferred to a sterile Sabouraud Dextrose Agar (SDA) plate. Dodine was added at a rate of 0.045% for semi-selection of Beauveria spp. All media supplemented with penicillin G (0.1 g/L media) and streptomycin sulfate (0.5 g/L media) were prepared in sterile distilled water and added after autoclaving media. After isolation it is usually necessary to ensure that the isolated fungus is free from contaminant microorganisms, thus the isolates obtained were sub cultured several times to acquire pure cultures. The cultures were incubated at 27 °C (all dark incubators) (Parker et al., 2003). Fungi were inspected under 40× magnification for identification. For isolation of effective fungal strains from soil, the samples were collected from the Fashand in Iran These samples were obtained by digging to 5 cm depth with sterile spoons and were collected in sterile plastic pots. The samples were transported to the laboratory and stored at 4 °C until use. A serial dilution technique was used for isolation of fungi. In this technique, a sample suspension was prepared by adding 10 g sample to 90 mL distilled water and shaking for 5 min in an orbital shaker (Nazir et al., 2007). Immediately afterward, each suspension was serially diluted to 10–6. From this sample, 0.1 mL was pipetted onto plates with potato dextrose agar media supplemented with Dodine, Penicillin G (0.1 g/L media); Streptomycin (0.5 g/L media), spread with a glass spreader and incubated at 25 °C for observation (Fig. 1).
Accession Number
Substrate
Location (Country)
IRAN 403C
Soil (with trapping method)
Karaj (Iran)
SPT 22
Eurygaster integriceps
Yavuzeli (Turkey)
SP 566
E. integriceps
Esfahan (Iran)
IR-K-40
E. integriceps
Tappeh Maran (Iran)
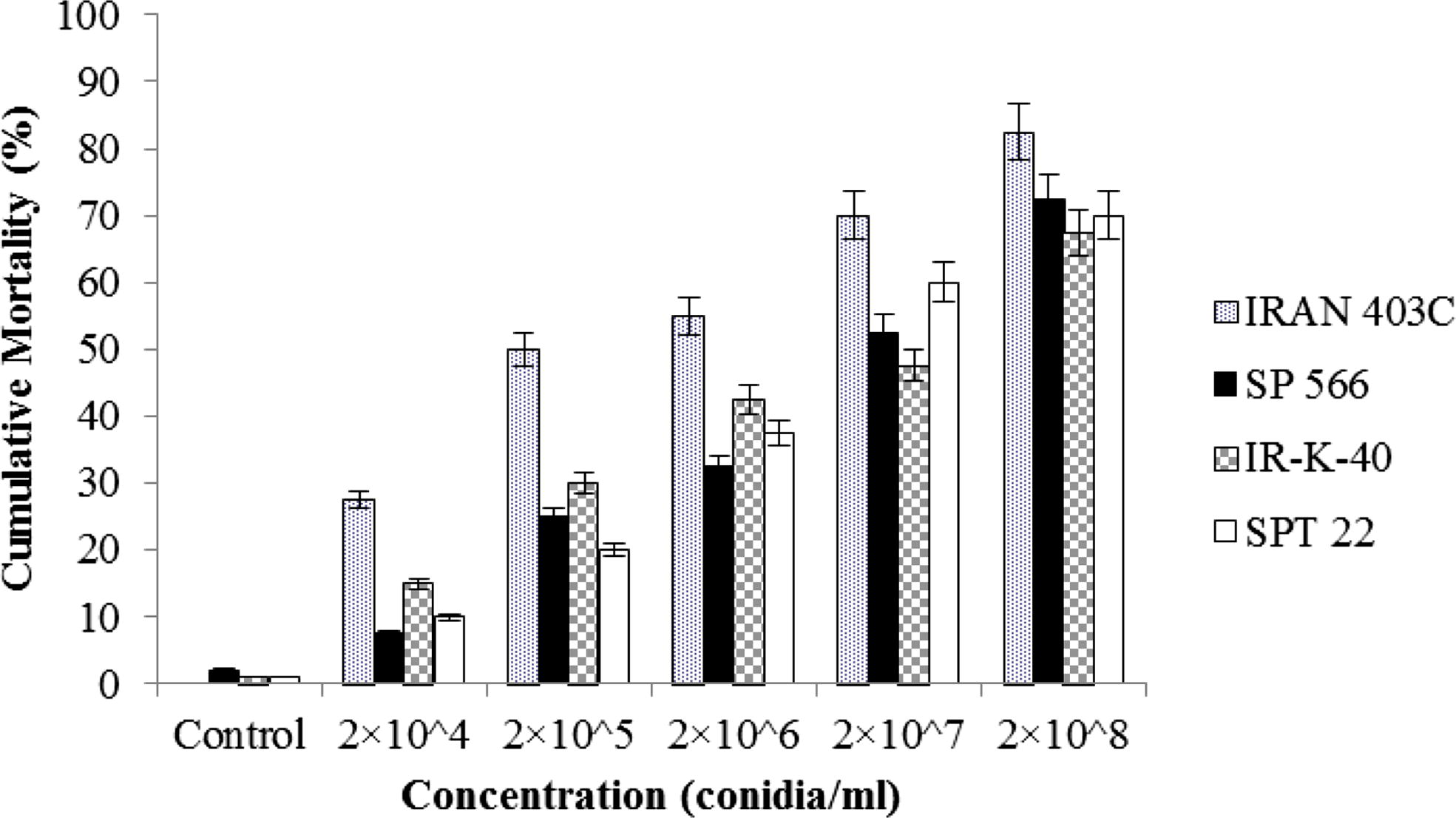
Cumulative corrected mortality (%) ± SE of Arge rosae larvae within 7 days after immersion in aqueous conidial suspensions of Beauveria bassiana isolates.
