Translate this page into:
Valorization of the Salvia officinalis L. of the Morocco bioactive extracts: Phytochemistry, antioxidant activity and corrosion inhibition
⁎Corresponding author: Tel.: +212624855571. khiya.zakaria@gmail.com (Zakaria Khiya)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present study aims to determine the chemical composition of Salvia officinalis L., collected in Khenifra region (Morocco), have a phytochemical screening and to evaluate its antioxidant activity, as well as its corrosion inhibiting powers using two methods: the potentiodynamic polarization and impedance spectroscopy (EIS) measurements.
The phytochemical screening helped us to highlight the presence of polyphenols, catechics and Gallic tannins, flavonoids, saponins and Terpenoids. It showed that the methanolic extract was very rich in total phenols (1.044 ± 0.004 mg GAE/g of extract) and flavonoids (0.037 ± 0.003 mg EQ/g of extract). Leaves of Salvia officinalis L. were steam distilled using a Clevenger apparatus. The yield of essential oil was 4.13 ± 0.01%, and its analysis by GC–MS has identified 105 components with the dominance of Trans-Thujone (17.74%), 1,8-cineol (12.63%), Camphor (12.24%), Caryophyllene (9.87%), α-pinene (7.82%), Dehydra-Aromadendrane (7.29%), and Guaiol (7.03%). The results of antioxidant activity allowed the determination of IC50 (IC50 = 309.42 mg/ml) for the oil. The latter has shown an inhibition efficiency of 83.06% and 70.58% for the both methods respectively at a concentration of 4 g/l, the methanolic extract which has shown an inhibition efficiency of 91.62% and 49.70% respectively at the same concentration.
Keywords
Salvia officinalis L.
HE
Polyphenols
GC–MS
Antioxidant activity
Iron corrosion inhibition
1 Introduction
Aromatic and medicinal plants have a considerable content of bioactive compounds with specific biochemical or organoleptic properties, allowing their use as bio-pesticide, or in pharmaceutical, cosmetic and food industries. Currently, the valuation of by-products of lignocellulosic materials is mainly studied in the field of green chemistry. The derivatives of chemical compounds of plants and micro-organisms that provide good protection for crops from weeds, pests and diseases (bio-pesticides active substances) have been used to formulate pesticides (Villaverde et al., 2016). In fact, in recent years, the vegetable biomass wastes have attracted great interest as a source of high-added-value compounds as a response of the depletion of fossil fuels, within the biomass refinery concept (Gullón et al., 2017). After the phenolic compound extraction, the residual bark of A. mollissima may also be used in the composting to produce organic fertilizer, or as biomass fuel (Foelkel, 2008).
Despite of all the progress made in synthetic drug research, plants and their products are still considered to be the major sources of medicaments and have an extensive use in the pharma-industry (Harvey, 2008; Meena et al., 2009). Mostly lots of modern medicines are derived from plants and their products are obtained by applying modern to traditional (Sucher and Carles, 2008).
Over the last decades, the various extracts and essential oils of plants have been of great interest in traditional medicine as they have been the sources of natural products. They have been preselected for their potential uses as alternative remedies for the treatment of many infectious diseases and the preservation of foods from the toxic effects of oxidants. Particularly, the oils and extracts of plants have formed the basis of many applications, including raw and processed food preservation, pharmaceuticals, alternative medicine and natural therapies (Lis-Balchin and Deans, 1997).
Among 1000 species of the genus Salvia (Lamiaceae), very well known since ancient times for their healing properties, Salvia officinalis L., is one of the first species traditionally used. It is a shrub with great commercial importance. Due to its flavouring and seasonings properties, this plant was widely used in the preparation of many foods. In medicine, it is popular in Asia and Latin America, and has been used for the treatment of different types of disorders, including seizure, ulcers, gout, rheumatism, inflammation, dizziness, tremors, paralysis, the diarrhea, and hyperglycemia (Garcia et al., 2016). S. officinalis L has been used in the European traditional medicine to treat mild dyspepsia (such as heartburn and bloating), excessive sweating, cognitive disorders related to aging, and inflammations in the throat and the skin (Adams et al., 2007). She is considered a stimulant for anaemic people, also for people stressed and depressed, and recommended for students during exam times. In addition, it is applied as a gargle against inflammation of the mouth, abscesses, and for cleaning and the healing of wounds. The commercial importance of the Salvia officinalis L. plant is due to its richness of phenolic and volatile compounds (such as essential oils). It occupies an important place in cosmetic and food industries because of its biological properties. Furthermore, the polyphenols and essential oils of Salvia officinalis L. also has biological properties such as antibacterial (Stagos et al., 2012), antioxidant et antitumor (Garcia et al., 2016; Kontogianni et al., 2013) antinociceptive and anti-inflammatory activities (Rodrigues et al., 2012), as much as cytotoxic and cytogenetic effects (Al-Barazanjy et al., 2013). The investigation of the polyphenolic profile of Salvia officinalis L. leaf extract and its related antioxidant and antimicrobial activities opens new possibilities of using this, already well-known, medicinal plant in the pharmaceutical and food industry as a source of natural food preservatives (Generalić et al., 2012). Due to the production of various secondary metabolites, many species of Salvia have different biological activities, such as antioxidant, anti-proliferation (Alimpi et al., 2017; Alimpić et al., 2015), anti-neurodegenerative (Orhana et al., 2012), anti-inflammatory, immunomodifiyng, cardioprotector (Kontogianni et al., 2013), and could be considered as a potential natural resources, drugs, cosmetics or food conservatives.
Recently the interest in natural antioxidants, in relation to their therapeutic properties, increased compared to previous years. In various specialties, scientific research has been developed to extract, identify, and quantify these compounds from several natural substances, including medicinal plants and food products (Huang et al., 2005; Marc et al., 2004; Nchez-Moreno, 2002). In addition to their important sensory properties, several studies have pointed out that many of them show biological activities related to their anti-free radical properties (Vinson and Hontz, 1995). Because of the mobility of phenolic hydrogens, phenolic compounds are capable to trap oxygen free radicals, in particular peroxide radicals (ROO·), alkoxyl radicals (RO•), superoxides (O2−) and hydroxyls (OH), permanently generated by our organism or trained in response to environmental stresses (cigarettes, pollutants, infections …) (Min and Ebeler, 2008). Indeed, their role as natural antioxidants allows the body to fight against a large number of diseases, which suggests new perspectives in the prevention and treatment of cancer (Weinguang et al., 2005), inflammatory diseases (Aruoma, 1998), cardiovascular diseases (Leifert and Abeywardena, 2008) and neurodegenerative diseases (Ramassamy, 2006). Evidence from several studies suggests that S. officinalis has potent antioxidant activity. The phenolic compounds are isolated from the extract of the Salvia officinalis L. such as carnosol, rosmarinic acid, and carnosic acid, followed by caffeic acid, rosmanol, rosmadial, genkwanin, and cirsimaritin which has the most effective antioxidant activity (Ghorbani and Esmaeilizadeh, 2017). In addition to rosmarinic acid, other flavonoids of S. officinalis L particularly quercetin and rutin have a strong antioxidant activity (Azevedo et al., 2013).
Another interest is credited to the use of these plants as a source of corrosion inhibitor. That are used to prevent or delay the corrosion of metals. Corrosion phenomenon is constant, continuous, and often difficult to eliminate. It affects several sectors (industry, buildings, etc.) and can cost billions of dollars each year (Hussin and Kassim, 2011). Most of the synthetic compounds used as inhibitors, have good anti-corrosive action. However, most of them are highly toxic to human beings and to the environment (Ostovari et al., 2009). The attempts to highlight environmentally friendly processes are reoriented to the use of natural products, which are known for their environmental and acceptable ecological properties. These organic compounds are synthesized or either extracted from aromatic herbs, spices and medicinal plants. We can cite here some research studies that have been used natural substances, either of essential oils or extracts as corrosion inhibition: natural honey extract (El-Etre and Abdallah, 2000), extract of Salvia officinalis L. (Soltani et al., 2012), essential oil of lavandula dentate (Ouadi et al., 2015), Athamanta sicula oil (Ouadi, 2015), Essential Oil of Eucalyptus globulus (Rekkab et al., 2012), Essential Oil of Warionia Saharea (Znini et al., 2011), Extract of Black Pepper (Dahmani et al., 2010), essential oil of Artemisia (Bammou et al., 2011), portulaca oleracea (Adejo et al., 2013), pricky pear (Ben Hmamou et al., 2012), piperanine (Dahmani et al., 2012), Dodonaca viscosa extract (Leelavathi and Rajalakshmi, 2013), ponagania pinnata extract (Singh and Quraishi, 2011), and Areca flower extract (Raghavendra and Ishwara Bhat, 2017).
Currently, Medicinal and Aromatic Plants (MAP) are getting a considerable asset and a popularity through the gradual return to natural substances. Their many uses make them now more demanded in world’s market. In this context, this work focused on the chemical characterization and evaluation of the antioxidant activity and corrosion inhibition of oils and alcohol extracts of Salvia officinalis L. in the Middle Atlas (Morocco).
2 Material and methods
2.1 Plant material
The plant of Salvia officinalis L. was collected in its natural habitat the month in March 2014. The harvest was made in the Middle Atlas in Khenifra region. Only the aerial parts (leaves) were collected and dried for 15 days in the shade at ambient temperature. Botanical identification of the plant was made in the Department of Botany and ecological plant of the Scientific Institute of Rabat.
2.2 Phytochemical screening
The phytochemical screening is the main way to highlight the great families of secondary metabolites in the studied plant extracts. This screening has focused either on the formation of insoluble complexes using precipitation reactions and the formation of a complex colored using colored reactions. The characterization of different chemical groups were done according to experimental protocols by Benmehdi et al. (2012), Dah-nouvlessounon et al. (2015), the most commonly used operations are: decoction, infusion and maceration.
2.3 Extraction of the essential oil and polyphenols of Salvia officinalis L.
Essential oils (EO) were extracted from 100 g of leaves of Salvia officinalis L. leaves by steam distillation for three hours using a Clevenger device. The EO was then dried on sulfate of sodium anhydrous and kept at a temperature of 4 °C protected from light until their use.
Concerning the extraction of polyphenols, the plant Salvia officinalis L. was dried in a dry and ventilated place, protected from sunlight, it was crashed completely and then weighed (M = 30 g). The plant material obtained was extracted by solvent using the maceration method in a hydro-alcoholic mixture (Methanol/Water: 70/30), for 24 h. Then, the solution obtained was concentrated in the rotavapeur until a solid residue was obtained. It is then recovered by a volume of hot distilled water and used subsequently, for determination of polyphenols, total flavonoids and for measures of antioxidant activity and corrosion inhibition in different concentrations.
2.4 Analysis and identification of the chemical composition of the essential oil of Salvia officinalis L.
Chromatographic analysis of the sample Salvia officinalis L. was made on a gas chromatograph (Trace GC Ultra, Thermo Electron) coupled to a mass spectrometer (Polaris Q MS, Thermo Electron), in the electron impact (EI) ionization mode (70 eV). The analysis was carried out using DB-5 (5% phenylmethylsiloxane) column (30 m × 0.25 mm × 0, film thickness 25 μm) using a temperature program of 50–200 °C at 4 °C/min for 5 min. The carrier gas at a constant flow rate of 1 ml/min. The injection mode is split (leak report: 1/70, flow ml/min).
