Translate this page into:
Vascular plants census linked to the biodeterioration process of the Portuguese city of Mazagan in El Jadida, Morocco
⁎Corresponding author. jamdahmani@gmail.com (Jamila Dahmani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Built in the early XVIth century, Mazagan is one of the first fortifications constructed in North Africa by Portuguese explorers on their way to India. Among the factors of degradation facing the Portuguese city, we were particularly interested in the action of plants that develop there. The objective of our study is to establish a list of plant species that develop on materials and to analyze the potential effects of that vegetation on substrate and thus on monument deterioration. We carried out a systematic sampling in Mazagan. After sampling and identification of plants, we established a list of 57 species belonging to 25 families and 54 genera. The Asteraceae family is the richest in species. The therophytes are the most represented in Mazagan with a proportion of nearly 53% followed by Hemicryptophytes that contain nearly 26% of the listed species. These two biological types may have a fasciculated or pivotal rooting that sinks into the substrate leading to its crumbling. Other biological types such as phanerophytes, chamaephytes and geophytes are no less harmful even if they are represented only in a small proportion: their generally pivoting roots destroy the substrate more quickly. The study site is colonized mainly by spontaneous plants, which represent nearly 81%. Naturalized plants like Lycium europaeum have become so invasive that they even settle on the side of walls. Maintenance measures must be undertaken in the city to control the spread of such destructive vegetation.
Keywords
Archeology
Biodeterioration
Historical monuments
Mazagan
Vascular flora
1 Introduction
Historical monuments are naturally subject to deterioration by the environment through especially rain, sun, temperature variations or pollution. Walls are subject to abiotic and microbial weathering that degrade the material over time. This progressive disintegration of building materials provides safe sites for the establishment of higher plants which can thus outcompete early colonizing organisms (Motti and Bonanomi, 2018). Most of the available knowledge about deterioration concerns alteration and weathering of stone caused by abiotic, anthropogenic, and microbial factors (Elharech et al., 2017; Motti and Bonanomi, 2018). Indeed, a number of works have focused on the role of microbiota, including bacteria, fungi, as well as lichens and mosses, on the biodeterioration of stone monuments (Elharech et al., 2017; Motti and Bonanomi, 2018). In recent decades, however, several studies have recognized the importance of higher plants in causing damage to stone monuments (Lisci and Pacini, 1993; Motti and Bonanomi, 2018).
Different types of mechanisms result in the biodeterioration of materials: physical or mechanical processes leading to phenomena such as loss of cohesion, rupture, or disaggregation; and chemical processes that lead to transformation, degradation, or decomposition of the substrate (Caneva et al., 2009a). The biodeterioration of materials is closely correlated to the chemical and chemical-physical nature of the substrate as well as to the characteristics of the surrounding environment (Caneva et al., 2009a).
Birds, insects and rodents are the most incriminated among animals (Benharbit, 2017a). However, it has been realized that, among the various biological agencies responsible for deterioration of stone monuments, the higher plants play a significant role (Mishra et al., 1995). Vascular plants, often associated with bryophyta, lichens, algae, and fungi, are the biodeteriogens causing most damage, such as cracks, collapse, and detachment of materials mainly due to biophysical and biochemical processes (Motti and Stinca, 2011). The damage caused by ruderal plants on monuments or archaeological sites is well known and is linked either to particular biological forms (woody species, shrubs, or grasses) or to the characteristics of the root system (Caneva et al., 2009a). The aerial parts and especially the roots of plants damage wall structure. The aerial part causes aesthetic and static alterations to wall structure. Roots can grow very large and go very deep, causing physical and/or chemical damage to wall structure (Caneva et al., 2009a). The roots tend to penetrate the areas offering the least resistance, such as mortars (Caneva et al., 2009a). Root secretions contain substances that attack building materials (Lisci and Pacini, 1993); mechanical stress due to root expansion produces cracking and scaling (Lisci and Pacini, 1993).
Higher plant colonization of stone monuments is limited by several factors, including the availability of safe sites for settlement, the hardness of the substratum, frequent disturbance, the large variability of microclimate in terms of temperatures and humidity, as well as the scarcity of water (Segal, 1969; Motti and Bonanomi, 2018).
The colonization of plant communities varies according to the pedological characteristics of the substrate and the amount of water, nitrates and light in the environment (Caneva et al., 1992). Vegetation can develop on a wall only after the conditions for settlement of plant species have become sufficiently favorable. Prerequisites are that the wall has been exposed to weathering long enough or that soil particles have accumulated (or both) (Segal, 1969).
