Translate this page into:
Vateria indica (Linn) resin based ointment for the topical treatment of Radiation-Induced burns in cancer patients
⁎Corresponding author. kmujeeb@ksu.edu.sa (Mujeeb Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cancer patients undergoing radiation-therapy (RT) suffer from many side-effects like dermatitis and mucositis. A simple economical and effective treatment for this condition required the preparation of an ointment to alleviate dermatological damage from radiation burns. Emphasis was laid upon finding an indigenous natural product therapy but fully authenticated by a thorough study under pharmacological guidelines. An ointment was prepared using the resin Sarja rasa (Ayurveda)/Vellaikungiliyam (Siddha)/Raal (Unani). A correlation between topical damage in RT patients and cellular dimensions was established. To circumvent poor quality controls or species contamination found in commercial sources it was felt important to locate the source of the resin and its botanical authentication. The particle size of the resin present in the ointment was ascertained using scanning electron microscopy (SEM). The resin was obtained by incising and tapping the V. indica L. tree which potentially possess wound-healing and has anti-sarcomic properties. This seminal study was carried out with the permission of ethics committee, targeting patients affected with head and neck cancers. An important aspect of this study was complete exclusion of microbial interference commonly found in indigenous, home-made and some commercial preparations. Therefore, the samples were microbiologically monitored to ensure absolute microbial exclusion from the ointment and thereafter, the stability studies were also conducted. A remarkable change was observed in terms of the decrease in severity of symptoms and shorter duration of healing when compared with the control group.
Keywords
Wound healing
Human health
Radiation burns
Nanopharmacology
Indigenous preparations
Endangered plants
1 Introduction
Cancer still leads in causing majority of global deaths, which is estimated to be in millions (∼10 millions), and most of the cases are reported in developing countries (Cao et al., 2021; García-Chico et al., 2023). Fortunately, in recent decades, significant advances have been made in early diagnosis, understanding and treatment modalities of a variety of cancers (Alshahrani and Ibrahim, 2022; Rajivgandhi et al., 2023; Smetana Jr and Masařík, 2022). Among various treatment methods, approximately half of the cancer patients are treated with radiation therapy alone or in combination with chemotherapy or surgery (Khan et al., 2016). During the radiation therapy (or radiotherapy, RT) high-energy radiations, such as X-rays, gamma rays, electron beams, or protons are employed to damage cancerous cells (Liu et al., 2016). This treatment is typically applied either in the case of primary therapy of localized solid tumors or as adjuvant and palliative therapies to reduce symptoms of advance stage or metastatic cancers (Chandra et al., 2021). Generally, the destruction of cancer cell by RT is not a selective phenomenon, therefore, together with cancerous cell, during the RT, there is high chance of damage to the normal cells (Kumari et al., 2021). Although, the modern advancements in RT, such as, the application of external-beam RT delivery, image-guided (i.e., magnetic resonance imaging [MRI]-guided or computed tomography [CT]-guided) and particle therapy (e.g., protons) offer better protection to crucial organs, while selectively targeting cancerous cells (Al-Hallaq et al., 2021). Neverthless, there are several side effects of RT, including toxicity and other radiation burn related injuries (Wang and Tepper, 2021). Particularly, the exposure to high-energy radiations causes considerable damage to the skin or other biological tissues (Waghmare, 2013).
Currently, modern radiation technologies such as, intensity-modulated radiation therapy (IMRT), causes less severe skin reactions (Pignol et al., 2008). Still, a substantial percentage (∼95%) of the treated patients develop relatively milder RT induced burn injuries (Haubner et al., 2012). These types of injuries, which often include inflammation and desquamation rarely develop into serious diseases such as, ulceration (Bontempo et al., 2021). Currently, the guidelines for the symptomatic treatment of radiation burns involve the application of topical corticosteroid creams like silver sulfadiazine, washing with soap and/or water and applying deodorants/antiperspirants (Finkelstein et al., 2022; Ginex, 1969; Hemati et al., 2012; Sunku et al., 2021; Zimmermann et al., 1998). Henceforth, an attempt to develop a remedy for the prophylaxis and management of radiation dermatitis is highly desirable.
