Translate this page into:
Vannilic acid ameliorates hyperglycemia-induced oxidative stress and inflammation in streptozotocin-induced diabetic rats
⁎Corresponding author. chixiaoyan2018@sina.com (Xiaoyan Chi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Diabetes mellitus (DM) is a metabolic illness and it is a result of hyperglycemia ensuing since a deficiency in insulin production, insulin function. Based on WHO statistics, more than 347 million peoples are suffered from the DM globally and it may increase to 694 million in 2030. Vanillic acid (VA) is a phenolic derived compound from dietary vegetations and fruits with the many biological acitivities. This current research was planned to investigate the antidiabetic and anti-inflammatory potential of VA on streptozotocin-challenged DM in rats.
Methods
Later than an overnight fast, the rats were induced diabetes via one intraperitoneal treatment of STZ (40 mg/kg b.wt) in a newly organized 0.1 M citrate buffer. We analyzed body weight, plasma glucose, liver marker enzymes, insulin, carbohydrate metabolic enzymes, glycosylated hemoglobin, protein profiles, oxidant/antioxidant levels, inflammatory mediators and also evaluate histopathology of pancreatic tissues in normal control and investigational animals.
Results
Diabetic animals were revealed an increased in HbA1c, blood glucose, food and fluid intake and relative weight of kidney and liver, whereas the reduced level of insulin and body weight. Furthermore, augmented levels of ALT, glucose-6-phosphatase, AST, TBARS, TNF-α, creatinine, LOOH, fructose 1,6-bisphosphatase, ALP, IL-6 and IL-1β whereas, reduced status of antioxidant enzymes were observed in diabetic rats. Histopathological analysis of pancreatic tissues was in support of the biochemical parameters.
Conclusions
Diabetic rats treated by VA significantly alter all these parameters toward normal levels. These results recommended that the VA is a potential antidiabetic and anti-inflammatory effect for the cure of DM.
Keywords
Vanillic acid
Inflammation
Diabetes mellitus
Hyperglycemia
Antioxidants
1 Introduction
As highly developed medical technology and better living standards broaden the expectation of human beings, chronic illness has become the most important risk to the healthiness of people (Megari, 2013). Diabetes mellitus (DM) is a cluster of metabolic illness, which described as a result of hyperglycemia ensuing from a deficiency in the production and function of insulin. The continual hyperglycemia of DM is related to the extensive time of injury, failure, and dysregulation of numerous organs, principally the nerves, kidneys, blood vessels, heart, and eyes. The symptoms of noticeable hyperglycemia contains weight loss, polydipsia, polyuria, occasionally with blurred vision and polyphagia (American Diabetes Association, 2002). The WHO has been informed that more than 347 million populace suffered due to the DM globally and expected this number would twofold (694 million) by 2030 (Cho et al., 2018).
Oxidative stress forms a metabolic system of damage in various kinds of vascular illnesses. The hyperglycemia enhances oxidative stress during the greatest formation of ROS has been informed (Hostalek, 2019). Free radicals are extremely reactive materials, which may show the way to oxidative damage to the living organism via damaging the macromolecules for example carbohydrates, lipids, nucleic acids, and proteins. There is a lot of support relating to the involvement of ROS to organ damage in the systems, for example, the central nervous system, liver, and heart, as well as that oxidative injury, is augmented in DM (Oguntibeju, 2019a,b). Along with the cellular damage of oxidative stress is dysregulation of NO synthesis, DNA oxidation, peroxidation of lipid and oxidation of protein (Samie et al., 2018). It has extended been respected that chronic stimulation of the immune system is related to DM. Increasing data support the connection of inflammatory processes with an irregular secretion of cytokines and stimulation of inflammatory signaling pathways in the growth of this metabolic disease (Khondkaryan et al., 2018).
Many antidiabetic agents are available, but all of them have several unwanted side effects like lactic acidosis, hyperglycemia, diarrhea or flatulence that result in augmented economic burden (Oguntibeju, 2019a,b). Thus, scientists around the global nations are executing a widespread researches to uncover the optional approaches for the treatment of DM with a minimal side effects and least cost. Remedial vegetation has been thought an admirable resource for substitute medicines to treat the DM by the benefit of their active phytochemicals. The beneficial outcomes of a natural medical product may because of their any single phyto-compound or more preferably, a synergistic effect of various phytochemicals. Up to now, more than 800 medicinal plants have been informed to display an antihyperglycemic activity (Rajagopal and Sasikala, 2008). The main frequent applications of medicinal plants are good effectiveness, enhanced safety levels, acceptability, wider accessibility and affordability (Sekhon-Loodu and Rupasinghe, 2019).
