Virulence and enzymatic activity of three new isolates of Beauveria bassiana (Ascomycota: Hypocreales) from the South American locust Schistocerca cancellata (Orthoptera: Acrididae)
⁎Corresponding author at: CEPAVE, Boulevard 120 s/n entre av. 60 y calle 64, La Plata 1900, Argentina. pelizza@cepave.edu.ar (S.A. Pelizza)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Schistocerca cancellata is a large-sized acridid, which has historically represented the greatest agricultural problem in southern South America, causing serious economic losses. Since 2015 S. cancellata entered in a state of outbreak condition of historical proportions, producing frequent and large swarms of up to 25 km2 in the north and central region of Argentina and areas of neigh boring Bolivia and Paraguay. At present, chemical insecticides are still the only means available for the control of S. cancellata. We analyzed under laboratory conditions the effectiveness of three fungal strains of Beauveria bassiana isolated from S. cancellata and also determined the relationship between chitinase, protease, and lipase levels at different temperatures of these fungi and their insecticidal activities. The pathogenicity assays were carried out by the sprayed method with concentrations of 1 × 104, 1 × 106 and 1 × 108 conidia/ml. We observed that isolate LPSc 1227 caused the highest mortality at each dose studied, ranging from 100% at a dose of 1 × 108 conidia/ml to 33.3 ± 3.2% at the lowest dose of 1 × 104 conidia/ml. Moreover, in this isolate the highest values of chitinolytic and proteolytic activity were recorded (2.31 ± 0.31 and 1.78 ± 0.04), respectively.
Keywords
Entomopatogenic fungi
Beauveria bassiana
Enzymatic activity
Mortality
Locust
1 Introduction
Among the acridid species of economic importance in Argentina, the South American locust Schistocerca cancellata (Serville) has historically represented the greatest agricultural problem, causing serious economic losses (Kohler 1962; Gastón, 1969; Liebermann, 1972). Schistocerca cancellata is a large-sized acridid (♂ = 28–49 mm, ♀ = 39–66 mm) that has a wide geographic distribution which covers central and northern Argentina, the whole of Uruguay and Paraguay, southern Brazil, south-eastern Bolivia, and central and northern Chile (De Wysiecki and Lange, 2005). This pest insect is polyphagous, feeding on a variety of wild and cultivated plants (COPR, 1982). Since 1954, after an intensive decade of controls involving 7000 people, airplanes, and more than 12,000 tons of insecticides, the plague entered into a state of recession (De Wysiecki and Lange, 2005), reducing its area of infestation, which had reached almost all of Argentina down to the southern province of Chubut in Patagonia. After 1954, when the population of S. cancellata was thought to be in check, upsurges were again recorded in 1961, 1989, and 2010 (Barrientos-Lozano et al., 2013). However, these upsurges were minimal when compared to what is currently under way. Since 2015 S. cancellata entered in a state of outbreak condition of historical proportions, producing frequent and large swarms of up to 25 km2 in the north and central region of the country (Medina et al., 2017). At present, chemical insecticides are still the only means available for the control of S. cancellata, but their use is of significant environmental concern (Gonzalez et al., 2010; Álvarez et al., 2013). In Australia and to a lesser extent in Africa (South Africa, Sudan, some west African countries), two commercial products, “Green Muscle” and Green Guard®, respectively, are used as biocontrol agents of locusts (Lawrence, 2006; Cuddeford, 2007; Moore, 2008). They were both developed from isolates of the entomopathogenic fungus Metarhizium acridum (Ascomycota: Hypocreales).
Unlike most other pathogens that need to be ingested, fungi usually infect insects by active penetration through the cuticle, being able to initiate invasion of the host independently of its feeding habits (Vega et al., 2012). Since the insect cuticle is the first barrier encountered by fungi, they synthesize a wide variety of extracellular enzymes involved in the degradation of protein, chitin, and lipids which are the principal components of the insect’s cuticle (Pedrini et al., 2007; Schrank and Vainstein, 2010). Given the relevance of enzymatic activities for fungal penetration and the importance of virulent strains for an efficient biological control, we analyzed under laboratory conditions the effectiveness of three fungal strains of Beauveria bassiana isolated from S. cancellata collected in Santiago del Estero province in northern Argentina. We also determined the relationship between chitinase, protease, and lipase levels of these fungi and their insecticidal activities.