Despite the fact that numerous topical wound care products are available in the market including corticosteroid creams for burn injuries. But well-designed randomized and controlled clinical trials regarding RT burn related wound care products are rare (Loonen, 2018; Sharquie and Jabbar, 2021). Therefore, for cancer patients and health-care community a new, clinically safe, and cost-effective method for the treatment of chronic RT related injuries would be undoubtedly welcome. In this regard, resins from various natural resources including plants are considered as effective substances due to their decent medicinal properties (Puttewar et al., 2010). Plant resins have been known to possess excellent biological potential such as, strong inhibiting effect against tumors, anti-inflammation properties against acute or chronic, surgical or nonsurgical wounds and pressure ulcers (Jokinen and Sipponen, 2016). Therefore, to address the problem of burn related injuries in patients undergoing RT, we explore the biological potential of Vateria indica resin, which possess excellent wound-healing properties (Ahmad et al., 2019).
Recently, different alternative medicine including, Ayurveda and Unani have reported wound and inflammation healing properties of Vateria indica resin in rats and episiotomy wound in women (Mokhtar et al., 2014; Shrikanth and Ashalatha, 2015; Sultana et al., 2021). Vateria indica resin is extracted from cow ghee, bees wax or sesame oil, and typically referred by various names like Marham-e-Raal in Unani Medicine (Khan et al.). Besides, this resin is also available in the market in different forms and as vital ingredients in a variety of topical therapeutics for the treatment of wound healing, haemorrhoids and anti-blemish cream. However, we have not used these products for the study of RT patients, since during the management of radiation dermatitis, a fat-free ointment base is recommended (Iacovelli et al., 2020). Additionally, such preparations can also form scabs hindering the dermal transportation of the resin. On the other hand, the raw resin of Vateria indica Linn. is also available in local dispensaries, which has been earlier applied in scientific studies such as, the dose dependent toxicity analysis in mice and found to be safe even at a daily oral administration of up to 1.0 g for 28 days (Venkateshwarlu et al., 2011). However, the application of Vateria indica Linn from local market is not safe for the study due to the authenticity of the locally available product, as similar resin such as, Shoria robusta resin (commonly referred as ‘raal’) is often sold as Vateria indica Linn. Therefore, it was imperative to identify the original source of pure Vateria indica resin, which was authenticated by the local government body (Telangana Forest Department), who has also offered guidance to locate the exact plant and extraction process.
Vateria indica Linn, also known as White Dammar, is a tropical tree typically found in East-Asian countries including India (Ito et al., 2003). The Vateria indica Linn is a tall (∼40 m), leathery tree consisting of glabrous leaves, and plenty of flavonoids and polyphenolic contents (Alshabi et al., 2022). The plant has been studied for a variety of remedies as an anthelminthic, anti-inflammatory, antiulcer and anticancer agent. The phytochemical constituents of leaves of Vateria indica or its bark extracted by using coconut water were used in the treatment of urinary tract infection. Some reports also exist on the gas chromatography and mass spectral data of the resin (Kavitha and Geethu, 2017). In this study, a pilot clinical trial is conducted to explore the safety and efficacy of the preparation of ointment using the resin of Vateria indica plant against radiation induced dermatitis. Possibly, the activity of the ointment is governed by particle size and skin porosity.
During this study, two primary aspects were explored; firstly, the sterility and stability i.e. shelf life of the preparation is investigated and secondly, optimal dermal transportation of the resin is studied. For this purpose, techniques of nanopharmacology were employed to achieve compatibility in resin particle size and skin porosity.
2 Materials and methods
All the materials required for the preparation of ointment were availed from Department of Pharmacology, Shadan Institute of Medical Sciences, Hyderabad. Packaging was performed by using the compressor machine at Shadan College of Pharmacy, Hyderabad. The stability and sterility studies of each and every ingredient used to prepare the ointment were carried out under the expert supervision of Department of Microbiology, Shadan Institute of Medical Sciences, Hyderabad. The microscope model used in study was OLYMPUS-CH20i/BIMF. The particle size of the pure resin after prolonged crushing using porcelain mortar and pestle was ascertained using Scanning Electron Microscope (SEM) at the Indian Institute of Chemical Technology, Tarnaka, Hyderabad. The model of SEM used for study is SEM Hitachi S-3000 N with magnification: 20X to 30,000X. The selected base Aloe vera gel was purchased from Ramayan company, Gujarat. Methyl paraben and propyl paraben from Nebula Health Care were kindly supplied by the Department of Pharmacy, Shadan College of Pharmacy, Hyderabad. The packaging materials were ordered from Alliance Containers Co., Athal Industrial Estate, Athal, Silvassa, Dadra and Nagar Haveli.