Vanillic acid (VA) is a phenolic compound derived from dietary vegetations and fruits well-known to have numerous pharmacological activities like antioxidant and antimicrobial effects to yeasts, molds and bacteria (Rupasinghe et al., 2006). VA is an intermediary source in the formation of vanillin as offerulic acid. Vanillin has also been informed to have antimutagenic, antitumor and anticlastogenic activities and thus it can be regarded as an utraceutical agent (Shyamala et al., 2007). Several researches have reported previously, the function of VA in different in vitro and several in vivo works. The in vivo methods implicated in a antidiabetic and anti-inflammatory properties of VA are moderately understand. The current study is evaluating the antidiabetic and anti-inflammatory potentials of VA in rat model.
2 Materials and methods
2.1 Chemicals
Vanillic acid (VA) and streptozotocin (STZ) were obtained from Sigma Chemical Co., Missouri, USA. The ELISA kits for IL-1β, IL-6, and TNF-α were acquired from R&D Systems Inc, Minneapolis, USA. The protein assay kit was procured form Randox, Crumblin, UK. All the additional chemicals were utilized by the analytical standard.
2.2 Animals
Male albino Wistar animals were procured and employed in this present work at the age of eight weeks (180–210 g). The rats were sustained in a clean confines, temperature 24 ± 2 °C under regular environmental setting on a 12 h light/12 h dark sequence. All rats were supplemented with an usual pellet diet and water ad libitum.
2.3 Induction of diabetes
After 12-h fasting, diabetes was stimulated to an animals by using STZ. The rats have received one intraperitoneal insertion of STZ (40 mg/kg). The STZ was newly organized in 0.1 M citrate buffer at the range of pH 4.5. For the i.p. insertion of STZ, the animals were detained in one hand in dorsal location, the insertion location was cleaned using povidone-iodine solution and the selected quantity of STZ was inserted in the caudal abdominal cavity with sterile 25 g needle.
2.4 Experimental design
In this research, totally 30 animals was separated into five groups of 6 each. VA and glibenclamide were mixed in DMSO (0.5%) and supplemented orally one time in a day for 45 days. The group-I rats were served as normal rats (0.5% of DMSO). The group II-V rats were induced diabetes above mentioned. Group II animals left with no additional treatments. The group III and IV diabetic rats received VA at different dosages (25 and 50 mg/kg b.wt) in 1 ml of saline. Group V animals received glibenclamide (0.1 mg/kg b.wt) in 1 ml of saline.
After the 6th week, plasma was used for the assessment of glucose. The food intake, b.wt and fluid intake of the entire group of animals were documented. After dissection of the kidney and liver, relative organ weight was calculated. Liver and pancreatic tissues were removed, cleaned with chilled buffer and stored at −20 °C until employed. The 10% of tissue homogenate was organized with Tris-HCl buffer (0.025 M), pH 7.5. After the 5 min centrifugation (1500g), the supernatant was utilized for different types of biochemical analyses.
2.5 Biochemical estimations
2.5.1 Determination of glucose, insulin, and HbA1c
The glucose level was determined via the manner of Trinder (1969) with employed a reagent kit. HbA1c was analysed through the way of Nayak and Pattabiraman (1981). The insulin was determined through the process of Bürgi et al. (1988).
2.5.2 Estimation of oxidant/antioxidant activities
The unstable hydroperoxides accreted due to the lipid peroxidation was examined by the method of Jiang et al. (1992). The LOOH present in the sample was react to the ferrous ions to generate the ferric ion and then it was combined with the xylenol to develop the chromogen. The intensity of developed colour was analysed at 560 nm using microtitre plate reader.
The status of lipid peroxidation was investigated via estimating the TBARS by the method of Ohkawa et al. (1979). The oxidative stress status in both control and investigational rats were investigated through estimating the malondialdehyde level in the serum. The malondialdehyde is a secondary product of lipid peroxidation and it was react to the thiobarbituric acid reactive substance (TBARS) to generate the pink chromogen that was determined at the 532 nm by using the microplate reader.
The catalase enzyme activity was determined via the technique of Sinha (1972) and the values were depicted as µmole of hydrogen peroxide decomposed/min. The status of superoxide dismutase activity was determined by using the procedure Kakkar et al. (1984) and the values were depicted as the enzyme level needed to inhibit the chromogen produced by 50% in one min under the standard condition. The gluthione peroxidase enzyme activity was determined via the procedure of Rotruck et al. (1973) and the values are illustrated as the µmole of GSH utilized/min.
2.5.3 Analysis of liver marker enzymes
The serum was gathered using centrifugation at 1500g for 5 min. Serum was next positioned in marked plastic tubes and it stores up at −20 °C until using. The levels of serum ALT and AST were measured via using the way of Reitman and Frankel (1957). The ALP was analyzed by using the way of Kind and King (1954).