The identification of the chemical composition of HE of Salvia officinalis L. was conducted based on the comparison of their kovats (IK) and Adams indices with those of the benchmark product known in the literature. It was complemented by a comparison of indices and the spectra of mass with different references (Adams, 2005). The Kovats indices compare the retention time of a product with a linear alkane of the same number of carbons. They are determined by injecting a mixture of alkanes (standard C7 – C40) under the same operating conditions.
2.5 Determination of the total polyhenols of Salvia officinalis L.
The presence of polyphenols was determined by the Folin-Ciocalteu method, this method, originally described by Singleton and Rossi (1965), to determine the total polyphenols content in a given sample.
0.1 ml of the hydromethanolic of Salvia officinalis L. extract is mixed with 1.5 ml of Folin-Ciocalteu reagent (10%) diluted 10 times and 1.5 ml of sodium carbonate (Na2CO3) at a concentration of 75 g/l. After incubation for 2 h at room temperature, the absorbance is measured at 760 nm. The calibration curve is performed by Gallic acid with a concentration of 50 µg/ml (5 mg/100 ml). Concentrations of total polyphenols of each extract were calculated from the equation of the regression of the calibration range with Gallic acid (y = 0.095× + 0.003) (Fig. 1b). The results are expressed in milligrams of the equivalent of Gallic acid per gram of extract (mg EAG/g of extract). These results were used to estimate the total content of polyphenols contained in the leaves of Salvia officinalis L., the total phenol content is calculated according to the following formula:
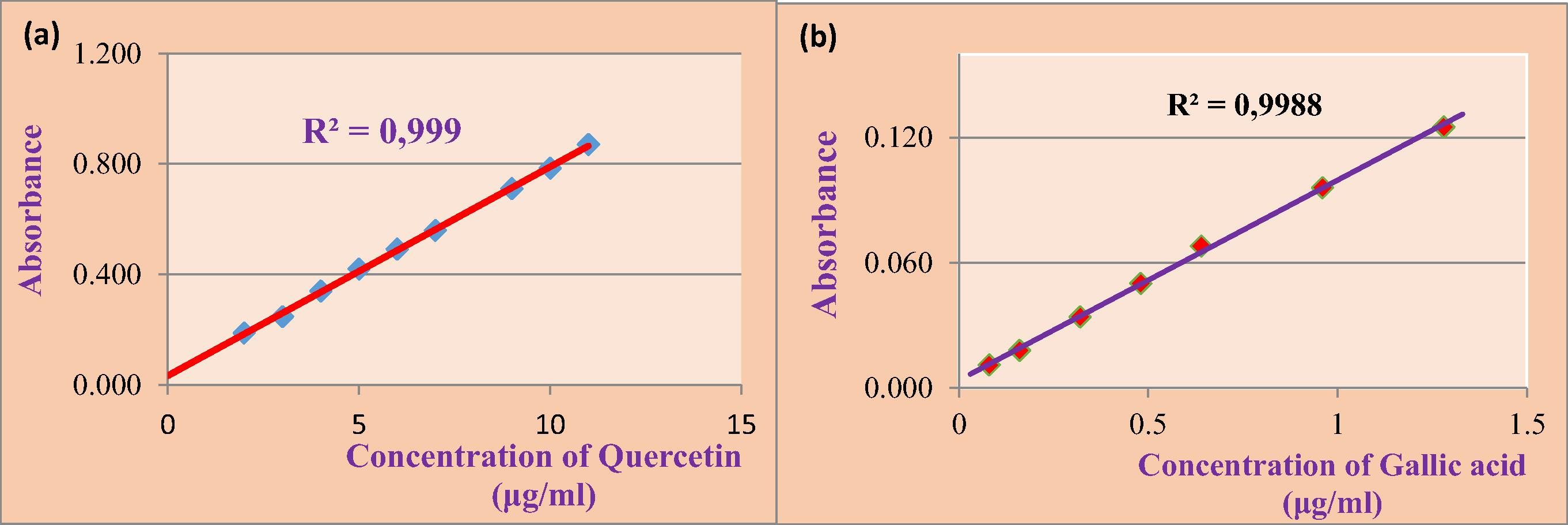
Calibration curves of Quercetin (a) and (b) Gallic acid.
2.6 Determination of the total flavonoids of Salvia officinalis L.
The content of total flavonoids of Salvia officinalis L. is estimated by the method of chloride (AlCl3) aluminum, according to the amended Protocol by Atanasova (2009).
0.1 ml of the extract from Salvia officinalis L. is mixed with 0.1 ml of chloride aluminum 10%, followed by 20 ml of distilled water and complete with absolute to 50 ml methanol, then left two hours in darkness. The absorbance of each solution is determined at 433 nm. Under the same conditions, the standard solution of the quercetin is prepared with a concentration equal to 0.1 mg/ml (25 mg/250 ml). Flavonoids of each extract concentration were calculated from the equation of the regression of the calibration range with quercetin (y = 0.073 × −0.081) established (Fig. 1a).
2.7 Evaluation of the antioxidant activity of the essential oil of Salvia officinalis L. by the free radical trapping: 2,2-diphenyl-1-picrylhydrazyl (DPPH•)
The DPPH• solution is prepared in advance by solubilizing 2.4 mg of DPPH• in 100 ml of absolute ethanol. The series of dilutions of the essential oil Salvia officinalis L. at different concentrations (18–360 mg/ml) are prepared in absolute ethanol. 200 μl of each extract at different concentrations are added to 2.8 ml of DPPH•. Reference antioxidant solutions (ascorbic acid) are also prepared under the same conditions. The control consists only of 2.8 ml of DPPH• and 200 μl of ethanol. The mixture is left in the dark for 30 min until discoloration occurs. The reading is performed by spectrophotometry at a wavelength of 515 nm. Results were expressed as a percentage of reduction of DPPH• (PI%) according to Sharififar et al. (2007).
With:
-
-
AControl: Absorbance of the solution containing only radical DPPH• solution
-
-
A Sample: Absorbance of the sample solution to be tested in the presence of DPPH•
The values of concentrations to inhibit or reduce 50% of the initial concentration of DPPH• (IC50) were determined graphically by linear regression.
As there is no absolute measure of the antioxidant capacity of a compound, the results are often worn over an antioxidant of reference, such as Ascorbic acid.
2.8 Electrochemical technics
2.8.1 Material preparation and solution
Materials used for the study were pure iron, prior to all measurements, iron specimens were abraded using SiC paper until 4000, washed thoroughly with double-distiller water degreased with ethanol and dried at room temperature. The surface in contact with the electrolyte is 1 cm2.
The aggressive solution 0.5 M sulfuric acid was prepared by dilution of an Analytical Grade 98% H2SO4 with double distilled water. All experiments were carried out in 0.5 M H2SO4 solution in the absence and presence of different concentrations (0.5–4 g/l) of Salvia officinalis L. extract.
2.8.2 Potentiodynamic polarization
Polarization measurements were carried out in a conventional three-electrode electrolytic cell. Saturated calomel electrode (SCE) and platinum electrode were used as reference and auxiliary electrodes respectively. The working electrode is in the form of a rectangular disk from iron on the surface 1 cm2. These electrodes are connected to Voltalab PGZ 100 piloted by ordinate associated with ‘‘Volta Master 4” software. The scan rate was 2 mV/s started from an initial potential of −800 to100 mV/SCE. Corrosion current densities were obtained from the polarization curves by linear extrapolation of the Tafel curves. Before recording each curve, a stabilization period of 30 min was allowed, which was proved satisfactory to attain a stable value for Ecorr. Tafel polarization curves were plotted at a polarization scan rate of 2 mV/s. Anodic and cathodic curve slopes were extrapolated to corrosion potential, for the determination of the corrosion current densities (Icorr). The Tafel equations predict a straight line for the variation of the logarithm of current density with potential. Therefore, currents are often shown in semi logarithmic plots known as Tafel plots. This type of analysis is referred to as Tafel Slope Analysis. The Tafel slope analysis tool provides a quick estimation of the corrosion rate and the polarization resistance. The corrosion rate is calculated from the estimated corrosion current, Icorr, obtained from the intercept of the two linear segments of the Tafel slope. Inhibition efficiency is determined by the following expression.
2.8.3 Electrochemical impedance spectroscopy
The Electrochemical impedance spectroscopy (EIS) is a method designed to avoid severe deterioration of the exposed surface of the structure studied and was widely used for monitoring the corrosion of a working electrode. Therefore, it is a non-destructive method for the evaluation of a wide range of materials, including coatings, anodized films and corrosion inhibitors. This method consists of applying frequencies and low amplitude sinusoidal voltage wave to produce perturbation signals on the working electrode. It can also provide detailed information of the systems under examination; parameters such as corrosion rate, electrochemical mechanisms and reaction kinetics, detection of localized corrosion, can all be determined from these data.
Electrochemical impedance spectroscopy (EIS) was carried out with the same equipment used for the polarization measurements, leaving the frequency response analyzer out of consideration. Quasi-potentiostatic polarization curves were obtained using a sweep rate of 2 mV s−1. After the determination of steady-state current at a given potential, sine wave voltage (10 mV/dec) peak to peak, at frequencies between 100 kHz and 100 mHz was superimposed on the rest potential. Computer programs automatically controlled the measurements performed at rest potential after 30 min of exposure. All potentials were reported versus saturated calomel electrode (SCE). The impedance diagrams are given in the Nyquist representation. From the impedance diagrams in the corrosion potential (Ecorr), we gain access to the charge transfer resistance (Rct), the capabilities of the double layer (Cdl) and therefore the rate of inhibition (η) in the operating conditions used.
Rt and are the charge-transfer resistance values without and with inhibitor, respectively.
3 Results and discussion
3.1 Phytochemical screening
The phytochemical screening results are reported in Table 1. This study showed that S. officinalis L. is rich in polyphenols, tannins (Gallic and catechics), flavonoids, sterols and catechols. However, the results show the absence of saponins, alkaloids, leucoanthocyanins and anthocyanins. All these results coincide with was obtained by Nacéra (2009). Another study focused on S. verbenaca where the same phytochemicals families were identified after extracting the aerial part of the plant, and this showed the presence of catechics tannins, flavonoids, cardiac glycosides and the absence of mucilage.
Chimicals group
Extracts of the flowering tops of S. officinalis L.
Gallic Tanins
+
Catechic Tanins
+
Flavonoid
+
Anthocyans
–
Leucoanthocyans
–
Catechols
+
Sterols and triterpens
+
Saponins
–
Alkaloids
–
(+): Presence (−): Absence
Nowdays, these secondary metabolites are considered, to be the pillars of a possible ecological intensification of agriculture, including the substitution of chemical inputs by natural plant defense mechanisms. From a pharmacological point of view, secondary metabolites represent the most active fraction of the chemical compounds present in plants, and it is now estimated that approximately 1/3 of the medications currently on the market contain a plant substance.
3.2 Content and identification of the chemical composition of S. officinalis L. essential oil
The yield of the essential oil obtained from the leaves of Salvia officinalis L. 4.13%, with a moisture content and a percentage of the dry matter are equal to 13.68% and 86.32% respectively. The yield of our species is much higher than the yield obtained from the same species in Tunisia (1.8%), France (2.05%), Portugal (2.9%) and Romania (2.3%) (Felleh et al., 2006).