Before original bioassays, to ensure the pathogenicity of these isolates, the Galleria mellonella larvae were treated with all isolates. The dead larvae were transferred to humid Petri dishes lined with moistened filter paper and incubated for five days and were observed. When the fungal isolates were grown, they were isolated from dead larvae under sterile conditions, cultured and then identified.
2.3 Preparation of conidial suspensions
Four isolates of B. bassiana were grown in sterile Petri dishes containing Potato Dextrose Agar (PDA) and incubated at 25 ± 1 °C and a photoperiod of 16:8 h (L:D) for 14 days. Spores were harvested from PDA plates with a sterile scalpel and were transferred to distilled water containing 0.03% Tween-80. The final concentrations were determined in distilled water using a haemocytometer (Improved Neubauer, 0.1 mm depth).
2.4 Fungal susceptibility tests
After preliminary assays, 5 different conidial concentrations (2 × 104, 2 × 105, 2 × 106, 2 × 107, and 2 × 108 conidia/ml) were prepared in sterile distilled water containing Tween-80 (0.03%). Fourth instar larvae were inoculated by immersing them for 10 Sec in 5 mL of conidia suspension. Individually treated larvae were transferred into Petri dishes covered with sterile filter paper, were provided with fresh rose leaves and sealed with parafilm. Four replicates were maintained for each treatment with 10 larvae in each replicate. Larvae treated with distilled water containing 0.03% Tween-80 served as a control. The experiments were conducted in laboratory condition (25 ± 1 °C) with 75 ± 5% relative humidity and 16:8 photoperiod. Mortality in treatments and controls was recorded daily until the death of all fungus-infected larvae. The dead larvae were transferred to humid Petri dishes lined with moistened filter paper and incubated for five days and observed. Only those larvae covered with white mycelia and spores were considered to have died as a result of fungal infection. Percent mortality was calculated according to Abbott’s formula (1925):
2.5 Statistical analysis
Probit analysis was used to estimate both LC25 and LC50 of the isolates with 95% confidence limits (CL) as well as LT50 values (Polo-PC, LeOra software).