2 Materials and methods
2.1 Collection of insects
Adult males and females of S. cancellata were collected with sweep nets in prime natural habitat of the species near Telaritos in La Rioja province, Argentina. The area is part of the Chaqueña Biogeographic province (Cabrera and Willink, 1973). Samples were immediately taken to the laboratory where they were kept in groups in wire-screened cages in a rearing room under controlled conditions (30 °C; 14:10, L:D photoperiod; 40% relative humidity). These conditions stimulated the copula and oviposition by females (Sanchez et al., 1997). Nymphs born from the resulting egg-pods were used in the pathogenicity bioassays.
2.2 Fungal isolates
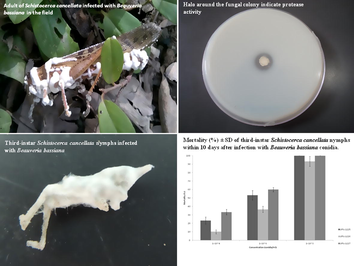
Three fungal isolates of B. bassiana obtained from specimens of S. cancellata found dead in the field were isolated and deposited in the Spegazzini-Institute-culture-collection with the following access numbers: LPSc 1225; LPSc 1226; LPSc 1227. The cadavers were collected near La Banda (27° 44′ 07″ S; 64° 14′ 36″ W), in Santiago del Estero province, Argentina, also within the Chaqueña Biogeographic province (Cabrera and Willink, 1973). Morphological species identification was corroborated by extracting DNA of the monosporic cultures according to Stenglein and Balatti (2006). PCRs were carried out in an XP thermal cycler (Bioer Technology Co, Hangzhou, China) to amplify the ITS rDNA region of B. bassiana using primer pairs ITS5 (5′-GGA AGT AAA AGT CGT AAC AAG G-3′)/ITS4 (5′-TCC TCC GCT TAT TGA TATGC-3′) (White et al., 1990). Fragments were sequenced and similarities with previously published sequence data were examined with BLASTn (Altschul et al., 1990) in the NCBI web page. The sequences generated in this study were submitted to GenBank (accession numbers MG012790, MG012791, and MG012792) for B. bassiana LPSc 1225, LPSc 1226, and LPSc 1227, respectively.
2.3 Pathogenicity assays
Conidia from different isolates were obtained from cultures on potato-dextrose-agar medium after incubation for 10 days at 25 °C in the dark. Conidia were harvested with disposable cell scrapers (Fisherbrand™) and placed in test tubes containing 0.01% (v/v) Tween 80™ (polyoxyethylene sorbitan monolaurate) (Merck). Suspensions were vortexed for 2 min, filtered through four layers of sterile muslin, and adjusted to 1 × 104, 1 × 106 and 1 × 108 conidia/ml, after cell counting in a Neubauer haemocytometer. The viability of the conidia from each isolate and the concentrations used in the tests were determined after 24 h as described by Goettel and Inglis (1997). This germination test was repeated for each stock suspension to maintain the constancy of the viability assessments. In all cases, the average viability of the conidia was over 95%. Three replicates (on different dates) of 10 third-instar S. cancellata nymphs each were sprayed with 1 ml of a suspension containing 1 × 104, 1 × 106 and 1 × 108 conidia/ml respectively (in 0.01% [v/v] Tween 80™) through the use of a 35-ml glass atomizer. Three additional replicates of 10 grasshoppers each for use as controls were sprayed in the same fashion, but with 1 ml of 0.01% [v/v] Tween 80™ only. The locusts were kept in groups of 10 individuals in 50 × 9-cm acetate tubes with screened ends (Henry, 1985) and fed with lettuce, cabbage, and wheat bran. Treated and control locusts were maintained at 30 °C, 60% relative humidity, and a 14:10-h light:dark photoperiod. The cumulative mortality was recorded daily for 10 days. Mycosis was confirmed by microscopic examination of dead locusts.