In this respect, we compared our essential oil yield to other plant yields belonging to the families Apiaceae and Lamiaceae, respectively. The Ferula Assafoetida specie has an essential oil yield of 0.94% (Dehpour et al., 2009), whereas Kargar et al. (2014) found a yield of essential oils that are equal to 2.78% and 2.37% for both species Satureja hortensis and Satureja hortensis respectively.
This variation of essential oils yield depends not only on the studied plant, but also on its environment, the stage and the date of harvest, genetic factors and extraction techniques (Felleh et al., 2006; Karousou et al., 2005).
3.2.1 Chemical composition of the essential oil of Salvia officinalis L. aerial part flowering
The chromatogram below shows the results of essential oil analysis of Salvia officinalis L. leaves. These results show that the essential oil consists of several chemical compounds with different proportions (Fig. 2).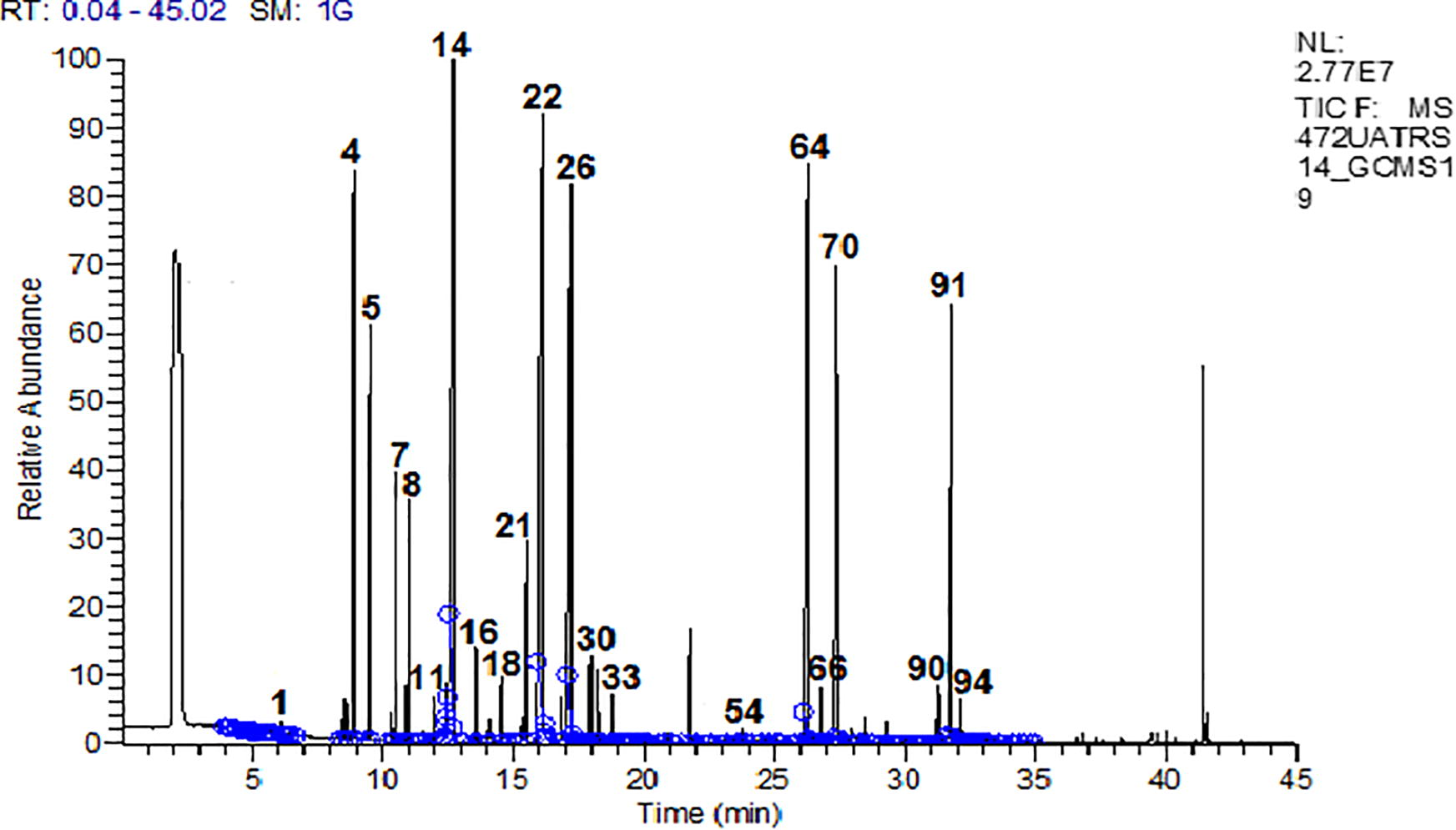
Chromatogram of the essential oil from the leaves of Salvia officinalis L.
Hydro-distilled of Salvia officinalis L. using a Clevanger permitted us to obtain an essential oil with a yield of 4.3%. Table 2 shows the identification of 105 compounds representing 98.38% of the chemical composition of Salvia officinalis L. essential oil. It should be noted that the class of oxygenated Monoterpenes represents the highest percentage (49.21%) followed by hydrocarbon Monoterpenes (20.58%). The sesquiterpenes represent only 28.59% of the total composition of the essential oil. The chemical profile of Salvia officinalis L. is predominated by the trans thujone (17.74%) followed by 1,8-cineol and camphor with percentages of 12.63% and 12.24%, respectively.
N°
RT
Area %
IK (adams)
Formul brut
Weight molecular
Compound
Reference
1
6.14
0.10
858
C9H16
124
E-Salvene
2
8.43
0.18
926
C10H16
136
Tricyclene
3
8.58
0.37
930
C10H16
136
α-Thujene
Kelen and Tepe (2008)
4
8.89
7.82
939
C10H16
136
α-Pinene
Hadian et al., 2014; Jouki et al. (2014)
5
9.49
4.03
954
C10H16
136
Camphene
Falsafi et al. (2015), Hadian et al. (2014)
6
10.33
0.24
975
C10H16
136
Sabinene
Hadian et al. (2014), Kelen and Tepe (2008)
7
10.51
2.51
979
C10H16
136
β-Pinene
Falsafi et al. (2015), Hadian et al. (2014)
8
10.97
2.31
990
C10H16
136
Myrcene
Falsafi et al. (2015), Hadian et al. (2014)
9
11.41
0.04
991
C8H18 O
130
Octanol
10
11.58
0.10
1002
C10H16
136
α-Phellandiene
11
11.97
0.42
1011
C10H16
136
δ-3-Carene
12
12.37
0.01
1024
C10H14
134
ρ-Cymene
Falsafi et al. (2015), Hadian et al. (2014), Jouki et al. (2014), Kargar et al. (2014)
13
12.45
0.00
1024
C10H14
134
Ο-Cymene
14
12.69
12.63
1031
C10H18O
154
1,8-Cineol
Ben et al. (2013), Falsafi et al. (2015), Kelen and Tepe (2008)
15
13.10
0.02
1050
C10H16
136
β-E-Ocimene
Hadian et al. (2014), Kargar et al. (2014)
16
13.55
0.82
1059
C10H16
136
γ-Terpinene
Falsafi et al. (2015), Hadian et al. (2014), Kargar et al., 2014)
17
14.08
0.33
1070
C10H18O
154
cis-Sabinene hydrate
Hadian et al. (2014)
18
14.55
0.59
1086
C10H16
136
Fenchone
19
14.81
0.11
1088
C10H16O
152
Terpinolene
20
15.35
0.37
1098
C10H18O
154
trans-Sabinene hydrate
Hadian et al. 2014), Kargar et al. (2014)
21
15.54
2.58
1102
C10H16O
152
Cis-Thujone
22
16.14
17.74
1114
C10H16O
152
Trans-Thujone
Kelen and Tepe (2008)
23
16.19
0.02
1126
C10H16O
152
α-Campholenal
24
16.33
0.01
1141
C10H16O
152
cis-Verbenol
25
16.84
0.49
1144
C10H18O
154
Cis-β-Terpineol
26
17.23
12.24
1146
C10H16O
152
Camphor
Ben et al. (2013), Kelen and Tepe (2008)
27
17.35
0.02
1159
C10H16O
152
Karahanaenone
28
17.56
0.02
1162
C10H16
152
Trans-Pinocamphone
29
17.63
0.01
1164
C10H16
152
Z-Isocitral
30
17.96
1.19
1169
C10H18O
154
Borneol
Kargar et al. (2014), Kelen and Tepe (2008)
31
18.22
0.68
1177
C10H18
154
Terpinen-4-ol
Falsafi et al. (2015), Hadian et al. (2014), Kargar et al. (2014)
32
18.58
0.03
1179
C10H14O
150
ρ-Cymen-8-ol
33
18.79
0.49
1188
C10H18O
154
α-terpineol
Kargar et al. (2014), Kelen and Tepe (2008)
34
19.09
0.09
1195
C10H14
150
Myrtenol
Kelen and Tepe (2008)
35
19.27
0.01
1196
C10H18O
154
Cis-Piperitol
Kelen and Tepe (2008)
36
19.54
0.01
1196
C10H22O
158
3-decanol
37
19.66
0.04
1215
C10H16O
152
Trans-Carveol
38
19.81
0.07
1230
C10H16
152
ρ-Mentha-1(7),8(10)-dien--ol
39
20.02
0.02
1229
C10H18O
158
Nerol
40
20.15
0.01
1230
C10H16O
152
Cis-ρ-Mentha-1(7),8-dien-2-ol
Kelen and Tepe (2008)
41
20.34
0.06
1237
C10H16O
152
Pulegone
Kargar et al. (2014)
42
20.66
0.02
1238
C12H18O2
194
Trans chrysanthenyl acetate
43
20.78
0.02
1247
C10H16O
152
Carvotanacetone
44
20.91
0.11
1254
C12H20O2
196
Linalool acetate
45
21.29
0.04
1263
C10H14O2
166
Cis-Carvone oxide
47
21.92
0.08
1265
C12H18O2
194
Cis-Chrysanthenyl acetate
48
22.07
0.09
1284
C10H12O
148
E-Anethol
49
22.22
0.03
1290
C10H14O
150
Thymol
Falsafi et al. (2015), Hadian et al., (2014), Jouki et al. (2014), Kargar et al. (2014), Kelen and Tepe (2008)
50
22.49
0.05
1299
C10H14O
150
Carvacrol
Falsafi et al. (2015), Hadian et al. (2014), Jouki et al. (2014)
51
23.11
0.05
1312
C12H18O2
194
Cis-Pinocarvyl acetate
52
23.44
0.04
1342
C12H18O2
194
Trans-Carvyl acetate
54
23.75
0.17
1326
C12H18O2
194
Myrthenyl acetate
55
23.87
0.01
1343
C10H14O
150
Piperitenone
Kelen and Tepe (2008)
56
24.25
0.06
1359
C10H12O2
164
Eugnol
Ben et al. (2013), Kelen and Tepe (2008)
57
24.49
0.05
1371
C15H14
204
Cyclosativene
58
24.69
0.06
1376
C15H24
204
α-copaene
Kelen and Tepe (2008)
59
24.97
0.10
1388
C15H24
204
β-Bourbonene
Kelen and Tepe (2008)
60
25.10
0.04
1391
C15H24
204
7-Epi-sesquithujene
62
25.71
0.07
1409
C15H24
204
α-Gurjunene
63
25.86
0.01
1433
C15H24
204
β-Gurjunene
64
26.25
9.87
1419
C15H24
204
Caryophyllene
Jouki et al. (2014), Kargar et al. (2014), Kelen and Tepe (2008)
65
26.50
0.12
1436
C15H24
204
γ-Elemene
66
26.62
0.06
1441
C15H24
204
Aromadendrene
Kelen and Tepe (2008)
67
26.77
0.54
1444
C15H24
204
6,9-Guaiadiene
68
26.99
0.08
1451
C15H24
204
α-Himachalene
69
27.14
0.02
1453
C15H24
204
Khusimene
70
27.40
7.29
1462
C15H24
204
Dehydra-Aromadendrane
71
27.49
0.11
1466
C15H24
204
α-Acrodiene
72
27.93
0.16
1472
C10H24
204
Dauca-5,8-diene
73
28.05
0.01
1476
C15H24
204
α-Neocallitropsene
74
28.13
0.09
1481
C15H24
204
Germacrene D
75
28.29
0.01
1493
C15H24
204
Cis-β-Guarene
76
28.43
0.31
1496
C15H24
204
Viridiflorene
77
28.60
0.10
1498
C15H24
204
α-Selinene