2.5.4 Estimation of carbohydrate metabolic enzymes
Hexokinase was estimated through the process of Brandstrup et al. (1957). Fructose 1,6-bisphosphatase was estimated according to the way of Gancedo and Gancedo (1971). Glucose-6-phosphatase was examined by the way of Koide and Oda (1959).
2.5.5 Estimation of protein profile
The blood was gathered and placed into anticoagulant free test tubes, continued in ice for 30 min and after that centrifuged at 1500g for 5 min at 4 °C. Then supernatant was eliminated as serum and store up at −20 °C for further analysis of biochemical parameters. Serum total protein and creatinine levels were analyzed by through the Randox protein kit (Crumlin, UK) through an automated Randox Daytona analyzer following protocols from the manufacturer.
2.5.6 Determination of inflammatory mediators
The ELISA was employed to quantify the statuses of IL-1β, IL-6, and TNF-α in pancreatic homogenate. A glass homogenizer was employed to made pancreatic homogenate in saline with an ice bath. After centrifugation, the supernatants were removed and instantly stock up at −80 °C. The quantities of IL-6 (SM6000B), IL-1β (SMLB00C), and TNF-α (SMTA00B) were investigated with commercial ELISA kits, employing the manufacturer’s information (R&D Systems Inc, Minneapolis, USA.).
2.6 Histopathological analysis
The pancreatic tissues were excised, preset in 10% formalin, dehydrated in a series of alcohol (80–100%) and fixed in paraffin. The tissue blocks were cut into 3–5 μm segments then placed on slides and stained with hematoxylin and eosin (H&E) and then slides were viewed by the Olympus BX40.
2.7 Statistical analysis
The statistical investigation was evaluated with SPSS 17 (SPSS, Inc., Chicago) statistical tool. Data are represented as mean ± SD. The one way ANOVA go after by DMRT quantity was employed to evaluate the separation among the variable groups. Data is calculated as significant if the p values are less than 0.05.
3 Results
3.1 Effect of VA on food and fluid intake, bodyweight and relative organ weight of liver and kidney
Bodyweight, food intake, organ weight of kidney and liver and fluid intake in control and STZ-induced diabetic animals were revealed in Fig. 1 (A&B). The diabetic animals demonstrated significantly (p < 0.05) lowered bodyweight and augmented fluid and food intake and also augmented the level of relative kidney and liver weight when evaluated to control animals. Whereas, oral supplementation of VA (25 and 50 mg/kg b.wt) changed these parameters near to the standard drug-treatedanimals.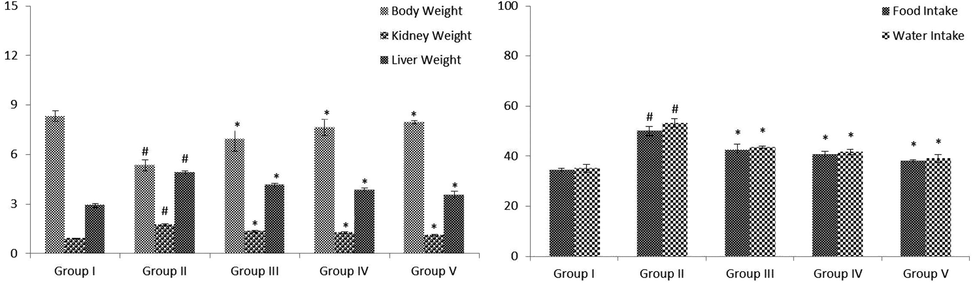
(A&B): Effect of VA on levels of body weight, fluid intake, food intake and relative weight of liver and kidney in control and experimental rats. Fig. 1 A were shown the body weight and relative weight of kidney and liver. Fig. 1 B was shown in the fluid and food intake. Values are given as mean ± SD from 6 rats. Values not sharing a common superscript letter (# - *) differ significantly at p < 0.05 (DMRT). # compare to the Group I & * compare to the group II.