2.1 Ointment preparation
Firstly the entire process of selection, authentication of Vateria indica Linn followed by its resin extraction and leading up to ointment preparation is described. Secondly on the clinical side the process of conducting the drug trials on patients displaying radiation burns at Bibi-KIMS cancer research centers is demonstrated. Vateria indica plant was located at Dulapally forest academy Hyderabad, but it was found to be too young to produce resin. For this study, an approximately 18 to 20 m long tree of over 60 years of age was selected, the selection and acquiring process was facilitated by the Telangana forest department, in the region of Sirsi forest, Karnataka. The taxonomic authentication from the twig and inflorescence was issued by the Botany department, Osmania University Hyderabad. The tree was injured with the axe by making semicircular incision on the stem through the cork cambium up to the surface of sapwood. The resin started oozing from the incisions in 3 or 4 days and continued for 60 to 90 days. By this procedure pure resin was procured and stored in an airtight plastic box.
2.2 Clinical trial
The exploratory clinical trials were conducted at BIBI-KIMS Cancer Hospital, Malakpet, Hyderabad after taking institutional ethics committee approval and obtaining the written informed consent and following the good clinical practice. Patients undergoing RT for head and neck cancers of either gender aged between 18 and 60 years receiving 3500 to 7000 CCGy dose of RT were eligible. Patients with any other skin disease, HIV and HBSAg positive were excluded. Twenty-six patients were studied, among them 17 were considered for test group and 9 were treated as control group. During the study the subjects were asked to use the ointment provided in collapsible tubes. The study was continued until the resolution of RT burn lesions which lasted for one and half months.
The patients undergoing radiation therapy (RT) applied the Vateria indica ointment provided over the radiation exposed area of the skin at night before going to bed. The following morning prior to leaving for the radiation fraction, the patient was asked to wash the affected area so that no ointment residues remain over the radiation exposed area. This procedure was followed daily during the course of RT as well as after its completion, until the burns disappear and the skin returns to normal. The patients were asked to visit on alternate days to assess the intensity of radiation burns. The radiation exposed area was evaluated for the time taken to onset of burns in days after the first dose of RT. And time taken to resolution of burns in days after completion of overall RT and the final stage of dermatitis attained as per Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0.
2.3 Statistical analysis
Demographic data was presented as mean ± SD. Descriptive data was presented as medians and ranges. Kaplan Meier estimates were used for time to events of onset and resolution of oral radiation dermatitis. For comparison of severity fisher exact test was done. A P < 0.05 was considered statistically significant. Power of the study was 80%. Statistical analysis was performed using IBM SPSS version 20.0. The sample size was calculated as 23 patients, according to outpatient load of head and neck cancers in the Department of Radiation Oncology at Bibi-KIMS Hospital.
3 Results
The particle size was monitored through scanning electron microscope until the optimal size (ranges 3–10 µm) (Garg and Piyush, 2012) was obtained following the crushing of the resin of Vateria indica with porcelain pestle and mortar. Scanning electron micrograph shown in Fig. 1, indicates that the after crushing the particles size has become less-than 10 µm, which is suitable for penetration. The high resolution SEM image exhibits a continuous smooth microstructure of the resin, which revealed that the griding of resin did not destroy the micro texture of the sample. The resin as such being crystalline and water free has good shelf life. On examining a few commercial preparations of Vateria indica resin that were based on fatty bases (ghee and waxes) we could identify bacterial growth. This was also observed in the ointment prepared by us. Further examination revealed that after 48 h of incubation gram positive bacilli was found, but the preparation was free from fungus for up to 14 days. On day 16 gross fungal growth was seen over the surface of ointment suggestive of Aspergillus species.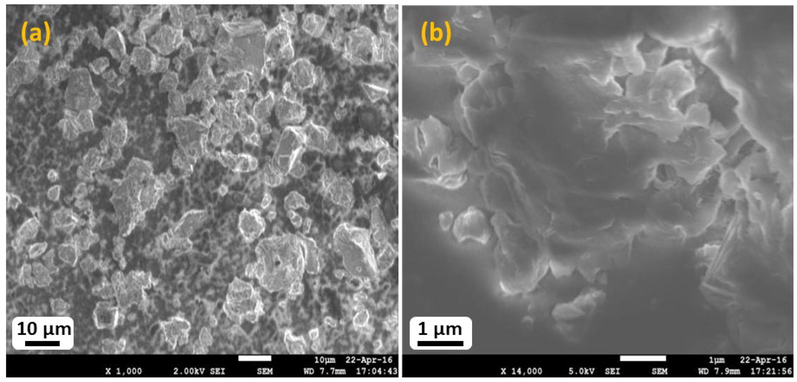
Scanning Electron Micrograph (SEM) resin of Vateria indica after crushing mortar and pestle.