Kelen and Tepe (2008)
78
28.79
0.02
1502
C15H24
204
β-Guaiene
79
29.14
0.07
1513
C15H24
204
γ-Cadinene
80
29.25
0.22
1523
C15H24
204
δ-Cadinene
Kelen and Tepe (2008)
81
29.39
0.05
1529
C15H24
204
Cis-calamenene
82
29.72
0.02
1534
C15H24
204
Trans-cadina-1,4-diene
83
29.85
0.01
1538
C15H24
204
α-Cadinene
84
29.98
0.04
1542
C15H22O3
250
Z-Laciniatafuranone
85
30.52
0.01
1544
C15H16O
222
Cis-sequisabinene hydrate
86
30.60
0.01
1556
C15H24
204
Trans Dauca-4(11),7-diene
87
30.67
0.02
1561
C15H24
204
Germaerene B
88
30.89
0.03
1967
C15H26O
222
Maaliol
89
31.17
0.25
1578
C15H24O
220
Spathulenol
Kargar et al. (2014), Kelen and Tepe (2008)
90
31.30
0.71
1583
C15H24O
220
Caryophyllene oxide
Kelen and Tepe (2008)
91
31.75
7.03
1600
C15H26O
222
Guaiol
Kelen and Tepe (2008)
92
31.83
0.05
1607
C15H28O
222
Z Sequilavandulol
93
31.98
0.08
1623
C15H26O
222
10-epi-γ-Eudesmol
94
32.15
0.46
1660
C15H24O
220
Gymnonutrol
95
32.32
0.12
1669
C15H24O
220
Z-14-hydroxy-9-epi-caryophyllene
96
32.93
0.03
1669
C15H22O
218
β-Atlantone
97
33.03
0.05
1663
C15H22O
222
7-epi-α-eudesmol
98
33.18
0.02
1687
C15H24O
220
Eudesma-4(15),7-dien-1-β-ol
99
33.44
0.12
1700
C15H26O
222
Eudesm-7(11)-en-4-ol
100
33.89
0.14
1713
C15H24O
220
Cedroxyde
101
34.17
0.02
1714
C15H26O
222
longifolol
102
34.30
0.04
1729
C15H26O
222
Iso-longifolol
103
34.40
0.01
1706
C15H24O
222
14-hydroxy-4,5-dihydro caryophyllene
104
34.46
0.02
1776
C15H24O
220
Z-α-hydroxy-Amorpha-4,7(11)-diene
105
34.81
0.02
1794
C15H26O2
262
Z-α-trans-bergamotol
Totale: 98,38%
Monoterpenes
hydrocarbon
20,58%
Oxygenated
49,21%
Sesquiterpenes
hydrocarbon
19,34%
Oxygenated
9,25%
Several researches have studied the chemical composition of Salvia officinalis L. essential oils and other species of salvia in different regions on both sides of the globe such as, Tunisia (Ben et al., 2013; Felleh et al., 2006), Turkey (Kelen and Tepe, 2008), Iran (Dehpour et al., 2009; Falsafi et al., 2015; Hadian et al., 2014; Kargar et al., 2014) and Benin (Jouki et al., 2014).
Felleh et al. (2006) have found the main compounds of Salvia officinalis L essential oils. collected in two different regions of Tunisia. The results were: α-thujone (26.49%–10.58%), β-thujone (13.09%–3.09%), 1.8-cineole (31.89%–8.58%), camphor (40%–2.10%), Viridiflorol (18,96%–1.73%) and caryophyllene (9.04%–0.5%) (Felleh et al., 2006). Thus, (Ben et al., 2013) found in their study that the major compounds of Salvia argentea essential oil growing in Tunisia are: manool and manoyl oxide characterized the vegetative stage while camphor, methyl eugenol, 1,8-cineole and viridiflorol prevailed during flowering and the fruiting phase was marked by the prevalence of α-humulene, viridiflorol, methyl eugenol and β-ionone.
Essential oils of three different Salvia species (Salvia aucherivar. aucheri (endemic), Salvia aramiensis and Salvia pilifera (endemic)) which were analyzed by Kelen and Tepe (2008). The major compounds for S. aucherivar. aucheri oil were 1,8-cineole (30.5%), camphor (21.3%) and borneol (8.50%), β-pinene (10.3%), was the main constituent of S. aramienesis together with 1,8-cineole (46.0%) and camphor (8.7%). In the case of S. pilifera oil, α-thujene (36.1%) and α-pinene (13.8%) were determined as the major compounds.
We compared our species to other genuses of different types that belongs to the same family Lamiaceae. The bibliographical research concerning different species showed a variation in the chemical compositions their essential oils. Indeed, a study by (Kargar et al., 2014) shows that the essential oil of S. sahandica collected in Iran is composed essentially of the thymol (31.53%), P-cymene (30.28%)، γ-Terpinene (19.37%), and α-terpinene (2.12%) as the main oil constituents. In addition, a study made by Hadian et al. (2014) in the same country of Iran, but different species showed that the carvacrol (89.20%) as a major component of Satureja rechingeri essential oil. The carvacrol (45,5%), thymol (27,9%), p-cymene (4,4%) and do γ-terpinene (4,0%) have been reported as major components of Satureja Bachitiarica essential oil (Falsafi et al., 2015). The Essential Oil of Ferula assafoetida analysis by Dehpour et al. (2009) has shown Phenol, 2-methyl-5-(1-methyl ethyl) (18.2%),Bisabolol (10.4%), Arsine triethyl (8.7%) and Cyclopropa [a] naphthaleneoctahydro-tetramethyl (6.6%), as the major components. The major constituents of Satureja hortensis essential oil are reported to be carvacrol, p-cymene, α-thujune, α-pinene, β-myrcene, β-terpinene, thymol, linalool, and β-caryophyllene (Jouki et al., 2014).
The variation detected in the chemical composition of the essential oil of Salvia officinalis L and other salvia species from different countries is linked to several parameters such as: the climate, which changes from one country to another, from the harvesting period, and the environmental factor (Felleh et al., 2006). The plant material used in this study as well as the one studied in Tunisia was collected up in March, while in Turkey it was collected at the flowering stage.
The extraction method also significantly influences the chemical composition of the essential oil and processes, which use water, can induce the hydrolysis of esters and also of oxidation, Isomerizations, rearrangements and Racemizations. In our study, we obtained the essential oil of Salvia officinalis L by distillation with a Clevenger device, it is the method adopted for the isolation of essential oil from other species of Salvia (Kelen and Tepe, 2008). (Ben et al., 2013; Felleh et al., 2006) used the distillation alone for the extraction of the essential oil from the sage collect in Tunisia.
3.3 Determination of polyphenols and total flavonoids
The results of the extraction/splitting of the total phenol and flavonoid content of Salvia officinalis L using various solvents is given in Fig. 3(a and b). From this Fig. 3, it was evident that plants extract contained noticeable amounts of extractable compounds. It is clear that the different solvents used for the extraction and fractionation of Salvia officinalis L, had different abilities to extract substances from this medicinal plant.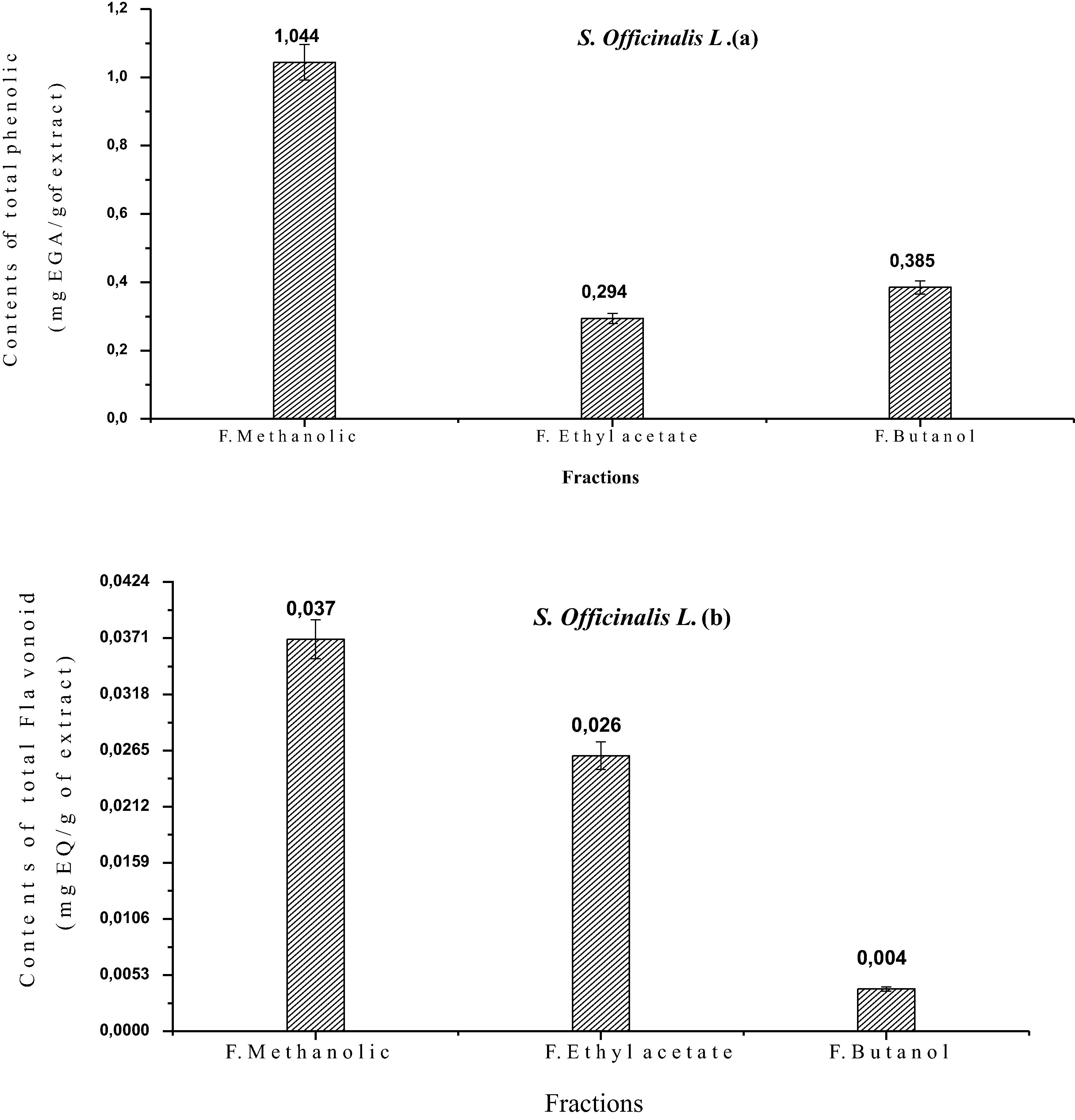
Contents in total polyphenols (a) and flavonoids (b) of the flowering tops of the Salvia officinalis L.