3.2 Effect of VA on hepatic marker enzymes
The quantities of ALP, AST, and ALT in the serum of normal and STZ-challenged diabetic animals were revealed in Fig. 2. The diabetic animals demonstrated notably (p < 0.05) enhanced ALT, AST, and ALP when evaluated with control animals. VA (25 and 50 mg/kg b.wt) supplemented rats illustrated markedly (p < 0.05) lowered these hepatic marker enzyme levels near to the standard drug-treated group when evaluated to STZ-treated animals.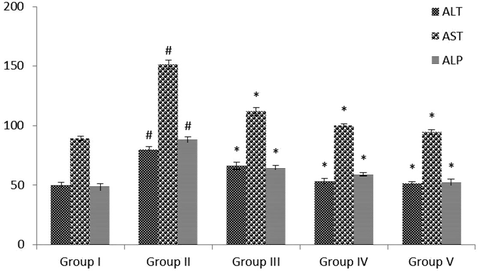
Effect of VA on the levels of hepatic marker enzymes in serum of control and experimental rats. Values are given as mean ± SD from 6 rats. Values not sharing a common superscript letter (# - *) differ significantly at p < 0.05 (DMRT). # compare to the Group I & * compare to the group II.
3.3 Effect of VA on protein profiles
The quantities of TP and creatinine in the serum of STZ-treated diabetic and control rats were revealed in Fig. 3. The level of creatinine was noticeably (p < 0.05) augmented, whereas the status of total protein were noticeably (p < 0.05) reduced in diabetic rats when evaluated with normal animals. These levels changed towards normal by administration with the VA (25 and 50 mg/kg bwt) to the glibenclamide administered rats when evaluated with diabetic animals.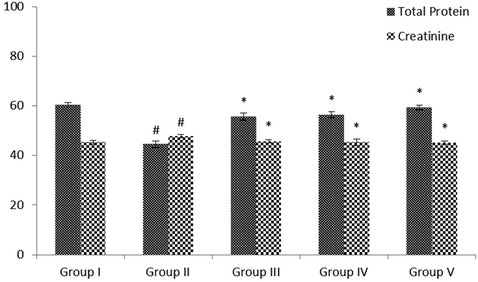
Effect of VA on the levels of serum total protein and creatinine activity in control and experimental rats. Values are given as mean ± SD from 6 rats. Values not sharing a common superscript letter (# - *) differ significantly at p < 0.05 (DMRT). # compare to the Group I & * compare to the group II.
3.4 Effect of VA on blood glucose, HbA1c and insulin
The effect of VA on HbA1c, insulin and blood glucose in control and STZ-treated animals were illustrated in Fig. 4 (A&B). The diabetic animals illustrated considerably (p < 0.05) augmented glucose and HbA1c and low level of insulin when evaluated with control animals. Oral supplementation of VA (25 and 50 mg/kg b.wt) was considerable (p < 0.05) enhanced the level of insulin and decreased glucose and HbA1c near to glibenclamide treated rats when evaluated with STZ-induced animals.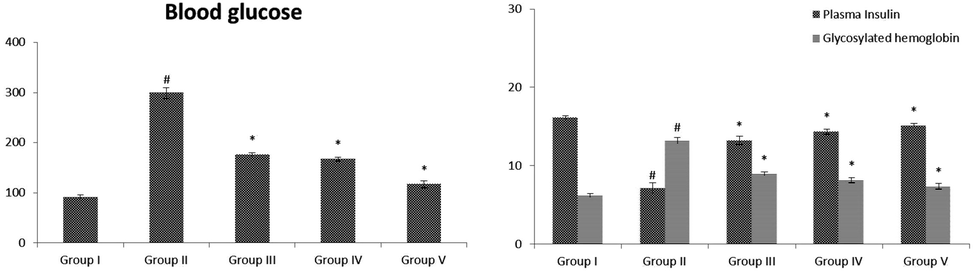
(A&B): Effect of VA on levels of blood glucose, insulin and glycosylated hemoglobin in control and experimental rats. Fig. 4 A were shown the level of glucose. Fig. 4 B was shown in the levels of insulin and glycosylated hemoglobin. Values are given as mean ± SD from 6 rats. Values not sharing a common superscript letter (# - *) differ significantly at p < 0.05 (DMRT). # compare to the Group I & * compare to the group II.