The porosity of building materials determines its water-retaining capacity and facilitates the formation of substrate suitable for plant growth. Travertine, limestone and sandstone are the most porous materials and are colonized more readily than compact materials like granite and other siliceous rocks. In the case of bricks, the plants grow in weathered interstitial lime mortar (Lisci and Pacini, 1993).
Indeed, the architecture of buildings can be conducive to biological colonization, especially on the facades concerned by moisture retention, capillary rise or prolonged stagnation of rainwater. The surface condition of the substrate namely porosity and chemical composition can also promote colonization (Benharbit, 2017b).
Plants causes various problems such as: mechanical damages and displacements, chemical corrosion by substances secreted by roots, threat of fire during the dry summer period, restriction of views and visitor access and obstruction of excavation and restoration works (Mishra et al., 1995; Papafotiou et al., 2017).
Mazagan, a historical city of Morocco that is threatened with biodeterioration, was among the first countertops built in North Africa by Portuguese explorers in the early sixteenth century. The Portuguese realized, indeed, two renaissance fortifications: Ceuta in the Mediterranean side of Morocco in the north and Mazagan overlooking the natural bay in south of El Jadida on the Atlantic coast (central part). They were both considered defenses that function in a system which incorporate the best defensive technics of that time (Carabelli, 2012).
Mazagan is now considered as a shared cultural heritage between Morocco and Portugal. In fact, it has been classified in 2004 in the UNESCO World Heritage List: «The Portuguese city of Mazagan is an exceptional example of the exchange of influences between the European and Moroccan cultures from the XVIth to the XVIIIth century» (UNESCO, 2004).
The spread of vascular plants is potentially the most important among the factors that cause the biodeterioration of Mazagan. The objective of our study is therefore to establish a list of plant species that develop on the masonry made of blocks of limestone Cenomanian grouted by a lime mortar and to analyze the potential effects of this vegetation on the deterioration of the monument.
2 Material and methods
2.1 Study area
The fortress of Mazagan, located at the Atlantic coast in south of the city of El Jadida (Fig. 1), is circumscribed by imposing walls and has currently four bastions (Fig. 2). From the Portuguese era, only few buildings remain in Mazagan: the church of the assumption and the cistern. This latter served as a warehouse for weapons before being turned into water reserve during the siege by the army led by the Moroccan King Sidi Mohamed Ben Abdallah and that resulted in the liberation of the city in 1769 (Chebri, 2012). The city was then abandoned and deserted for several decades before the Sultan Sidi Abderrahmane decided, in 1823, to turn it into a main port leading to the current city of El Jadia.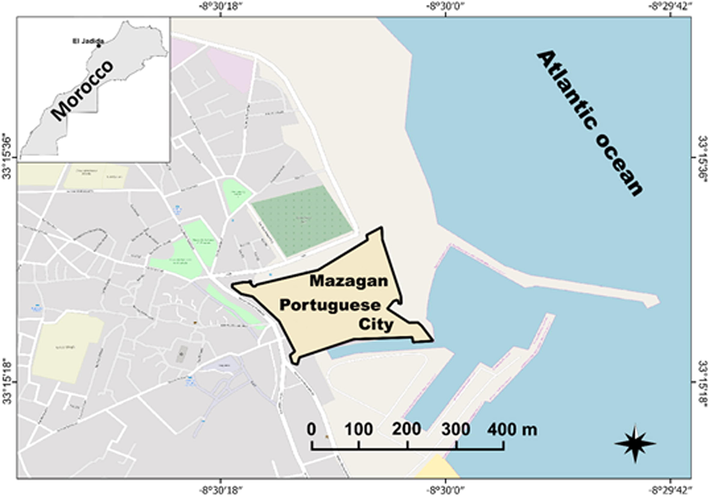
Location map of the Portuguese city of Mazagan in El Jadida, Morocco.

Aerial photo of Mazagan (Derif, 2016).