2.4 Production of enzymes at different temperatures
The level of hydrolase activity in each fungal strain tested was estimated on different agar media supplemented with the following specific substrates: Chitin Azure (Sigma, C 3020), Casein, and Tween 20 (Merck)™, for chitinase, protease, and lipase activity, respectively. Chitinase activity was determined in a Potato Dextrosa Agar media at 0.08% Chitin Azure final concentration, autoclaved and incubated for two weeks according to Howard et al. (2003), Casein 0.5% medium was prepared according to Koneman and Roberts (1987). For lipolytic activity, an agar medium containing sorbitan monolaurato (Tween 20) as the lipid substrate according Hankin and Anagnostakis (1975) was used. A 6-mm agar plug with mycelium from cultures grown on Malt extract agar (MEA) was inoculated onto the agar surface of every plate and incubated at 4 ± 1 °C, 15 ± 1 °C and 26 ± 1 °C for 15 days (Kathiresan and Manivannan 2006; Saparrat et al., 2008; Sabu et al., 2012; Sudarkodi et al., 2015). Three replicates were carried out for each temperature studied and enzyme activity. Fungal growth was estimated by measuring colony diameters. The hydrolytic activity was estimated qualitatively according to the development of a halo around the fungal colony and was expressed semi quantitatively as the ratio between the hydrolytic halo and the colony diameter (Saparrat et al., 2008).
2.5 Statistical analysis
A two-way ANOVA was performed to determine whether the differences were significant among isolates with respect to each of the variables studied (chitinolytic, proteolytic, and lipolytic activity at different temperatures). Thereafter, we used the Treamed Spearman Karber analysis to estimate LC50 of the isolates with 95% confidence limits (CL).
3 Results
In assessing the pathogenicity of the three fungal isolates of B. bassiana on third-instar nymphs of S. cancellata, we observed that isolate LPSc 1227 caused the highest mortality at each dose studied, ranging from 100% at a dose of 1 × 108 conidia/ml to 33.3 ± 3.2% at the lowest dose of 1 × 104 conidia/ml (Fig. 1). Moreover, in this isolate the highest values of chitinolytic and proteolytic activity at 4 °C were recorded (2.31 ± 0.31 and 1.78 ± 0.04, respectively) (Table 1). We observed that isolate LPSc 1226 caused lower mortality, ranging from 93.3 ± 5.9% at a dose of 1 × 108 conidia/ml to 10 ± 2.3% at the lowest dose of 1 × 104 conidia/ml, and showed lower values of chitinolytic and proteolytic activity at all temperatures tested (Table 1). Isolate B. bassiana LPSc 1225 showed the highest lipolytic activity at all temperatures tested (Table 1). We observed significant differences in lipolytic activity for each of the studied variables, isolates, temperature, and interaction between them isolates*temperature (Table 2). Nevertheless, we did not observe significant differences for any of the studied variables or the interaction between them for chitinolytic and proteolytic activity (Table 2). The relationship between the conidial concentrations of each fungal isolates and nymphal mortality are depicted using Treamed Spearman Karber analysis. The LC50 values and confidence limits of different isolates of B. bassiana against S. cancellata are presented in Table 3. The lowest LC50 value (1.71 × 105 conidia/ml) was observed in isolate LPSc 1227. Based on the LC50 values, isolate LPSc 1227 was the most effective against nymphs of S. cancellata, followed by isolates LPSc 1225 and LPSc 1226.