The respective concentrations of total phenolics and flavonoids have been estimated from the equivalent of the amount of Gallic acid and quercetin, in which solutions are prepared in advance and concentrations are known, were used for the realization of calibration curves (Fig. 1).
According to the Fig. 3, we found that the methanolic fraction (F0) is very rich in total phenols with a value of 1.044 mg EAG/g of extract, thus, the same fraction was also rich in flavonoids with a rate of 0.037 mg EQ/g of extract. Indeed, the total phenol content is not constant; it differs from one plant to another and between the species of the same genre. (Miliauskas et al., 2004) found that the total phenol content of salvia species is between 9.7 ± 0.4 and 24 ± 1.1 mg EAG/g of extract.
Liquid-liquid extraction, by solvents of increasing polarity (ethyl acetate and n-butanol), revealed that the total phenol content was lower in the two fractions of ethyl acetate and butanol compared to the methanol fraction, with values of: 0.294 and 0.385 mg EAG/g extracted, respectively (Fig. 3 a), the rate of total flavonoids for both fractions ethyl acetate and n-butanol are respectively in the order of 0.026 and 0.004 mg EQ/g of extract (Fig. 3b).
The phenolic content of a plant depends on a number of factors such as weather conditions, the time of harvest, the extraction solvent, extraction methods and storage conditions (Wonlee et al., 2003).
According to Athamena et al. (2010), the phenolic content of a plant depends on a number of intrinsic and extrinsic factors.
3.4 Antioxidant activity
3.4.1 DPPH• radical scavenging activity.
DPPH• is a stable radical, which presents an absorbing characteristic in 515 nm solution giving a violet color to its solution. This color disappears when the DPPH• is reduced by a of free radicals and becomes pale yellow, which is translated by a change of optical absorption.
In Fig. 4 below, shows the results as a percentage of inhibition of free radicals based on the concentration of S. officinalis L. essential oil and ascorbic acid. They show that the percentage of inhibition of the free radicals increases with the increase in the concentration of plant essential oils and ascorbic acid (Fig. 4). The values IC50 (mg/ml) determined graphically and expressing the inhibitory concentration of the antioxidant extract required for trapping and reducing 50% of DPPH• radicals. The lower the IC50 value, the more the extract is considered a powerful antioxidant. The results show that the essential oil has an important antioxidant activity with IC50 = 309.42 mg/ml, but remains low comparing to ascorbic acid which that is more effective with an IC50 = 3, 53 mg/ml (Fig. 5). This value can be compared to other species (Kelen and Tepe, 2008). These authors found that S. aramienesis is more active with an IC50 value of 12.26 ± 1.09 μg/ml followed by S. aucheri var. Aucheri (18.82 ± 1.27 μg/ml-1) and S. pilifera (24.19 ± 0.76 μg/ml).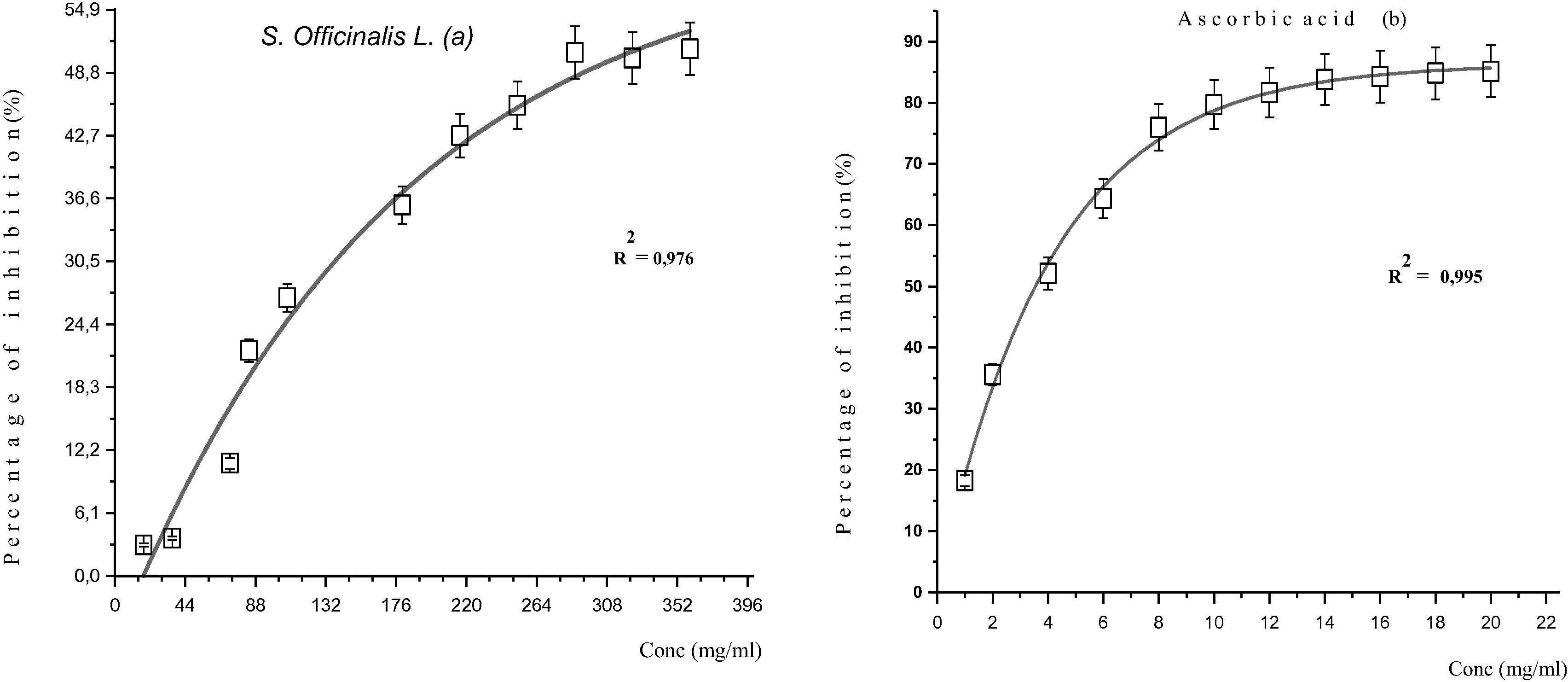
Percentage of inhibition of the DPPH• radical different concentrations of the essential oil of S. officinalis L (a) and Ascorbic acid.
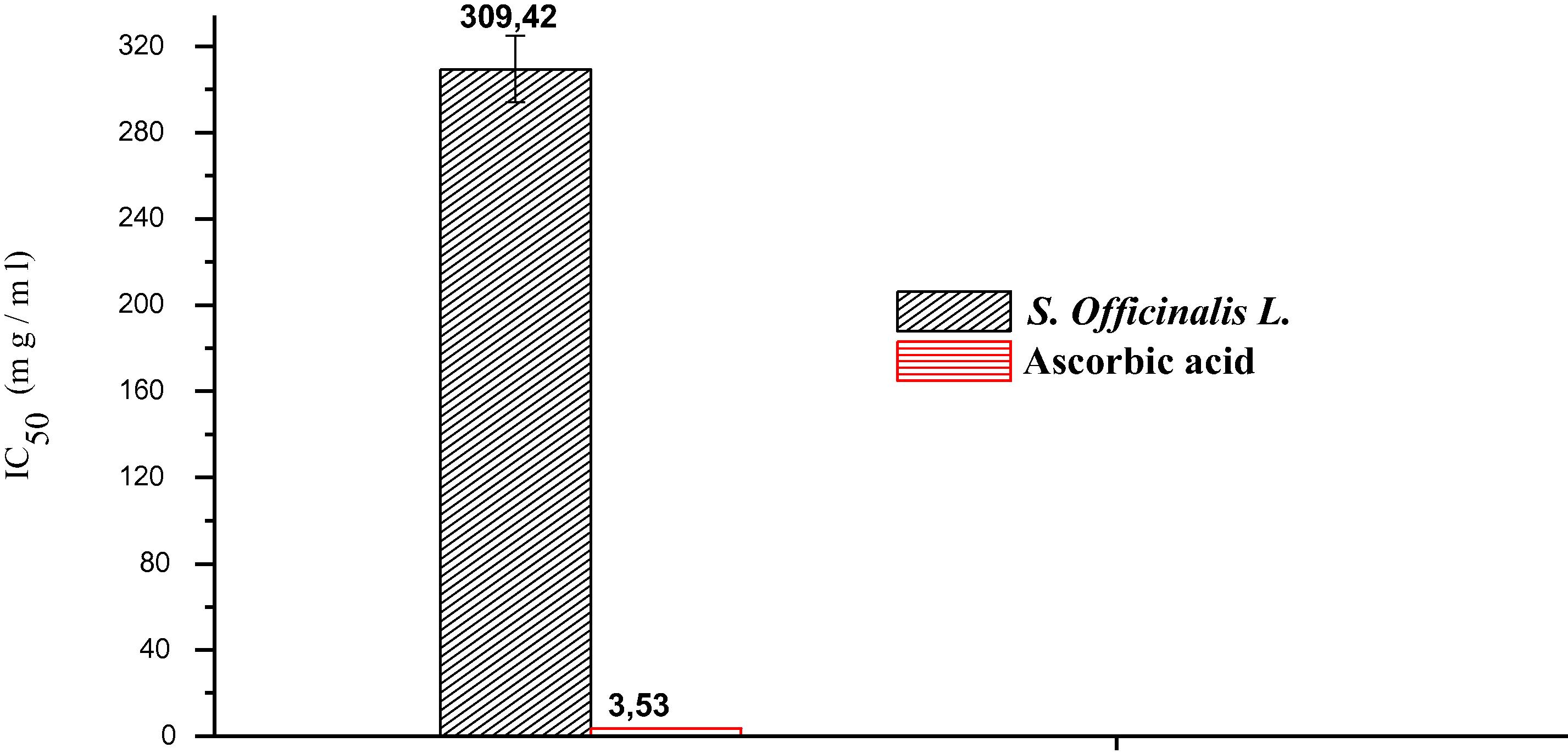
Value IC50 of the essential oil of S. officinalis L. and Ascorbic acid.
The antioxidant activity of essential oils of different species of the genus Satureja has been the subject of several studies (Bagheri et al., 2013; Bukvički et al., 2014; Cherrat et al., 2014; Giweli et al., 2012). Ozturk has highlighted an important antioxidant activity of Satureja. Thymbra of Turkey as well as its major compounds thymol and Carvacrol (Öztürk, 2012). The antioxidant power of Satureja. Cuneifolia essential oil was also explained by the high content in Carvacrol (Oke et al., 2009). It has been reported that essential oils of the genus Satureja are rich in isopropanoids such as Thymol, Carvacrol, β-caryophyllene, γ-Terpinene, P-cymene and Linalool which are known for their antioxidant potentials (Abdollahi, 2010).