The climate in El Jadida is warm and temperate and is classified as semi-arid bioclimate meaning a Mediterranean climate with dry summer (Fig. 3). The average temperature in El Jadida is 17.4 °C and for rainfall 372 mm (climate–data, 2018).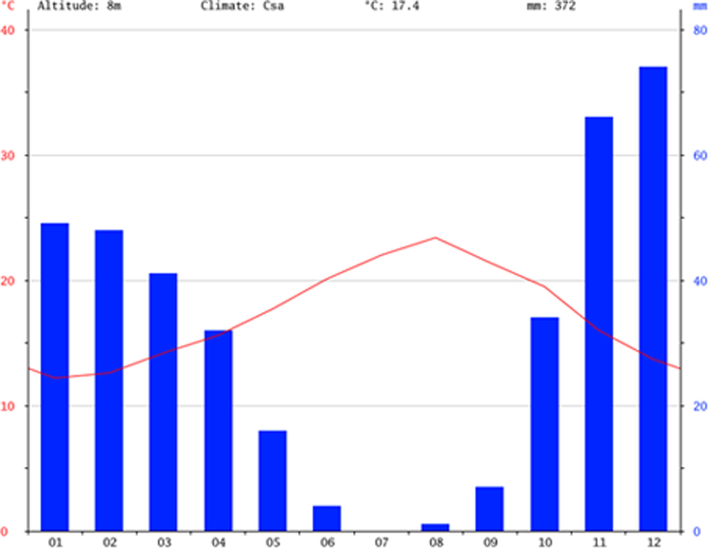
Climate diagram of El Jadida (climate-data, 2018).
2.2 Sampling
We adopted the systematic sampling: all the plants observed at the base of walls, on their outer or inner side and on their summit were sampled carefully to not damage the substrate; inaccessible plants are photographed. A herbarium has been realized. The harvest took place between April 2016 and January 2017.
Herbaceous plants are harvested completely (root, stem, leaves, flowers and if possible fruits). Shrubs and deep-rooted plants are not removed to avoid damaging walls; only branches with flowers and, if possible, fruits are harvested. We took many photographic documentation of substrates colonized by plants for illustration.
The identification of species was carried out based on some documents such as “la Flore Pratique du Maroc” (Fennane et al., 1999, 2007, 2014), “la nouvelle flore d'Algérie et des régions désertiques méridionales” (Quezel and Santa, 1962–1963) and thanks to some well-known websites such as Tela Botanica (www.tela-botanica.org).
3 Results and discussion
By exploring the Portuguese city of Mazagan, we identified 57 plant species belonging to 25 families with that of Asteraceae ranking the first place with 10 species. The species diversity is then important showing very heterogeneous vegetation that are difficult to control to limit their proliferation.
Most harvested species are light demanding and prefer warm to moderately hot and relatively humid environments. Outside Mazagan, they normally settle on soils mainly sandy, loamy, clayey or even stony.
Dissemination by wind and animals leads the seeds on very different supports. They germinate when conditions are favorable. In the city of Mazagan, the plants are on pavement of the walkways, at the base of walls or on the grouting mortars that they contribute to loosen and whose sand-clay composition in some places becomes more humic. Walls of fortifications, built with limestone, are also colonized.
4 Floristic list of the Portuguese city of Mazagan
AMARANTHACEAE
Scandix pecten-veneris L.
Amaranthus deflexus L.
ARACEAE
Beta maritima L.
Arisarum vulgare O. Targ.Tozz.
Chenopodium album L.
ARECACEAE
Chenopodium murale L.
Trachycarpus fortunei (Hook.) H.Wendl.
ANACARDIACEAE
ASTERACEAE
Schinus terebinthifolius Raddi
Anacyclus coronatus Murb.
APIACEAE
Centaurea calcitrapa L.
Ferula communis L.
Malva parviflora L.
Erigeron bonariensis L.
MORACEAE
Leontondon saxatilis L.
Ficus carica L.
Pallenis spinosa subsp. aurea (Willk.) Nyman
OLEACEAE
Scolymus hispanicus L.
Olea europaea L.
Senecio vulgaris L.
OXALIDACEAE
Sonchus oleraceus L.
Oxalis pes-caprae L.
Symphyotrichum squamatum (Spreng.) G.L.Nesom
PLANTAGINACEAE
Volutaria tubuliflora (Murb.) Sennen
Cymbalaria muralis P.Gaertn., B.Mey. & Scherb.
BRASSICACEAE
Kickxia commutata (Bernh. ex Rchb.) Fritsch
Brassica tournefortii L.
Plantago coronopus L.
Diplotaxis catholica L.
POACEAE
Diplotaxis tenuifolia L.
Anisantha rubens L.
Lepidium coronopus L.
Catapodium rigidum L.