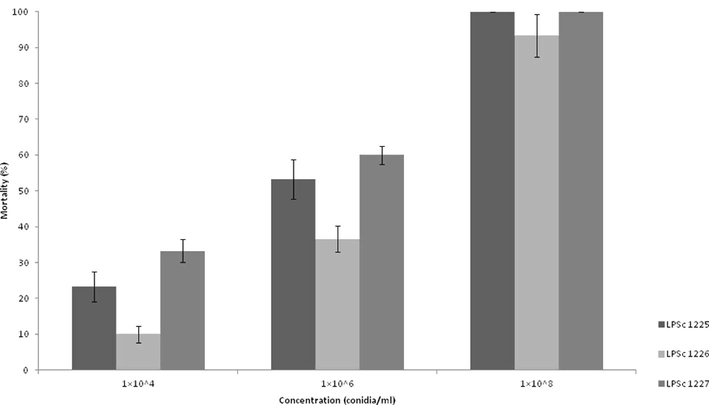
- Cumulative mortality (%) ± SD of third-instar Schistocerca cancellata nymphs within 10 days after inoculation with 1 × 104; 1 × 106 and 1 × 108 conidia/ml of Beauveria bassiana (LPSc 1225; LPSc 1226 and LPSc 1227).
| Isolates (LPSc) | Lipolytic | Proteolytic | Quitinolytic | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 °C | 15 °C | 26 °C | 4 °C | 15 °C | 26 °C | 4 °C | 15 °C | 26 °C | |
| 1225 | 4.23 ± 0.3 | 1.18 ± 0.02 | 1.18 ± 0.05 | 1.27 ± 0.14 | 1.11 ± 0.04 | 1.15 ± 0.1 | 0 ± 0 | 0 ± 0 | 1.30 ± 1.2 |
| 1226 | 3.05 ± 0.2 | 1.15 ± 0.03 | 1.13 ± 0.04 | 1.10 ± 0.08 | 1.05 ± 0.02 | 1.04 ± 0 | 0 ± 0 | 0 ± 0 | 0.69 ± 0.1 |
| 1227 | 2.78 ± 0.3 | 0 ± 0 | 0 ± 0 | 1.78 ± 0.04 | 1.27 ± 0.15 | 2 ± 0 | 2.31 ± 0.31 | 0.5 ± 0.2 | 1.01 ± 0 |
| Lipolytic | Proteolytic | Quitinolytic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DF | F | P | DF | F | P | DF | F | P | |
| Isolates | 2 | 41.95 | <.0001 | 2 | 0.71 | .5060 | 2 | 14.71 | .0002 |
| Temperature | 2 | 278.99 | <.0001 | 2 | 1.52 | .2446 | 2 | 8.36 | .0027 |
| Isolates*Temperature | 4 | 6.71 | <.0017 | 4 | 0.81 | .5329 | 4 | 7.94 | .0007 |
| Isolates | LC50 | Confidence limits (95%) |
|---|---|---|
| LPSc 1225 | 4.53 × 105 | 9.82 × 105–2.10 × 106 |
| LPSc 1226 | 1.99 × 106 | 1.99 × 106–5.82 × 106 |
| LPSc 1227 | 1.71 × 105 | 2.31 × 104–1.27 × 106 |
4 Discussion
For the first time three strains of B. bassiana were isolated from field, naturally infected individuals of S. cancellata. Although B. bassiana is an ubiquitous fungus among grasshoppers and locusts worldwide (Goettel, 1992), previous detections of fungal species associated to S. cancellata did not include B. bassiana isolates (Pelizza et al., 2010). Fungal penetration of the insect cuticle can be mediated by mechanical processes or enzymatic attack as well as a combination of both (Vega et al., 2012). Entomopathogenic fungi such B. bassiana penetrate the cuticle with the aid of a battery of extracellular cuticle-degrading enzymes including proteases, chitinases, and lipases (Binod et al., 2007). In entomopathogenic fungi these hydrolases might be key virulence factors since their substrata are structural components in the insect cuticle, which are used as C and N sources for fungal growth, contributing so fungal penetration (Pedrini et al., 2007). We observed differences in the mortality produced by the three B. bassiana isolates on S. cancellata nymphs. This could be related to the ability to overcome the insect immune response, molecular, and physiological mechanisms such as excretion of extracellular enzymes (Valero-Jimenez et al., 2014). We observed that the isolate LPSc 1227 produced the highest mortality at each of the doses used. This may be related to the higher values of chitinolytic and proteolytic activity at the different temperatures studied. Chitinase is the most important enzyme degrading the chitin polymer of the insect cuticle. Therefore, virulence of entomopathogenic fungi can be correlated with the chitinase activity (Dhawan and Joshi, 2017). Our results agree with those of Khosravi et al. (2015) and Dhawan and Joshi (2017)who reported that those fungal isolates