Antioxidants play a role in the capture of free radicals and a role to stabilize a radical by the transfer of a hydrogen atom. This process of neutralization of these highly reactive compounds to reduce their harmful effects on the body (Bourrel, 1993).
(Ozer et al., 2007) have shown that the antioxidant activity of essential oils is heavily influenced by their chemical composition, mainly the phenolic compounds (thymol, carvacrol) or compounds with double bonds (chamazulene), while (Karoui et al., 2016), have confirmed that essential oils devoid of phenolic compounds also showed a relatively high antioxidant activity. The antioxidant activity of essential oils is, therefore, in close collaboration with the chemical structure of its components (Boyd et al., 2003).
3.5 Electrochemical measurements
3.5.1 Polarization measurements
In the potentiostatic method, the electrode potential is fixed at the value chosen for the time necessary to the achievement of the balance. Current is measured between the working electrode and the electrode of Platinum.
The curve of polarization cathodic and anodic pure iron in the workplace H2SO4 0.5 M at 25 °C and the scanning speed of 2 mV·s−1 in absence and in the presence of the essential oil (a) and the methanolic extract (b) of the Salvia officinalis L. at different concentrations, are shown in (Fig. 5), the values of electrochemical parameters determined from the obtained curves of polarization for essential oil (a) and the methanolic extract (b) of the Salvia officinalis L. (Table 3) to know the density of the corrosion (Icorr), the potential of corrosion (Ecorr) current, as well as inhibition efficiency corrosion (EI%).
Inhibitors
Conc(g/l)
−Ecorr (mV/ECS)
Icorr (mA/cm2)
Rp (ohm/cm2)
EI%
ERp%
Θ
H2SO4 0.5M
–
496.90
0.37
16.27
–
–
–
Salvia officinalis L. essential oil (a)
0.5
581.40
0.34
29.65
9.81
45.13
0.09
1
578.90
0.18
60.72
51.35
73.20
0.51
1.5
583.60
0.14
91.32
62.16
82.18
0.62
2
580.30
0.12
59.19
67.57
72.51
0.67
3
583.68
0.08
72.68
78.38
77.61
0.78
4
577.10
0.06
96.03
83.78
83.06
0.83
Methanolic extract of the Salvia officinalis L. (b)
0.5
503.8
0.11
44.65
69.72
63.56
0.69
1
516.3
0.07
85.39
79.18
80.95
0.79
1.5
512
0.05
107
85.94
84.79
0.85
2
523
0.03
380.12
90.27
95.72
0.90
3
514
0.03
410.59
90.54
96.04
0.90
4
520
0.03
178.66
91.62
90.89
0.91
The curves log I = f (E) depending on the concentrations of the inhibitor show the corrosion potential moving towards more cathode values. The cathodic Tafel slope decreases suggesting that the extract inhibits the corrosion of iron through the adsorption of its secondary metabolites and it acts as a mixed type inhibitor (Fig. 6). The values of Ecorr change slowly to negative values and the value of Icorr decreases and hence the value of inhibition efficiency increases with the concentration of inhibitors and reaches values of 83.78% and 91.62% respectively in the essential oil (a) and the methanolic extract (b) of the Salvia officinalis L. (Table 3). The inhibition efficiency is usually explained by adsorption of the inhibitor at the interface solution/metal. For information on molecules that can act qualitative and quantitative analysis of Salvia officinalis L. essential oil were conducted using GC and GC/MS. The relative percentage of each component and the retention values were shown in Table 2. It can be suggested that Trans-Thujone (17.74%), 1,8-cineol (12.63%), Camphor (12.24%), Caryophyllene (9.87%), α-pinene (7.82%), Dehydra Aromadendrane (7.29%), and Guaiacol (7.03%) are responsible for the inhibition efficiency process in the acid solution. This observation shows that essential oils and extracts of the plants are classified as an inhibitor of cathodic corrosion (Table 3).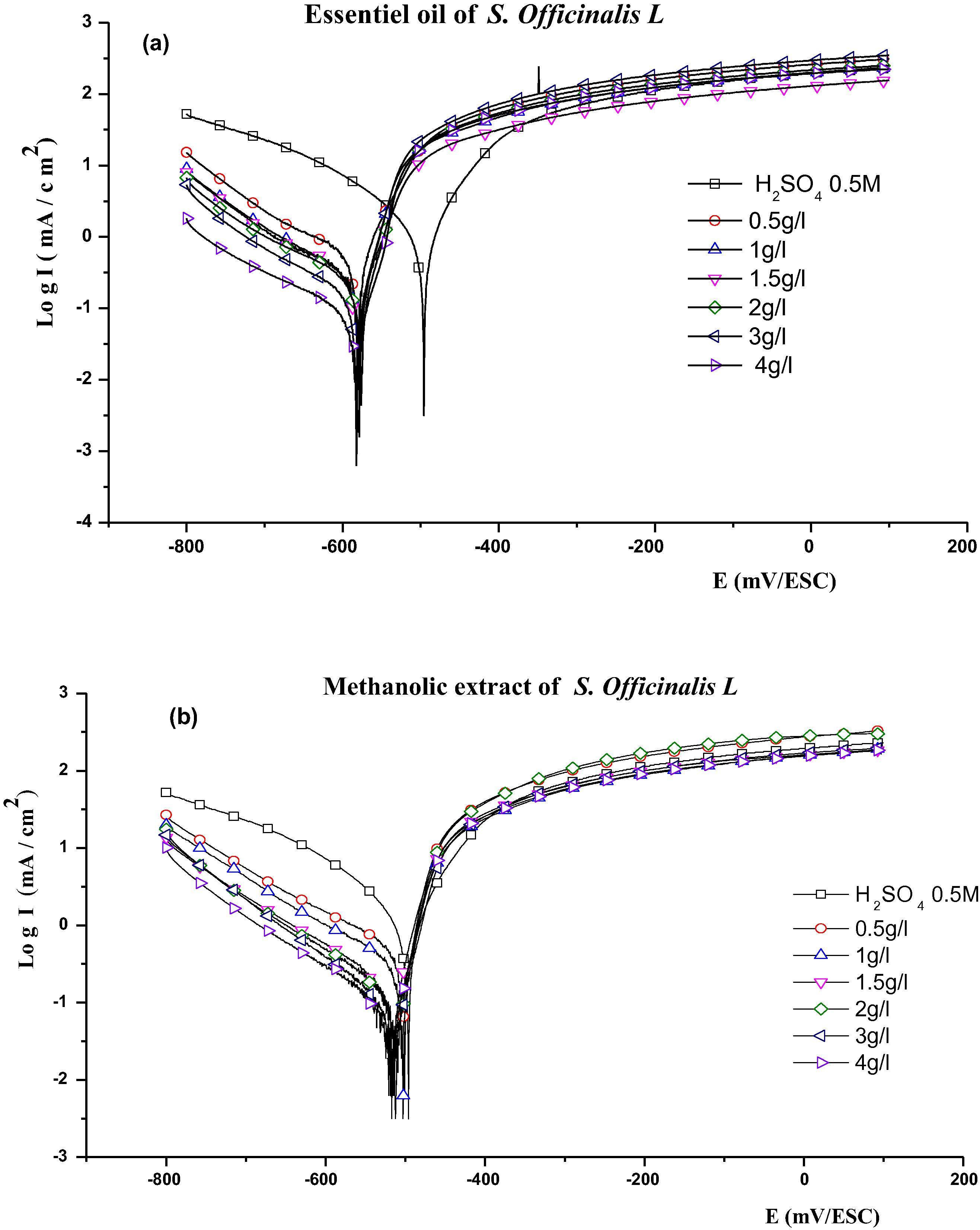
Curves of polarization of iron in H2SO4 0.5 M in absence and in presence of essential oil (a) and the methanolic extract (b) of the Salvia officinalis L. in different concentrations at room temperature.
These results coincide with other results obtained by other researchers (Soltani et al., 2012). Indeed, the extract of Salvia officinalis L. leaves was used as an inhibitor of corrosion for stainless steel 304 in HCl solution.
3.5.2 Electrochemical impedance spectroscopy measurements
Some of the parameters obtained from of EIS data analysis using the equivalent circuit model embedded in Fig. 7 are shown in Table 4. The obtained curves in the Nyquist plot (Fig. 7) are not perfect semicircles and this difference has been attributed to frequency dispersion (Bayol et al., 2007; Zarrok et al., 2012). This can generally be due to the corrosion reaction that is controlled by a process of transfer of loads on a heterogeneous solid and irregular electrode surface (Larabi et al., 2006; Umoren, 2016). The similarity of the shape of the observed curves of Salvia officinalis L. methanolic extract and essential oil indicates that the corrosion mechanism is the same regardless of the influence of the inhibitor. However, the presence of the inhibitor affects the sizes of the curves diameters. The highest diameter is obtained with the concentration 4 g/l from the essential oil and the methanolic extract; it gradually decreases when the concentration decreases. This indicates that the concentration of the test solutions influences the corrosion rate of iron by inhibition. The influence can be seen as being dependent on concentration since the diameters increase with the increase of the concentration of Salvia officinalis L. essential oil and methanolic extract.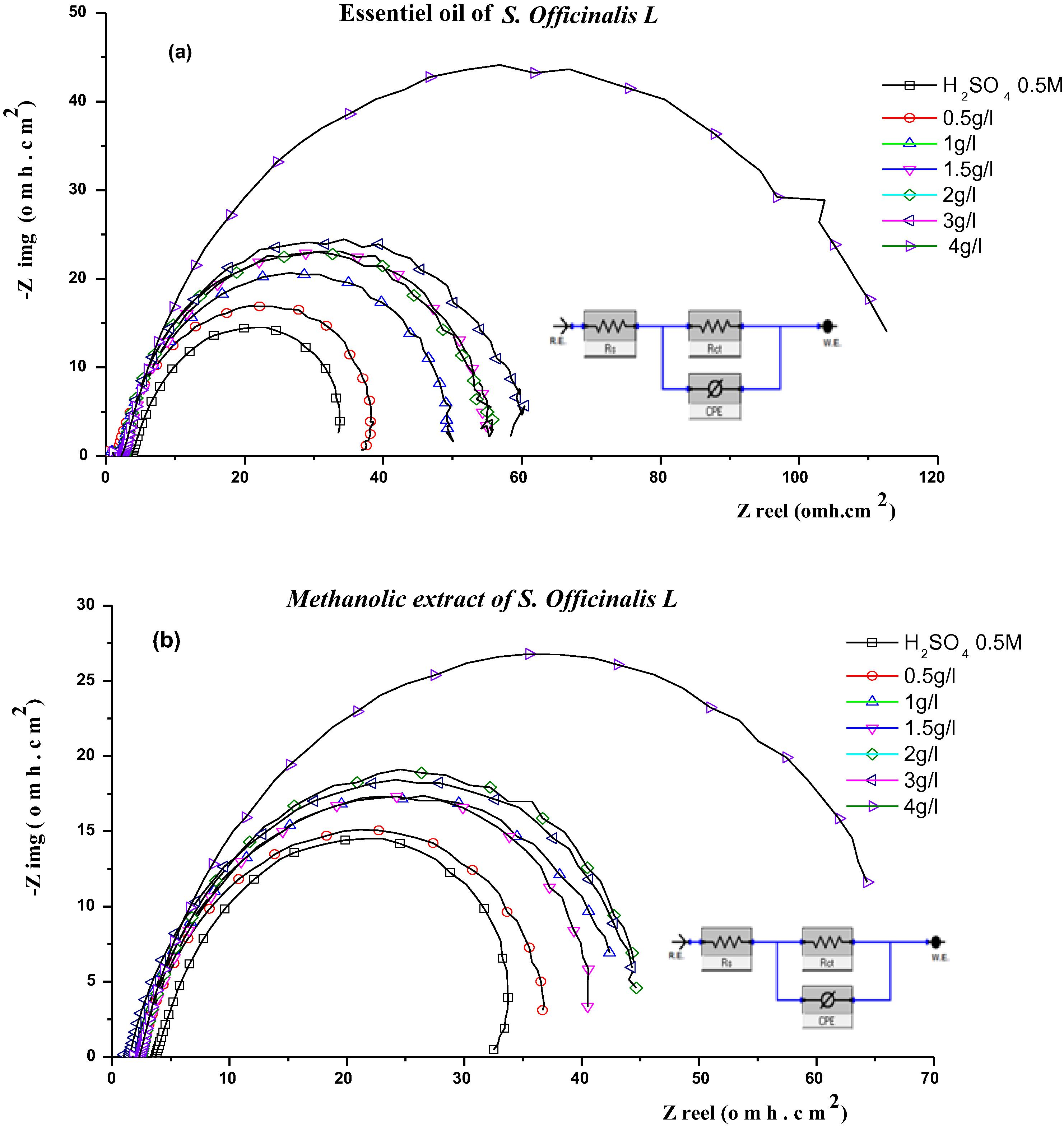
Diagrams of impedance of iron in H2SO4 0.5 M in absence and in presence of essential oil (a) and the methanolic extract (b) of the Salvia officinalis L. at different concentration at room temperature and Equivalent circuits used to fit the impedance spectra data.