3.5 Effect of VA on carbohydrate metabolic enzymes
The status of carbohydrate metabolic enzyme activities in the liver of normal and STZ-challenged animals demonstrated in Fig. 5 (A&B). The amount of hexokinase was considerably (p < 0.05) lowered, while the levels of carbohydrate metabolic enzyme were remarkable (p < 0.05) improved in STZ administrated animals when evaluated with normal animals. The values altered toward normal on administration with the VA (25 and 50 mg/kg b.wt) to the standard drug supplemented groups when evaluated with diabetic animals.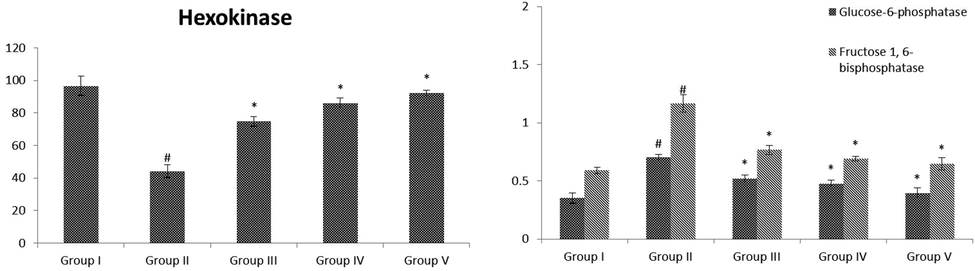
(A&B): Effect of VA on activities of hepatic carbohydrate metabolic enzymes in control and experimental rats. Fig. 5 A were shown the activity of hexokinase. Fig. 5 B was shown in the activities of glucose-6-phosphatase and fructose 1,6-bisphosphatase. Values are given as mean ± SD from 6 rats. Values not sharing a common superscript letter (# - *) differ significantly at p < 0.05 (DMRT). # compare to the Group I & * compare to the group II.
3.6 Effect of VA on lipid peroxidative markers
The quantities of lipid peroxidative markers in the pancreas of normal and STZ stimulated diabetic animals were demonstrated in Fig. 6. The diabetic animals exhibited notably (p < 0.05) enhanced TBARS and LOOH when evaluated with control rats. VA (25 and 50 mg/kg b.wt) administrated rats showed markedly (p < 0.05) lowered these lipid peroxidation levels near to the standard drug-treated groups when compared to STZ-induced animals.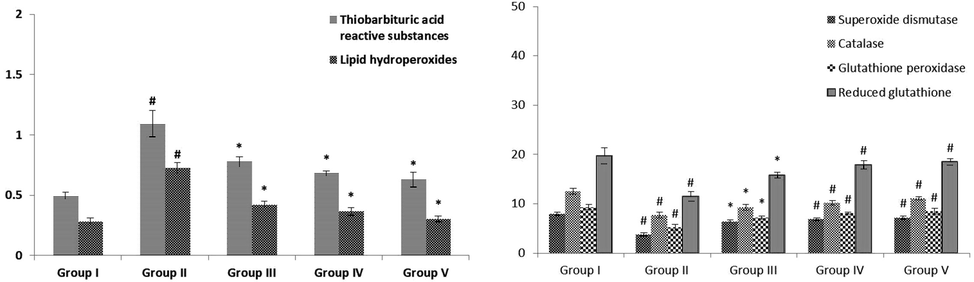
Effect of VA on levels of pancreatic lipid peroxidative markers, enzymatic and non-enzymatic antioxidants in control and experimental rats. Values are given as mean ± SD from 6 rats. Values not sharing a common superscript letter (# - *) differ significantly at p < 0.05 (DMRT). # compare to the Group I & * compare to the group II.
3.7 Effect of VA on non-enzymatic and enzymatic antioxidant
The levels of antioxidants like GPx, SOD, GSH, and CAT in the pancreas of control and STZ-induced diabetic animals were demonstrated in Fig. 6. The diabetic animals revealed remarkably (p < 0.05) reduced antioxidant levels when evaluated with control animals. VA (25 and 50 mg/kg b.wt) supplemented rats illustrated notably (p < 0.05) enhanced these antioxidant activities near to the glibenclamide treated groups when evaluated to STZ-induced animals.
3.8 Effect of VA on inflammatory markers
The quantities of inflammatory mediators in the pancreas of normal and STZ stimulated diabetic animals were revealed in Fig. 7 (A-C). The status of inflammatory markers were considerable (p < 0.05) enhanced in diabetic animals when evaluated with control. These quantities were reverted back normally treated by VA (25 and 50 mg/kg b.wt) to the standard drug administrated rats.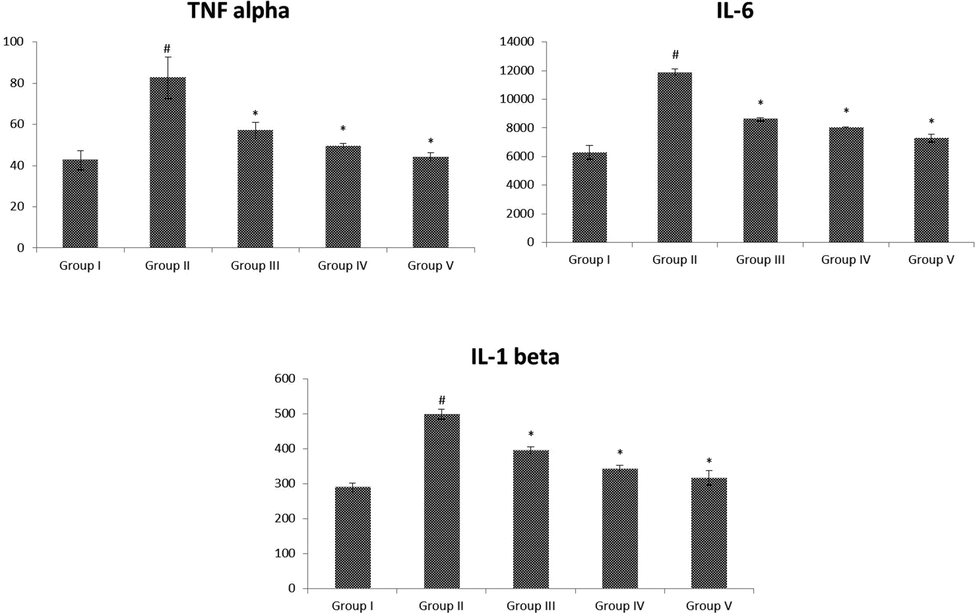
(A-C): Effect of VA on the levels of pancreatic inflammatory markers in control and experimental rats. Fig. 8 A-C were shown in the inflammatory markers of TNF-α, IL-6 and IL-1β, respectively. Values are given as mean ± SD from 6 rats. Values not sharing a common superscript letter (# - *) differ significantly at p < 0.05 (DMRT). # compare to the Group I & * compare to the group II.