CARYOPHYLLACEAE
Cynodon dactylon L.
Polycarpon tetraphyllum L.
Hordeum murinum L.
Spergularia fimbriata L.
POLYGONACEAE
CONVOLVULACEAE
Emex spinosa L.
Convolvulus althaeoides L.
Polygonum maritimum L.
Convolvulus arvensis L.
RUBIACEAE
Ipomoea imperati L.
Asperula arvensis L.
CRASSULACEAE
SCROPHULARIACEAE
Sedum sediforme (Jacq.) Pau
Misopates orontium L.
CYPERACEAE
Verbascum sinuatum L.
Cyperus rotundus L.
SOLANACEAE
EUPHORBIACEAE
Hyoscyamus albus L.
Euphorbia terracina L.
Lycium europaeum L.
Mercurialis ambigua L.
Nicotiana glauca Graham
FABACEAE
Solanum nigrum L.
Lotus arenarius L.
URTICACEAE
Medicago polymorpha L.
Urtica urens L.
GERANIACEAE
Parietaria judaica L.
Erodium ciconium L.
MALVACEAE
4.1 Species richness by family
The following Fig. 4 illustrates the specific richness for each of the 25 families encountered in Mazagan.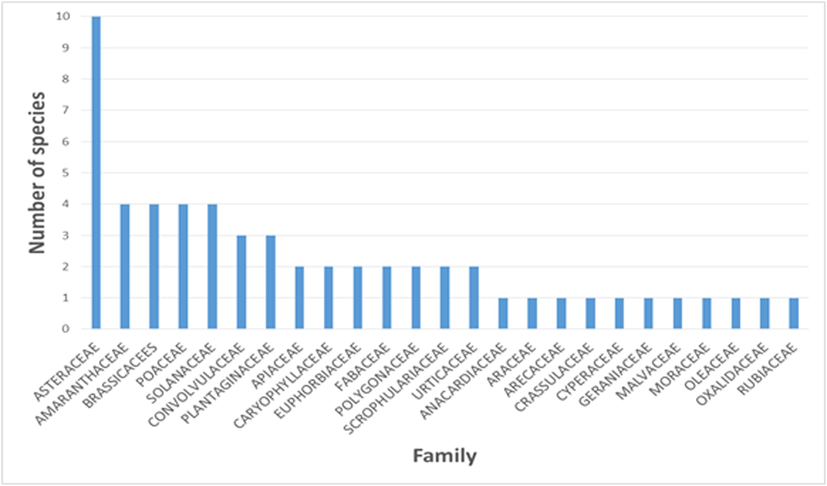
Specific richness by family.
The most represented families are: Asteraceae (10 species), Poaceae (4 species), Amaranthaceae (4 species), Brassicaceae (4 species) and Solanaceae (4 species). In Morocco, the family of Asteraceae that occupies the first rank in specific richness is present with nearly 550 taxa (Fennane and Ibn Tattou, 2012).
4.2 Biological spectrum
The listed species belong to 5 biological types: therophytes (52.63%), hemicryptophytes (26.32%), phanerophytes (10.53%), geophytes (8.77%) and chamaephytes (1.75%) (Fig. 5).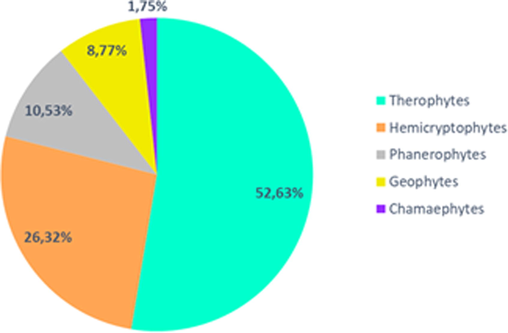
Biological spectrum.
The therophytes are predominant in the city with more than half of the identified species. They are annual plants that complete their life cycle during the favorable season and stay during winter in the form of seeds. This is a strategy of adaptation to the rigor of winter period. These plants are distinguished by the ease of dissemination of their seeds that greatly increases their covering. The seeds germinate where moisture accumulates. The therophytes colonize with hemicryptophytes, important surfaces of walls, bastions and paths of the Portuguese city. In fact, the identified species are generally undemanding plants and install on substrate poor in organic matter.