3 Results
In the present study, four different isolates of B. bassiana were tested against rose sawfly, A. rosae. The linear relationships between the conidial concentrations of each fungal isolates and the larvae mortality are depicted using probit analysis. The LC25, LC50 values, confidence limits, slope and Chi-square (χ2) of different tested isolates of fungi against A. rosae larvae are presented in Table 2. The lowest LC50 value 5.54 × 105 conidia/ml was observed in the IRAN 403C isolate. Based on the LC50 values, the IRAN 403C isolate was the most effective to A. rosae larvae followed by SP 566, SPT 22, and IR-K-40. LC25 and LC50 values based on conidia/ml and CL 95% confidence limits are considered significantly different when the 95% CL fail to overlap.
Isolates
LC25 (95% CL)
LC50 (95% CL)
Slope ± SE
χ2 (df)
IRAN 403C
0.71 × 104 (0.43 × 104–3.39 × 104)
5.54 × 105 (1.19 × 105–1.80 × 106)
0.356 ± 0.06
0.95 (3)
SPT 22
3.32 × 105 (1.12 × 105–1.24 × 106)
9.19 × 106 (4.86 × 106–4.21 × 107)
0.468 ± 0.07
1.02 (3)
SP 566
3.67 × 105 (8.59 × 104–9.99 × 105)
9.99 × 106 (3.92 × 106–1.99 × 107)
0.47 ± 0.07
0.53 (3)
IR-K-40
1.27 × 105 (0.99 × 104 -5.17 × 105)
1.25 × 107 (3.56 × 106–7.65 × 107)
0.339 ± 0.06
0.95 (3)
The mortality within the control group was very low and no fungal growth was observed on dead control larvae. All isolates of B. bassiana were capable of infecting larvae of A. rosae. Percentage mortality of larvae was dose dependent and increased with increasing concentration. Percentage mortality at the end of experiment caused by different concentrations of each isolates has been shown graphically. The cumulative mortality in A. rosae larvae exposed to isolates of B. bassiana ranged between 27.5% and 82.5%, 7.5% and 72%, 10% and 70%, 15% and 67.2%, for different concentrations of IRAN 403C, SP 566, SPT 22, and IR-K-40 respectively (Fig. 1).
LT50 values decreased whenever the concentration increased. There was a difference among LT50s of the isolates. At a concentration of 108 conidia/ml the lowest LT50 was obtained by IRAN 403C isolate, followed by SPT 22, SP 566 and IR-K-40 (Table 3).
Isolates
LT50 (days)
Confidence limit (95%)
IRAN 403C
3.92
3.51–4.36
SPT 22
5.23
4.71–5.95
SP 566
5.4
4.91–6.08
IR-K-40
5.63
4.93–6.74
4 Discussion
Entomopathogenic fungi are important key factors to regulate insect population in nature. Numerous and diverse isolates of entomopathogenic fungi have been tested for their potential to control insect pests (Feng et al., 2004).
This study demonstrated that the isolates used are capable of causing infection and mortality against A. rosae via contact. All fungal isolates show potential as control agent for this pest. Study on Iranian isolates of B. bassiana, Metarhizium anisopliae and Lecanicillium psalliotae by Pirali-Kheirabadi et al. (2007) for biological control of Rhipicephalus annulatus demonstrated that all isolates were pathogenic and 90% adult’s mortality, 89.1% decrease in egg hatchability and 88.69% reduction in reproductive efficiency of the ticks were observed using 1 × 107 conidia/ml of IRAN 403C isolate. Sabbour and Sahab (2005) demonstrated that B. bassiana exhibited larvicidal activity against Plutella xyllostella, Pieris rapae and Spodoptera exigua. Similarly other biological agents like Bacillus thuringiensis reduced the attack of Spodoptera litura on groundnut (Krishna et al., 2008). Wraight et al. (2010) compared virulence of B. bassiana isolates against lepidopteran pests of vegetable crops. They showed that all lepidopteran species tested were susceptible to B. bassiana, Helicoverpa zea and S. exigua were the most susceptible to fungal infection, and Spodoptera frugiperda was least susceptible.