3.9 Histopathology of the pancreatic tissues
The effect of VA on the histological alterations of the pancreas in control and STZ-stimulated diabetic animals were illustrated in Fig. 8 (A-E). The histological alterations of diabetic groups II (B) revealed the selective demolition of β-cells of islets of Langerhans. Conversely, group III (C) and group IV (D) VA (25 and 50 mg/kg b.wt) supplemented animals illustrated normal islet cells of Langerhans and also standard drug treatment group V (E) rats showed normal islet cells evaluated with the normal control group I (A).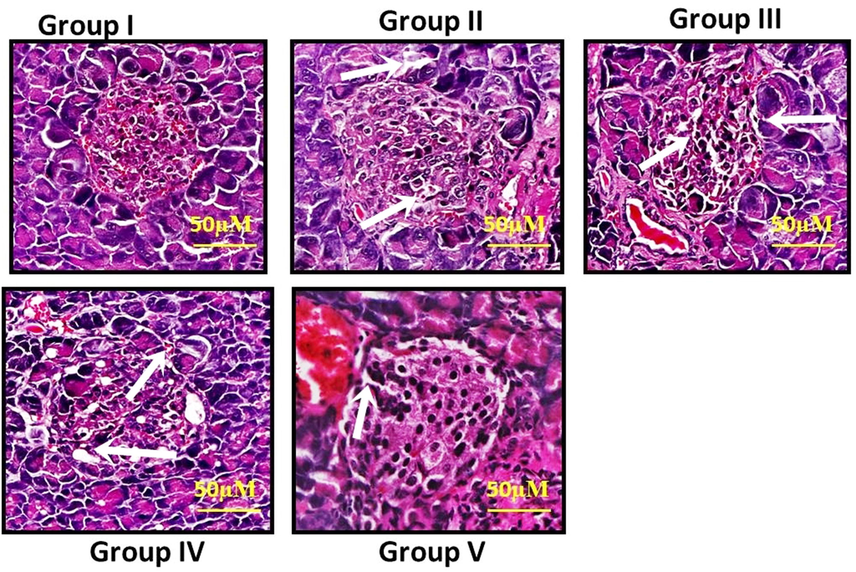
(A-E): Effect of VA on histological changes of pancreatic tissues in control and experimental rats. The histological changes of diabetic groups II showed selective destruction of β-cells of islets of Langerhans. Group III and group IV rats treated with VA (25 and 50 mg/kg bwt) showed normal islet cells of Langerhans and also standard drug treatment group V rats showed normal islet cells when compared with control group I.
4 Discussion
The DM has frequently simply regarded as diabetes, which is a condition of disordered metabolism with unusually elevated blood glucose levels leads to hyperglycemia. DM is a complex of various groups of metabolic circumstances described by augmented levels of blood glucose due to low down in insulin action and/or insulin secretion. Insulin is the most important hormone that controls the intake of glucose from the blood into the majority of cells, contain skeletal muscle and adipocytes (Seko et al., 2017). Babukumar et al. (2017) reported that lowered body weight, insulin, and augmented glucose and glycosylated hemoglobin in diabetic animals. Histopathological results revealed that diabetes results the selective demolition of β-cells of islets of Langerhans consequential in a noticeable diminish in the status of insulin. Conversely, oral supplementation of VA of diabetic rats had a significantly improved the secretion of insulin level and normal islets of β-cells of langerhans. Oxidative stress plays a vital function in chronic problems of diabetes and is suggested to be related toan improved TBARS. Antioxidants are the primary protection mechanism that controls the toxicity related to free radicals. The statuses of this protection system are changed in diabetes. As a result, the unsuccessful scavenging of ROS acting a critical role in analyzing the level of tissue damage (Ramachandran et al., 2004). Previous studies informed that the VA is decreased lipid peroxidation and enhanced antioxidants are agreeing with the present investigations [29] due to the free radical scavenging action. Previous data reported that VA inverted the SOD and CAT level in palmitic acid-treated oxidative stress in HUVE cells (Ma et al., 2019).