The resin in the powdered form and Aloe vera gel were cultured separately in the culture media that were kept for incubation. After 48 h when the culture was observed both the media showed the development of bacterial colonies. These were gram positive bacilli in the resin and the gram negative bacilli in the Aloe vera gel. Therefore, the ointment prepared was periodically checked for the sterility and stability and summarized in Table 1.
Sample
Preservative
Smear-wet mount
(48 Hours)
Culture
(48 Hours)
Inference
Resin
None
No motile form and hyphae observed
Bacteria
Gram positive Bacilli
Gel
None
No motile form and hyphae observed
Bacteria
Gram negative Bacilli
Gel + Resin
None
No motile form but hyphae observed
Bacteria
Gram positive Bacilli and hyphae
Gel + Resin
0.2 % Methyl paraben
No motile form and hyphae observed
Bacteria
Gram positive Bacilli
Gel + Resin
0.5 % Methyl paraben
No motile form and hyphae observed
Bacteria
Gram positive but very few Bacilli compared to 0.2%
Gel + Resin
0.4% propyl paraben and 0.5 % methyl paraben
No motile form and hyphae observed
None
No bacteria or fungal growth.
In this regard, two samples with doses of 0.2% and 0.5% methyl paraben were made, which is used as excipient (Khan et al., 2010). The sample with 0.2 % methyl paraben did not show any fungal growth even after 45 days of ointment preparation but showed positive for bacterial growth when culture was performed after 14 days. The bacteria were suggested as gram positive bacilli. It was inferred that 0.2% ointment was sufficient to overcome the fungal growth but was not sufficient to control bacterial development. The sample with 0.5% methyl paraben also did not show any fungal development and the colonies formed were very small with only few gram positive bacteria. The sample prepared with 0.4 % propyl paraben did not show much activity against the bacteria and the result of culture suggested the presence of gram positive bacilli bacterial in the ointment. However, the fungal growth was well controlled. On the other hand, the sample containing 0.5% methyl paraben and 0.4% propyl paraben did not show any fungal growth, and in addition, the gram stain study of the ointment showed negative report for both gram positive and gram negative bacteria. Therefore, the combination of methyl paraben and the propyl paraben which demonstrated good compatibility and stability, was selected for the preparation of ointment. This as-prepared ointment, when stored at room temperature and away from direct sun light in the collapsible tubes remained stable even after the 6 months, which was offered to the patients.