Inhibitors
Conc. (g/l)
Re (ohm·cm2)
Rct (ohm·cm2)
n
Q (S·cm−2·sn) × 10−4
Cdl (µf/cm2)
η(%)
Θ
H2SO4 0.5M
–
3.64
34.59
0.84
2.37
60.64
–
Salvia officinalis L. essential oil (a)
0.5
1.43
40.47
0.83
2.63
51.93
14.52
0.14
1
2.06
50.2
0.85
1.84
45.49
31.09
0.31
1.5
2.42
56
0.85
1.90
48.49
38.23
0.38
2
2.46
53.77
0.89
1.24
45.34
35.67
0.35
3
2.53
58.65
0.88
1.42
47.95
41.02
0.41
4
2.21
117.60
0.82
2.41
45.88
70.58
0.70
Methanolic extract of the Salvia officinalis L. (b)
0,5
2.65
37.43
0.84
2.5
61.20
7.58
0.07
1
1.35
45.48
0.81
3.44
56.46
23.94
0.23
1.5
2.74
42.22
0.84
2.3
55.83
18.07
0.18
2
2.39
45.61
0.86
1.89
75.77
24.16
0.24
3
1.42
46.44
0.84
2.55
56.05
25.55
0.25
4
2.44
68.78
0.83
2.18
46.21
49.70
0.49
This trend is also in line with the trend of charge transfer resistance Rtc values and of the corresponding inhibition efficiency calculated. Increasing the concentrations of S. officinalis L tmethanolic extracts and essential oil increases the charge transfer resistance and decrease of the value double layer capacity Cdl (Table 4).
This decrease is attributed to the adsorption of the inhibitor molecules to the iron surface, forming a protective layer (Al-Turkustani et al., 2016; Anejjar et al., 2013; Bentiss et al., 2000).
The constant phase elements CPE associated with the first time constant (with an impedance Z = [Q (jω)n]−1) show similar values whatever the corrosive medium and immersion duration. Q is in the order of magnitude of 100–200 µS sn cm−2 which is higher than the value of a double layer capacitance usually observed on bare metal (from 10 to 50 µF cm−2) (Orazem and Tribollet, 2008). However, the corrosion process occurring on iron in the H2SO4 corrosive medium leads to a heterogeneous attack of the extracts (Fig. 7), inducing a two-dimensional distribution of current and potential at the surface and therefore a n factor lower than 1 (around 0.8 in all cases). In this case, an effective capacitance Ceff can be calculated using the relation (6) (Brug et al., 1984):
The calculated effective capacitances given in Table 4 correspond to double layer capacitances (Cdl).
The extracts inhibitory effect of corrosion of can be attributed to different constituents such as phenolic compounds. The corrosion inhibition is initiated by the movement of water molecules adsorbed species of inhibitor leading to a specific adsorption on the surface of the metal (Solmaz et al., 2008).
The different components can react with Fe2+ ions freshly generated on a metal surface organometallic complexes [Fe-INH]. The effect of inhibitors of these complexes, depends on their stability and solubility in the corrosive environment. The corrosion inhibition process is complex in nature. This complexity is increased by several orders of magnitude when considering extracts of plants with their complex chemical compositions.
3.5.3 Mechanism of corrosion inhibition
The inhibiting action of S. officinalis L (SO) extract towards the acid corrosion of steel can be attributed to the adsorption of the leaf extract components onto the steel surface. Phytochemical screening results also display that leaves extract of S. officinalis L oxygen and nitrogen atoms in functional groups (O–H, N–H, C–N, C–O) and aromatic ring, which meet the general consideration of typical corrosion inhibitors. In aqueous acidic solutions, the components of the plant parts extracts exist either as neutral molecules or in the form of cations (protonated species). In general, two modes of adsorption could be considered. The neutral species may adsorb on metal surface via the chemisorption mechanism, involving the displacement of water molecules from the metal surface and the sharing electrons between the N, O and C = C atoms and Fe. The S. officinalis L (SO) components can also adsorb on the surface of the metal based on donor–acceptor interactions between π-electrons of aromatic ring and vacant d-orbits of Fe. The large number of different chemical compounds in plant extracts of S. officinalis L (SO) may react with the iron, which is firstly dissolved from the metal surface, forming organo-metallic complex, such as Fe–plant extract [Fe–SO] according to the following mechanism (Abdel-Gaber et al., 2006):
4 Conclusion
Our work was devoted to the study of S. officinalis L leaves extracts and their antioxidant activity and corrosion inhibition. The quantitative and qualitative analysis of essential oil identified Trans-Thujone (17.74%), 1,8-cineol (12.63%), Camphor (12.24%), Caryophyllene (9.87% %), Dehydra-Aromadendrane (7.29%), Guaiol (7.03%) and Camphene (4.03%) as the major compounds. The phytochemical screening reveals the presence of tannins (Gallic and Catechic), flavonoids, sterols and terpenes. The dosage showed that the methanol extract is very rich in total phenols and flavonoids, their contents are on the order of 1.044 ± 0.004 mg GAE/g of extract and 0.037 ± 0.003 mg QE/g of extract. The essential oil of this plant showed a significant antioxidant capacity with an inhibitory concentration IC50 = 309.42 mg/ml. The anti-corrosive action of the essential oil and methanol extract of this plant reaches an efficiency of 83.78% and 91.62%.To evaluate the therapeutic properties of this plant, isolation and identification of the bioactive compounds responsible for these activities are necessary.
References
- Natural honey as corrosion inhibitor for metals and alloys. II. C-steel in high saline water. Corros. Sci.. 2000;42:731-738.
- [CrossRef] [Google Scholar]
- Inhibitive action of some plant extracts on the corrosion of steel in acidic media. Corros. Sci.. 2006;48:2765-2779.
- [CrossRef] [Google Scholar]
- Abdollahi, S.M., M., 2010. An Update on Pharmacology of Satureja Spacies; From Antioxidant, Antimicrobial, Antidiabetes and Anti-hyperlipidemic to Reproductive Stimulation.
- Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. J. Am. Soc. Mass Spectrom.. 2005;16:1902-1903.
- [CrossRef] [Google Scholar]
- Plants traditionally used in age related brain disorders — a survey of ethnobotanical literature. J. Ethnopharmacol.. 2007;113:363-381.
- [CrossRef] [Google Scholar]
- Inhibitory effect and adsorption parameters of extract of leaves of Portulaca oleracea of corrosion of aluminium in H2SO 4 solution. Arch. Appl. Sci. Res.. 2013;5:25-32.
- [Google Scholar]
- Cytotoxic and cytogenetic effects of salvia officinalis on different tumor cell lines. Middle East J. Int. Med.. 2013;6:15-25.
- [CrossRef] [Google Scholar]
- Extr. Ind. Crop. Prod.. 2017;105:1-9.
- [CrossRef]
- The in vitro antioxidative and cytotoxic effects of selected Salvia species water extracts. J. Appl. Bot. Food Qual.. 2015;88:115-119.
- [CrossRef] [Google Scholar]
- Evaluating the behavior of aluminum corrosion in hydrochloric acid in the presence aqueous extract of olive seeds evaluating the behavior of aluminum corrosion in hydrochloric acid in the presence aqueous extract of olive seeds. Int. J. Innov. Res. Sci. Eng. Technol.. 2016;4
- [CrossRef] [Google Scholar]
- Inhibition of carbon steel corrosion in 1 M HCl medium by potassium thiocyanate Inhibition of carbon steel corrosion in 1 M HCl medium by potassium thiocyanate. J. Assoc. Arab. Univ. Basic Appl. Sci. 2013
- [CrossRef] [Google Scholar]
- Free radicals, oxidative stress, and antioxidants in human health and disease. JAOCS. 1998;75:199-212.
- [Google Scholar]
- Atanasova, M., 2009. Revue de Génie Industriel Phénols et flavonoïdes totaux dans les extraits secs des feuilles des bouleaux argentés bulgares (Betula pendula). Rev. Génie Ind. 4, 21–25.
- Athamena, S., Chalghem, I., Kassah-Laouar, A., Khebri, S.L.E.S., 2010. Activite Anti-Oxydante Et Antimicrobienne D’Extraits De Cuminum Cyminum L. Leban. Sci. J. 11, 69–81.
- The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol. Pain. 2013;9 1744–8069–9–53
- [CrossRef] [Google Scholar]
- Antioxidant properties and inhibitory effects of Satureja khozestanica essential oil on LDL oxidation induced-CuSO(4) in vitro. Asian Pac. J. Trop. Biomed.. 2013;3:22-27.
- [CrossRef] [Google Scholar]
- Inhibition effect of natural artemisia oils towards tinplate corrosion in HCl solution: chemical characterization and electrochemical study. Int. J. Electrochem.. 2011;6:1454-1467.
- [Google Scholar]
- The inhibitive effect of hexamethylenetetramine on the acid corrosion of steel. Mater. Chem. Phys.. 2007;104:74-82.
- [CrossRef] [Google Scholar]
- Prickly pear seed oil extract: a novel green inhibitor for mild steel corrosion in 1 M HCl Solution. Int. J. Electrochem. Sci.. 2012;7:1303-1318.
- [Google Scholar]
- Essential oil constituents of salvia argentea l. from tunisia: phenological variations. Med. Aromat. Plant Sci. Biotechnol.. 2013;7:40-44.
- [Google Scholar]
- Phytochemical investigation of leaves and fruits extracts of Chamaerops humilis L. J. Mater. Environ. Sci. 2012;3:320-327.