The liver plays a main function in the control of carbohydrate metabolism, as it uses glucose as energy; it has the potential to accumulates glucose as glycogen and also secrete glucose from noncarbohydrate sources. This type of role creates the liver more susceptible to diseases in subjects having a metabolic disorder, especially for the DM (Marchesini et al., 2001). This present study demonstrates that the diabetic rats, hexokinase levels were remarkably reduced, whereas the augmented status of glucose-6-phosphatase and fructose-1,6-bisphosphatase. VA treatment brings these enzyme statuses to the near-normal level in the present research. The function of the liver was the maintenance of standard glucose levels during the postprandial period, fasting and its function in the pathogenesis of DM has involved greater concern. Certainly, hepatic dysregulation follow-on from insulin resistance syndrome can show the way to the growth of the DM (Hohmann et al., 2013). Liver marker enzymes containing ALP, AST, and ALT are commonly utilized to evaluate liver injury (Calixto-Campos et al., 2015). Previous data reported that the treatment with the VA does not provoke liver injury (Sujithra et al., 2019). Although the positive control of STZ induced diabetic animals demonstrated a considerable augment of these enzymes. Conversely, VA administration is a considerable reduction in these liver marker enzyme levels in the present investigations supported on the above-mentioned findings. Diabetic rats showed an improved creatinine and decreased total protein leads to disturbed liver functions due to the deep loss of serum protein levels, which are investigated in diabetes, whereas treatment with the VA reverted to all these protein profiles.
Inflammation was documented as a symptom of oxidative stress that produces cytokines, like interleukins and adhesion molecules, which are stimulated by oxidative stress. The inflammatory reactions are related to pro-inflammatory markers, which are formed by the stimulation of transcription mediators containing NF-κB. The NFκB plays a vital responsibility in the appearance of several genes implicated in inflammatory and immune processes (Ahn et al., 2014). Certainly, VA decreased the stimulation of NF-κB and formation of IL-1β, TNF-α, and IL-6 treated by lipopolysaccharide in vitro (Kim et al., 2011). Karatas et al. (2019) demonstrated that VA derivatives possess promising anti-inflammatory activity on ligature-stimulated periodontal disease in animals. Previous data highlighted that the anti-inflammatory values of VA on the lysolecithin mediated neurodegenerative disease in the mouse model (Siddiqui et al., 2019). Bai et al. (2019) demonstrated that VA attenuated the manifestation of ovalbumin-induced asthma during the inhibition of inflammatory cytokines in rats. In this present study also reported that VA inhibited the overproduction of these inflammatory cytokines in STZ challenged diabetic animals due to the anti-inflammatory responses.
5 Conclusion
VA is an evidently occurring phenolic acid broadly presented in different plant foods. The current study has demonstrated that the protective properties of VA through decreasing hepatic marker enzymes, reducing lipid peroxidation, altering carbohydrate metabolic enzymes, enhancing antioxidant levels, altering protein profiles, alleviating inflammation and also altering histopathological changes in STZ challenged diabetic animals. Our findings supported the therapeutic value of VA and can be used in clinical medication or as a nutritional supplement for inhibiting the development of DM.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The association between liver enzymes and risk of type 2 diabetes: the Namwon study. Diabetol. Metab. Syndr.. 2014;6:14.
- [Google Scholar]
- American Diabetes Association: clinical practice recommendations, 2002. Diabetes. Care. 25, 1–147.
- Geraniol, a natural monoterpene, ameliorates hyperglycemia by attenuating the key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Pharm. Biol.. 2017;55(1):1442-1449.
- [Google Scholar]
- Vanillic acid mitigates the ovalbumin (OVA)-induced asthma in rat model through prevention of airway inflammation. Biosci. Biotechnol. Biochem.. 2019;83(3):531-537.
- [Google Scholar]
- The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J. Gerontol.. 1957;12:166-171.
- [Google Scholar]
- One-step sandwich enzyme immunoassay for insulin using monoclonal antibodies. Clin. Biochem.. 1988;21:311-314.