4.3 Status
Among the 57 species recorded, 81% belong to the Moroccan flora. They may be nitrophiles such as Paretaria judaica, Chenopodium album, Malva parviflora, Hyoscyamus albus, Solanum nigrum and Urtica urens, which develop particularly in nitrate-rich areas such as places where trash is deposited. Some species are remarkable for their abundance in the city as Cynodon dactylon, a rhizome geophyte whose flowering is possible all the year. This plant represents a threat by the high level of produced seeds and by its rhizome whose horizontal development leads to the alteration of the superficial layer of the substrate that is thus weakened.
Moreover, 3 species are endemic: Pallenis spinosa subsp. aurea endemic to Morocco and the Iberian Peninsula, Anacyclus coronatus, endemic to Morocco and the Canary Islands and Lotus arenarius endemic to Morocco, the Iberian Peninsula and the Canary Islands.
This endemism shows the biogeographical relations between Morocco and the Iberian Peninsula, on the one hand, and between Morocco and the Canary Islands on the other. Fennane (2004) in his survey of endemic plant species found 209 endemic species to Morocco and the Iberian Peninsula and 11 endemic to Morocco and the Canary Islands.
The naturalized species are Lycium europaeum, Nicotiana glauca, Symphyotrichum squamatum, Cymbalaria muralis, Oxalis pes-caprae and Erigeron bonariensis. Lycium europaeum is the most frequently encountered phanerophyte; it settles on the side of walls, on their top and on the pavement. These plants have been able to adapt perfectly to the climate of Morocco so that they can sometimes become invasive. According to Ozenda (1964), the various Oxalis are from Cape Town and are very invasive throughout the Mediterranean region.
4.4 Root system
The phanerophytes identified in the city are characterized by a relatively deep, strong and vivacious root system. It is pivoting or fasciculated. The main root bundles carry lateral secondary roots that increase the root volume and subsequently the exchange surface with the substrate. By sinking into the substrate, as seen, the root system causes cracks and can even burst walls.
In the case of therophytes, the root system is shallower and much weaker. These plants survive bad season in the form of seeds; the vegetative parts is destroyed by desiccation due to frost or drought. These annuals plants are short-lived so they develop rapidly; germination of seeds depends strictly on moisture. Poaceae, for example, have fasciculate roots much weaker than those of phanerophytes but whose threat consists in the number of individuals that is considerable due to the great capacity of dissemination of these herbaceous plants. The seeds are easily spread by birds (zoochory) and wind (anemochory).
The other therophytes encountered in the city possess a pivoting rooting of variable thickness and depth. This type of root is also found in hemicryptophytes such as Beta maritima, Centaurea calcitrapa, Leontondon saxatilis, Ferula communis, Pallenis spinosa subsp. aurea, Scolymus hispanicus, Symphyotrichum squamatum, Polygonum maritimum and Verbascum sinuatum. These latter are perennial by their underground part; they therefore participate in the deterioration of the substrate more actively and more continuously in time than therophytes. Other hemicryptophytes have underground stolons that propagate horizontally on the substrate; this kind of propagation cause significant damage.
Regarding the geophytes, the root system is either a bulb (Oxalis pes-caprae), a rhizome (Cynodon dactylon), or several tubers (Arisarum vulgare). Thus, the geophytes survive during bad season buried in the ground. The plant is therefore unapparent during a few months of its annual cycle.
Rhizome geophytes such as Cynodon dactylon, which is characterized by rapid growth, Cyperus rotundus and Sedum sediforme are harmful in that their horizontal spread crushes a larger substrate surface than a rotational rooting whose nuisance is punctual and deep. This crumbled surface enriched with organic matter will more easily accommodate new plant species. The bulbous geophytes of the city are mainly Oxalis pes-caprae and Arisarum vulgare. They have an important mechanical effect due to their type of roots.
Chammephytes are characterized by a root system not as deep as that of Phanerophytes but more harmful for the substrate since they are perennial. This biological type is represented only by Sedum sediforme, a taxon of the family of Crassulaceae whose recovery is very limited in the city.
4.5 Substrate
Biological colonization occurs on different supports. On limestone walls of the ramparts (Fig. 6) grow plants whose roots penetrate the joints.
Development of vegetation on limestone walls.
The base of the internal side of walls of the ramparts, covered with a coating of lime, is often covered with plants due to water retention (Fig. 7). Important vegetation that flourishes at the expense of joints, which are less resistant and relatively rich in nutrients often, invades the walkways pavement.
Proliferation of vegetation at the base of the walkways walls of the Portuguese city.