- [Google Scholar]
- The substituted 1, 3, 4-oxadiazoles: a new class of corrosion inhibitors of mild steel in acidic media. Corros. Sci.. 2000;42:127-146.
- [Google Scholar]
- Analyse chimique, activités biostatiques et antioxydantes d’extraits de plantes aromatiques sélectionnées. Thèse présentée en vue l’obtention du grade Docteur en. Biochim. Inst. Natl. Polytech. Toulouse. Fr. 1993
- [Google Scholar]
- Etude pilote ouverte de l’effet antioxydant d'ambrotose AOTM sur des personnes en bonne santé. Gluco Sci. Nutr.. 2003;4:7.
- [Google Scholar]
- The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem.. 1984;176:275-295.
- [CrossRef] [Google Scholar]
- Satureja horvatii essential oil: in vitro antimicrobial and antiradical properties and in situ control of Listeria monocytogenes in pork meat. Meat Sci.. 2014;96:1355-1360.
- [CrossRef] [Google Scholar]
- Chemical composition, antioxidant and antimicrobial properties of Mentha pulegium, Lavandula stoechas and Satureja calamintha Scheele essential oils and an evaluation of their bactericidal effect in combined processes. Innov. Food Sci. Emerg. Technol.. 2014;22:221-229.
- [CrossRef] [Google Scholar]
- Corrosion Inhibition of C38 Steel in 1 M HCl: a comparative study of black pepper extract and its isolated piperine. Int. J. Electrochem.. 2010;5:1060-1069.
- [Google Scholar]
- Investigation of Piperanine as HCl Ecofriendly Corrosion Inhibitors for C38 Steel. Int. J. Electrochem.. 2012;7:2513-2522.
- [Google Scholar]
- Phytochemical analysis and biological activities of Cola nitida Bark. Biochem. Res. Int.. 2015;12 hindawi Publ. Coporation
- [Google Scholar]
- Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas y Aceites. 2009;60:405-412.
- [CrossRef] [Google Scholar]
- Effect of Athamanta sicula oil on inhibition of mild steel corrosion in 1M HCl. Der Pharma Chem.. 2015;7:103-111.
- [Google Scholar]
- Chemical composition and anti-Helicobacter pylori effect of Satureja bachtiarica Bunge essential oil. Phytomedicine. 2015;22:173-177.
- [CrossRef] [Google Scholar]
- Extraction et etudes des huiles essentielles de la salvia officinalis L. cuieillie dans deux régions differentes de la tunisie. J. Soc. Alger. chim. 2006;16:193-202.
- [Google Scholar]
- Foelkel, C., 2008. THE EUCALYPTUS AND THE LEGUMINOSAE Part 01: Acacia mearnsii. Eucalyptus Online B. Newsl. 1–87.
- Pharmacological perspectives from Brazilian Salvia officinalis (Lamiaceae): antioxidant, and antitumor in …. Ann. Braz. Acad. Sci. 2016
- [CrossRef] [Google Scholar]
- Seasonal variations of phenolic compounds and biological properties in sage (Salvia officinalis L.) Chem. Biodivers.. 2012;9:441-457.
- [CrossRef] [Google Scholar]
- Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med.. 2017;7:433-440.
- [CrossRef] [Google Scholar]
- Antimicrobial and antioxidant activities of essential oils of Satureja thymbra Growing Wild in Libya. Molecules. 2012;17:4836-4850.
- [CrossRef] [Google Scholar]
- Optimization of solvent extraction of antioxidants from Eucalyptus globulus leaves by response surface methodology: Characterization and assessment of their bioactive properties. Ind. Crops Prod.. 2017;108:649-659.
- [CrossRef] [Google Scholar]
- Essential oil characterization of Satureja rechingeri in Iran. Ind. Crops Prod.. 2014;61:403-409.
- [CrossRef] [Google Scholar]
- Natural products in drug discovery. Drug Discovery Today. 2008;13:894-901.
- [CrossRef] [Google Scholar]
- The chemistry behind antioxidant capacity assays. J. Agric. Food Chem.. 2005;53:1841-1856.
- [CrossRef] [Google Scholar]
- The corrosion inhibition and adsorption behavior of Uncaria gambir extract on mild steel in 1 M HCl. Mater. Chem. Phys.. 2011;125:461-468.
- [CrossRef] [Google Scholar]
- Phenol antioxidant index: comparative antioxidant effectiveness. J. Agric. Food Chem. 1995;43:401-403.
- [Google Scholar]
- Optimization of extraction, antioxidant activity and functional properties of quince seed mucilage by RSM. Int. J. Biol. Macromol.. 2014;66:113-124.
- [CrossRef] [Google Scholar]
- Essential oil constituents of Satureja sahendica Bornm. and Satureja hortensis L. cultivated in Iran. Int. J. Farming Allied Sci. 2014
- [Google Scholar]
- Bioactive compounds and antioxidant activities of thyme-enriched refined corn oil. J. Agric. Sci. Tech. 2016;18:79-91.
- [Google Scholar]
- Essential oil composition is related to the natural habitats: Coridothymus capitatus and Satureja thymbra in NATURA 2000 sites of Crete. Phytochemistry. 2005;66:2668-2673.
- [CrossRef] [Google Scholar]
- Chemical composition, antioxidant and antimicrobial properties of the essential oils of three Salvia species from Turkish flora. Bioresour. Technol.. 2008;99:4096-4104.
- [CrossRef] [Google Scholar]
- Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem.. 2013;136:120-129.
- [CrossRef] [Google Scholar]
- 2-Mercapto-1-methylimidazole as corrosion inhibitor for copper in hydrochloric acid. Appl. Surf. Sci.. 2006;253:1371-1378.
- [CrossRef] [Google Scholar]
- Dodonaea viscosa (L.) leaves extract as acid corrosion inhibitor for mild steel–a green approach. J. Mater. Environ. Sci.. 2013;4:625-638.
- [Google Scholar]
- Cardioprotective actions of grape polyphenols. Nutr. Res.. 2008;28:729-737.
- [CrossRef] [Google Scholar]
- Bioactivity of selected plant essential oils against Listeria monocytogenes. J. Appl. Microbiol.. 1997;82:759-762.
- [Google Scholar]
- D’ Évaluation Du Potentiel Antioxydant Dans Les Aliments. médicine Sci.. 2004;20:458-463.
- [Google Scholar]
- Plants-herbal wealth as a potential source of ayurvedic drugs. Distribution. 2009;4:152-170.
- [Google Scholar]
- Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem.. 2004;85:231-237.
- [CrossRef] [Google Scholar]
- Flavonoid effects on DNA oxidation at low concentrations relevant to physiological levels. Food Chem. Toxicol.. 2008;46:96-98.
- [CrossRef] [Google Scholar]
- Nacéra, B., 2009. Etude phytochimiq ue, activités antimicrobiennes et antioxydantes de Saccocalyx sarureioïdes, Salvia verbenaca et Teucriumpolium de la région Ouest d’Algérie. thése.
- Food science and technology international review: methods used to evaluate the free radical scavenging. Food Sci. Technol. Int.. 2002;8:121-137.
- [CrossRef] [Google Scholar]
- Essential oil composition, antimicrobial and antioxidant activities of Satureja cuneifolia Ten. Food Chem.. 2009;112:874-879.
- [CrossRef] [Google Scholar]
- Profiling of in vitro neurobiological effects and phenolic acids of selected endemic Salvia species. Food Chem.. 2012;132:1360-1367.
- [CrossRef] [Google Scholar]
- Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: a comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, ??-d-Glucose and Tannic acid) Corros. Sci.. 2009;51:1935-1949.
- [CrossRef] [Google Scholar]
- Chemical composition and inhibitory effect of essential oil of Lavande (Lavandula Dentata) LD on the Corrosion of mild steel in hydrochloric acid (1M) Arab. J. Chem. Environ. Res.. 2015;1:49-65.
- [Google Scholar]
- Chemical composition and antimicrobial and antioxidant activities of the essential oil and methanol extract of Hippomarathrum microcarpum (Bieb.) from Turkey. J. Agric. Food Chem. 2007
- [CrossRef] [Google Scholar]
- Anticholinesterase and antioxidant activities of Savoury (Satureja thymbra L.) with identified major terpenes of the essential oil. Food Chem.. 2012;134:48-54.
- [CrossRef] [Google Scholar]
- Inhibition of Al corrosion in 0.5MHCl solution by Areca flower extract. J. King Saud Univ. Eng. Sci. 2017
- [CrossRef] [Google Scholar]
- Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur. J. Pharmacol.. 2006;545:51-64.
- [CrossRef] [Google Scholar]
- Green Corrosion inhibitor from essential oil of eucalyptus globulus (Myrtaceae) for C38 steel in sulfuric acid solution. J. Mater. Environ. Sci. 2012;3:613-627.
- [Google Scholar]
- Antinociceptive and anti-inflammatory potential of extract and isolated compounds from the leaves of Salvia officinalis in mice. J. Ethnopharmacol.. 2012;139:519-526.
- [CrossRef] [Google Scholar]
- In vitro evaluation of antibacterial and antioxidant activities of the essential oil and methanol extract of endemic Zataria multiflora Boiss. Food Control. 2007;18:800-805.
- [CrossRef] [Google Scholar]
- Inhibition effect of environmentally benign Karanj (Pongamia pinnata) seed extract on corrosion of mild steel in hydrochloric acid solution. J. Solide State Electrochem.. 2011;15:1087-1097.
- [CrossRef] [Google Scholar]
- Colorimetryof total phenolics with phosphomolybdic-phosphotungstic acid reagent. Am. J. Encol. Viticul. 1965;16:144-158.
- [Google Scholar]
- Adsorption and corrosion inhibition effect of 1, 1 thiocarbonyldiimidazole on mild steel in H2SO4 solution and synergistic effect of iodide ion. Acta Phys. Chim. Sin.. 2008;24:1185-1191.
- [Google Scholar]
- Green approach to corrosion inhibition of 304 stainless steel in hydrochloric acid solution by the extract of Salvia officinalis leaves. Corros. Sci.. 2012;62:122-135.
- [CrossRef] [Google Scholar]
- Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek …. Food Chem. Toxicol.. 2012;50:4115-4124.
- [CrossRef] [Google Scholar]
- Genome-based approaches to the authentication of medicinal plants. Planta Med.. 2008;74:603-623.
- [CrossRef] [Google Scholar]
- Polypropylene glycol: a novel corrosion inhibitor for ×60 pipeline steel in 15% HCl solution. J. Mol. Liq.. 2016;219:946-958.
- [CrossRef] [Google Scholar]
- Biopesticides from natural products: current development, legislative framework, and future trends. BioResource. 2016;11:5618-5640.
- [Google Scholar]
- Study of anticancer activities of muscadine grape phenolics in vitro. J. Agric. Food Chem. 2005;53:8804-8812.
- [Google Scholar]
- Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J. Agric. Food Chem.. 2003;51:7292-7295.
- [Google Scholar]
- Investigation of the inhibition effect of N-1-Naphthylethylenediamine dihydrochloride monomethanolate on the C38 steel corrosion in 0.5M H 2SO 4. Der Pharma Chem.. 2012;4:407-416.
- [Google Scholar]
- Chemical composition and anticorrosive activity of Warionia Saharea essential oil against the corrosion of mild steel in 0.5 M H2SO4. Int. J. Electrochem.. 2011;6:5940-5955.
- [Google Scholar]