that produced the highest values of chitinolytic and proteolytic activity were those that also produced higher mortality on the rose sawfly, Arge rosae and the caterpillar Pieres brassicae, respectively. Although our results appear encouraging from a biocontrol perspective, particularly those relative to isolate B. bassiana LPSc 1227, they should obviously be treated as preliminary because they were all obtained under laboratory conditions. A more complete or realistic assessment of isolate LPSc 1227 would require further work under natural conditions which is our next envisaged goal.
5 Conclusion
In these work three strains of B. bassiana could be isolated, which were found naturally infected individuals of S. cancellata. This is the first finding of this magnitude for Argentina. Although this is a first step, we think, through the results we have obtained in this work, that we can be in the presence of an entomopathogenic fungus that is able to biologically control this pest insect and be an alternative to the chemical control of S. cancellata.
Acknowledgements
This study was partially supported by Consejo Nacional de Investigaciones Científicas y Tecnológicas (PIP 0018), Comisión de Investigaciones Científicas de la provincia de Buenos Aires (CICPBA), Universidad Nacional de La Plata (UNLP, 11/N 651) and Fondo para la Investigación Científica y Tecnológica (FONCYT; PICT 2015-1146).
References
- Behavior of insecticide chlorpyrifos on soils and sediments with different organic matter content from provincia de buenos aires, República Argentina. Water Air Soil Pollut.. 2013;224:1453.
- [Google Scholar]
- Ecología y dinámica poblacional de Schistocerca cancellata (Orthoptera: Cytrtacanthacridinae) Entomología Mexicana. 2013;12(1):485-490.
- [Google Scholar]
- Evaluation of fungal culture filtrate containing chitinase as a biocontrol agent against Helicoverpa armigera. J. Appl. Microbiol.. 2007;103:1845-1852.
- [Google Scholar]
- Cabrera, A.L., Willink, A., 1973. Biogeography of Latin America. Monogr. 13, Ser. Biol. Washington, DC: OEA.
- COPR (Centre for overseas pest research), The Locust and Grasshopper Agricultural Manual 1982 CORP London 690.
- Cuddeford, V., 2007. Biocontrol files. Co-published by World Wildlife Fund, the Biocontrol Network and Agriculture and Agri-food Canada, pp. 4-5.
- De Wysiecki, M.L., Lange, C.E., 2005. The locust Schistocerca cancellata Serville (Orthoptera: Acrididae: Cyrtacanthacridinae) in Argentina: biology, ecology, history and control. In: Memories of the second international course: Integrated management of the Central American locust (Schistocerca piceifrons piceifrons, Walker) and acridoideos pest in Latin América. pp. 151-156.
- Enzymatic comparison and mortality of Beauveria bassiana against cabbage caterpillar Pieris brassicae. Braz. J. Microbiol.. 2017;48:522-529.
- [Google Scholar]
- Síntesis histórica de las invasiones de langosta en la Argentina. Secretaría Estado Agricultura y Ganadería, Publicación Miscelánea. 1969;433:1-30.
- [Google Scholar]
- Fungal agents for biocontrol. In: Lomer C.J., Prior C., eds. Biological Control of Locusts and Grasshoppers. Wallingford: CABI; 1992. p. :122-132.