For the clinical trial, out of the forty patients that were screened, twenty-six eligible subjects were enrolled for the study. Two patients from test arm and one patient from control arm withdrew during the study. Finally, only a total of twenty-three patients completed the study. The CONSORT diagram and the demographic details of the patients for the full study is shown in Fig. 2 and Table 2, respectively. The demographic details depicted in Table 2 demonstrate that no significant differences were observed between the treatment and control group in the baseline characteristics, including age and radiation dose.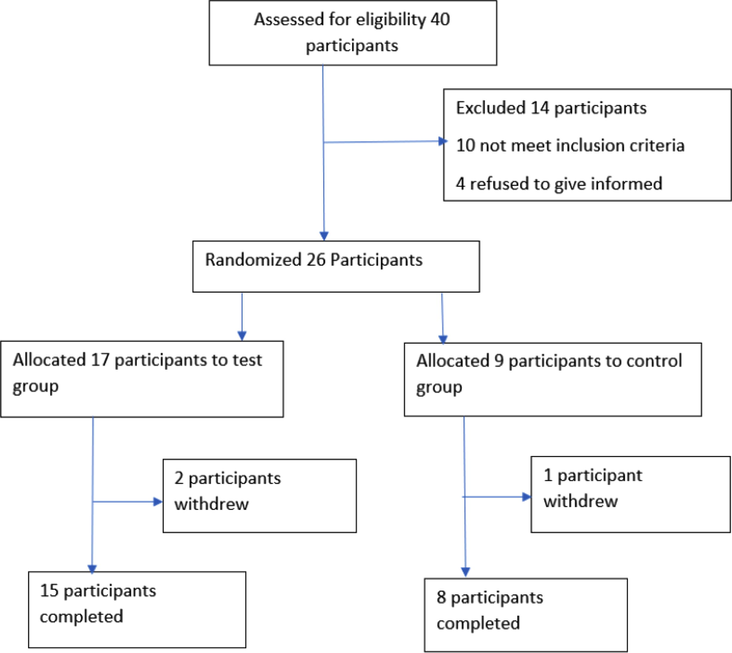
CONSORT Diagram and the demographic details of the patients analyzed in this study.
Test
Control
Total Number
15
8
Gender Male: female
10:7
4:4
Age Range
25–58
49–59
RT dose (Grey)
50–70
50–70
Post-surgery
5
6
Weekly Cisplatin
15
8
Median time of onset of radiation burns was found to be 6 days in control group whereas its onset took 7 days in the test group (p greater than 0.05). This indicates that there was a negligible difference between Vateria indica ointment and control patients as far as onset of radiation burns are concerned. Survival curve is shown in Fig. 3. However, there was a significant difference between Vateria indica ointment and control patients in the resolution of radiation burns. Median time of resolution was 20 days in control group and 16 days test group (p < 0.05). The survival curve is shown in Fig. 4.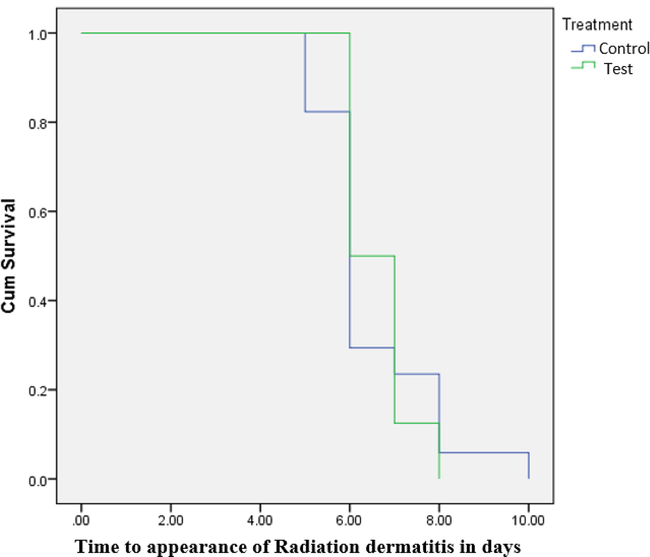
Time taken for appearance of radiation dermatitis during RT.
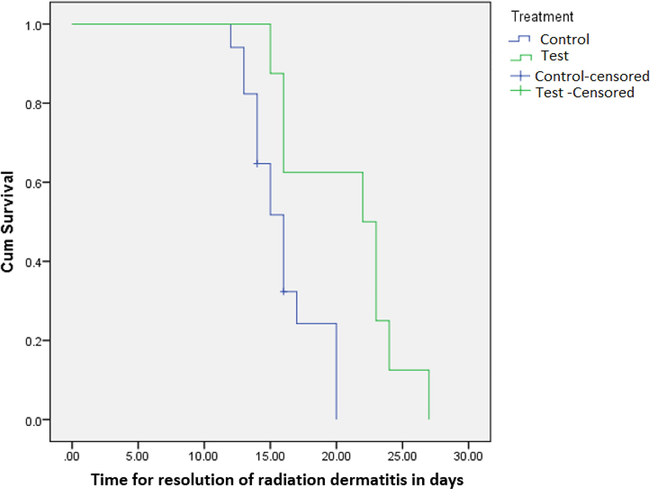
Time taken for complete resolution of radiation dermatitis post-RT.