- [Google Scholar]
- Jr. Vanillic acid inhibits inflammatory pain by inhibiting neutrophil recruitment, oxidative stress, cytokine production, and NFκB activation in mice. J. Nat. Prod.. 2015;78:1799-1808.
- [Google Scholar]
- IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes. Res. Clin. Pract.. 2018;138:271-281.
- [Google Scholar]
- Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch Mikrobiol.. 1971;76:132-138.
- [Google Scholar]
- Hohmann, M.S., Cardoso, R.D., Pinho-Ribeiro, F.A., Crespigio, J., Cunha, T.M., Alves-Filho, J.C., Da Silva, R.V., Pinge-Filho, P., Ferreira, S.H., Cunha, F.Q., Casagrande, R., 2013. 5-lipoxygenase deficiency reduces acetaminophen-induced hepatotoxicity and lethality. Biomed. Res. Int.
- Global epidemiology of prediabetes-present and future perspectives. Clin. Diabetes. Endocrinol.. 2019;5:5.
- [Google Scholar]
- Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem.. 1992;202:384-389.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian. J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- The effect of vanillic acid on ligature-induced periodontal disease in Wistar rats. Arch. Oral. Biol.. 2019;103:1-7.
- [Google Scholar]
- Impaired Inflammatory Response to LPS in type 2 diabetes mellitus. Int. J. Inflam.. 2018;2018:2157434.
- [Google Scholar]
- Vanillic acid inhibits inflammatory mediators by suppressing NF-κB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immuno. Pharmacol. Immuno. toxicol.. 2011;33:525-532.
- [Google Scholar]
- Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J. Clin. Pathol.. 1954;7:322-326.
- [Google Scholar]
- Pathological occurrence of glucose-6-phosphatase in serum in liver diseases. Clin. Chim. Acta. 1959;4:554-561.
- [Google Scholar]
- Vanillic acid alleviates palmitic acid-induced oxidative stress in human umbilical vein endothelial cells via Adenosine Monophosphate-Activated Protein Kinase signaling pathway. J. Food. Biochem.. 2019;43(7):12893.
- [Google Scholar]
- Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850.
- [Google Scholar]
- A new colorimetric method for the estimation of glycosylated hemoglobin. Clin. Chim. Acta. 1981;109:267-274.
- [Google Scholar]
- Hypoglycaemic and anti-diabetic activity of selected African medicinal plants. Int. J. Physiol. Pathophysiol. Pharmacol.. 2019;11(6):224-237.
- [Google Scholar]
- Type 2 diabetes mellitus, oxidative strees and inflammation: examining the links. Int. J. Physiol. Pathophysiol. Pharmacol.. 2019;11(3):45-63.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Antihyperglycaemic and antihyperlipidaemic effects of Nymphaeastellata in alloxan-induced diabetic rats. Singapore. Med. J.. 2008;49:137.
- [Google Scholar]
- Effect of macrocyclic binuclear oxovanadium complex on tissue defense system in streptozotocin-induced diabetic rats. Clinic.achimicaacta.. 2004;345:141-150.
- [Google Scholar]
- A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol.. 1957;28:56-63.
- [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588-590.
- [Google Scholar]
- Vanillin inhibits pathogenic and spoilage microorganisms in vitro and aerobic microbial growth in fresh-cut apples. Food. Res. Int.. 2006;39:575-580.
- [Google Scholar]
- Hesperetin, a citrus flavonoid, attenuates testicular damage in diabetic rats via inhibition of oxidative stress, inflammation, and apoptosis. Life. Sci.. 2018;210:132-139.
- [Google Scholar]
- Evaluation of antioxidant, antidiabetic and antiobesity potential of selected traditional medicinal plants. Front. Nutr.. 2019;6:53.
- [Google Scholar]
- Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol. Res.. 2017;47:1072-1078.
- [Google Scholar]
- Studies on the antioxidant activities of natural vanilla extract and its constituent compounds through in vitro models. J. Agric. Food. Chem.. 2007;55:7738-7743.
- [Google Scholar]
- Gallic and vanillic acid suppress inflammation and promote myelination in an in vitro mouse model of neurodegeneration. MolBiol Rep.. 2019;46(1):997-1011.
- [Google Scholar]
- Allyl methyl sulfide, a garlic active component mitigates hyperglycemia by restoration of circulatory antioxidant status and attenuating glycoprotein components in streptozotocin-induced experimental rats. Toxicol. Mech. Methods. 2019;29(3):165-176.
- [Google Scholar]
- Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J. Clin. Pathol.. 1969;22:158-161.
- [Google Scholar]