The base of the walls and the joints of the paving often have a loose appearance (Fig. 8). The substrate is then sandy to clay and has locally varying contents of granular elements sometimes rich in fragments of lime coming directly from the wall coatings.
The vegetation invades the joints of less resistance.
The structural heights of rough stone or rubble covered with plaster are also colonized in favor of high water retention.
4.6 Biodeterioration
As seen, the vascular plants are able to develop on supports a priori unfavorable to their development such as manmade structures in general and the walls of historical monuments in particular. The proliferation of these plants in this case in particular is a significant disorder factor.
Since then, several studies have focused on the identification and inventory of plants that invade historical monuments (Caneva et al., 1993; Krigas et al., 1999; Celesti Grapow et al., 2001; Caneva et al., 2002; Celesti Grapow and Blasi, 2003; Ceschin et al., 2006; Motti and Stinca, 2011), as well as on the ecological factors (humidity, temperature, …) and edaphic (nature of substrates, porosity, cracks, slopes, …) which favor their development and growth (Lisci and Pacini,1993; Duchoslav, 2002; Kumbaric et al., 2012).
If doubts related to the harmfulness of plants such as lichens, algae (Seaward et al., 1989; Danin and Caneva, 1990; Bartoli et al., 2014), or ivy (Bartoli et al., 2016) have been definitely ruled out, the adverse impact of higher plants is not disputed in the field of historical monuments (Schaffer, 1972; Caneva and Roccardi, 1989; Caneva et al., 1991; Griffin et al., 1991; Mishra et al., 1995; Krigas et al., 1999; Mydans, 2001; Tiano, 2002; Caneva et al., 2009b).
All these plants get the water and the mineral salts necessary for their growth thanks to their roots. When these elements are lacking, the roots, by hydrotropism, lengthen and gain depth to reach other reserves thus weakening the structure and risk of damaging the wall irreversibly. The growth of the roots then exerts significant pressure in the interstices between stones, up to 15 atmospheres (Mishra et al., 1995), which is a triggering factor for cracks, loosening of the blocks, and a fortiori a threat to the structural stability of the wall.
In addition to this physical aspect of biodeterioration, chemical processes are also taking place involving acidity of the root tips and the acidic and chelating abilities of the various root exudates that chemically react with substrates (Mortland et al. 1956; Carroll, 1970; Caneva and Altieri, 1988; Caneva et al., 1991).
According to Fisher (1972) and Mishra et al. (1995), the plant growth may increase the risk of fire in dry conditions and may also influence other factors of decay by causing changes in the microclimate which that may favor the growth of other forms of bio-based organisms or by harboring pests and microorganisms.
5 Conclusion
The universal heritage of the Portuguese city of Mazagan is subject to strong anthropic and natural pressures leading to its gradual deterioration. The destructive action of vegetation is the most important natural factor. The roots of plants crack the substrate and the acidic secretions dissolve it leading to it degradation and the infiltration of water.
In Mazagan, we identified 57 plant species belonging to 25 families with that of Asteraceae ranking the first place with 10 species. Almost all the encountered species are spontaneous or subspontaneous with 3 endemics: Pallenis spinosa subsp. aurea, Anacyclus coronatus and Lotus arenarius. The therophytes and the hemicryptophytes are the richest in species and cover important areas in the city compared to phanerophytes, geophytes and chamaephytes. The high dissemination of seeds of therophytes and hemicryptophytes, their high germination capacity and the low nutrient requirement of these plants compared to other biological types explain this predominance. The seeds scattered by wind, birds and animals germinate in cracks and crevices where a soil blank has already been initiated and some moisture has accumulated.
The root system of the listed species depends on the biological type. Phanerophytes are characterized by strong and high branched roots that have a remarkable effect on the degradation of the historical monument. Therophytes, hemicryptophytes and chamaephytes that have a less swiveling or fasciculated root system than phanerophytes are also detrimental to old substrates by their high ability to colonize walls. Geophytes, whose roots are perennials and in the form of rhizomes, tubers or bulbs, will exert significant pressure on the substrate leading to its destruction and favoring in particular the infiltration of water. The installed plants exploit and help to create microenvironments suitable for plant growth; if left undisturbed, a succession takes place of plants of increasing diversity and size (Motti and Stinca, 2011).