- [Google Scholar]
- Fungi: hyphomycetes. In: Lacey L.A., ed. Manual of Techniques in Insect Pathology. San Diego, CA: Academic Press; 1997. p. :213-248.
- [Google Scholar]
- Assessing pesticide leaching and desorption in soils with different agricultural activities from Argentina (Pampa and Patagonia) Chemosphere. 2010;81:351-358.
- [Google Scholar]
- The use of solid media for detection of enzyme production by fungi. Mycologia. 1975;67:597-607.
- [Google Scholar]
- Melanoplus spp. In: Singh P., Moore R.F., eds. Handbook of Insect Rearing. Amsterdam: Elsevier; 1985. p. :451-464.
- [Google Scholar]
- Detection and characterization of chitinases and other chitin-modifying enzymes. Appl. Microbiol. Biotechnol.. 2003;30:627-635.
- [Google Scholar]
- Cellulase production by Penicillium fellutanum isolated from coastal mangrove rhizosphere soil. Res. J. Microbiol.. 2006;1(5):438-444.
- [Google Scholar]
- Virulence of four Beauveria bassiana (Balsamo) (Asc., Hypocreales) isolates on rose sawfly, Arge rosae under laboratory condition. J. King Saud. Univ. Sci.. 2015;27:49-53.
- [Google Scholar]
- Ecología de la zona central y de gregarización de la langosta en la República Argentina. Idia Suppl.. 1962;7:108.
- [Google Scholar]
- Practice laboratory Mycology. Buenos Aires-Argentina: Editorial Médica Panamericana S.A; 1987.
- A new green control for locust now readily available to farmers. Biocontrol News Inf.. 2006;27:18-19.
- [Google Scholar]
- The current state of the locust and grasshoper problem in Argentina. London: Proc. Int. Study Conf. Current and Future Problems of Acridiol; 1972. p. :191-198.
- The resurgence of the South American locust (Schistocerca cancellata) Metaleptea. 2017;37(3):17-21.
- [Google Scholar]
- Nuevos registros de hongos entomopatógenos en acridios (Orthoptera: Acridoidea) de la República Argentina. Rev. Soc. Entomol. Argent.. 2010;69:287-291.
- [Google Scholar]
- Biochemistry of insect epicuticle degradation by entomopathogenic fungi. Com. Biochem. Physiol.. 2007;146:124-137.
- [Google Scholar]
- Solid-State Fermentation for Production of Phytase by Rhizopus oligosporus. Appl. Biochem. Biotechnol.. 2012;103:251-260.
- [Google Scholar]
- Life history parameters of the gregarious phase of the Southamerican locust Schistocerca cancellata (Serville) (Orthoptera: Acrididae), under laboratory conditions. J. Orthoptera Res.. 1997;6:121-124.
- [Google Scholar]
- Celtis tala and Scutia buxifolia leaf litter decomposition by selected fungi in relation to their physical and chemical properties and the lignocellulolytic enzyme activity. Eur. J. Soil Biol.. 2008;44:400-407.
- [Google Scholar]
- Genetic diversity of Phaeoisariopsis griseola in Argentina as revealed by pathogenic and molecular markers. Physiol. Mol. Plant Path.. 2006;68:158-167.
- [Google Scholar]
- Production and Optimization of Protease by Filamentous Fungus isolated from Paddy Soil in Thiruvarur District Tamilnadu. J. A. B. B.. 2015;3(06):066-069.
- [Google Scholar]
- Natural variation in virulence of the entomopathogenic fungus Beauveria bassiana against malaria mosquitoes. Malaria J.. 2014;13:479-487.
- [Google Scholar]
- Fungal entomopathogenes. In: Vega F.E., Kaya H.K., eds. Insect Pathology. London: Elsevier; 2012. p. :171-220.
- [Google Scholar]
- Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. San Diego CA: PCR protocols: a guide to methods and applications Academic Press; 1990. p. :315-322.