The IRAN 403C of B. bassiana had the highest virulence against larvae of rose sawfly because it had lower LC50 and LT50 and caused the highest mortality (90%) at 2 × 108 conidia/ml concentration as compare with other isolates. Our results were in agreement with Shams et al. (2011), who reported that the LC50 values of B. bassiana on the day 9 post-treatment were 3.17 × 106 and 6.08 × 107 conidia/ml for Callosobruchus maculatus and Sitophilus granarius, respectively.
External white mycelial growth from all dead bodies was evident within 48–72 h of death. Post- mortem mycelial and conidial growth demonstrated that the fungal pathogens were the reason of insect’s death.
Differences in potential of different strain’s toxicity depended on physiological and enzymatic properties of each isolate (Leland et al., 2005). The fungal pathogenesis is a complex process and is dependent upon the attributes of both, the pathogen and the host. The cuticle appears to influence all stages of the infection process; adhesion, germination and aspersorium differentiation (Butt et al., 2001). Significant differences were found in mortality among the B. bassiana isolates tested on rose sawfly, A. rosae. Isolate IRAN 403C caused high mortality and other isolates demonstrated a different degree of specify. Relationships between enzyme activities and the virulence of B. bassiana toward G. mellonella L. and Trichoplusia ni (Hübner) have been demonstrated by Gupta et al. (1994) and many other researchers, but this may not be the case in all instances (Gillespie et al., 1998). Cuticle-degrading enzymes (CDEs) may determine not only virulence but also the host specificity of fungal isolate (Gupta et al., 1994). Cuticle types in various insects differ in their protein composition and degree of sclerotization (Charnley, 2003) and many CDEs are induced by cuticular components (Paterson et al., 1994; Butt et al., 1998) and some of them are produced under nutrient-poor conditions and repressed by excess nutrients (St. Leger et al., 1992). Mortality caused by B. bassiana isolates was not significantly different between the isolates SP 566, IR-K-40 and SPT 22. The isolates IRAN 403C appear the most promising for biological control utilization because it was highly virulent by 8 d post infection to the A. rosae.
Laboratory assessment of entomopathogenic fungi is an essential step in identifying virulent strain prior to field or large scale use. Entomopathogenic fungi are being developed worldwide for the control of insect pests and some products are already available commercially (Ekessi et al. 2001). Initial stages of A. rosae larvae are gregarious feeders but their feeding is very low and most damaged caused by the last larval instar. For this reason and because there was no possibility of bioassay with early instar larvae in the laboratory, we selected fourth instar larvae for bioassays. However, this point should be kept in mind that gregarious feeding habitat may cause spread of infection among pest population. It can be considered in integrated pest management programs and better be tested in field conditions. In integrated pest management programs, it is important to use most effective agents. The most effective isolate can be selected by these works and improved by genetic research and manipulation.
References
- A method of computing the effectiveness of an insecticide. J. Econ. Entomol.. 1925;18:265-267.
- [Google Scholar]
- A preliminary evaluation of the potential of Beauveria bassiana for bed bug control. J. Invertebr. Pathol.. 2012;111:82-85.
- [Google Scholar]
- Variation in the subtilisins of fungal pathogens of insects and nematodes. In: Bridge P., Couteaudier Y., Clarkson J., eds. Molecular Variability of Fungal Pathogens. Wallingford: CAB International; 1998. p. :149-169.
- [Google Scholar]
- Introduction-fungal biological control agents: progress, problems and potential. In: Butt T.M., Jackson C.W., Magan N., eds. Fungi as Biocontrol Agents: Progress, Problems and Potential. Wallingford, UK: CABI Publishing; 2001.
- [Google Scholar]
- Fungal diseases. In: Fux A., Tanada Y., eds. Epizootiology of Insect Diseases. New York: John Wiley and Sons; 1987.
- [Google Scholar]
- Fungal pathogens of insects: cuticle degrading enzymes and toxins. Adv. Bot. Res.. 2003;40:241-321.