In Vateria indica group, only 33 % patients developed high grade burns, whereas, in case of control group 62% patients exhibited high grade burns. This difference was found to be statistical significance (p < 0.05). The details are depicted in Table 3.
Grade-I & II
Grade-III & IV
Total
Test
10 (67%)
5 (33%)
15
P < 0.05
Control
3 (38%)
5 (62%)
8
Total
13
10
23
All the patients continued application of ointment without any gap and the study was carried out under the direct supervision of the staff nurse. Apart from radiation burns, the other common adverse events such as mucositis, pharyngitis, laryngitis and oral thrush were observed. The frequency of these adverse events was equally distributed in both the groups. Two participants from the treatment group complained of mild itching after use of ointment and they were withdrawn from the study.
4 Discussion
Radiation therapy which uses ionizing radiations is the first form of treatment introduced for cancer therapy to eliminate the atypical neoplastic cells. The normal but actively dividing cells of the body are also affected especially the stem cells of bone marrow, gastrointestinal tract, skin and mucosa (Mirza et al., 2022). As in the case of head and neck cancers, the patients undergoing RT experience skin aberrations ranging from desquamation and inflammation to ulceration and infections. This study was made keeping in view the expected cooperation and outcome from the patients on radiation exposed parts.
Oral, topical, and intravenous therapies are in use and out of which the topical method showed more effect (Omidvari et al., 2007; Schmuth et al., 2002). A study of the existing preparations reveals that no ointment exists specifically to treat radiation induced burns in cancer patients. The parameters for the remedies selection was focused upon treating inflammation and to facilitate wound healing (Salvo et al., 2010). We have chosen Vateria indica resin for its anti-inflammatory role as a topical remedy and its anti-microbial activity that is reported for treatment in urinary tract and gastrointestinal ailments (Venkateshwarlu et al., 2011). In this program the work pertaining with procuring the resin to the final application in an ointment form through a battery of tests ensuring safety, sterility and stability of the final product i.e. ointment, is described.
In this study, out of 23 patients, 15 patients were treated with Vateria indica ointment and the results were compared with results of 8 other patients who were offered just standard treatment (without Vateria indica ointment). From a range of branded and unbranded commercially available ointments, we determined presence of bacteria when fatty bases (e.g., ghee, waxes etc.) were used, which possibly promote microbial growth. A commercially available sample of Vateria indica resin was examined and its culture revealed a gross development of colonies. On gram staining, we found gram positive bacilli with spore forming bacteria. Surprisingly, the locally available ointment under the name of Marham-e-Raal which stands for Vateria indica resin, which is known for its wound healing qualities, is not found to be safe and sterile. Earlier, in many cases, just Aloe vera was applied to treat radiation induced dermatitis, which is however, not found to be effective (Garg and Piyush, 2012). Recently, herbo-medicinal formulation of Vateria indica, Marham-e-Raal for acute and chronic wounds was reviewed. Therefore, in this work Aloe vera gel was selected, since it consists of non–fatty aqueous base and ∼ 98% water, it is famous as a cosmetic ingredient and also known to offer soothing effect on the skin. Thus, the ointment is prepared using an indigenous and low cost natural product but fully authenticated Vateria indica resin and favorable dermal uptake to ensure proper pharmacological absorption.
Particle size influences the bioavailability and efficacy of a topical drug. Optimal particle size for penetration over the skin falls in the range of 3–10 µm, those particles more than 10 µm remains on the skin (Garg and Piyush, 2012). The resin of Vateria indica was subjected to crushing with porcelain pestle and mortar until a fine talcum-powder like particles were achieved. The particle size was monitored through scanning electron microscope until the optimal size was obtained. Secondly, the coarse size particle forming a crust was attributed to inability to enter the skin [16]. This was rectified in this work by ensuring nano particle sized of resin in our ointment. Therefore, in formulation of topical ointments particle size plays vital role in improving the efficacy of the product.