To preserve this historic monument, it is important to fight against the proliferation of plants. The environmentally friendly methods should be favored. Traditional mechanical methods such as grubbing are an effective means for plants that have a small cover and whose root system is not strong or branched as the case of therophytes, hemicryptophytes and some geophytes. To this process, which can deteriorate the substrates and lead to the loss of mortar that makes it necessary to strengthen the structures by filling the voids inside the masonry, the conservators-restorers prefer more and more cutting to the base of the roots and renew this action until the disappearance of the plants. However, this technique does not guarantee the definitive cessation of the vegetative activity.
The chemical control by using herbicides, if effective in short term, is highly harmful to the treated site. Chemical products can be toxic to living beings and, in long term, can be very dangerous for nature through their dispersal in the environment. The selective application of herbicides is an excellent solution to the aggravating problem of mechanical damages to architectural parts caused by perennial woody species. In fact, the method of delivery ensures the application of the minimum amount of herbicide necessary, while avoiding dispersion to architectural parts of the monument or the environment (Papafotiou et al., 2017).
Other ecological processes, such as the use of lime that limits the proliferation of fungi and bacteria, could contribute to the maintenance and protection of historical monuments while ensuring a limited impact on the environment.
Finally, many methods can be used to control vegetation, but often a combined program (manual and chemical methods) is required to solve the problem (Lisci and Pacini, 1993). Monitoring is the most important factor and regular inspection and timely curative measures could greatly reduce the threat posed by plants to monuments (Mishra et al., 1995).
References
- Biological colonization patterns on the ruins of Angkor temples (Cambodia) in the biodeterioration vs bioprotection debate. Int. Biodeterioration Biodegradation. 2014;96:157-165.
- [CrossRef] [Google Scholar]
- Aggressiveness of Hedera helix L. growing on monuments: evaluation in Roman archaeological sites and guidelines for a general methodological approach. Plant Biosyst.. 2016;151: 5:866-877.
- [CrossRef] [Google Scholar]
- Benharbit, M., 2017a. Le site archéologique de Chellah. Panorama des phénomènes de dégradation. Ed. Toppress, Rabat. 94p. ISBN : 978-9954-690-86-4.
- Benharbit, M., 2017b. La pierre, Vade-mecum des facteurs d’altération. Ed. Toppress, Rabat. 74p. ISBN: 978-9954-690-89-5.
- Biochemical mechanisms of stone weathering induced by plant growth. Torun: Proceedings of the VIth International Congress on Deterioration and Conservation of Stone; 1988. p. :32-44.
- Analysis of the Colosseum’s floristic changes during the last four centuries. Plant Biosyst.. 2002;136(3):291-312.
- [Google Scholar]
- The wall vegetation of the roman archaeological areas. Sci. Technol. Cultural Heritage. 1992;I:217-226.
- [Google Scholar]
- Plant communities on the walls of Venosa castle (Basilicata, Italy) as biodeteriogens and bioindicators. Paris: Int. UNESCO Congress on Conservation of stone and other materials; 1993. p. :263-270.
- Tree roots and damages in the Jewish catacombs of Villa Torlonia (Roma) J. Cultural Heritage. 2009;10:53-62.
- [Google Scholar]
- Plant biology for cultural heritage: biodeterioration and conservation. Getty Publ.. 2009;400p ISBN: 978-0-89236-939-3
- [Google Scholar]
- “Harmful flora in the conservation of roman monuments”. Luknow (India): Proc. Int. Conf. “Biodeterioration of Cultural Property”; 1989. p. :212-218. Ed. ICCROM-INTAC
- L’héritage portugais au Maroc. Mutual Heritage, Citeres: Un patrimoine d'actualité; 2012.
- Rock Weathering. New York: Springer, Monographs in Geoscience Plenum Press; 1970. p. :204. 10.1007/978-1-4684-1794-4
- I siti archeologici nella conservazione della biodiversita‘ in ambito urbano: La flora vascolare spontanea delle Terme di Caracalla a Roma. Webbia. 2003;58(1):77-102.
- [Google Scholar]
- Contributo alla conoscenza della vegetazione ruderale: delle aree archeologiche romane (Roma) Fitosociologia. 2006;43(1):1-43.
- [Google Scholar]
- Doukkala et environs. Région Doukkala-Abda, histoire et patrimoine. Basma Print; 2012. p. :188.
- Climate-Data, 2018. Climate data for cities around the world. Website: https://en.climate-data.org/; accessed May 2018.
- Deterioration of limestone walls in Jerusalem and marble monuments in Rome caused by cyanobacteria and cyanophilous lichens. Int. Biodeterior. Biodegr.. 1990;26:397-417.