- [Google Scholar]
- Laboratory evaluation of the entomopathogenic fungus, Metarhizium anisopliae for the control of the groundnut bruchid, Caryedon serratus on groundnut. J. Strored Prod. Res.. 2001;37:313-321.
- [Google Scholar]
- Trials of Beauveria bassiana, Paecilomyces fumosoroseus and imidacloprid for management of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on greenhouse grown lettuce. Biocontrol Sci. Technol.. 2004;14:531-544.
- [Google Scholar]
- Role of cuticle- degrading proteases in the virulence of Metarhizium spp. for the desert locust,Schistocerca gregaria. J. Invertebr. Pathol.. 1998;71:128-137.
- [Google Scholar]
- Relationships among enzyme activities and virulence parameters in Beauveria bassiana infections of Galleria mellonella and Trichoplusia ni. J. Invertebr. Pathol.. 1994;64:13-17.
- [Google Scholar]
- Use of hyphomycetous fungi for managing insect pests. In: Butt T.M., Jackson C.W., Magan N., eds. Fungi as Biocontrol Agents: Progress, Problems and Potential. Wallingford, United Kingdom: CABI International/AAFC; 2001. p. :23-69.
- [Google Scholar]
- Efficacy of certain new insecticide molecules against groundnut defoliator, Spodoptera litura (Fab.) (Noctuidae: Lepidoptera) Curr. Biotica. 2008;2:173-180.
- [Google Scholar]
- Strain selection of fungal entomopatogen, Beauveria bassiana, for control of plant bugs (Lygus sp.) (Heteroptera: Miridae) Biol. Control. 2005;35:104-114.
- [Google Scholar]
- Some studies of thermophilic and thermotolerant fungi from Lahore, Pakistan. Mycopathologia. 2007;5:95-100.
- [Google Scholar]
- Entomopathogenic fungi of Eurygaster integriceps Puton (Hemiptera: Scutelleridae) collection and characterization for development. Biol. Control. 2003;27:260-272.
- [Google Scholar]
- Partial characterization of specific inducers of a cuticle-degrading protease from the insect pathogenic fungus Metarhizium anisopliae. Microbiology. 1994;140:3153-3159.
- [Google Scholar]
- Biological control of Rhipicephalus (Boophilus) annulatus by different strains of Metarhizium anisopliae, Beauveria bassiana and Lecanicillium psalliotae fungi. Parasitol. Res.. 2007;100:1297-1302.
- [Google Scholar]
- Entomogenous fungi. In: Cole G.T., Kendrick B., eds. Biology of Conidial Fungi. New York: Academic Press; 1981. p. :201-236.
- [Google Scholar]
- Deep space and hidden depths: understanding the evolution and ecology of fungal entomopathogens. BioControl. 2010;55:1-6.
- [Google Scholar]
- Efficacy of some microbial control agents against cabbage pests in Egypt. Pak. J. Biol. Sci.. 2005;8:1351-1356.
- [Google Scholar]
- Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol.. 2003;61:413-423.
- [Google Scholar]
- A laboratory assessment of the potential of the entomopathogenic fungi Beauveria bassiana (Beauvarin®) to control Callosobruchus maculatus (F.) Coleoptera: Bruchidae) and Sitophilus granarius (L.) (Coleoptera: Curculionidae) Afr. J. Microbiol. Res.. 2011;5(10):1192-1196.
- [Google Scholar]
- Molecular cloning and regulatory analysis of the cuticle-degrading protease structural gene from the entomopathogenic fungus Metarhizium anisopliae. Eur. J. Biochem.. 1992;204:991-1001.
- [Google Scholar]
- Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biol. Control. 2000;17:203-217.
- [Google Scholar]
- Comparative virulence of Beauveria bassiana isolates against lepidopteran pests of vegetable crops. J. Invertebr. Pathol.. 2010;103(3):186-199.
- [Google Scholar]
- Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Technol.. 2007;17:553-596.
- [Google Scholar]