In the case of the control group, where Vateria indica treatment was not given in comparison with the study group, the time of onset of dermatitis was not significantly different. However, in the control population the healing period ranged from 18 to 26 days, whereas, for the test ointment treated patients the period of resolution fell in the range of 13 to 20 days (Figs. 3 & 4). This shows that despite the self-resolving but the delayed resolution of radiation burns our Vateria indica treatment makes a clear impact in its treatment. This result can be attributed to a sterile environment which is facilitated by the resin. The results also confirmed the role of particle size selected for the resin in the ointment ensuring drug delivery through nano-pharmacology for the first time in the treatment of radiation induced burns. In this way patient confidence is resumed from an early stage. Notably, the research under pharmacological guidelines using Vateria indica resin and current legislation seems lacking as revealed by our study from literature survey. Therefore, the use of Vateria indica ointment offers reassurance to the patient about their condition which is considerably alleviated by applying the product.
5 Conclusions
Herein, the Vateria indica resin was used to prepare an ointment for treating RT induce burn, which was obtained in its pure form, while the ointment was developed using Aloe vera gel as a base. The final product demonstrated early healing when compared to standard treatment without the ointment. The effectiveness of the ointment is possibly associated with the optimized particle size of resin, which is compatible with skin pores and hence, demonstrated enhanced dermal intake of the ointment. Although, a large number of indigenous medicines are currently available in the market for burn injuries. However, the proper pharmacological approach adopted in this study, for the preparation and application of Vateria indica resin based ointment, has rendered the resulting product relatively more effective compared to the available products. Besides, in this study, other indigenously prepared ointments, such as, Marham-e-Raal (Unani), obtained from other sources were also applied to head and neck cancer patients receiving RT. However, their application did not demonstrate any signs of dermal absorption and formed a crusty layer. Hence, for the first time an ointment was prepared using natural resin, which demonstrated high compatibility with skin pores for topical therapy, due to the presence of nano-size particles in the sample. The sterility and stability of the ointment and the proper application procedures applied during this study may potentially enhance the applicability of Vateria indica drug discovery and development.
Funding
The authors acknowledge the funding from Researchers Supporting Project number (RSPD2023R665), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
The authors acknowledge the funding from Researchers Supporting Project number (RSPD2023R665), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Herbo-medicinal formulation; Marham-e-raal: A potent ointment for acute and chronic wounds–A review. Turkish Journal of Plastic Surgery. 2019;27:77.
- [Google Scholar]
- The role of surface-guided radiation therapy for improving patient safety. Radiother. Oncol.. 2021;163:229-236.
- [Google Scholar]
- The antiepileptic potential of Vateria indica Linn in experimental animal models: Effect on brain GABA levels and molecular mechanisms. Saudi Journal of Biological Sciences. 2022;29:3600-3609.
- [Google Scholar]
- Gold nanoparticles (AuNPs) and Rosmarinus officinalis extract and their potentials to prompt apoptosis and arrest cell cycle in HT-29 colon cancer cells. J. King Saud Univ. Sci.. 2022;34:102304
- [Google Scholar]
- Bontempo, P.d.S.M., Ciol, M.A., Menêses, A.G.d., Simino, G.P.R., Ferreira, E.B., Reis, P.E.D.d., 2021. Acute radiodermatitis in cancer patients: incidence and severity estimates. Revista da Escola de Enfermagem da USP 55.
- Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-791.
- [Google Scholar]
- Comparison of clinical practice guidelines on radiation dermatitis: a narrative review. Support. Care Cancer 2022:1-12.
- [Google Scholar]
- Physical Exercise and the Hallmarks of Breast Cancer: A Narrative Review. Cancers. 2023;15:324.
- [Google Scholar]
- Garg, S., Piyush, G., Resent advances in semisolid Dosage forms for Dermatological Applications Pharmaceutical Technology. March: 2012.
- Ginex, P.K., 1969. Radiodermatitis in patients with cancer: Systematic review and meta-analysis. Number 6/November 2020 47, E225-E236.
- Wound healing after radiation therapy: review of the literature. Radiat. Oncol.. 2012;7:1-9.
- [Google Scholar]
- Topical silver sulfadiazine for the prevention of acute dermatitis during irradiation for breast cancer. Support. Care Cancer. 2012;20:1613-1618.