- [Google Scholar]
- Derif, J., 2016. La ville portugaise de Mazagan: Patrimoine Mondial. Website: https://ame2p.wordpress.com/2016/02/27/la-forteresse-de-mazagan-1-un-apecu- historique/; accessed December 2017.
- Flora and vegetation of stone walls in East Bohemia (Czech Republic) Preslia. 2002;74:1-25.
- [Google Scholar]
- Study of the bryological flora at the archaeological site of Chellah, Morocco. Int. J. Environ. Agric. Biotechnol.. 2017;2(4):1631-1643.
- [CrossRef] [Google Scholar]
- Statistiques et commentaires sur l'inventaire actuel de la flore vasculaire du Maroc. Bulletin de l’Institut Scientifique. Rabat, section Sciences de la Vie. 2012;34(1):1-9.
- [Google Scholar]
- Propositions de zones importantes pour les plantes au Maroc (ZIP Maroc). Rabat, Morocco: Atelier National, Zones importantes de plantes au Maroc. Institut Scientifique; 2004.
- Flore pratique du Maroc: Manuel de détermination des plantes vasculaires. Rabat, Morocco: Travaux de l’Institut Scientifique, série botanique; 1999.
- Flore pratique du Maroc: Manuel de détermination des plantes vasculaires. Rabat, Morocco: Travaux de l’Institut Scientifique, série botanique; 2007.
- Flore pratique du Maroc: Manuel de détermination des plantes vasculaires. Rabat, Morocco: Travaux de l’Institut Scientifique, série botanique; 2014.
- The Biodeterioration of Stone: A Review of Deterioration Mechanisms, Conservation Case Histories, and Treatment. International Biodeterioration. England: Elsevier Science Publishers Ltd; 1991. p. :187-207.
- Main ecological parameters conditioning the colonization of higher plants in the biodeterioration of stone embankments of Lungotevere (Rome) Int. Biodeterioration Biodegradation. 2012;72:31-41.
- [Google Scholar]
- The vascular flora of the Byzantine walls of Thessaloniki (N Greece) Willdenowia. 1999;29(1–2):77-94.
- [Google Scholar]
- Plants Growing on the Walls of Italian Towns; 1. Sites and Distribution. Phyton (Horn, Austria). 1993;33(1):15-26.
- [Google Scholar]
- Role of higher plants in the deterioration of historic buildings. Sci. Total Environ.. 1995;167:375-392.
- [Google Scholar]
- Alteration of biotite to vermiculite by plant growth. J. Soil Sci.. 1956;82:477-481.
- [Google Scholar]
- Analysis of the biodeteriogenic vascular flora at the Royal Palace of Portici in southern Italy. Int. Biodeterioration Biodegradation. 2011;65(8):1256-1265.
- [CrossRef] [Google Scholar]
- Vascular plant colonisation of four castles in southern Italy: effects of substrate bioreceptivity, local environment factors and current management. Int. Biodeterioration Biodegradation. 2018;133(2018):26-33.
- [CrossRef] [Google Scholar]
- In the jungle, saving temples from doom. Newspaper article in the International Herald Tribune; 2001. August 21
- Ozenda, P., 1964. Biogéographie végétale. Editions Doin. 374p.
- Integrated design and management of vegetation at archaeological sites to protect monuments and enhance the historical landscape. Acta Horticulturae. 2017;1189:1-10.
- [CrossRef] [Google Scholar]
- Quezel P., Santa S., Nouvelle flore d'Algérie et des régions désertiques méridionales. 2 tomes Editions CNRS 1962–1963. Paris, France 1170 p.
- The Weathering of Natural Building Stones. In: Building Research Special Report No. 18. UK: Building Research Station; 1972.
- [Google Scholar]
- The role of lichens in the biodeterioration of ancient monuments with particular reference to Central Italy. Int. Biodeterior. Biodegrad.. 1989;25:49-55.
- [Google Scholar]
- Ecological notes on wall vegetation. Springer; 1969. p. :352. 10.1007/978-94-017-6232-8
- Biodegradation of Cultural Heritage: Decay Mechanisms and Control Methods. Proceedings ARIADNE Workshop 9 – historic materials and their diagnostic; 2002.
- La liste du patrimoine mondial : Ville portugaise de Mazagan (EL Jadida) 2004. accessed on September 2017
- [Google Scholar]