- [Google Scholar]
- Topical treatment of radiation-induced dermatitis: current issues and potential solutions. Drugs in Context. 2020;9
- [Google Scholar]
- Two new oligostilbenes with dihydrobenzofuran from the stem bark of Vateria indica. Tetrahedron. 2003;59:1255-1264.
- [Google Scholar]
- Refined spruce resin to treat chronic wounds: rebirth of an old folkloristic therapy. Adv. Wound Care. 2016;5:198-207.
- [Google Scholar]
- In vitro study on anti-inflammatory activity of aqueous extract of Vateria indica resin. International of Pharmacy and Biological Sciences. 2017;7:129-135.
- [Google Scholar]
- Crystal engineering of pharmaceutical co-crystals: application of methyl paraben as molecular hook. J. Am. Chem. Soc.. 2010;132:5254-5263.
- [Google Scholar]
- Khan, M.R., Ahmad, T., Jamal, M.A., Clinical efficacy of Safoof Ushba and Marhame Raal in Narfarsi (Eczema).
- Apoptosis inducing ability of silver decorated highly reduced graphene oxide nanocomposites in A549 lung cancer. Int. J. Nanomed.. 2016;11:873.
- [Google Scholar]
- Advances in cancer therapeutics: Conventional thermal therapy to nanotechnology-based photothermal therapy. Pharmaceutics. 2021;13:1174.
- [Google Scholar]
- Nanoscale metal− organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials. 2016;97:1-9.
- [Google Scholar]
- Outpatient Burn Treatment: A Conservative and Effective Personal Approach. Cosmetol & Oro Facial Surg. 2018;4:2.
- [Google Scholar]
- Efficacy of Bacillus clausii UBBC-07 spores in the amelioration of oral mucositis in head and neck cancer patients undergoing radiation therapy. Cancer Treatment and Research Communications. 2022;31:100523
- [Google Scholar]
- Wound healing effect of a Unani formulation Marham-e-Ral in albino rats. Int J Adv Pharm Med Bioallied Sci. 2014;2:20-24.
- [Google Scholar]
- Topical betamethasone for prevention of radiation dermatitis. Indian J. Dermatol. Venereol. Leprol.. 2007;73
- [Google Scholar]
- A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J. Clin. Oncol.. 2008;26:2085-2092.
- [Google Scholar]
- Formulation and evaluation of orodispersible tablet of taste masked doxylamine succinate using ion exchange resin. J. King Saud Univ. Sci.. 2010;22:229-240.
- [Google Scholar]
- Anti-cancer ability of chitosan loaded plant essential oils evaluated against A549 human lung cancer cells through invitro approaches. J. King Saud Univ. Sci. 2023:102598.
- [Google Scholar]
- Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr. Oncol.. 2010;17:94-112.
- [Google Scholar]
- Topical corticosteroid therapy for acute radiation dermatitis: a prospective, randomized, double-blind study. Br. J. Dermatol.. 2002;146:983-991.
- [Google Scholar]
- Medical therapy of burn scar before any plastic surgery by using topical corticosteroid combined with oral zinc sulfate. Journal of the Turkish Academy of Dermatology. 2021;15:37.
- [Google Scholar]
- Evaluation of Wound Healing Activity of Four samples of Sarjarasaby Excision wound in Albino Rats. International Ayurveda Medical Journal. 2015;3:381-387.
- [Google Scholar]
- Sultana, A., Joonus, A.F.M., Rahman, K., 2021. Effect of Marham-i-Raal on Episiotomy Wound Healing: A Single-Arm pre-and post-treatment study. CELLMED 11, 17.11-17.14.
- Effect of corticosteroid ointment on radiation induced dermatitis in head and neck cancer patients: A prospective study. Indian J. Cancer. 2021;58:69.
- [Google Scholar]
- Preliminary physicochemical evaluation of Sarja rasa (resin of Vateria indica Linn.) and it's traditional medicinal formulation. International Journal of Research in Ayurveda and Pharmacy (IJRAP). 2011;2:334-337.
- [Google Scholar]
- Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J. Clin.. 2021;71:437-454.
- [Google Scholar]
- Individual skin care during radiation therapy. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft...[et al]. 1998;174:74-77.
- [Google Scholar]







